Abstract
B-cell chronic lymphocytic leukemia (B-CLL) remains an incurable disease that requires innovative new approaches to improve therapeutic outcome. Honokiol is a natural product known to possess potent antineoplastic and antiangiogenic properties. We examined whether honokiol can overcome apoptotic resistance in primary tumor cells derived from B-CLL patients. Honokiol induced caspase-dependent cell death in all of the B-CLL cells examined and was more toxic toward B-CLL cells than to normal mononuclear cells, suggesting greater susceptibility of the malignant cells. Honokiol-induced apoptosis was characterized by the activation of caspase-3, -8, and -9 and cleavage of poly(adenosine diphosphate-ribose) polymerase (PARP). Exposure of B-CLL cells to honokiol resulted in up-regulation of Bcl2-associated protein (Bax) and down-regulation of the expression of the key survival protein myeloid-cell leukemia sequence 1 (Mcl-1), which is associated with response to treatment in B-CLL patients. In addition, B-CLL cells pretreated with interleukin-4 (IL-4), a cytokine known to support B-CLL survival, underwent apoptosis when subsequently incubated with honokiol, indicating that honokiol could also overcome the prosurvival effects of IL-4. Furthermore, honokiol enhanced cytotoxicity induced by fludarabine, cladribine, or chlorambucil. These data indicate that honokiol is a potent inducer of apoptosis in B-CLL cells and should be examined for further clinical application either as a single agent or in combination with other anticancer agents. (Blood. 2005;106:690-697)
Introduction
The use of plants for medicinal purposes in traditional Chinese medicine and Japanese herbal medicine has been effective in the treatment of many diseases. The root and stem bark of Magnolia species have been used for centuries in the treatment of anxiety and nervous disorders, fever, gastrointestinal symptoms, and stroke.1 One of the major chemical constituents of Magnolia species to which its therapeutic benefits are attributed is the phenolic compound honokiol.2 Honokiol can be extracted from the root, stem bark, and seed cones of several magnolia species and has a number of pharmacologic benefits, including antithrombocytic, antibacterial, anti-inflammatory, and anxiolytic effects.3-6
Honokiol is known to possess potent antineoplastic and antiangiogenic properties. In vitro studies revealed that honokiol inhibited cell proliferation and induced cytotoxicity in the human leukemia cell line HL-60.7 Previous reports have demonstrated that honokiol induces apoptosis in numerous cell lines, including murine endothelial SVR cells, human leukemia MOLT 4B cells, human colorectal carcinoma RKO cells, and human squamous lung cancer CH27 cells.8-11 Honokiol has also been reported to act as an inhibitor of reactive oxygen species,5 a property that may be of therapeutic benefit in certain neoplasias.12 In vivo studies further support the observation that honokiol is a potent antineoplastic compound. Honokiol demonstrated significant inhibitory effects in the classical 2-stage model for chemically induced skin carcinogenesis.13 Moreover, honokiol exhibited antitumor activity in mice, as demonstrated by inhibition on the in vivo growth of SVR angiosarcoma cells in nude mice.8
B-cell chronic lymphocytic leukemia (B-CLL) is a clinically heterogeneous disease characterized by the accumulation of CD5+ B lymphocytes with significant resistance to apoptosis and, therefore, prolonged survival. The impaired apoptosis that occurs in B-CLL is attributed to several mechanisms, including overexpression of B-cell CLL lymphoma 2 (Bcl-2) family members, impaired activity of cell death receptors, and/or overexpression of cytokines and angiogenic factors that support the survival of B-CLL cells.14-23 Current treatment regimens employ alkylating agents, purine analogs, monoclonal antibodies, or combinations thereof. Such combination therapies result in greater response rates than those seen with single agent-based therapy alone, but none of these therapies is curative. Thus, continued preclinical studies on innovative therapeutic strategies are warranted. The identification of new agents that interfere with the survival of B-CLL cells by promoting their apoptosis is one critical approach to improving therapeutic outcome.
Given the ability of honokiol to induce apoptosis in tumor cells, we investigated the in vitro efficacy of honokiol in primary B-CLL cells. We demonstrate that honokiol exhibits cytotoxic activity toward B-CLL cells and that B-CLL cells were more susceptible to the cytotoxic effects of honokiol compared with normal hematopoietic cells. Honokiol-induced cytotoxicity was characterized by caspase-mediated programmed cell death, which was maintained in the presence of the prosurvival cytokine interleukin-4 (IL-4). Moreover, honokiol potentiated the cytotoxicity of fludarabine, cladribine, and chlorambucil. These preclinical studies suggest that honokiol could be useful for the treatment of B-CLL.
Materials and methods
Cell isolation, culture, and reagents
Approval for these studies was obtained from the Dana Farber Cancer Institute institutional review board. Patients provided written informed consent in accordance with the Declaration of Helsinki. Peripheral blood was collected in heparin-coated tubes from patients fulfilling diagnostic criteria for B-CLL following informed consent. Mononuclear cells were isolated by Ficoll density-gradient centrifugation. Flow cytometric analysis of coexpression of CD5 and CD19 determined that the mean purity of the isolated B-CLL cells was 92% plus or minus 3.9% (range, 85.0%-97.7%). Normal peripheral blood mononuclear cells (PBMCs) were obtained from donors following informed consent and isolated in the same manner. Fresh and cryopreserved B-CLL cells were studied. The viability of fresh cells was determined by trypan blue counting and was 98.1% to 99%. For studies in which frozen cells were used, B-CLL cells were frozen in fetal bovine serum (FBS) containing 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen. The viability of cryopreserved cells was determined by trypan blue counting immediately upon thawing. Only cells whose viability exceeded 93% (range, 93.4%-99%) were used in this study. B-CLL cells were cultured in RPMI 1640 containing 10% FBS. Honokiol was obtained from Wako Chemical (Tokyo, Japan), and its purity was determined to be a minimum of 99% by high-performance liquid chromatography. Honokiol was solubilized in DMSO at a concentration of 10 mg/mL.
Reagents
Benzoyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk) (BD Pharmingen, San Diego, CA) was dissolved in DMSO and used at a concentration of 50 μM. Recombinant human IL-4 (R&D Systems, Minneapolis, MN) was solubilized in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin and used at a concentration of 25 ng/mL.
Viability assay
B-CLL cells or normal PBMCs were cultured at a density of 5 × 106/mL in RPMI 160 containing 10% FBS. Cells were left untreated or treated with various concentrations of honokiol for 6 or 24 hours, at which time 100 μL of the cell culture was transferred to a 96-well opaque-walled plate. Cell viability assays were performed in duplicate using CellTiter-Glo (Promega, Madison, WI) to measure the luminescent output from the adenosine triphosphate (ATP) present in viable cells according to the manufacturer's instructions.
Apoptosis assays
For flow cytometric analysis of apoptosis, B-CLL cells were treated with honokiol for 16 hours, and then 5 × 105 cells were removed from the culture, washed once with cold PBS, and evaluated for apoptosis by double staining with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI; Beckman Coulter, Miami, FL) in binding buffer followed by fluorescence-activated cell sorter (FACS) analysis using the FL1 and FL3 channels using a Beckman-Coulter EPICS-XL-MCL flow cytometer. To avoid nonspecific fluorescence from dead cells, live cells were gated tightly using forward and side scatter. For studies using z-VAD-fmk (BD Pharmingen) to inhibit caspase activity, 5 × 106 B-CLL cells were incubated with 50 μM z-VAD-fmk for 30 minutes prior to addition of honokiol. Cells were treated with 40 μM honokiol and were further incubated for 16 hours and analyzed for apoptosis using the annexin V-FITC/PI staining method described for apoptosis assays. To measure caspase-3 activity, B-CLL cells were treated with honokiol for 16 hours, and then 5 × 105 cells were collected by centrifugation and resuspended in 50 μL of 10 μM substrate solution containing a caspase-3 peptide substrate molecule homodoubly labeled with FITC (OncoImmunin, Gaithersburg, MD). The cell mixture was incubated at 37°C for 60 minutes followed by detection of the cleaved substrate by flow cytometric analysis using the FL1 channel.
Real-time PCR
Total cellular RNA was isolated using the RNEasy Mini kit (Qiagen, Valencia, CA). Complementary DNA was synthesized from 300 ng total RNA using the SuperScript First-Strand Synthesis kit (Invitrogen Life Technologies, Carlsbad, CA). Polymerase chain reaction (PCR) was performed in triplicate in 50-μL reaction volumes using 1.5 μL cDNA, 1 × SYBR Green master mix (Stratagene, Cedar Creek, TX), 1 μL carboxy-x-rhodamine (ROX) reference dye (1:400 dilution), and 5 pmol each primer. PCR was performed using myeloid-cell leukemia sequence 1 (Mcl-1) primers (5′-GAG ACC TTA CGA CGG GTT-3′ and 5′-TTT GAT GTC CAG TTT CCG-3′) and β-actin primers (5′-TCC CTG GAG AAG AGC TAC GA-3′ and 5′-AGC ACT GTG TTG GCG TAC AG-3′). Primers for Mcl-1 spanned exons 1 and 2, and primers for β-actin spanned exons 4 and 5. Samples were amplified in a 7500 Real Time PCR System (Applied Biosystems, Foster City, CA) for 40 cycles using the following PCR parameters: 95°C for 30 seconds, 57°C for 1 minute, and 72°C for 1 minute. Gene expression was quantitated using the comparative CT method of relative quantification using 7500 System SDS software (Applied Biosystems). The mean fold changes plus or minus SEM of the 3 replicates were calculated.
Western blot analysis
B-CLL cells were lysed in buffer containing 50 mM Tris (tris(hydroxymethyl) aminomethane)-Cl, pH 80, 250 mM NaCl, 0.5% Nonidet P-40 (NP-40), 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 2 μg/mL pepstatin. For Western analysis, 80 μg protein was resolved on 7% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to nitrocellulose membranes. Blots were incubated with antibodies that recognize caspase-8 (including procaspase-8 and cleaved forms of caspase-8; 1:5; a kind gift from Dr Junying Yuan, Harvard Medical School), caspase-9 (1 μg/mL; EMD Biosciences, San Diego, CA), bcl-2 (1 μg/mL; EMD Biosciences), Bcl2-associated protein (Bax) (0.5 μg/mL; EMD Biosciences), poly(adenosine diphosphate-ribose) polymerase (PARP) (1: 7000; a kind gift from Dr David Fisher, Dana-Farber Cancer Institute), or Mcl-1 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA; SC-819) for 1 hour at room temperature. The blots were stripped and reprobed with antibodies that recognize signal transducer and activator of transcription-3 (STAT3) (1:10 000; Santa Cruz Biotechnology; C-20) or β-actin (1:5000; Sigma-Aldrich, St Louis, MO) as loading controls. Blots were incubated with goat antirabbit, goat antirat, or goat antimouse horseradish peroxidase-conjugated secondary antibodies (Calbiochem, La Jolla, CA), and detection was performed using the Renaissance chemiluminescent ECL kit (NEN Dupont, Boston, MA).
IL-4 studies
B-CLL cells were cultured at a density of 5 × 106/mL in RPMI 160 containing 10% FBS and incubated with 25 ng/mL IL-4. After 24 hours, 40 μM honokiol was added to cultures. After an additional 24 hours, apoptosis was analyzed using the annexin V-FITC/PI staining method described above.
Combination treatments
B-CLL cells were cultured at a density of 5 × 106/mL in RPMI 160 containing 10% FBS and treated with the cell-permeable form of fludarabine (fludarabine des-phosphate) at concentrations from 0.2 to 20 μM, cladribine (2-CdA) at concentrations from 1 to 100 μM, or with chlorambucil at concentrations from 1 to 30 μM (all from Sigma-Aldrich) in the presence or absence of 25 μM honokiol. Cells were incubated for 24 hours, and then viability was assessed on 100 μL of cell culture using the luminescence-based assay as described above.
Statistical analysis
The independent Student t test was used for statistical significance between 2 groups, and paired data were evaluated by the paired Student t test. The P value was considered significant when it was less than .05.
Results
Honokiol-induced cytotoxicity toward B-CLL cells is dose and time dependent
To determine whether honokiol can induce B-CLL cell death, CLL cells from 19 untreated patients with B-CLL (Table 1 shows clinical characteristics) were incubated in vitro with various concentrations of honokiol for 6 to 24 hours and assayed for viability using a luminescent-based ATP assay. Each patient's cells were sensitive to the cytotoxic effects of honokiol (Figure 1A). The concentration of honokiol that caused death of 50% of the cells (LC50) after 6 hours of incubation with honokiol was 49 μM. Exposure to honokiol for 24 hours resulted in an LC50 of 38 μM. The viability of B-CLL cells treated with 40 μM honokiol for 24 hours was determined for each patient (Table 1). There were no apparent correlations between sensitivity to honokiol and clinical characteristics. To compare the cytotoxicity of honokiol toward B-CLL cells with normal PBMCs, viability was measured in either B-CLL cells or PBMCs isolated from healthy donors incubated with different doses of honokiol for 6 to 48 hours (Figure 1B). Although honokiol was cytotoxic toward normal PBMCs, B-CLL cells were more susceptible to the cytotoxic effects of 40 μM and 60 μM honokiol. Cell death occurred faster in the B-CLL cells compared with normal PBMCs. The viability decreased to less than 20% by 24 hours in B-CLL cells treated with 40 μM and 60 μM honokiol, whereas the viability remained above 20% in normal PBMCs until 48 hours. These findings demonstrate that honokiol induces death of B-CLL cells in a dose- and time-dependent manner and that B-CLL cells are more susceptible to honokiol-induced cell death compared with normal PBMCs.
Clinical characteristics of patients with B-CLL
Patient no. . | Sex . | Age, y . | Rai stage . | Cytogenetics . | Mutation status . | Percent viability after 24-h honokiol treatment . |
|---|---|---|---|---|---|---|
| 1 | Male | 78 | III | — | — | 11 |
| 2 | Male | 36 | I | Trisomy 12; normal p53; IgH rearranged | UM | 59.2 |
| 3 | Female | 52 | I | — | M | 5.5 |
| 4 | Male | 50 | II | del 13q | M | 26.9 |
| 5 | Male | 39 | IV | del 13q; del 17p | UM | 38 |
| 6 | Male | 57 | IV | del 9q | UM | 24.9 |
| 7 | Female | 65 | III | Trisomy 12 | — | 22.6 |
| 8 | Female | 52 | I | del 13q | M | 11.1 |
| 9 | Male | 51 | I | del 13q | M | 8.3 |
| 10 | Male | 57 | I | Normal | UM | 60 |
| 11 | Female | 52 | III | Normal | — | 30 |
| 12 | Female | 44 | II | Normal | M | 49 |
| 13 | Male | 51 | II | Normal | M | 17.2 |
| 14 | Female | 64 | I | Trisomy 12 | UM | 35.2 |
| 15 | Female | 49 | II | Normal | M | 40 |
| 16 | Male | 61 | III | del 12 centromere; del 13q | M | 44.6 |
| 17 | Female | 50 | I | Normal | UM | 31.8 |
| 18 | Male | 50 | I | Normal | UM | 11.6 |
| 19 | Female | 60 | III | — | — | 9.1 |
Patient no. . | Sex . | Age, y . | Rai stage . | Cytogenetics . | Mutation status . | Percent viability after 24-h honokiol treatment . |
|---|---|---|---|---|---|---|
| 1 | Male | 78 | III | — | — | 11 |
| 2 | Male | 36 | I | Trisomy 12; normal p53; IgH rearranged | UM | 59.2 |
| 3 | Female | 52 | I | — | M | 5.5 |
| 4 | Male | 50 | II | del 13q | M | 26.9 |
| 5 | Male | 39 | IV | del 13q; del 17p | UM | 38 |
| 6 | Male | 57 | IV | del 9q | UM | 24.9 |
| 7 | Female | 65 | III | Trisomy 12 | — | 22.6 |
| 8 | Female | 52 | I | del 13q | M | 11.1 |
| 9 | Male | 51 | I | del 13q | M | 8.3 |
| 10 | Male | 57 | I | Normal | UM | 60 |
| 11 | Female | 52 | III | Normal | — | 30 |
| 12 | Female | 44 | II | Normal | M | 49 |
| 13 | Male | 51 | II | Normal | M | 17.2 |
| 14 | Female | 64 | I | Trisomy 12 | UM | 35.2 |
| 15 | Female | 49 | II | Normal | M | 40 |
| 16 | Male | 61 | III | del 12 centromere; del 13q | M | 44.6 |
| 17 | Female | 50 | I | Normal | UM | 31.8 |
| 18 | Male | 50 | I | Normal | UM | 11.6 |
| 19 | Female | 60 | III | — | — | 9.1 |
All patients were untreated at the time of this study. Mutation status refers to immunoglobulin VH (IgVH) gene mutation status. B-CLL cells were treated in vitro with 40 μM honokiol for 24 hours, and viability was determined by measuring the level of ATP as an indicator of metabolic activity using a luminescence-based assay.
UM indicates unmutated; M, mutated; del, deletion; and -, information unavailable.
To determine whether the cytotoxicity of honokiol toward B-CLL cells was caused by apoptosis, annexin V-FITC/PI double staining was analyzed. CLL cells were incubated with various concentrations of honokiol for 16 hours, and then apoptosis was assayed using annexin V-FITC/PI double staining. Although there was little increase in apoptosis at the 20-μM concentration, exposure to 40 μM honokiol resulted in an increase in the percentage of annexin V-positive/PI-negative cells from 11.8% in the medium control compared with 23.1% in treated cells (Figure 1C). Further evidence for an increase in apoptosis is the increase in the total percentage of annexin V-positive cells, a measurement that includes secondary apoptotic cells, apparent at both the 40-μM and 50-μM concentrations.
To compare the level of apoptosis induced by honokiol in B-CLL cells with that of normal PBMCs, apoptosis was measured in either B-CLL cells or normal PBMCs incubated with different doses of honokiol for 16 hours (Figure 1D). Flow cytometric analysis of annexin V-FITC/PI double staining of multiple B-CLL samples revealed that honokiol treatment increased the mean percentage of apoptotic cells from 28.9% plus or minus 9.1% (range, 13.8%-39.7%) to 64.1% plus or minus 18.2% (range, 29.1%-88.9%) at a concentration of 40 μM and to 83.4% plus or minus 12.8% (range, 58.3%-94.7%) at a concentration of 50 μM. This corresponds to an average increase in honokiol-induced apoptosis over spontaneous apoptosis levels of 35.1% and 54.5% at concentrations of 40 μM and 50 μM honokiol, respectively. In contrast, honokiol increased the mean percentage of apoptotic cells in PBMC cultures from 22.9% plus or minus 5.2% (range, 17.1%-32.4%) to 30.1% plus or minus 9.4% and to 44.9% plus or minus 15.0% (range, 25.5%-67%) at concentrations of 40 μM and 50 μM, respectively. These data support the conclusion that B-CLL cells are more susceptible to honokiol-induced apoptosis compared with normal PBMCs.
Honokiol induces caspase-dependent apoptosis of B-CLL cells
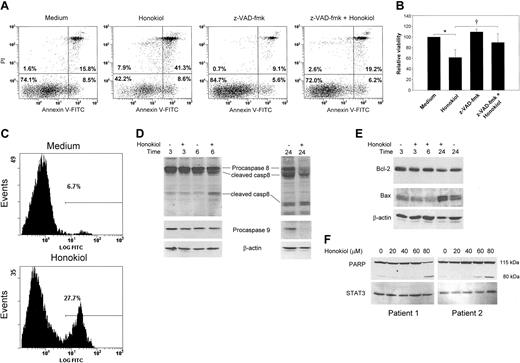
To determine whether honokiol-induced apoptosis was dependent upon caspase activation, B-CLL cells were cultured in the presence and absence of the broad-spectrum caspase inhibitor z-VAD-fmk and analyzed by annexin V-FITC/PI double staining. Pretreatment of B-CLL cells with z-VAD-fmk resulted in inhibition of honokiol-induced apoptosis (Figure 2A-B), indicating that its ability to induce cell death is dependent upon caspases. Our data also confirm previous observations24 that z-VAD-fmk also inhibited spontaneous apoptosis of B-CLL cells.
Honokiol induces B-CLL cell death and apoptosis in a dose- and time-dependent manner. (A) B-CLL cells from 19 patients were isolated and treated with varying concentrations of honokiol for 6 or 24 hours. Viability was determined by measuring the level of ATP as an indicator of metabolic activity using a luminescence-based assay. The assay was performed in duplicate, and each sample was normalized to cells incubated without drug to determine the LC50. (B) PBMCs from 19 B-CLL patients or from 7 healthy donors were isolated and treated with the indicated concentrations of honokiol for 6, 24, or 48 hours. Viability was measured as described above. Results were normalized to cells incubated without drug. Error bars represent 95% confidence intervals. (C) B-CLL cells were incubated with the indicated concentrations of honokiol for 16 hours, and then apoptosis was determined by flow cytometric analysis of annexin V-FITC/PI-stained cells. Numbers in each quadrant indicate the percentage of cells labeled with annexin V-FITC (bottom right), PI (top left), annexin V-FITC and PI (top right), or unlabeled (bottom left). (D) PBMCs from 8 B-CLL patients or from 6 healthy donors were isolated and treated with the indicated concentrations of honokiol for 16 hours, and then apoptosis was determined by flow cytometric analysis of annexin V-FITC/PI-stained cells. Apoptosis was determined by the percentage of annexin V-positive cells. Error bars represent 95% confidence intervals.
Honokiol induces B-CLL cell death and apoptosis in a dose- and time-dependent manner. (A) B-CLL cells from 19 patients were isolated and treated with varying concentrations of honokiol for 6 or 24 hours. Viability was determined by measuring the level of ATP as an indicator of metabolic activity using a luminescence-based assay. The assay was performed in duplicate, and each sample was normalized to cells incubated without drug to determine the LC50. (B) PBMCs from 19 B-CLL patients or from 7 healthy donors were isolated and treated with the indicated concentrations of honokiol for 6, 24, or 48 hours. Viability was measured as described above. Results were normalized to cells incubated without drug. Error bars represent 95% confidence intervals. (C) B-CLL cells were incubated with the indicated concentrations of honokiol for 16 hours, and then apoptosis was determined by flow cytometric analysis of annexin V-FITC/PI-stained cells. Numbers in each quadrant indicate the percentage of cells labeled with annexin V-FITC (bottom right), PI (top left), annexin V-FITC and PI (top right), or unlabeled (bottom left). (D) PBMCs from 8 B-CLL patients or from 6 healthy donors were isolated and treated with the indicated concentrations of honokiol for 16 hours, and then apoptosis was determined by flow cytometric analysis of annexin V-FITC/PI-stained cells. Apoptosis was determined by the percentage of annexin V-positive cells. Error bars represent 95% confidence intervals.
Caspase-3 is an effector caspase that plays a central role in the mitochondrial-mediated cell death pathway and is responsible for the breakdown of several cellular components involved in DNA repair and regulation. To determine whether apoptosis induced by honokiol was associated with activation of caspase-3, a fluorogenic peptide substrate that emits light upon caspase-3-dependent cleavage was incubated with B-CLL cells treated with honokiol. Flow cytometric analysis revealed that honokiol treatment resulted in a marked increase in caspase-3 activity after 16 hours (Figure 2C). Caspase-3 activity was detectable as early as 4 hours after honokiol treatment (data not shown).
To further characterize honokiol-induced apoptosis, we examined whether honokiol activates the extrinsic or intrinsic apoptotic pathway in B-CLL cells. To determine which apoptotic pathway honokiol activates, we examined patterns of proteolytic processing of caspase-8 and -9, the apical proteases in the extrinsic and intrinsic pathways, respectively, by Western analysis. Levels of procaspase-8 decreased, and levels of the cleaved, active form of caspase-8 accumulated within 6 hours of honokiol treatment (Figure 2D). Procaspase-8 levels continued to decrease, and levels of cleaved caspase-8 continued to increase through 24 hours of honokiol treatment. Levels of procaspase-9 also decreased upon honokiol treatment; however, this was not detectable until 24 hours. These data suggest that both caspase-8 and -9 are activated by honokiol but that caspase-8 activation precedes caspase-9 activation, which can be activated by caspase-8 activity in a feedback loop.25,26 The proteolytic processing patterns and kinetics are consistent with activation of the extrinsic pathway.
To further examine whether important proapoptotic and antiapoptotic regulatory proteins could be modulated by honokiol in B-CLL cells, we analyzed Bcl-2 and Bax levels by Western blotting. Bcl-2 is an antiapoptotic regulatory protein that is overexpressed in B-CLL cells.14,27 Western blot analysis revealed that exposure of B-CLL cells to honokiol had no effect on Bcl-2 levels (Figure 2E). In contrast, the expression of Bax, a protein that can promote apoptosis, increases following honokiol treatment. The combination of caspase-3 activation, caspase-8 activation, decreased levels of caspase-9, and up-regulation of Bax explains, in part, the onset of apoptosis induced by honokiol in B-CLL cells.
To further characterize the cytotoxic action of honokiol, we examined cleavage of the DNA repair enzyme poly(adenosine diphosphate-ribose) polymerase (PARP), the substrate for activated caspase-3. The amount of cleaved PARP, as demonstrated by the appearance of the 85 kDa cleavage product, increased following honokiol treatment of B-CLL cells in a dose-dependent manner (Figure 2F). Caspase activity and PARP cleavage are intracellular signs of activation of the apoptotic machinery. Altogether, these data demonstrate that honokiol induces cytotoxicity of B-CLL cells through an apoptotic mechanism dependent upon caspase activation.
Honokiol modulates the level of the antiapoptotic protein Mcl-1
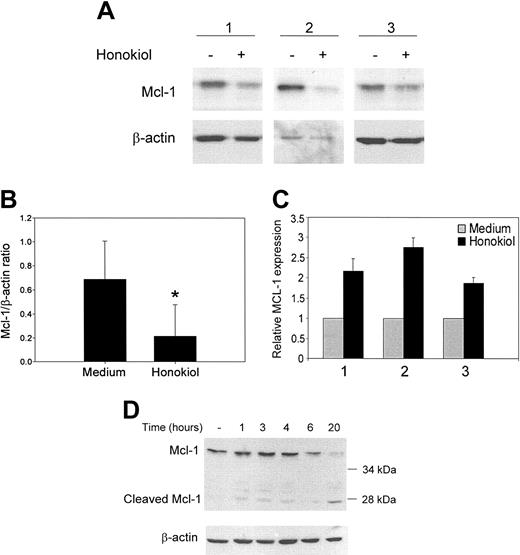
Mcl-1 is a prosurvival member of the Bcl-2 family of apoptotic regulatory proteins whose expression levels in B-CLL cells are associated with response to treatment both in vitro and in vivo.15,28 Previous studies in tissue culture have shown that persistent Mcl-1 expression is strongly associated with resistance to chlorambucil and fludarabine.15 Clinical studies have demonstrated that low Mcl-1 levels are found in patients who achieve complete remission after treatment.28 The level of Mcl-1 expression was found to be the best measure of clinical response in B-CLL patients. Thus, we examined whether honokiol could modulate the level of Mcl-1 in B-CLL cells in vitro. B-CLL cells were incubated with honokiol for 24 hours and examined for changes in Mcl-1 expression by Western analysis. Honokiol treatment caused approximately a 3-fold decrease in Mcl-1 expression in 6 of 7 patients' cells over the time period during which honokiol induces apoptosis (Figure 3A-B).
Honokiol induces caspase-dependent apoptosis of B-CLL cells. (A-B) PBMCs from B-CLL patients were pretreated with or without 50 μM z-VAD-fmk for 30 minutes and then incubated in the presence or absence of 40 μM honokiol for 16 hours. Apoptosis was determined by annexin V-FITC/PI double staining. The numbers in each quadrant indicate the percentage of cells labeled with annexin V-FITC (bottom right), PI (top left), or annexin V-FITC and PI (top right), or the percent unlabeled (bottom left). A representative example is shown in panel A, and panel B represents the means of PI-negative cells ± standard deviation from 5 patients' cells. Results were normalized to cells incubated without drug. *P < .001 when cells treated with honokiol were compared with cells incubated in medium alone. †P < .05 when cells treated with honokiol were compared with cells pretreated with z-VAD-fmk and honokiol. (C) B-CLL cells were incubated with or without 40 μM honokiol for 16 hours. Cells were analyzed for caspase-3 activity using a fluorogenic peptide substrate. The histogram is representative of 4 experiments. The gate indicates the percentage of cells containing active caspase 3. (D-E) B-CLL cells were incubated with or without 40 μM honokiol for the indicated times. Cells were lysed, and Western analysis was performed using antibodies that recognize (D) the proform of caspase-8 as well as caspase-8 cleavage products or the proform of caspase-9 and (E) Bcl-2 or Bax. Blots were stripped and reprobed for β-actin as a loading control. One representative example of 4 is shown. (F) CLL cells were incubated for 4 hours with 20, 40, 60, and 80 μM honokiol or left untreated. Cells were lysed, and Western analysis was performed using antibodies that recognize the caspase-3 substrate, PARP. The proform of PARP is 115 kDa, and the cleaved, activated form is 80 kDa. The blot was stripped and reprobed for STAT3 as a loading control. Two representative examples of 6 are shown.
Honokiol induces caspase-dependent apoptosis of B-CLL cells. (A-B) PBMCs from B-CLL patients were pretreated with or without 50 μM z-VAD-fmk for 30 minutes and then incubated in the presence or absence of 40 μM honokiol for 16 hours. Apoptosis was determined by annexin V-FITC/PI double staining. The numbers in each quadrant indicate the percentage of cells labeled with annexin V-FITC (bottom right), PI (top left), or annexin V-FITC and PI (top right), or the percent unlabeled (bottom left). A representative example is shown in panel A, and panel B represents the means of PI-negative cells ± standard deviation from 5 patients' cells. Results were normalized to cells incubated without drug. *P < .001 when cells treated with honokiol were compared with cells incubated in medium alone. †P < .05 when cells treated with honokiol were compared with cells pretreated with z-VAD-fmk and honokiol. (C) B-CLL cells were incubated with or without 40 μM honokiol for 16 hours. Cells were analyzed for caspase-3 activity using a fluorogenic peptide substrate. The histogram is representative of 4 experiments. The gate indicates the percentage of cells containing active caspase 3. (D-E) B-CLL cells were incubated with or without 40 μM honokiol for the indicated times. Cells were lysed, and Western analysis was performed using antibodies that recognize (D) the proform of caspase-8 as well as caspase-8 cleavage products or the proform of caspase-9 and (E) Bcl-2 or Bax. Blots were stripped and reprobed for β-actin as a loading control. One representative example of 4 is shown. (F) CLL cells were incubated for 4 hours with 20, 40, 60, and 80 μM honokiol or left untreated. Cells were lysed, and Western analysis was performed using antibodies that recognize the caspase-3 substrate, PARP. The proform of PARP is 115 kDa, and the cleaved, activated form is 80 kDa. The blot was stripped and reprobed for STAT3 as a loading control. Two representative examples of 6 are shown.
Previous studies have demonstrated that Mcl-1 mRNA is up-regulated as part of an initial rapid cellular response to cytotoxic stimuli such as chemotherapeutic agents, UV irradiation, calcium ionophores, and pneumococcal infection.29-31 To determine whether honokiol could similarly affect Mcl-1 gene expression in B-CLL cells, we examined Mcl-1 mRNA levels. Real-time PCR was performed on B-CLL cells from 3 patients treated with honokiol for 3 hours. In each case, Mcl-1 mRNA increased in response to honokiol (Figure 3C). Thus, honokiol falls into the category of apoptosis-inducing agents that initially up-regulate Mcl-1 mRNA. To further characterize the finding of increased Mcl-1 mRNA, we examined the expression of the Mcl-1 protein in a more detailed time course between 1 hour and 24 hours after exposure to honokiol (Figure 3D). A modest increase in Mcl-1 protein was observed 3 to 4 hours following honokiol exposure followed by a decrease in Mcl-1 protein within 6 hours that continued through 20 hours. The expression of Mcl-1 protein following honokiol exposure appeared to be biphasic, again consistent with previous studies demonstrating a transient increase in Mcl-1 protein during the initial stages of an apoptotic response. Down-regulation of full-length Mcl-1 protein was likely due to posttranslational regulation by caspases because we observed the accumulation of an approximately 28-kDa cleavage product, consistent with caspase cleavage. Altogether, these data indicate that honokiol treatment modulates Mcl-1 expression at both the transcriptional and posttranslational levels.
Honokiol overcomes IL-4-mediated B-CLL cell survival
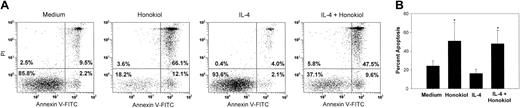
IL-4 is a cytokine known to be important in promoting the survival of B-CLL cells in vitro at concentrations between 1 and 25 ng/mL as well as in vivo and may be an important factor in resistance to therapy.24,32-34 Also, the combination of IL-4 and fludarabine, a nucleoside analog used in the clinical management of B-CLL, causes most B-CLL cells to become less susceptible to fludarabine-induced apoptosis.35 To determine whether honokiol could induce apoptosis in B-CLL cells in the presence of IL-4, B-CLL cells were pretreated with IL-4 for 24 hours, after which time honokiol was added. Twenty-four hours later, cells were analyzed for apoptosis. IL-4 treatment decreased the percentage of apoptotic cells from 24% to 16%. Yet the prosurvival effects of IL-4 failed to prevent honokiol-induced apoptosis (Figure 4). In cultures pretreated with IL-4, honokiol induced apoptosis in 48% of the cells, which was nearly equivalent to the percentage of apoptotic cells in cultures treated with honokiol alone (ie, 50%). These data indicate that honokiol remained active in the presence of IL-4. Thus, IL-4 does not increase the in vitro drug resistance of B-CLL cells exposed to honokiol, in contrast to other drugs that induce apoptosis of B-CLL cells, such as fludarabine.
Honokiol down-regulates the survival protein Mcl-1. (A) B-CLL cells were incubated with 40 μM honokiol for 24 hours. Cells were lysed, and Western analysis was performed using antibodies that recognize Mcl-1. Blots were stripped and reprobed for β-actin as a loading control. Three representative patients' samples are shown out of 6 examined. (B) Films from the immunoblots were scanned, and mean intensities were calculated using Kodak Digital Science 1D image analysis software (Eastman Kodak, New Haven, CT). The ratio of Mcl-1 to β-actin was then calculated for cells treated with or without honokiol, and data are shown as mean ± standard deviation. *P < .02 when compared with cells treated with medium alone. (C) B-CLL cells treated for 3 hours with or without 40 μM honokiol were analyzed for Mcl-1 mRNA expression by real-time PCR using SYBR green technology Mcl-1 levels were normalized to β-actin. (D) B-CLL cells were incubated with 40 μM honokiol for the indicated times and then lysed and analyzed for Mcl-1 expression by Western blotting as described above.
Honokiol down-regulates the survival protein Mcl-1. (A) B-CLL cells were incubated with 40 μM honokiol for 24 hours. Cells were lysed, and Western analysis was performed using antibodies that recognize Mcl-1. Blots were stripped and reprobed for β-actin as a loading control. Three representative patients' samples are shown out of 6 examined. (B) Films from the immunoblots were scanned, and mean intensities were calculated using Kodak Digital Science 1D image analysis software (Eastman Kodak, New Haven, CT). The ratio of Mcl-1 to β-actin was then calculated for cells treated with or without honokiol, and data are shown as mean ± standard deviation. *P < .02 when compared with cells treated with medium alone. (C) B-CLL cells treated for 3 hours with or without 40 μM honokiol were analyzed for Mcl-1 mRNA expression by real-time PCR using SYBR green technology Mcl-1 levels were normalized to β-actin. (D) B-CLL cells were incubated with 40 μM honokiol for the indicated times and then lysed and analyzed for Mcl-1 expression by Western blotting as described above.
Honokiol induces apoptosis in the presence of IL-4. PBMCs from B-CLL patients were pretreated with 25 ng/mL IL-4 for 24 hours and then treated with 40 μM honokiol and examined for apoptosis using annexin V-FITC double staining. The numbers in each quadrant indicate the percentage of cells labeled with annexin V-FITC (bottom right), PI (top left), or annexin V-FITC and PI (top left), or the percentage unlabeled (bottom left). A representative example is shown in panel A, and data from 7 patients are shown in panel B. Apoptotic cells were defined as the percentage of cells that were annexin-V positive, and data are shown as mean ± standard deviation. *P < .005 when cells incubated with either honokiol or honokiol and IL-4 were compared with cells incubated in medium alone.
Honokiol induces apoptosis in the presence of IL-4. PBMCs from B-CLL patients were pretreated with 25 ng/mL IL-4 for 24 hours and then treated with 40 μM honokiol and examined for apoptosis using annexin V-FITC double staining. The numbers in each quadrant indicate the percentage of cells labeled with annexin V-FITC (bottom right), PI (top left), or annexin V-FITC and PI (top left), or the percentage unlabeled (bottom left). A representative example is shown in panel A, and data from 7 patients are shown in panel B. Apoptotic cells were defined as the percentage of cells that were annexin-V positive, and data are shown as mean ± standard deviation. *P < .005 when cells incubated with either honokiol or honokiol and IL-4 were compared with cells incubated in medium alone.
Honokiol enhances the cyotoxicity of fludarabine, cladribine, and chlorambucil. B-CLL cells were incubated with the indicated concentrations of fludarabine, cladribine, or chlorambucil in the presence or absence of 25 μM honokiol for 24 hours, at which time cell viability was assessed. The assay was performed in duplicate, and each sample was normalized to untreated cells. Data from 6 patients' samples (patients 1, 4, 6, 9, 11, and 17) are represented as mean ± SEM. *P < .05 when cells incubated with the combination of honokiol and nucleoside analog or chlorambucil were compared with cells incubated with nucleoside analog or chlorambucil alone.
Honokiol enhances the cyotoxicity of fludarabine, cladribine, and chlorambucil. B-CLL cells were incubated with the indicated concentrations of fludarabine, cladribine, or chlorambucil in the presence or absence of 25 μM honokiol for 24 hours, at which time cell viability was assessed. The assay was performed in duplicate, and each sample was normalized to untreated cells. Data from 6 patients' samples (patients 1, 4, 6, 9, 11, and 17) are represented as mean ± SEM. *P < .05 when cells incubated with the combination of honokiol and nucleoside analog or chlorambucil were compared with cells incubated with nucleoside analog or chlorambucil alone.
Low-dose honokiol augments the cytotoxicity of fludarabine, cladribine, and chlorambucil
Given that honokiol appeared to lower the apoptotic threshold of B-CLL cells, we considered the possibility that low doses of honokiol might potentiate the effects of chemotherapeutic agents used in the clinical management of B-CLL. B-CLL cells were incubated for 24 hours with a nucleoside analog, either fludarabine or cladribine, or an alkylating agent, chlorambucil, in the presence or absence of low-dose honokiol (Figure 5). There was virtually no cytotoxicity induced by 0.2 μM fludarabine alone; however, addition of honokiol caused a 35% decrease in cell viability. At each concentration of fludarabine tested, the addition of low-dose honokiol (25 μM) enhanced cell killing. Similarly, the combination of honokiol and cladribine, at concentrations ranging from 1 to 100 μM, caused a progressive increase in cytotoxicity over that seen with cladribine alone. The cytotoxic effect of chlorambucil was enhanced by honokiol to an even greater extent compared with the effects of fludarabine and cladribine. While virtually no cell death was caused by the lowest dose of chlorambucil, there was a 44% decrease in viability when combined with honokiol, which was 30% more cell death than in cells treated with honokiol alone. This trend in greater than additive effects was continued at both of the higher concentrations of chlorambucil as well. Thus, the combination of a low concentration of honokiol with nucleoside analogs as well as the alkylating agent chlorambucil enhances their cytotoxic effects.
Discussion
B-CLL is a disease characterized by the accumulation of apoptotic-resistant B cells. The natural product honokiol was reported to induce apoptosis in a variety of tumor cells. Therefore, we investigated whether honokiol could overcome the apoptotic resistance inherent in B-CLL cells. We found that honokiol acts directly on B-CLL cells to induce cytotoxicity in a manner that causes caspase-dependent apoptosis within 16 hours. Our data, the first in primary patient tumor cells, support previous work demonstrating the activity of honokiol in cell lines and tumor models. Honokiol induced cytotoxicity toward B-CLL cells at concentrations that are minimally toxic to normal PBMCs. Investigating the mechanism by which B-CLL cells undergo apoptosis in response to honokiol treatment, we found that apoptosis occurred in manner that depended upon caspase activation. Furthermore, exposure of B-CLL cells to honokiol modulated the expression of key apoptotic regulatory proteins, including the important Bcl-2 family member, Mcl-1, which is a molecular change associated with positive clinical outcome. Finally, honokiol-induced cytotoxicity could not be overcome by preincubation of the cells with IL-4, an antiapoptotic survival factor for B-CLL cells.
Natural products have been the source of many medically beneficial drugs, and their importance in the prevention and treatment of cancer is becoming increasingly apparent. Natural products, including bryostatin 1, triterpenoids, and (-)-epigallocatechin gallate (EGCG), a polyphenol found in green tea, and their synthetic derivatives have previously been reported to demonstrate activity against B-CLL cells, and some of these compounds have entered clinical trials for B-CLL and other indolent B-cell malignancies.36-39 In addition, a Chinese herbal extract associated with a sustained complete remission in a B-CLL patient showed direct cytotoxicity to B-CLL cells in vitro.40 Thus, there is reason to consider the use of medicinal botanicals and other natural compounds, perhaps in combination with existing therapies, in the treatment of B-CLL.
To determine whether honokiol modulated the expression of proteins important in promoting the survival of B-CLL cells and whose expression is associated with prognosis, we focused on Mcl-1. Mcl-1 is a member of the Bcl-2 gene family, which includes both proapoptotic and antiapoptotic factors. Alterations in the balance of the corresponding proteins are commonly found in hematologic malignancies. A high ratio of Bcl-2/Bax expression and high expression of Mcl-1 are considered to contribute to the pathogenesis of B-CLL.15,41 Of importance, an association between high Mcl-1 expression and the inability to achieve complete remission has been reported, indicating that higher Mcl-1 protein expression is an indicator of adverse outcome for B-CLL patients.28 In one study, Mcl-1 was the only protein among a panel of antiapoptotic proteins studied found to be associated with chemoresistance in vitro and failure to achieve complete response in B-CLL patients.15 Conversely, patients who achieved complete remission exhibited low Mcl-1 levels, and in vitro exposure to chemotherapeutic agents caused a reduction in its relative levels. Thus, it was important to perform in vitro studies as a first step toward determining whether honokiol could affect Mcl-1 expression in B-CLL patients. We found that Mcl-1 levels were reduced in honokiol-treated B-CLL cells, ranging from 1.3-fold to 12-fold in 6 of 7 patients' cells, and that Mcl-1 undergoes a complex multistep regulatory process. Down-regulation of Mcl-1 presumably alters the balance of proapoptotic and antiapoptotic proteins and would facilitate mitochondrial activation of programmed cell death. Mcl-1 expression is emerging as a prognostic determinant of outcome and response.42 Our finding that honokiol overcomes the apoptosis resistance present in B-CLL cells at least in part by down-regulation of Mcl-1 bears potential significant clinical relevance.
Another clinically relevant observation of our study was that IL-4 does not protect against the proapoptotic effects of honokiol. IL-4 is a major survival factor for B-CLL cells and protects cells against spontaneous apoptosis in tissue culture. The prosurvival effect of IL-4 is thought to be due to its ability to up-regulate Bcl-2 protein levels in B-CLL cells in vitro.20,43 In the present study, cells were cultured for 24 hours with IL-4 to allow sufficient time to induce prosurvival effects before honokiol was added; yet honokiol remained active and could still induce apoptosis in the presence of the cytokine. This is in contrast to other treatments, such as fludarabine, chlorambucil, and prednisone, whose cytotoxicity is diminished by IL-4.32 Our findings suggest that the cytotoxic effects of honokiol may be able to overcome the supportive role of the tumor microenvironment, which provides cytokines and factors that support the survival of B-CLL cells.
An important observation of our study is that honokiol, perhaps by lowering the apoptotic threshold, enhances the cytotoxic effects of other chemotherapeutic agents commonly used in the treatment of B-CLL. This reduced viability was evident when honokiol was used in combination with nucleoside analogs or with chlorambucil. This suggests that the combining of a low dose of honokiol with other anticancer drugs may be a potential therapeutic strategy.
Further investigation is needed to determine whether honokiol can be applied clinically for the treatment of B-CLL; however, pharmacokinetic studies in animals and studies from clinical trials investigating herbal remedies that contain honokiol suggest that honokiol may be a safe and potent chemotherapeutic agent. Pharmacokinetic studies in mice revealed that honokiol is readily absorbed and maintained in the plasma for more than 10 hours.44 The plasma concentration attainable in mice administered 10 mg/kg or 250 mg/kg honokiol was 5 μg/mL and 1000 μg/mL, respectively, which corresponds to concentrations that approximate or exceed the levels that induce cytotoxicity of tumor cells in vitro.44,45 Administration of 3 mg/d honokiol in mice (ie, 120 mg/kg) for 30 days was well tolerated.8 Honokiol is one of the active compounds in the Japanese herbal medicine, Saiboku-to, which has traditionally been consumed as a tea for its antianxiety effects and has undergone clinical evaluation in Japan for the treatment of asthma.46 Symptomatic improvement was reported in the honokiol-treated asthma patients; however, higher-quality clinical trials that address both the efficacy as well as the potential adverse effects that occur with the use of herbal medicines are needed.
In conclusion, honokiol may be an effective therapeutic agent in the treatment of B-CLL, and thus clinical studies with honokiol may be appropriate. Given its ability to overcome apoptotic resistance, honokiol may also be effective in other hematopoietic malignancies. Further investigation of honokiol in mouse models of B-CLL and other leukemias will contribute to additional understanding of its in vivo activity toward malignant cells and its potential toxicity toward normal tissues. Finally, efforts to elucidate the molecular target of honokiol are ongoing and will aid in its clinical application.
Prepublished online as Blood First Edition Paper, March 31, 2005; DOI 10.1182/blood-2004-11-4273.
Funding support for this work was donated by the family and friends of Emma Paige Carchia-Tullo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal