The metalloprotease ADAMTS13 (a disintegrin and metalloprotease with thrombospondin motif) converts the hyperreactive unusually large (UL) forms of von Willebrand factor (VWF) that are newly released from endothelial cells into less active plasma forms by cleaving a peptide bond in the VWF A2 domain. Familial or acquired deficiency of this metalloprotease is associated with thrombotic thrombocytopenic purpura (TTP). ADAMTS13 belongs to the ADAMTS metalloprotease family, but, unlike other members, it also contains 2 C-terminal CUB domains (complement component Clr/Cls, Uegf, and bone morphogenic protein 1). Mutations in the CUB region have been found in congenital TTP, but deletion of the region did not impair enzyme activity in conventional in vitro assays. We investigated the functions of the CUB domain in ADAMTS13 activity under flow conditions. We found that recombinant CUB-1 and CUB-1+2 polypeptides and synthetic peptides derived from CUB-1 partially blocked the cleavage of ULVWF by ADAMTS13 on the surface of endothelial cells under flow. The polypeptide bound immobilized and soluble forms of ULVWF, and blocked the adhesion of ADAMTS13-coated beads to immobilized ULVWF under flow. These results suggest that the CUB-1 domain may serve as the docking site for ADAMTS13 to bind ULVWF under flow, a critical step to initiate ULVWF proteolysis.
Introduction
In response to stimulation, vascular endothelial cells release unusually large (UL) and hyperactive von Willebrand factor (VWF) multimers1,2 that spontaneously bind the platelet glycoprotein (GP) Ib-IX-V complex in the absence of any modulators or high fluid shear stress.3,4 Because of this hyperactivity, ULVWF multimers released into the bloodstream spontaneously aggregate platelets and are responsible for the systemic microvascular thrombosis seen in patients with thrombotic thrombocytopenic purpura (TTP). To prevent such disastrous consequences, ULVWF multimers undergo limited proteolysis immediately upon release from endothelial cells by the zinc- and calcium-dependent metalloprotease ADAMTS13 (a disintegrin and metalloprotease with thrombospondin motif).5-7 ADAMTS13 cleaves VWF at a single peptide bond between Y842 and M843 in the VWF A2 domain, generating 176-kDa and 140-kDa fragments detectable on reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels.8-10 ADAMTS13 deficiency has been demonstrated in patients with TTP,11 caused either by mutations in the ADAMTS13 gene6,12-15 or by acquired antibody inhibitors of the metalloprotease.16-19
ADAMTS13 has a domain structure similar to that of other family members, with the exception that it contains unique domains at its C-terminus, known as CUB domains.5 “CUB” is an acronym for the first 3 proteins described that contain this domain: complement components C1r/C1s, Uegf (sea urchin fibropellins), and bone morphogenic protein 1 (Bmp1). CUB domains have been found in functionally diverse proteins from prokaryotes to humans and are known to function primarily in protein-protein interactions.20-22 Two individual mutations within the CUB domains of ADAMTS13 have been identified in association with congenital TTP,6,13 suggesting that this domain is critical for the biosynthesis or activity of the metalloprotease. A potential functional role for the ADAMTS13 CUB domains is also suggested by a study showing that 64% of plasma samples from patients with acquired TTP contain antibodies against the CUB domains (all of them also contain antibodies against the Cys-rich and Spacer domains).23 Nevertheless, the precise roles of the CUB domains in ADAMTS13 cleavage of ULVWF remain unknown, particularly in the conditions found in flowing blood.
Using a parallel-plate flow chamber system, we previously showed that in the absence of ADAMTS13, ULVWF multimers secreted from histamine-stimulated endothelial cells are anchored to the cell surface to form extraordinary long stringlike structures that spontaneously bind platelets.4,24 Perfusion of plasma or recombinant ADAMTS13 over the endothelial cells rapidly cleaves these ULVWF strings under physiologic flow, suggesting that the cleavage of newly released ULVWF in vivo occurs predominantly on the endothelial surface. These anchored ULVWF strings containing adherent platelets are exposed to tensile stress applied by the flowing blood and are stretched to expose sites for ADAMTS13 docking (VWF A1 and A3 domains) and cleavage (the VWF A2 domain).25 It is still unknown, however, which domains or sequences within ADAMTS13 attach the enzyme from the blood to the relatively stationary ULVWF strings. We hypothesize that it is the CUB domains, alone or in combination with other domains, that carry out this docking function. Here, we examined this hypothesis and found that the CUB domains, in particular CUB-1, bind ULVWF under both static conditions and under flow.
Materials and methods
Platelet and plasma preparations
Freshly drawn blood from 20 healthy donors (12 women and 8 men; age range, 24-46 years) was used as the source of plasma and platelets under a protocol approved by the institutional review board of the Baylor College of Medicine. All donors signed informed consent before blood was drawn. Washed platelets were obtained from blood in 10% acid-citrate dextrose buffer (ACD; 85 mM sodium citrate, 111 mM glucose, and 71 mM citric acid) by centrifuging the blood first at 150g for 15 minutes at 24°C to obtain platelet-rich plasma (PRP), and then centrifuging the PRP at 900g for 10 minutes to collect platelets. The platelets were washed once with a CGS buffer (13 mM sodium citrate, 30 mM glucose, and 120 mM sodium chloride, pH 7.0) and resuspended in Ca++ and Mg++-free Tyrode buffer (138 mM sodium chloride, 5.5 mM glucose, 12 mM sodium bicarbonate, 2.9 mM potassium chloride, and 0.36 mM dibasic sodium phosphate, pH 7.4).
As the source of ADAMTS13, plasma was obtained from blood anticoagulated with d-Phe-Pro-Arg-chloromethylketone, HCl (PPACK; 75 μM final concentration; Calbiochem, La Jolla, CA).
Expression of recombinant ADAMTS13 and isolated CUB domains
ADAMTS13 cDNA7 was subcloned into the mammalian expression vector pSectag (Invitrogen, Carlsbad, CA) that carries the C-terminal His- and Myc-tag for purification and detection. The cDNA was introduced to Hela cells, which contain endogenous furin to cleave propeptide and release the mature metalloprotease,26,27 by liposome-mediated DNA transfer (Lipofectamine TM 2000; Invitrogen). Transfected cells were maintained in Dulbecco-modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 mM l-glutamine at 37°C with 95% air and 5% CO2. Cells expressing the recombinant ADAMTS13 (rADAMTS13) were first selected in medium containing hygromycin-B (400 μg/mL) and cells expressing high levels of rADAMTS13 were then selected by single-cell cloning. Recombinant ADAMTS13 was collected from the supernatant of confluent cells grown in serum-free medium (Opti-Pro SFM; Invitrogen) and purified through the C-terminal His-tag using a Y-per 6xHis Fusion Protein Purification Kit (Pierce Chemicals, Rockford, IL).
To express the recombinant CUB domains, DNA fragments encoding CUB-1 (A1191 to E1298), CUB-2 (C1299 to T1427), and CUB-1+2 (A1191 to T1427) were amplified from the ADAMTS13 cDNA by polymerase chain reaction. To preserve structural integrity and remove the vector sequence (there are more than 30 nucleotides between the vector-derived signal cleavage site and the start of CUB-1 sequence), we have included a furin-cleavage site sequence RQRR to the N-terminus of each fragment. When the constructs are expressed in Hela cells, which express furin, the CUB-1 polypeptide can be generated without the foreign sequence. The CUB-1 and CUB-1+2 fragments were amplified using the same forward primer, ATGCAAGCTTCAGGCAGAGGAGGGCCTGTGGCAGGCAGC, and the reverse primers ATGCCTCGAGCTTCTCTGTAGAAGGTTTCAGG and ATGCCTCGAGCGGTTCCTTCCTTTCCCTTCC, respectively. The CUB-2 was amplified with forward ATGCAAGCTTCAGGCAGAGGAGGGAATGTGACATGCAGCTC and reverse ATGCCTCGAGCGGTTCCTTCCTTTCCCTTCC primers. The amplified fragments were digested with HindIII and Xho 1, subcloned into the mammalian expression vector pSecTag2, and expressed in Hela cells as described for the wild-type rADAMTS13. The recombinant CUB polypeptides were again purified using a Y-per 6xHis Fusion Protein Purification Kit (Pierce Chemicals).
Synthetic CUB peptides
Five peptides derived from overlapping sequences of the CUB-1 domain (Table 1) were made by SigmaGenosys (Woodlands, TX). These peptides were chosen because their sequences are least homologous to that of CUB-2. Three control peptides were also synthesized: a scrambled peptide with the same amino acid composition as the peptide CUB-1A, and 2 peptides from the catalytic domain of ADAMTS13, which did not interfere with cleavage of ULVWF (data not shown). The peptides were dissolved in phosphate-buffered saline (PBS) to a stock concentration of 2 mM and stored at -20°C as small aliquots until use.
Synthetic peptides used in the studies
Name . | Sequence . | Region . |
|---|---|---|
| CUB-1A | HLEPTGT | CUB-1 domain |
| CUB-1As | PELHTTG | Scrambled CUB-1A sequence |
| CUB-1B | GTIDMRG | CUB-1 domain |
| CUB-1C | GPGQADCA | CUB-1 domain |
| CUB-1D | AVAIGRP | CUB-1 domain |
| CUB-1E | APETFYRE | CUB-1 domain |
| MET1 | GHSFGLEH | Catalytic domain |
| MET2 | GILHLE | Catalytic domain |
Name . | Sequence . | Region . |
|---|---|---|
| CUB-1A | HLEPTGT | CUB-1 domain |
| CUB-1As | PELHTTG | Scrambled CUB-1A sequence |
| CUB-1B | GTIDMRG | CUB-1 domain |
| CUB-1C | GPGQADCA | CUB-1 domain |
| CUB-1D | AVAIGRP | CUB-1 domain |
| CUB-1E | APETFYRE | CUB-1 domain |
| MET1 | GHSFGLEH | Catalytic domain |
| MET2 | GILHLE | Catalytic domain |
Endothelial culture
Human umbilical vein endothelial cells (HUVECs) were obtained under a protocol approved by the institutional review board of the Baylor College of Medicine. The umbilical cords were washed with phosphate buffer and then incubated with 0.02% of collagenase (Invitrogen) for 30 minutes at room temperature. Endothelial cells were collected by centrifuging the elutes at 250g for 10 minutes, then plated in a culture dish coated with 1% gelatin and grown in Medium 199 (Invitrogen) containing 20% heat-inactivated fetal bovine serum and 0.2 mM l-glutamine.4 Before use, the cells were stimulated with 25 μM histamine (Sigma-Aldrich, Saint Louis, MO) for 10 minutes at room temperature.
HUVECs were also used to produce ULVWF.28-30 For this, the confluent HUVECs were first incubated with a serum-free M199 medium containing 5 μg/mL to 10 μg/mL insulin, 5 μg/mL transferrin, and 1% glutamine for 48 to 72 hours, and then treated with 100 μM histamine for 30 minutes at 37°C to induce the release of ULVWF. The conditioned medium was collected and centrifuged at 350g for 10 minutes to remove cell debris and the supernatant was used as the source of ULVWF. The multimeric composition of ULVWF from this preparation was evaluated by 1% SDS agarose gel electrophoresis and immunoblotting using a polyclonal VWF antibody (DakoCytomation, Carpinteria, CA).
Parallel-plate flow chamber
Culture dishes (35 mm) containing confluent monolayes of HUVECs served as the bottom of the chamber during assembly of the parallel-plate flow chamber. When necessary, HUVECs were stimulated with histamine before assembly of the chamber. The chamber was connected to a syringe pump to draw Tyrode buffer containing washed platelets through the chamber at a flow rate of 0.2 mL/min, which generates a wall shear stress of 2.5 dyne/cm2 for buffer or PRP (viscosity up to 1 centipoise). ULVWF strings were quantitated by counting the number of strings in 20 continuous viewfields (× 400) following a 2-minute perfusion of platelet suspension.
Cleavage of ULVWF in the absence of flow
ADAMTS13 activity was measured under static conditions using a method31 modified from that of Furlan et al,8 with ULVWF multimers as the substrate. Briefly, normal plasma was diluted (1:5) with low-ionic-strength Tris-saline buffer and ADAMTS13 was then activated with 1 mM BaCl2 for 5 minutes. The plasma was then mixed with ULVWF, dialyzed against 1.5 M urea for 24 hours at 37°C, and then subjected to electrophoresis on a 1% nonreducing SDS agarose gel. The separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane and VWF multimers detected by Western blot using a polyclonal anti-VWF antibody (DakoCytomation) and chemiluminescence. We measured the amount of peptides recovered after a 24-hour dialysis and found it to be 90% and 40% for the recombinant CUB-1 and synthetic CUB-1A peptide, respectively (data not shown).
ULVWF-CUB interaction
Interactions of ULVWF with wild-type ADAMTS13, CUB domains, or a truncation mutant that lacks the sequence C-terminal to the Spacer domain (TSP-2)32 were measured in the absence and presence of flow. For the static enzyme-linked immunosorbent assay (ELISA), the recombinant ADAMTS13, CUB polypeptides (200 nM coating concentration), and the truncation mutant were immobilized onto the wells of microtiter plates (2 hours, room temperature). The coated plates were incubated with 5% bovine serum albumin (BSA) for 60 minutes to block nonspecific sites, washed with phosphate-buffered saline (PBS), and then incubated with ULVWF for an additional 30 minutes at room temperature. After washing the wells with PBS, bound ULVWF was detected using a polyclonal VWF antibody (DakoCytomation) and chemiluminescence. The ADAMTS13-ULVWF interaction was also examined in the presence of an antibody against the ADAMTS13 CUB domains (10 μg/mL; Bethyl Laboratory, Houston, TX). This antibody was generated using, as immunogen, a synthetic peptide that contains the sequence of 3 peptides used in the blocking studies (peptide CUB-1A-C).
To examine the interaction of ADAMTS13 with ULVWF under flow conditions, ADAMTS13-coated polystyrene beads (0.5 μm in diameter; Polysciences, Warrington, PA) were perfused over immobilized ULVWF at a shear stress of 2.5 dyne/cm2 and the adherent beads in 10 random review fields (× 400) were counted after 5 minutes of perfusion.25
Protein elution assay
ULVWF was incubated with either biotinylated synthetic CUB-1A peptide or a scrambled peptide of the same length and amino acid composition for 15 minutes at room temperature and the mixture was then passed through a gel filtration column with a molecular weight cut-off at 5000 (PD-10 column; Amersham Biosciences, Piscataway, NJ). Because of its very high molecular weight, ULVWF was normally eluted from the void volume at early fractions (fractions 3-7), whereas the peptides, which have a molecular weight of less than 1000, eluted at later fractions (after fraction 8). However, if the peptide formed a complex with ULVWF, it would appear in the ULVWF fraction. Fourteen fractions of 1 mL each were collected and 200 μL of each fraction was applied to a nitrocellulose membrane by dot blot and the membrane was then probed for ULVWF and the CUB-1A peptides using a polyclonal anti-VWF antibody (DakoCytomation) and a monoclonal antibiotin antibody (Pierce Biotechnology), respectively.
Statistical analysis
All experimental data are presented as the mean plus or minus the standard error of the mean (SEM). The unpaired 2-tailed Student t test was used for data analysis. Statistical significance was defined as a P value of less than .05.
Results
Recombinant CUB-1, but not CUB-2, blocked the cleavage of ULVWF strings by ADAMTS13 under flow
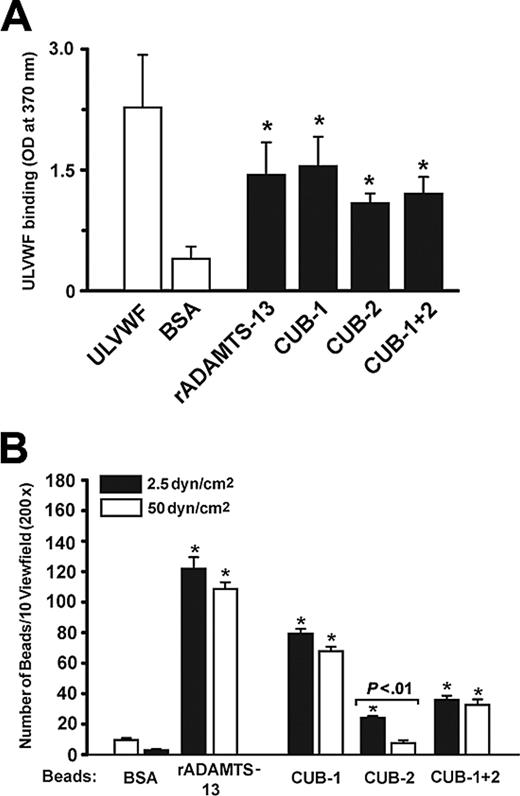
We previously demonstrated that washed platelets in buffer perfused over stimulated HUVECs form stringlike structures on newly released ULVWF multimers.4 No such structures formed if the platelets were suspended in plasma, a source of ADAMTS13. Using this system, we examined the role of the ADAMTS13 CUB domains in ULVWF cleavage by incubating PRP with recombinant CUB-1, CUB-2, or CUB-1+2 polypeptides before perfusion over histamine-stimulated HUVECs (2.5 dyne/cm2 shear stress). The CUB-1 and CUB-1+2 polypeptides inhibited string cleavage by 72.4% ± 11.6% and 39.8% ± 8.4%, respectively, whereas the isolated CUB-2 did not (Figure 1A). Inhibition was dose-dependent, with maximal inhibition at 100 nM of the recombinant CUB-1 polypeptides (Figure 1B).
Consistent with this observation, we found that 5 synthetic peptides derived from the CUB-1 domain (CUB-1 peptides A through E, each at 200 μM) also partially inhibited ULVWF cleavage by ADAMTS13, whereas cleavage was not inhibited by a scrambled peptide for CUB-1A or either of 2 peptides from the catalytic domain (Figure 2A). As with the recombinant CUB polypeptides, CUB-1A inhibition was partial, even up to a concentration of 1 mM (Figure 2B).
Recombinant CUB polypeptides did not block ULVWF cleavage under static conditions
Compared with the results obtained under flow, ULVWF proteolysis was not inhibited by any of the recombinant CUB polypeptides or synthetic peptides in a static assay of ADAMTS13 activity, using ULVWF as the substrate (Figure 3). This finding is consistent with previous studies that the ADAMTS13 CUB domains are dispensable for cleaving plasma VWF under static conditions.26,33
ULVWF interacts with CUB polypeptides under static conditions
The observation that recombinant CUB-1 blocked the cleavage of ULVWF under flow, but not under static conditions, suggests that the CUB-1 domain may dock ADAMTS13 to ULVWF in flowing blood. We therefore examined the interaction of ULVWF with ADAMTS13 or CUB polypeptides under static and flow conditions. Using ELISA, we found that ULVWF bound wild-type ADAMTS13 and each of the 3 recombinant CUB polypeptides (Figure 4A). Of interest, ULVWF bound the CUB-2 polypeptide to a similar extent as it bound wild-type ADAMTS13, CUB-1, and CUB-1+2, even though CUB-2 did not inhibit ULVWF cleavage under flow. One possible explanation for the observation is that the CUB-2–ULVWF interaction is too weak to withstand fluid shear stress. To test this possibility, we examined the adhesion of polystyrene beads coated with either wild-type ADAMTS13 or with each of the CUB polypeptides to immobilized ULVWF under flow. Because the beads were unable to attach directly to ULVWF from the flowing buffer, we allowed the CUB-coated beads to settle onto the surface of immobilized ULVWF for 3 minutes before beginning the perfusion. Buffer was then perfused over the bead-covered surface for 2 minutes (shear stress 2.5 dyne/cm2) and the beads remaining adherent after this interval were counted. The hierarchy of bead binding was as follows: ADAMTS13 more than CUB-1 more than CUB-1+2 more than CUB-2. The difference between the binding of CUB-1 beads and CUB-2 beads was statistically significant (Student t test, n = 10, P < .01; Figure 4B, black bars). We then determined the detachment of the adherent beads after a 2-minute perfusion under a high shear stress of 50 dyn/cm2. The numbers of adherent beads coated with ADAMTS13, CUB-1, or CUB-1+2 remained relatively unchanged, whereas more than 65% of CUB-2–coated beads were detached (Figure 4B, white bars). Thus, the bonds formed by CUB-1 were stronger and more shear resistant than those formed by CUB-2. The data also indicate that the presence of CUB-2 in the CUB-1+2 polypeptide attenuates the interaction with ULVWF.
Recombinant CUB-1 polypeptide inhibits UVLWF cleavage under flow conditions. (A) Recombinant CUB polypeptides (100 nM) were incubated with PRP for 10 minutes and then perfused over histamine-stimulated HUVECs at 2.5 dyn/cm2 of shear stress. ULVWF strings formed when buffer was perfused (0% activity), but were cleaved when PRP was perfused (100% activity). The ULVWF strings were detected in PRP that was pretreated with CUB-1 or CUB-1+2, but not when pretreated with CUB-2. (B) The inhibition of ULVWF cleavage by CUB-1 polypeptide was dose-dependent with maximal inhibition at 100 nM. The data are mean ± SEM (Student t test, *P < .01 compared with untreated samples).
Recombinant CUB-1 polypeptide inhibits UVLWF cleavage under flow conditions. (A) Recombinant CUB polypeptides (100 nM) were incubated with PRP for 10 minutes and then perfused over histamine-stimulated HUVECs at 2.5 dyn/cm2 of shear stress. ULVWF strings formed when buffer was perfused (0% activity), but were cleaved when PRP was perfused (100% activity). The ULVWF strings were detected in PRP that was pretreated with CUB-1 or CUB-1+2, but not when pretreated with CUB-2. (B) The inhibition of ULVWF cleavage by CUB-1 polypeptide was dose-dependent with maximal inhibition at 100 nM. The data are mean ± SEM (Student t test, *P < .01 compared with untreated samples).
Synthetic CUB-1 peptides block the cleavage of ULVWF under flow. (A) Each of the synthetic peptides (final concentration 200 μm) was incubated with PRP for 10 minutes at room temperature and the mixture was then perfused over the histamine-stimulated endothelial cells. All of the peptides corresponding to CUB sequences (CUB-1A to E) partially inhibited the cleavage of ULVWF strings, but the scrambled peptide CUB-1As did not. Two peptides corresponding to sequences in the catalytic domain (Met1 and Met2) also did not inhibit cleavage. (B) Inhibition of proteolysis by recombinant CUB-1 was dose-dependent with a maximal effect at 200 μM. The data are presented as mean ± SEM (Student t test, *P < .01 compared with untreated samples).
Synthetic CUB-1 peptides block the cleavage of ULVWF under flow. (A) Each of the synthetic peptides (final concentration 200 μm) was incubated with PRP for 10 minutes at room temperature and the mixture was then perfused over the histamine-stimulated endothelial cells. All of the peptides corresponding to CUB sequences (CUB-1A to E) partially inhibited the cleavage of ULVWF strings, but the scrambled peptide CUB-1As did not. Two peptides corresponding to sequences in the catalytic domain (Met1 and Met2) also did not inhibit cleavage. (B) Inhibition of proteolysis by recombinant CUB-1 was dose-dependent with a maximal effect at 200 μM. The data are presented as mean ± SEM (Student t test, *P < .01 compared with untreated samples).
Neither recombinant CUB domains nor a CUB-1 synthetic peptide inhibit the cleavage of ULVWF under static conditions. ULVWF was incubated with barium-treated plasma in the presence of recombinant polypeptides (CUB-1, CUB-2, and CUB-1+2) or synthetic peptide (CUB1A) or its scrambled form (CUB1As) for 24 hours in the presence of 1.5 M urea. The cleavage of ULVWF was then assessed by 1% agarose gel electrophoresis and immunoblotting. Plasma ADAMTS13 cleaved the ULVWF multimers and the cleavage was not affected by pretreatment of plasma with any of the CUB polypeptides or synthetic peptides. The figure is representative of 3 separate experiments.
Neither recombinant CUB domains nor a CUB-1 synthetic peptide inhibit the cleavage of ULVWF under static conditions. ULVWF was incubated with barium-treated plasma in the presence of recombinant polypeptides (CUB-1, CUB-2, and CUB-1+2) or synthetic peptide (CUB1A) or its scrambled form (CUB1As) for 24 hours in the presence of 1.5 M urea. The cleavage of ULVWF was then assessed by 1% agarose gel electrophoresis and immunoblotting. Plasma ADAMTS13 cleaved the ULVWF multimers and the cleavage was not affected by pretreatment of plasma with any of the CUB polypeptides or synthetic peptides. The figure is representative of 3 separate experiments.
ULVWF binds recombinant CUB polypeptides under static and flow conditions. (A) Static assay: ULVWF was incubated with immobilized wild-type ADAMTS13 or CUB domain polypeptides for 30 minutes and bound ULVWF was detected using a polyclonal VWF antibody. The data are expressed as mean ± SEM (Student t test, n = 5, *P < .001 for each peptide compared with BSA). (B) Flow assay: Polystyrene beads coated with ADAMTS13 or CUB polypeptides were allowed to settle on immobilized ULVWF and then perfused with buffer for 2 minutes. The beads remaining adherent were counted (▪). The wall shear stress was then increased to 50 dyn/cm2 for 2 minutes perfusion and the beads that remained were again counted (□). The data are mean ± SEM of beads bound in 10 random × 400 review fields (Student t test, n = 4, *P < .001 compared with BSA beads).
ULVWF binds recombinant CUB polypeptides under static and flow conditions. (A) Static assay: ULVWF was incubated with immobilized wild-type ADAMTS13 or CUB domain polypeptides for 30 minutes and bound ULVWF was detected using a polyclonal VWF antibody. The data are expressed as mean ± SEM (Student t test, n = 5, *P < .001 for each peptide compared with BSA). (B) Flow assay: Polystyrene beads coated with ADAMTS13 or CUB polypeptides were allowed to settle on immobilized ULVWF and then perfused with buffer for 2 minutes. The beads remaining adherent were counted (▪). The wall shear stress was then increased to 50 dyn/cm2 for 2 minutes perfusion and the beads that remained were again counted (□). The data are mean ± SEM of beads bound in 10 random × 400 review fields (Student t test, n = 4, *P < .001 compared with BSA beads).
Because immobilization of either ULVWF or CUB-1 onto a solid surface could artifactually influence their interaction, we also examined the interaction of polypeptides in solution by gel filtration. Based on their vastly different molecular masses, the CUB-1A synthetic peptide and ULVWF should elute in widely separated fractions in a gel filtration column if added together to the column. However, when mixed and applied to the column, both eluted in the same fractions, indicating that they bind each other (Figure 5). By contrast, when ULVWF was mixed with the scrambled CUB-1A peptide, the peptide eluted alone in the later fractions.
Recombinant CUB-1 polypeptide and synthetic peptides block adhesion of ADAMTS13 beads to immobilized ULVWF under flow
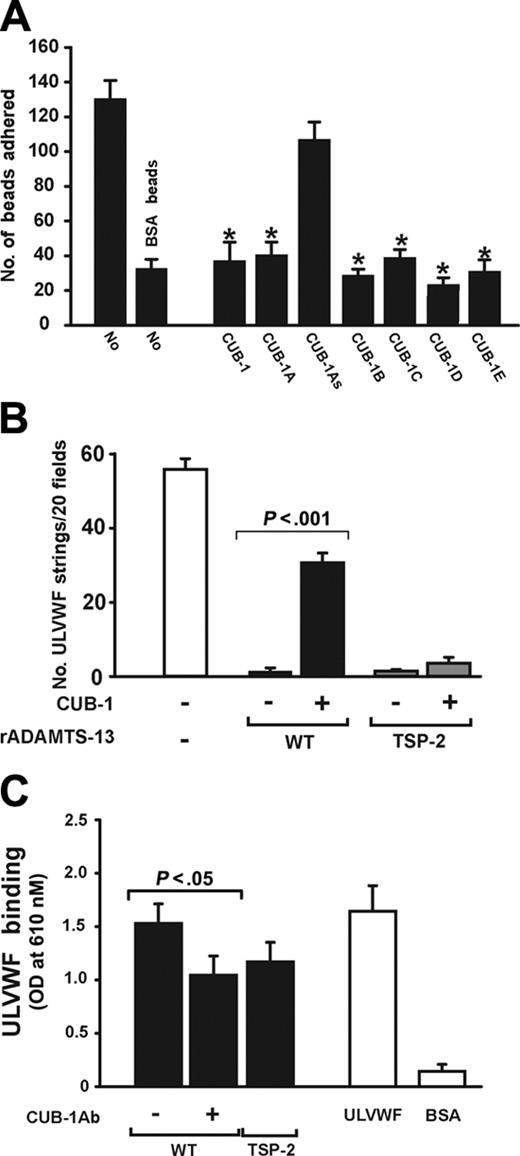
We next investigated whether the recombinant CUB-1 polypeptide and CUB-1–derived synthetic peptides could interfere with the attachment of ADAMTS13-coated beads to ULVWF under flow. The beads were incubated with recombinant CUB-1 or each of the synthetic peptides for 3 minutes before being allowed to settle on the ULVWF surface. After the chamber was perfused with buffer (containing the polypeptide or peptide) for 2 minutes (shear stress 2.5 dyn/cm2) the adherent beads were counted. In the absence of peptides, 131 ± 18 adherent ADAMTS13 beads remained per ×400 view (Figure 6A). Adhesion was significantly reduced in the presence of either the synthetic CUB-1 peptides (A-E) or the recombinant CUB-1 polypeptides, but not in the presence of the scrambled synthetic peptide.
ULVWF interaction with C-terminal truncated ADAMTS13
The results presented thus far suggest that CUB-1 binds ULVWF to dock the ADAMTS13 to the substrate and facilitate its proteolysis in flowing blood. If this were the only mechanism for ADAMTS13 attachment to ULVWF, one would expect that high concentrations of the polypeptide would be capable of completely inhibiting both ULVWF proteolysis and ADAMTS13 bead attachment to ULVWF. In both cases, inhibition appears to reach a plateau. Furthermore, the inhibition data also appear to be in conflict with our earlier observation that a truncated mutant ADAMTS13 that lacks the sequence C-terminal to the Spacer domain (TSP-2) also cleaves ULVWF strings under flow.32 One explanation that could resolve these apparent discrepancies is that ADAMTS13 contains a second binding site for ULVWF that is normally cryptic and can be exposed by truncation of C-terminal sequences. We tested this possibility by perfusing washed platelet suspensions containing either wild-type ADAMTS13 or TSP-2 mutant over histamine-stimulated endothelial cells and assessing for ULVWF string formation in the presence or absence of recombinant CUB-1 (200 nM). In the absence of CUB-1, both polypeptides completely cleaved ULVWF strings at a shear stress of 2.5 dyn/cm2. CUB-1 partially inhibited the cleavage of ULVWF strings by wild-type ADAMTS13, but had no effect on the activity of the TSP-2 mutant (Figure 6B). Consistent with the functional data, we also detected binding of ULVWF to TSP-2 by ELISA (Figure 6C).
The CUB-1A peptide coelutes with ULVWF from a gel filtration column. Biotinylated CUB-1A peptide and CUB-1As were incubated with UVLWF for 15 minutes and then allowed to pass through a gel filtration column at a flow rate of 1 mL/min. The eluate was collected in 14 fractions of 1 mL each and probed for the peptide with a polyclonal antibiotin antibody and for ULVWF with a monoclonal VWF antibody. The CUB-1A peptide coeluted with ULVWF (A), whereas the CUB-1As peptide did not (B). The figure is representative of 3 separate experiments.
The CUB-1A peptide coelutes with ULVWF from a gel filtration column. Biotinylated CUB-1A peptide and CUB-1As were incubated with UVLWF for 15 minutes and then allowed to pass through a gel filtration column at a flow rate of 1 mL/min. The eluate was collected in 14 fractions of 1 mL each and probed for the peptide with a polyclonal antibiotin antibody and for ULVWF with a monoclonal VWF antibody. The CUB-1A peptide coeluted with ULVWF (A), whereas the CUB-1As peptide did not (B). The figure is representative of 3 separate experiments.
CUB-1 peptides blocked ADAMTS13 binding to immobilized ULVW. (A) Beads coated with ADAMTS13 were perfused over immobilized ULVWF in the presence or absence of recombinant CUB-1 polypeptide (CUB-1, 100 nM) or synthetic peptides (CUB-1A-E, 200 μM). ADAMTS13 beads adhered to immobilized ULVWF and the adhesion was blocked by the recombinant CUB-1 polypeptide and CUB-1A-E peptides, but not by the CUB-1As polypeptide (Student t test, n = 4, *P < .01 compared with untreated ADAMTS13 beads). (B) Washed platelets were perfused over histamine-activated HUVECs in the presence of either wild-type ADAMTS13 or the truncation mutant TSP-2. The numbers of strings detected after 2 minutes of perfusion were counted. CUB-1 polypeptide partially blocked the cleavage of ULVWF by wild-type ADAMTS13 as demonstrated by the increased numbers of ULVWF strings, but not by the TSP-2 truncation mutant (Student t test, n = 4). (C) Binding of ULVWF to wild-type ADAMTS13 or TSP-2 mutant was measured by ELISA. ULVWF bound wild-type ADAMTS13 and TSP-2 mutant. The binding to wild-type ADAMTS13 can be partially blocked by an anti-ADAMTS13 antibody (10 μg/mL) generated using as an immunogen a synthetic peptide from the CUB-1 domain (Student t test, n = 7). All data are expressed as mean ± SEM.
CUB-1 peptides blocked ADAMTS13 binding to immobilized ULVW. (A) Beads coated with ADAMTS13 were perfused over immobilized ULVWF in the presence or absence of recombinant CUB-1 polypeptide (CUB-1, 100 nM) or synthetic peptides (CUB-1A-E, 200 μM). ADAMTS13 beads adhered to immobilized ULVWF and the adhesion was blocked by the recombinant CUB-1 polypeptide and CUB-1A-E peptides, but not by the CUB-1As polypeptide (Student t test, n = 4, *P < .01 compared with untreated ADAMTS13 beads). (B) Washed platelets were perfused over histamine-activated HUVECs in the presence of either wild-type ADAMTS13 or the truncation mutant TSP-2. The numbers of strings detected after 2 minutes of perfusion were counted. CUB-1 polypeptide partially blocked the cleavage of ULVWF by wild-type ADAMTS13 as demonstrated by the increased numbers of ULVWF strings, but not by the TSP-2 truncation mutant (Student t test, n = 4). (C) Binding of ULVWF to wild-type ADAMTS13 or TSP-2 mutant was measured by ELISA. ULVWF bound wild-type ADAMTS13 and TSP-2 mutant. The binding to wild-type ADAMTS13 can be partially blocked by an anti-ADAMTS13 antibody (10 μg/mL) generated using as an immunogen a synthetic peptide from the CUB-1 domain (Student t test, n = 7). All data are expressed as mean ± SEM.
Discussion
We have shown that recombinant CUB-1 polypeptides and synthetic peptides derived from the CUB-1 domain partially inhibited ULVWF cleavage by ADAMTS13 under flow (Figures 1 and 2). The isolated recombinant CUB-2 domain, in contrast, did not. Neither the recombinant CUB polypeptides nor the synthetic peptides inhibited ULVWF proteolysis under static conditions (Figure 3). We also showed that beads coated with each of the recombinant CUB polypeptides bound directly to ULVWF, but attachment of beads coated with the isolated CUB-2 domain was unable to withstand elevated shear stresses (Figure 4B). Finally, we showed that recombinant CUB-1 inhibits the interaction of ADAMTS13 with ULVWF to a greater extent under flow than under static conditions (Figure 4). Taken together, these results suggest a critical role for CUB-1 in docking ADAMTS13 to ULVWF under flow.
In addition to supporting the data generated using recombinant CUB-1, the CUB-1 synthetic peptides also help to define the region of the CUB-1 domain that interacts with ULVWF. Alignment of the CUB1 domain sequence with the sequences of CUB domains of 2 spermadhesions (Figure 7A) with defined x-ray crystal structures34 shows that the CUB-1 peptides A through D correspond to sequences within the third and fourth β-strands in the known CUB domain structures (Figure 7B). These 2 β-strands are therefore likely to interact directly with ULVWF. In addition, the peptide CUB-1E, derived from the C-terminus of the CUB-1 domain, also inhibits the binding of ADAMTS13 to immobilized ULVWF to a similar extent as the other 4 peptides, making it likely that the C-terminal residues are in proximity with the third and fourth β-strands (Figure 7B), and also make up part of the binding interface.
A model of 2-site binding of ADAMTS13 to ULVWF. (A) Alignments of the sequences of the CUB-1 domain of ADAMTS13 and the CUB domains of 2 spermadhesins. (B) Four of the 5 peptides were located in the third and fourth β-strands of the CUB fold, indicating that this region is involved in binding ULVWF. One peptide was derived from the C-terminus that potentially interacts with the third and fourth β-strands. (C) A 2-site model of ADAMTS13 binding to ULVWF. ADAMTS13 exists in plasma in open and closed states that are in equilibrium, with the closed state being the dominant form. The binding of ADAMTS13 through its CUB-1 domain to ULVWF anchored to the surface of endothelial cells is facilitated by fluid shear stress. Once bound, ADAMTS13 assumes the open conformation that exposes the second cryptic binding site to stabilize the interaction with ULVWF.
A model of 2-site binding of ADAMTS13 to ULVWF. (A) Alignments of the sequences of the CUB-1 domain of ADAMTS13 and the CUB domains of 2 spermadhesins. (B) Four of the 5 peptides were located in the third and fourth β-strands of the CUB fold, indicating that this region is involved in binding ULVWF. One peptide was derived from the C-terminus that potentially interacts with the third and fourth β-strands. (C) A 2-site model of ADAMTS13 binding to ULVWF. ADAMTS13 exists in plasma in open and closed states that are in equilibrium, with the closed state being the dominant form. The binding of ADAMTS13 through its CUB-1 domain to ULVWF anchored to the surface of endothelial cells is facilitated by fluid shear stress. Once bound, ADAMTS13 assumes the open conformation that exposes the second cryptic binding site to stabilize the interaction with ULVWF.
Docking of ADAMTS13 is necessary to bring the circulating metalloprotease to the ULVWF cleavage site, which is likely to be exposed only transiently when the ULVWF strand experiences a threshold tensile stress.35 The enzyme would thus be poised to cleave the strand at the appropriate moment when the cleavage site is exposed. This scenario is consistent with our observation that ULVWF strands are most often cleaved near their proximal site of attachment on HUVECs,4 the point of maximum tensile stress. Also consistent with this mechanism is the early observation by Tsai and coworkers36 that shear stresses corresponding to those found in human arteries applied to plasma ex vivo enhance the proteolysis of VWF. This observation has a pathophysiologic correlate: enhanced proteolysis of VWF in vivo was shown to produce acquired von Willebrand disease in patients with severe aortic stenosis, a condition in which the blood is continually exposed to elevated shear stress at the site of valve stenosis.37
The conditions in the static assays are very different. In the most commonly used assays, chaotropic agents such as urea are required, presumably to denature the substrate. Under these conditions, one would expect the cleavage site to remain exposed, obviating the need for a docking mechanism. Furthermore, enzyme docking may be less important for cleavage of ULVWF under static conditions because prolonged incubation of VWF and ADAMTS13 would allow repeated contacts between the substrate and the metalloprotease, the duration of which would be limited only by molecular diffusion and not by fluid flow.
The situation is not that simple, however. Available in vitro data are conflicting regarding the role of the CUB domain in the binding of the metalloprotease to VWF. On one hand, CUB domains have been demonstrated to directly bind to VWF38 or modulate the ADAMTS13-ULVWF interaction.39 Further, Majerus et al39 showed that deletion of the CUB domains reduces ADAMTS13 binding to VWF 3-fold. On the other hand, we recently reported that an ADAMTS13 truncation mutant lacking the sequence C-terminal to the Spacer (without CUB domains) actually cleaved ULVWF more efficiently than wild-type ADAMTS13 under flow.32 The same truncation mutant was also found to be active under static conditions.26 What these seemingly contradictory data imply, however, is that ADAMTS13 contains a second binding site for ULVWF. This presumption is suggested by the data presented in Figure 6.
We therefore propose a working model of ADAMTS13 activity that takes into account all of the data cited above (Figure 7C). The model posits that ADAMTS13 in solution is in equilibrium between a closed state in which the second ULVWF-binding domain, and possibly the catalytic site, are cryptic, and an open state in which both the primary and secondary binding sites (and the catalytic site) are available for interacting with the substrate. In this model, the closed conformation is favored when ADAMTS13 is in solution. However, when ADAMTS13 binds VWF (through the CUB domains) the equilibrium shifts toward the open conformation (by fluid shear stress for instance), revealing both the second binding site, which reinforces the interaction (note the difference in ULVWF binding between intact ADAMTS13 and the isolated CUB-1 domain) and the catalytic site. In this way, engagement of the second site is almost always preceded by engagement of the first site, explaining the ability of the isolated CUB-1 domain to inhibit the binding of ADAMTS13-coated beads to an ULVWF-coated surface. However, ADAMTS13 may occasionally adapt the open conformation in solution and could therefore bind ULVWF through the usually cryptic second site. This would explain why recombinant CUB-1, even at very high concentrations, cannot completely inhibit ULVWF cleavage (Figures 1 and 2). The second binding site remains to be identified. One likely candidate is the spacer domain, given the findings of Majerus and coworkers39 of a 10-fold drop in the affinity of ADAMTS13 for ULVWF when the enzyme was truncated before the spacer domain. Other studies have shown that the spacer domain is required for ADAMTS13 activity under both static and flow conditions.26,32
We wish to emphasize that the proposed interaction between ADAMTS13 and ULVWF is a working model that was developed based on currently available data, but the existence of open and closed states remain to be further investigated. In addition to this model, other explanations for the data may also be possible. For example, the isolated CUB domains may function distinctively from those in the native enzyme. This is, however, unlikely for several reasons. First, ULVWF binds similarly to the wild-type ADAMTS13 and isolated CUB domain polypeptides (Figure 4). Second, tethering and adhesion of ADAMTS13-coated beads to immobilized ULVWF under flow was blocked by the recombinant CUB-1 polypeptide, as well as the synthetic peptides (Figure 2) that are derived from 2 separate regions of CUB-1. Finally, the inhibitory effect is domain-specific for CUB-1, even though CUB-1 shares a high degree of homology with CUB-2.
In summary, we have demonstrated that the recombinant CUB-1 domain of ADAMTS13 and peptides derived from it inhibit ULVWF proteolysis under flow conditions, likely by preventing ADAMTS13 docking to ULVWF. These data highlight an important function for the CUB domains of ADAMTS13, the only member of the ADAMTS family so far described that contains CUB domains. These results also suggest that ULVWF proteolysis could be impaired by defective docking of ADAMTS13 to ULVWF under flow (by CUB domain antibodies, for example), a defect that would not be detectable by the conventional static assays currently in use.
Prepublished online as Blood First Edition Paper, September 1, 2005; DOI 10.1182/blood-2005-05-2029.
Supported by National Institutes of Health (NIH) grants P50-HL65 967 and HL71 895, a grant-in-aid from the American Heart Association-Texas Affiliate, and the Mary R. Gibson Foundation. J.F.D is an Established Investigator of the American Heart Association.
Z.T. and Y.P. contributed equally to this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal