Abstract
The activation of phosphoinositide metabolism represents a critical step in the signaling pathways leading to the activation of cytolytic machinery, but its regulation is partially understood. We report here that the stimulation of the low-affinity receptor for immunoglobulin G (IgG) (FcγRIIIA, CD16) on primary human natural killer (NK) cells induces a phosphatidylinositol 3-kinase (PI3K)–dependent activation of the small G protein Arf6. We first demonstrate a functional role for Arf6-dependent signals in the activation of the antibody-dependent cellular cytotoxicity (ADCC) attributable to the control of secretion of lytic granule content. We also show that Arf6 couples CD16 to the lipid-modifying enzymes phosphatidylinositol4phosphate 5-kinase type I alpha (PI5KIα) and phospholipase D (PLD) that are involved in the control of granule secretion; Arf6, but not Rho family small G proteins RhoA and Rac1, is required for receptor-induced PI5KIα membrane targeting as well as for PI5KIα and PLD activation. Our findings suggest that Arf6 plays a crucial role in the generation of a phosphatidylinositol4,5-bisphosphate (PIP2) plasma membrane pool required for cytolytic granule-mediated target cell killing.
Introduction
CD16, the low-affinity receptor for the Fc fragment of immunoglobulin G (IgG), is a major surface structure on natural killer (NK) cells capable of triggering the antibody-dependent cellular cytotoxicity (ADCC) and the expression of genes encoding surface activation molecules and cytokines upon engagement by IgG-opsonized targets.1,2 CD16 represents the prototype of the so-called immunoreceptor tyrosine-based activation motif (ITAM)–containing NK receptors whose clustering results in a cascade of signaling events responsible for cytolytic granule-mediated target cell killing.2 This process involves several steps, including the formation of a cytolytic synapse between NK and target cells, the rapid reorientation of the Golgi complex and the microtubule-organizing center (MTOC), the polarization of lytic granules toward the target contact site followed by granule docking and fusion at specialized secretory domains within the cytolytic synapse leading to the release of granule content.3 Most of the signaling components controlling secretion of the granule content after their polarization have yet to be identified.
The activation of the lipid-modifying enzymes phospholipase C gamma (PLCγ) and phosphatidylinositol 3-kinase (PI3K) plays a central role in the activation of cytolytic machinery2,4 ; notably, they share the common substrate phosphatidylinositol4,5-bisphosphate (PIP2).5 Furthermore, PIP2 is also required for the latest steps of the secretory pathway by marking membrane microdomains for specific structural and functional modifications required for granule docking and fusion and for the disassembly of cortical actin barrier.5-7
Little is known about how PIP2 levels are regulated in cells of the immune system. PIP2 is mainly synthesized by the phosphorylation of phosphatidylinositol4-phosphate (PI4P) at the D-5 position of the inositol ring by phosphatidylinositol4phosphate 5-kinase type I (PI5KI).5,7 Three isoforms of PI5KI have been described, PI5KIα, PI5KIβ, and PI5KIγ, with distinct subcellular localization and functions. PI5KIγ targets to focal adhesion and nerve terminals where PIP2 is required for focal adhesion assembly and for recycling of synaptic vesicle membrane, respectively8,9 ; PI5KIβ is present in the vesicular perinuclear region7 and is required for constitutive receptor endocytosis10 ; whereas PI5KIα specifically localizes in the nucleus and membrane ruffles7 and regulates ligand-dependent PIP2 generation at the plasma membrane.11
Evidence indicates that Arf6 can stimulate the production of PIP2 by regulating the activation of PI5KI12-15 and phospholipase D (PLD)16,17 whose product, phosphatidic acid (PA), contributes to the enzymatic activation of PI5KI.
Arf6 belongs to the class III ARF (ADP [adenosine diphosphate] ribosylation factor) family of Ras-related small guanosine triphosphate (GTP)–binding proteins and functions as a switch that cycles through GTP for guanosine diphosphate (GDP) exchange and GTP hydrolysis. Arf6 has been involved in the regulation of membrane trafficking, receptor endocytosis, endosomal recycling, and secretion in a number of cellular systems.18,19
Whether Arf6-regulated pathways are coupled to receptors triggering the cytotoxic function is presently unknown. Here, we report that endogenous Arf6 becomes transiently activated upon CD16 stimulation in human NK cells and plays a functional role in the regulation of CD16-induced cytotoxicity. We show that Arf6, although not essential for lytic granule polarization, is required for their secretion. We also provide evidence that Arf6 is responsible for CD16-induced PI5KIα and PLD activation. Active Arf6, by interacting with PI5KIα, shuttles and activates the kinase at the plasma membrane in response to CD16 aggregation, thus ensuring local PIP2 synthesis required for the cytotoxic function.
Materials and methods
Reagents
The following antibodies (Abs) were used: anti–major histocompatibility complex (MHC) class I (W6.32), used as negative control, and anti-CD16 (B73.1) were provided by Dr G. Trinchieri (Schering Plough, Dardilly, France). Goat polyclonal antibodies anti-PI5KIα (C17, N20), anti-Arf6 monoclonal antibody (mAb), anti–lymphocyte-specific kinase (Lck) mAb, and anti-hemagglutinin (HA) mAb were from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti–mouse (GAM) F(ab′)2 was from Cappel (Aurora, OH). Anti-HA mAb used for confocal microscopy analysis was from Babco (Richmond, CA). Fluorescein isothiocyanate (FITC)–conjugated GAM F(ab′)2 was from Zymed (South San Francisco, CA). FITC-conjugated anti–perforin mAb was from Ancell (Bayport, MN). Purified human IgG and IgG F(ab′)2 were from Cappel. Anti–β-tubulin mAb, PI4,5P2, PI4P, LY294002, and p-nitrophenyl N-acetyl-βD-glucosaminide were from Sigma-Aldrich (Milan, Italy).
Preparation of human NK cells
NK cell populations were obtained from 10-day cocultures of peripheral blood mononuclear cells (PBMCs) with irradiated Epstein-Barr virus positive (EBV+) RPMI 8866 lymphoblastoid cell line as previously described.20 On day 10, cell population was routinely 80% to 95% CD56+, CD16+, CD3–, as assessed by immunofluorescence and cytofluorimetric analysis. The experiments were performed on NK cell populations that were more than 90% pure. Ab-mediated CD16 stimulation was performed as described.20 For IgG-coupled bead stimulation, human IgG- or CD16-coupled polystyrene beads (2.5-μm diameter; Interfacial Dynamic, Portland, OR) were incubated with human NK cells resuspendend in RPMI 1640 serum-free medium, as described.20
Pull-down assay
Primary cultured human NK cells were incubated overnight in RPMI medium containing 0.1% fetal calf serum (FCS). Cells (25 × 106 cells/sample) were stimulated with anti-CD16 or control mAb. When indicated, cells were preincubated with LY294002 (50 μM, final concentration). Cells were then lysed in 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl pH 7.5, 200 mM NaCl, 20 mM MgCl2, 0.1% SDS (sodium dodecyl sulfate), 0.5% sodium deoxycholate, 10% glycerol, 1% Triton X-100. Postnuclear supernatants were precleared with glutathione Sepharose beads (Amersham Pharmacia, Uppsala, Sweden) for 5 minutes at 4°C. The pull-down assay was performed with glutathione Sepharose beads conjugated with glutathione S-transferase–Golgi-localized, γ ear-containing, Arf-binding protein 3 (GST-GGA3) fusion protein (50 μg) or GST alone (50 μg) for 45 minutes at 4°C. The beads were washed 3 times (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 2 mM MgCl2, 10% glycerol, 1% NP-40 (Nonidet P-40, [Octylphenoxy]polyethoxyethanol)). The precipitated Arf6GTP was resolved by SDS–polyacrylamide gel electrophoresis (PAGE) and revealed by Western blot. The GST-GGA3 fusion protein was kindly provided by Dr J. Bonifacino (NIH, Bethesda, MD).21
Vaccinia virus infection and lysate preparation
Wild-type and recombinant vaccinia viruses encoding HA-tagged wild-type (WT) or the GTP hydrolysis-deficient Arf6 mutant (Q67L) were kindly provided by Dr G. Thomas (Vollum Institute, Portland, OR).22 Recombinant vaccinia viruses encoding for the GDP-bound Rac1 (N17Rac) or RhoA (N19Rho) mutant were kindly provided by Dr P. J. Leibson (Mayo Clinic, Rochester, MN).23 Semipurified vaccinia viruses were used to infect human NK cells for 5 hours at a multiplicity of infection of 15:1. When required, postnuclear cell lysates were prepared in 50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EGTA (ethylenediaminetetraacetic acid), 1 mM MgCl2, 50 mM NaF, 1% NP-40, 1 mM PMSF (phenylmethylsulfonyl fluoride), 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 1 mM orthovanadate.
Cytotoxic and degranulation assay
Uninfected or recombinant vaccinia virus–infected human NK cells were assayed in a 51Cr release reverse ADCC assay, as described.20 Lytic units were calculated as reported.1 For N-α-benzyloxycarbonyl-L-lysine thiobenzyl ester (BLT) esterase activity, cells (5 × 106/mL) were incubated for 4 hours with anti–CD16-, anti–MHCI-, human IgG-, or human IgG F(ab′)2-coated polystyrene beads in serum-free Hanks Balanced Buffered Solution (HBBS) medium containing 0.1% bovine serum albumin (BSA). Cell-free supernatant was assayed for BLT esterase (granzyme A) activity, as previously described.24 Briefly, the specific release of BLT esterase was measured on incubation of 45 μL cell-free supernatant with 180 μL 0.2 M Tris-HCl, pH 8.1 containing 10–4 M BLT and 2.2 × 10–4 M dithio-bisnitrobenzoic acid. The percentage of specific enzymatic release was calculated on the basis of the total enzymatic content solubilized with 0.5% NP-40. Absorbance was read at 405/690 nm.
CD16 down-modulation analysis
Human NK cells were infected with recombinant vaccinia viruses, treated with anti-CD16 mAb at 4°C, resuspended in serum-free prewarmed RPMI medium, and incubated for various times at 37°C. Endocytosis was stopped by addition of ice-cold phosphate-buffered saline (PBS)–0.1% NaN3. Cells were then incubated with FITC-conjugated GAM F(ab′)2 and analyzed by FACS star (BD Biosciences, Milan, Italy). Data were expressed as the percentage of the mean fluorescence intensity of cells stained as described here and kept on ice in the presence of PBS-0.1% NaN3.
Membrane and cytosolic fraction preparation
Unstimulated or anti–CD16-stimulated NK cells (1 × 108) were gently sonicated in ice-cold lysis buffer (25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.2, 10% glycerol, 1 mM EDTA, 1 mM EGTA, 1 mM DTT (dithiothreitol)). After centrifugation at 500g for 10 minutes at 4°C the postnuclear supernatants were ultracentrifuged at 15 000g for 60 minutes at 4°C to separate the cytosolic and membrane fractions. Membrane pellets were lysed in 20 mM Tris pH 7.5, 150 mM NaCl, 1% NP-40 or 1% Triton X-100. Protein content was determined by colorimetric assay. SDS-PAGE was loaded with an equal amount of proteins derived from each fraction or with immunoprecipitates derived from an equal amount of protein recovered from each fraction.
PI5K activity assay
PI4P was resuspended in assay buffer (30 mM HEPES pH 7.4, 100 mM KCl, 1 mM EGTA, 2 mM MgCl2, 0.05% NP-40) and sonicated. PI5KIα was immunoprecipitated from membrane or cytosolic proteins derived from uninfected NK cells or, alternatively, from total lysates derived from recombinant vaccinia virus–infected NK cells. Beads were incubated with 50 μL assay buffer containing PI4P, MgATP (50 μM) and 32γ-ATP (adenosine triphosphate) for 15 minutes at 37°C. The organic phase, containing PIP2 was separated by thin-layer chromatography (TLC) on Silica gel 20 × 10 plates (Merck, Darmstadt, Germany) as previously described.25 The radioactive lipids were visualized by autoradiography. The identity of PIP2 and PI4P was confirmed by comparison with standard phospholipids revealed by iodine vapor. The spot corresponding to PIP2 was quantified by densitometric analysis (Imagequant; Molecular Dynamics, Amersham Bioscience, Cologno Monzese, Italy).
Phospholipase D activity assay
NK cells (5 × 108) were preincubated with 1 mCi (37 MBq) 3H oleic acid in serum-free medium for 2 hours at 37°C. After labeling cells were infected as described here and left untreated or stimulated with anti-CD16 or control mAb in medium containing 0.5% ethanol. Stimulation was stopped by the addition of 2 volumes of ice-cold methanol. Lipids were extracted and separated by TLC as described.26 Standards were chromatographed in parallel. TLC plates were then autoradiographed, and the phospholipid bands of interest were scraped and counted in a beta counter. PLD activity was quantitated by evaluating the production of phosphatidylethanol (PetOH).
Microscopic analysis of granule polarization and Arf6 distribution
To analyze cytolytic granule polarization, recombinant vaccinia virus–infected NK cells were treated at 4°C with anti-CD16 or control mAb. After washing cells were mixed to FcγR+ P815 target cells (effector-to-target [E/T] ratio, 2:1), briefly pelleted, and incubated in the dark for 15 minutes at 37°C. The pellet was gently resuspended and spun onto ice-cold poly-l-lysine–coated glass slides, fixed in 4% paraformaldehyde, permeabilized in 0.5% saponin, blocked in PBS containing 1% FCS and 0.05% saponin, and stained with FITC-conjugated antiperforin mAb. Granule polarization was assessed in NK cells forming conjugates with target cells using a fluorescence microscope. A total of 100 conjugates were evaluated per slide.
To analyze Arf6 localization in NK cells infected with recombinant vaccinia virus encoding HA-tagged Arf6-WT, cells were fixed, permeabilized as described here, and stained with anti-HA mAb followed by FITC-labeled GAM F(ab′)2. The slides were analyzed using a Leica TCS4D laser scanning confocal fluorescence microscope equipped with an argonkrypton laser, double-dichroic splitters (488/518 nm), 520 nm barrier for FITC-fluorochrome, with a 100×/0.75 objective lens (Leica Microsystem, Heidelberg, Germany). Images were processed with Photoshop 7.0 (Adobe, San Jose, CA).
Results
CD16 ligation induces PI3K-dependent Arf6 activation in human NK cells
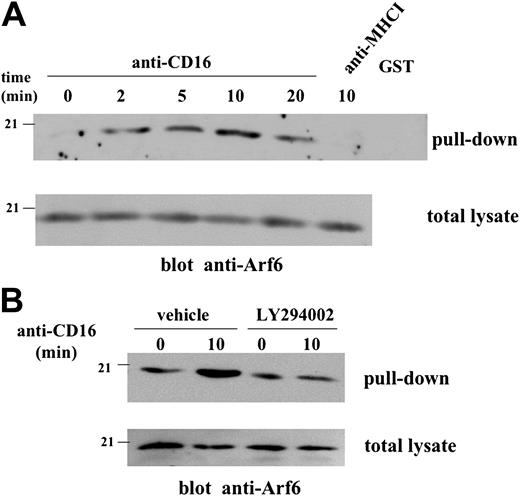
To evaluate whether CD16 stimulation induces the activation of Arf6, we monitored the activation levels of endogenous Arf6 in CD16-stimulated human NK cells. Arf6GTP was detected by a pull-down assay based on GST domain fused to the Arf-binding domain of GGA3.21 MAb-mediated CD16 cross-linking induces up-regulation of Arf6GTP that was already detectable at 2 minutes, reached a maximum at 10 minutes, and was down-regulated at 20 minutes after stimulation (Figure 1A). No precipitation of Arf6 was detected in the presence of GST. Anti-Arf6 blot of an aliquot of total lysates indicates that the variations observed after precipitation were not due to different protein content between the samples. We then analyzed whether CD16-induced Arf6 activation could depend on PI3K activation status. LY294002 compound was used to specifically block PI3K activity. Despite a variable degree of basal level of active Arf6 observed in our experiments, the data in Figure 1B clearly demonstrate that inhibition of PI3K activity completely prevents CD16-induced Arf6GTP; on the contrary, the basal active status is not affected by LY294002 treatment.
Arf6 is required for CD16-dependent cytotoxicity and granzyme release
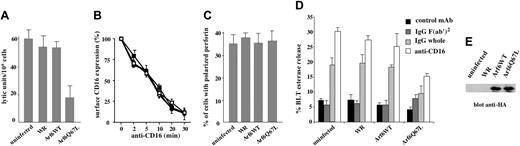
To functionally define the role for Arf6 in CD16-mediated NK cell cytotoxicity, we overexpressed Arf6-WT or the GTP-hydrolysis–deficient mutant, Q67L, which has been shown to interfere with the proper compartmentalization of Arf6 effector molecules.15 Human NK cells were infected with HA-tagged recombinant vaccinia viruses, and Arf6 expression was assessed by Western blot (Figure 2E). By comparing the levels of endogenous and overexpressed Arf6, we estimated that the neo-expressed protein was almost twice the amount of endogenous Arf6 (not shown); the overexpression levels were comparable among different experiments. Uninfected, Arf6-WT, Q67L, or control vector-infected NK cells were tested in a reverse ADCC assay using FcγR+ P815 target cells in the presence of anti-CD16 mAb. Q67L-expressing cells exhibited a significant down-regulation of CD16-mediated killing when compared with uninfected or empty virus infected cells (mean of lytic unit inhibition 69%) (Figure 2A). The lack of functional effects in Arf6-WT–overexpressing cells has been reported15 and may be related to the high level of endogenous Arf6.
CD16 stimulation induces PI3K-dependent Arf6 activation in human NK cells. (A) Cell lysates derived from unstimulated, anti–CD16- or anti–MHCI-stimulated cultured human NK cells (2.5 × 107/sample) were incubated with glutathione Sepharose beads conjugated with GST-GGA3 fusion protein or GST alone. The precipitated protein (top) or an aliquot of the samples derived from the same experiment (bottom) was separated by 12% SDS-PAGE. Western blot analysis with anti-Arf6 mAb was performed. (B) NK cells were pretreated with LY294002 (50 μM) or control medium (vehicle) and left unstimulated or stimulated with anti-CD16 mAb for 10 minutes. Cell lysates were precipitated and analyzed as above. One experiment representative of 4 performed is shown.
CD16 stimulation induces PI3K-dependent Arf6 activation in human NK cells. (A) Cell lysates derived from unstimulated, anti–CD16- or anti–MHCI-stimulated cultured human NK cells (2.5 × 107/sample) were incubated with glutathione Sepharose beads conjugated with GST-GGA3 fusion protein or GST alone. The precipitated protein (top) or an aliquot of the samples derived from the same experiment (bottom) was separated by 12% SDS-PAGE. Western blot analysis with anti-Arf6 mAb was performed. (B) NK cells were pretreated with LY294002 (50 μM) or control medium (vehicle) and left unstimulated or stimulated with anti-CD16 mAb for 10 minutes. Cell lysates were precipitated and analyzed as above. One experiment representative of 4 performed is shown.
Since Arf6 has been involved in the control of ligand-induced receptor internalization in a variety of cellular systems18,19,27 and, in particular, the Q67L mutant has been shown to prevent receptor trafficking,22 we analyzed whether the reduced CD16-mediated killing could be related to an alteration of receptor expression levels. In Figure 2B the kinetics of CD16 down-modulation induced by mAb-mediated receptor cross-linking is shown. No major differences were observed in control or Arf6-mutant–expressing cells, demonstrating that Arf6 is not involved in the control of CD16 surface expression. We then assessed the ability of lytic granules to move and polarize toward the area of cytolytic synapse in NK cells stimulated by means of reverse ADCC, and we observed that lytic granules polarized toward the area of contact with the target cells as in control conjugates (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). We also investigated the role of Arf6 in CD16-induced cytolytic granule secretion by measuring BLT esterase activity in the supernatant of NK cells stimulated with human IgG- or anti–CD16-coupled polystyrene beads. Our findings show that Arf6 mutant expression induces a significant reduction of CD16-triggered granule release as compared with control virus-infected cells (Figure 2D); total enzymatic activity was comparable between the experimental groups (not shown).
These data demonstrate that Arf6 regulates CD16-induced cytotoxic function by controlling granule secretion, but it is not required for the microtubule-mediated granule movement to the site of target cell contact.
CD16 stimulation induces membrane translocation and activation of PI5KIα
PI5KIα plasma membrane relocalization is necessary for kinase activation and to ensure the spatially regulated PIP2 generation.7
Arf6 is involved in the regulation of CD16-dependent cytotoxicity. (A) Human NK cells were left uninfected or infected with empty virus (WR) or with recombinant vaccinia viruses encoding HA-tagged Arf6 wild-type (Arf6-WT) or Arf6 mutant Q67L and were assessed in a 4-hour 51Cr release assay against P815 target cells in the presence of anti-CD16 mAb. No detectable cytotoxicity against P815 targets was observed in the absence of anti-CD16 mAb at the indicated E/T ratios (not shown). Data are expressed as lytic units/106 cells ± SD referred to 3 independent experiments. (B) Cells were left uninfected (□) or infected with empty virus (WR, ▪), or with recombinant vaccinia viruses encoding HA-tagged Arf6 wild-type (Arf6-WT, •) or Arf6 mutant Q67L (○). Surface receptor levels were evaluated as described in “Materials and methods.” Data derived from 3 independent experiments are expressed as the percentage of the mean fluorescence intensity ± SD with respect to the control cells. Each experimental group has its own control sample. (C) NK cells were infected as indicated and stimulated by means of reverse ADCC as in panel A. The percentage of NK cells conjugated with target cells containing polarized granules is shown (see also Figure S1). Data from 3 independent experiments ± SD is shown. (D) NK cells were infected as indicated and stimulated with anti–MHCI-(control mAb), IgG F(ab′)2-, IgG-, or anti–CD16-coated polystyrene beads. Cell supernatants were collected and assayed for BLT esterase release. Data represent the percentage ± SD of specific release (sample/total release) from 3 independent experiments. (E) Overexpressed Arf6 constructs of a representative experiments are shown.
Arf6 is involved in the regulation of CD16-dependent cytotoxicity. (A) Human NK cells were left uninfected or infected with empty virus (WR) or with recombinant vaccinia viruses encoding HA-tagged Arf6 wild-type (Arf6-WT) or Arf6 mutant Q67L and were assessed in a 4-hour 51Cr release assay against P815 target cells in the presence of anti-CD16 mAb. No detectable cytotoxicity against P815 targets was observed in the absence of anti-CD16 mAb at the indicated E/T ratios (not shown). Data are expressed as lytic units/106 cells ± SD referred to 3 independent experiments. (B) Cells were left uninfected (□) or infected with empty virus (WR, ▪), or with recombinant vaccinia viruses encoding HA-tagged Arf6 wild-type (Arf6-WT, •) or Arf6 mutant Q67L (○). Surface receptor levels were evaluated as described in “Materials and methods.” Data derived from 3 independent experiments are expressed as the percentage of the mean fluorescence intensity ± SD with respect to the control cells. Each experimental group has its own control sample. (C) NK cells were infected as indicated and stimulated by means of reverse ADCC as in panel A. The percentage of NK cells conjugated with target cells containing polarized granules is shown (see also Figure S1). Data from 3 independent experiments ± SD is shown. (D) NK cells were infected as indicated and stimulated with anti–MHCI-(control mAb), IgG F(ab′)2-, IgG-, or anti–CD16-coated polystyrene beads. Cell supernatants were collected and assayed for BLT esterase release. Data represent the percentage ± SD of specific release (sample/total release) from 3 independent experiments. (E) Overexpressed Arf6 constructs of a representative experiments are shown.
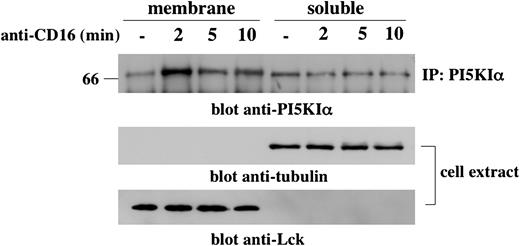
We first investigated whether CD16 cross-linking induces PI5KIα membrane recruitment. To this purpose, we prepared cytosol and membrane fractions from unstimulated or CD16-stimulated NK cells, and PI5KIα distribution was analyzed by Western blotting (Figure 3). In unstimulated NK cells the kinase was mainly present in the soluble fraction. Two minutes after receptor stimulation, PI5KIα was recruited to the membrane compartment where it was present until 10 minutes of stimulation, albeit at lower concentrations, and returned to basal levels at 15 minutes after stimulation (R.G., unpublished data May 2004). Lck and tubulin immunoreactivity being present in membrane and cytosolic fractions, respectively, regardless of the cellular activation status, indicates the correct fractioning.
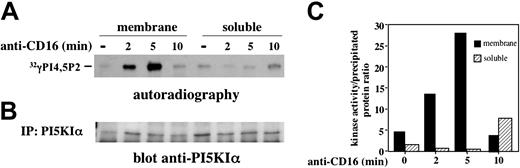
We then analyzed the activation status of PI5KIα in membrane and soluble compartments. Endogenous PI5KIα was precipitated from unstimulated or CD16-stimulated NK cells, and the kinase activity was evaluated (Figure 4A). Membrane-associated kinase exhibited a transient activation upon CD16 stimulation; it became active at 2 minutes, further increased at 5 minutes, and returned almost to basal level at 10 minutes after stimulation. On the contrary, enzymatic activity was barely detected in the cytosolic fraction with some increase at the later time points of stimulation. Figure 4B shows an aliquot of precipitated kinase. Figure 4C shows the kinase activity normalized for the quantity of precipitated enzyme and clearly indicates that membrane-associated kinase activity peaks at 5 minutes of stimulation.
CD16 stimulation induces a transient PI5KIα membrane translocation. Unstimulated or CD16-stimulated cultured NK cells (1 × 108/sample) were fractionated as described in “Materials and methods.” (Top) PI5KIα immunoprecipitates were obtained from protein extract derived from membrane and soluble fraction, loaded on 8% SDS-PAGE, and analyzed by immunoblotting with anti-PI5KIα Ab. (Middle and bottom) Total cell extract derived from the samples of the same experiment was loaded on 8% SDS-PAGE and analyzed by immunoblotting. The equivalent protein amount within the lanes was checked by Ponceau S red staining. One experiment representative of 3 performed is shown.
CD16 stimulation induces a transient PI5KIα membrane translocation. Unstimulated or CD16-stimulated cultured NK cells (1 × 108/sample) were fractionated as described in “Materials and methods.” (Top) PI5KIα immunoprecipitates were obtained from protein extract derived from membrane and soluble fraction, loaded on 8% SDS-PAGE, and analyzed by immunoblotting with anti-PI5KIα Ab. (Middle and bottom) Total cell extract derived from the samples of the same experiment was loaded on 8% SDS-PAGE and analyzed by immunoblotting. The equivalent protein amount within the lanes was checked by Ponceau S red staining. One experiment representative of 3 performed is shown.
Arf6 controls PI5KIα plasma membrane recruitment and activation upon CD16 stimulation
Since both the Ras-related GTPase Arf612-15 and the Rho family small G proteins RhoA and Rac128-31 have been previously reported to control PI5KI activation, we were interested in understanding whether CD16 stimulation is coupled to kinase activation in a small G protein-dependent manner. To this purpose we analyzed the kinase activity of endogenous PI5KIα in NK cells expressing Arf6-WT, the Q67L mutant, or the dominant negative N17Rac and N19Rho. PI5KIα was precipitated and the kinase activity was tested in vitro.
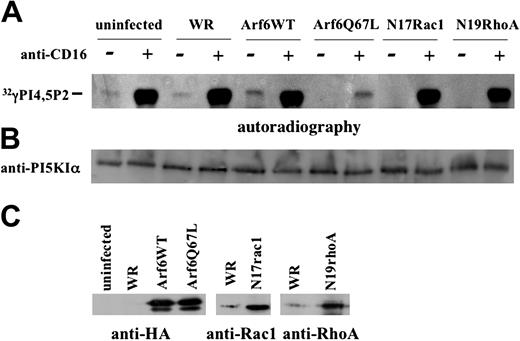
The data shown in Figure 5A reveal a constitutive low level of PI5KIα activity that is up-regulated upon CD16 triggering, both in uninfected and control virus-infected NK cells. Strikingly, Q67L-expressing cells exhibited a dramatic down-regulation of CD16-induced activity that was not observed in N17Rac- and N19Rho-expressing cells. Furthermore, a complete inhibition of constitutive kinase activity was found in mutant Arf6-, Rac1-, and RhoA-expressing cells, while Arf6-WT overexpression induced up-regulation of the basal kinase activity. An aliquot of precipitated protein was used for sample normalization (Figure 5B). The overexpressed proteins are shown in Figure 5C.
CD16 stimulation induces membrane-associated PI5KIα activation. (A) PI5KIα was immunoprecipitated from membrane and soluble fraction of unstimulated or CD16-stimulated cultured NK cells. Immunoprecipitates were assayed for PI5KIα activity, and the reaction products were subjected to TLC followed by autoradiography. Anti–MHCI-stimulated samples (not shown) showed a kinase activity that was superimposable to the unstimulated sample. (B) An aliquot of the immunoprecipitates from the same experiment was analyzed by immunoblot with anti-PI5KIα Ab. (C) The ratio between kinase activity and the amount of the kinase precipitated from the samples of the same experiment obtained by densitometric analysis, is shown. One representative experiment of 3 performed is shown.
CD16 stimulation induces membrane-associated PI5KIα activation. (A) PI5KIα was immunoprecipitated from membrane and soluble fraction of unstimulated or CD16-stimulated cultured NK cells. Immunoprecipitates were assayed for PI5KIα activity, and the reaction products were subjected to TLC followed by autoradiography. Anti–MHCI-stimulated samples (not shown) showed a kinase activity that was superimposable to the unstimulated sample. (B) An aliquot of the immunoprecipitates from the same experiment was analyzed by immunoblot with anti-PI5KIα Ab. (C) The ratio between kinase activity and the amount of the kinase precipitated from the samples of the same experiment obtained by densitometric analysis, is shown. One representative experiment of 3 performed is shown.
Arf6 but not Rho family small G proteins controls PI5KIα activation upon CD16 stimulation. (A) Cultured human NK cells were left uninfected or infected with the indicated recombinant vaccinia viruses. Lysates from 2 × 107 unstimulated or CD16-stimulated cells (5 minutes) were precipitated with anti-PI5KIα Ab. Immunoprecipitates were assayed for kinase activity. The panel derives from the autoradiography of 2 different TLC plates of the same experiment. (B) An aliquot of cell lysate derived from the same experiment was analyzed by immunoblotting. (C) The overexpressed constructs are shown. One representative experiment of 3 performed is shown.
Arf6 but not Rho family small G proteins controls PI5KIα activation upon CD16 stimulation. (A) Cultured human NK cells were left uninfected or infected with the indicated recombinant vaccinia viruses. Lysates from 2 × 107 unstimulated or CD16-stimulated cells (5 minutes) were precipitated with anti-PI5KIα Ab. Immunoprecipitates were assayed for kinase activity. The panel derives from the autoradiography of 2 different TLC plates of the same experiment. (B) An aliquot of cell lysate derived from the same experiment was analyzed by immunoblotting. (C) The overexpressed constructs are shown. One representative experiment of 3 performed is shown.
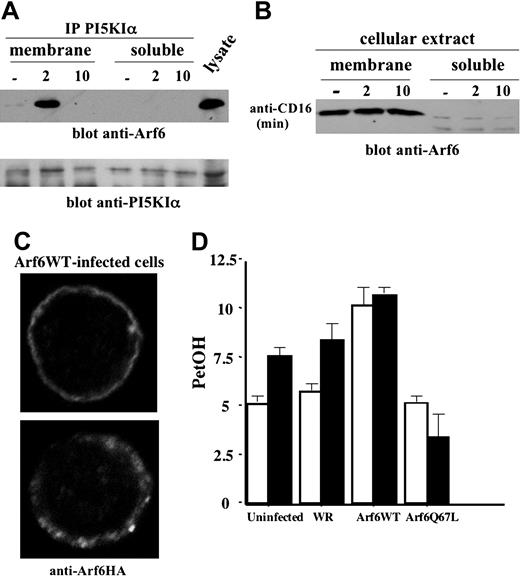
We then addressed the mechanisms responsible for Arf6-mediated PI5KIα activation, and we first explored whether Arf6 associates with PI5KIα. The kinase was precipitated from the cytosolic and membrane fractions derived from unstimulated or CD16-stimulated NK cells. We observed (Figure 6A) a transient PI5KIα/Arf6 association that selectively occurred in the membrane fraction at 2 minutes of receptor stimulation; this complex was still evident at 5 minutes (not shown), but not at 10 minutes of stimulation. No detectable Arf6/PI5KIα complex was observed in the soluble fraction. To further understand whether Arf6 could be responsible for PI5KIα plasma membrane recruitment, we analyzed Arf6 subcellular distribution in CD16-stimulated NK cells. Our experiments show that Arf6 is constitutively present in the membrane compartment and is virtually absent in the soluble fraction; receptor stimulation did not affect Arf6 subcellular distribution (Figure 6B). We also analyzed Arf6 subcellular distribution in NK cells overexpressing HA-tagged Arf6-WT by confocal microscopy. Anti-HA staining reveals that the majority of infected NK cells (up to 80%) exhibit a plasma membrane distribution of Arf6 with a discrete punctate pattern (Figure 6C).
Arf6 controls PLD activation in CD16-stimulated NK cells
Since CD16 ligation in human NK cells stimulates PLD activity that is required for receptor-induced degranulation and cytotoxicity,26 we investigated whether Arf6 could be responsible for CD16-induced PLD activation as previously reported in a variety of cellular systems.16,17,32 Radiolabeled Arf6-WT and Q67L mutant-expressing NK cells were stimulated with anti-CD16 mAb in the presence of primary alcohol. In vivo–produced radiolabeled PetOH was analyzed as a measure of PLD activity. Q67L expression completely prevented CD16-induced PLD activation, while Arf6-WT–expressing cells exhibited increased basal PetOH levels that were not further augmented by CD16 stimulation (Figure 6D). PetOH production in cells stimulated with control mAb was superimposable to that observed in unstimulated cells (not shown).
Discussion
Herein, we identify a novel PI3K-dependent pathway involved in the regulation of CD16-mediated NK cytotoxicity. We first demonstrate that CD16 engagement on primary cultured human NK cells induces a transient activation of endogenous Arf6 that peaks at 10 minutes after receptor stimulation with a kinetics consistent with previous evidence in human macrophages stimulated through FcγRI.33 In line with a number of observations indicating that the recruitment and activation of several pleckstrin homology (PH) domain-containing Arf6 exchange factors such as general receptor for phosphoinositides-1 (Grp1), ARF nucleotide-binding site opener (Arno) and Cytohesin 1, are strictly dependent on phosphatidylinositol3,4,5trisphosphate (PIP3),34,35 we also show that CD16-induced Arf6 activation is PI3K dependent.
Our results also demonstrate a role for Arf6 in the regulation of NK cell-mediated ADCC. We observed a significant down-regulation of reverse ADCC in NK cells expressing the GTP-hydrolysis deficient, Q67L, Arf6 mutant. Although the dominant interfering function of Q67L mutant may be related to its ability to block endogenous Arf6 from cycling between the GTP and GDP binding status, and/or to sequester Arf6 effector molecules,15,22,36 we have evidence favoring the latter possibility: in fact we did not observe cytotoxicity down-regulation by using T27N GTP-binding defective Arf6 mutant (R.G., unpublished data January 2004) that is known to block endogenous Arf6 cycling and to localize in a perinuclear tubulovesicular area.37
Arf6 mediates PI5KIα membrane recruitment and PLD activation upon CD16 stimulation. (A) PI5KIα immunoprecipitates were obtained from protein extract of membrane and soluble fraction of unstimulated or CD16-stimulated cultured human NK cells. Immunoprecipitated samples were divided into 2 equal aliquots and loaded on 12% (top) or 8% (bottom) SDS-PAGE and analyzed by immunoblotting as indicated. (B) Protein extracts derived from membrane and soluble fraction of unstimulated or CD16-stimulated cultured human NK cells were loaded on 12% SDS-PAGE and analyzed by immunoblotting. (C) Arf6-WT recombinant vaccinia virus–infected NK cells were stained with anti-HA mAb and analyzed by confocal microscopy (original magnification, × 600). (D) 3H oleic acid–radiolabeled NK cells were left uninfected or infected as indicated. Cells were left unstimulated (□) or stimulated with anti-CD16 (▪) in the presence of 0.5% ethanol. Lipids were separated by TLC, and the percentage of counts per minute of PetOH with respect to the total counts per minutes of phospholipids was quantified by liquid scintillation counted as a measure of PLD activity. Results are expressed as arbitrary units. Data from 3 independent experiments ± SD is shown.
Arf6 mediates PI5KIα membrane recruitment and PLD activation upon CD16 stimulation. (A) PI5KIα immunoprecipitates were obtained from protein extract of membrane and soluble fraction of unstimulated or CD16-stimulated cultured human NK cells. Immunoprecipitated samples were divided into 2 equal aliquots and loaded on 12% (top) or 8% (bottom) SDS-PAGE and analyzed by immunoblotting as indicated. (B) Protein extracts derived from membrane and soluble fraction of unstimulated or CD16-stimulated cultured human NK cells were loaded on 12% SDS-PAGE and analyzed by immunoblotting. (C) Arf6-WT recombinant vaccinia virus–infected NK cells were stained with anti-HA mAb and analyzed by confocal microscopy (original magnification, × 600). (D) 3H oleic acid–radiolabeled NK cells were left uninfected or infected as indicated. Cells were left unstimulated (□) or stimulated with anti-CD16 (▪) in the presence of 0.5% ethanol. Lipids were separated by TLC, and the percentage of counts per minute of PetOH with respect to the total counts per minutes of phospholipids was quantified by liquid scintillation counted as a measure of PLD activity. Results are expressed as arbitrary units. Data from 3 independent experiments ± SD is shown.
Upon engagement of ITAM-containing activating receptors the development of NK cytotoxic activity critically requires the activation of a number of signaling pathways involving Src, spleen tyrosine kinase–ζ-associated protein 70 (Syk/Zap70) family tyrosine kinases, PI3K, and different members of the Rho family GTP-binding protein.2,4 Rac1, RhoA, and Cdc42, by activating specific downstream effectors, regulate cytoskeleton rearrangement that is required for the coalescence of lipid rafts in the area of the cytolytic synapse, conjugate formation, and MTOC-directed granule polarization.4,23,38-42 A key mechanism of target-cell killing mediated by cytotoxic T lymphocyte (CTL) and NK cells is represented by the exocytosis of granules containing lytic mediators and apoptosis-inducing proteins.43
We observed that Arf6 does not perturb the ability of NK cells to form conjugates with target cells (R.G., unpublished data October 2003) and to polarize lytic granules toward the cytolytic synapse triggered by CD16 engagement by means of reverse ADCC. Notably, however, we show that Arf6 mutant expression impairs the release of cytolytic granule content induced by CD16 cross-linking, indicating that Arf6-regulated signals may control the cytolytic secretory pathway. This observation is consistent with several reports demonstrating a main role for Arf6 in the control of regulated secretion in neuronal or endocrine cellular systems14,15 ; similar to our study in PC12 neuroendocrine cell line Arf6 has been reported to control vesicle secretion without affecting actin-dependent movement of secretory granules.17 Our results also indicate that, despite the well-defined role of Arf6 in the regulation of surface receptor trafficking,22,27 the rate of cross-linking–induced CD16 endocytosis is not affected by Arf6 mutant overexpression, thus excluding that the perturbation of surface receptor levels may be implicated in the down-regulation of cytotoxic function.
Herein, we also investigate Arf6 effector molecules possibly involved in the regulation of cytolytic function. The lipid-modifying enzymes PI5KIα and PLD may represent good candidates. In particular, the requirement of PIP2 during stimulated secretion has been demonstrated.44 Localized generation of PIP2 at the plasma membrane marks membrane microdomains for specific structural and functional modifications required for Ca2+-dependent fusion and for the disassembly of cortical actin barrier.5-7 In particular, Arf6-mediated PIP2 generation has been shown to be implicated the regulation of secretion in neuroendocrine and pancreatic β cells.14,15 PIP2 also represents the common substrate of PI3K and PLCγ, responsible for the generation of the second messengers PIP3, and inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG), respectively,5,7 that are required for the propagation of intracellular signaling pathways induced by most surface-activating receptors and for the activation of cytolytic machinery.4,45 The in vivo kinetic analysis of receptor-activated PIP2 turnover in cellular membrane revealed that its levels fall within the first 30 seconds and is followed by a rapid replenishment of the mediator over the following 2 to 3 minutes.46 The signaling mechanisms responsible for PI5K regulation in the immune cells are poorly elucidated. In mouse B cells the Bruton tyrosine kinase (Btk) has been shown to regulate PI5KI activity and cell activation in response to B-cell–receptor (BCR) stimulation47 ; on the other hand, Arf6 and Rac1 have been reported to control PI5KIα activity required for β1 integrin-dependent Yersinia pseudotuberculosis uptake in human macrophages.13 Thus, distinct mechanisms may be involved in PI5KI activation, depending on receptor specificity and/or PIP2-regulated cell functions.
No evidence is presently available on how PIP2-producing enzymes are regulated in cytotoxic lymphocytes. We show here that CD16 stimulation on NK cells induces the rapid and transient PI5KIα membrane recruitment where it undergoes enzymatic activation. Moreover, our data first demonstrate a critical role for Arf6 in mediating CD16-induced PI5KIα membrane targeting and activation. Consistent with previous evidence,48 Arf6 is found in the membrane fraction and is localized at the plasma membrane regardless of receptor engagement. Upon CD16 stimulation, an Arf6-PI5KIα complex is formed, and, based on the time course of Arf6 activation, it likely involves its GTP-bound form. Accordingly, the colocalization of Arf6 with PI5KIα and PIP2 has been previously reported.12 We also provide a direct demonstration of Arf6 involvement in the control of PI5KIα activation as Arf6 mutant-expressing cells exhibited a dramatic reduction of PI5KIα enzymatic activity. Surprisingly, we found that CD16-induced PI5KIα enzymatic activity was not regulated by Rho family GTPases as shown in other cellular systems,28,30 including thrombin-stimulated platelets29 and B lymphocytes in response to BCR plus CD19 stimulation.49 However, it is still possible that Rho family small G proteins control CD16-induced PIP2 production in distinct spatial and temporal contexts and/or mediated by other PI5KI enzyme isoforms. In this regard, we show that the basal activity of PI5KIα is regulated by Rac1 and RhoA, and we also found a constitutive PI5KIα/Rac1 association in membrane compartment (R.G., unpublished data June 2004).
Our results also provide evidence that Arf6 is required for PLD activation in response to CD16 stimulation. PLD regulation by Arf family proteins, including Arf6, has been previously described in a variety of cells, including mast cells and T lymphocytes.16,18,19,32 PLD activity plays a role in enhancing PIP2 production by generating PA, a known activator of PI5KI, and this effect may amplify the direct Arf6-mediated control of PI5KIα.12,32 In accordance, we show that, although membrane PI5KIα recruitment peaks at 2 minutes upon receptor stimulation, maximal activity is observed at later time points, thus suggesting that CD16-induced PA generation is an additional mechanism required for full kinase activation. PLD activation is involved in CD16-triggered degranulation and cytotoxicity in human NK cells,26 and its requirement in CD16-induced granule secretion may be related to its ability of potentiating PIP2 production. We cannot rule out, however, that PA may have additional roles in granule secretion that could be related to the regulation of calcium levels and protein kinase C activation32 or to the direct effect on membrane budding and fusion as proposed in neuroendocrine cells and mast cells.17,50
Overall our data suggest that Arf6 may control CD16-triggered NK cell cytotoxicity by the regulation of the PI5KIα and PLD activation.
The characterization of the molecular events involved in the spatial control of cytolytic granule trafficking and exocytosis have begun to be elucidated43,51 ; the further analysis of the molecular basis of Arf6 function will contribute to the knowledge of the mechanisms underlying the secretory pathway in cytotoxic lymphocytes.
Prepublished online as Blood First Edition Paper, April 7, 2005; DOI 10.1182/blood-2004-10-4100.
Supported by grants from Italian Association for Cancer Research (AIRC), the Italian Ministry for University and Research (MIUR), and the Center of Excellence (BEMM).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr C. Ramoni for confocal microscopy studies and Dr V. Hsu for sharing some reagents. We also thank D. Milana, A. M. Bressan, P. Birarelli, and A. Procaccini for expert technical assistance; Raffaella Centicolella and Paola Di Russo for manuscript editing. We are grateful to Prof C. Montecucco for critical review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal