Abstract
Autoantibodies neutralizing human ADAMTS13 (a disintegrin-like and metalloproteinase with thrombospondin type 1 motif), the metalloprotease that physiologically cleaves von Willebrand factor, are a major cause of severe deficiency of the protease and of acquired thrombotic thrombocytopenic purpura (TTP). We evaluated prevalence of anti-ADAMTS13 antibodies in 59 patients with thrombotic microangiopathies (TMAs) and in 160 patients with immunologic or thrombocytopenic diseases different from TTP, using an enzyme-linked immunosorbent assay (ELISA). Immunoglobulin G (IgG) antibodies directed against ADAMTS13 were found in 97% of untreated patients with acute acquired TMA who had plasma levels of ADAMTS13 activity below 10%. The corresponding prevalence of IgM antibodies was 11%. In contrast, anti-ADAMTS13 antibodies of G or M isotypes were detected in 20% of patients with TMA with ADAMTS13 activity above 10%. The ELISA was more sensitive than the standard functional inhibitor assay for detecting antibodies against ADAMTS13. Patients with thrombocytopenia from various causes (n = 50), systemic lupus erythematosus (SLE; n = 40), and the antiphospholipid antibody syndrome (APS; n = 55) had prevalences of IgG antibodies of 8%, 13%, and 5% respectively, only slightly higher than the prevalence in 111 healthy donors (4%). A rather high prevalence of anti-ADAMTS13 IgM antibodies was found in patients with SLE and APS (18% each). The clinical significance of IgM antibodies in these groups is unclear. In conclusion, the ELISA method detected anti-ADAMTS13 IgG antibodies in a very large proportion of patients with acquired TMA associated with severe ADAMTS13 deficiency, and was more sensitive than the inhibitor assay.
Introduction
Thrombotic microangiopathies (TMAs) are a heterogeneous group of diseases characterized by microangiopathic hemolytic anemia and thrombocytopenia due to platelet clumping in the microcirculation leading to ischemic organ dysfunction with neurologic symptoms and renal impairment. The 2 principal forms of TMA are the hemolytic-uremic syndrome (HUS), in which severe renal failure prevails, and thrombotic thrombocytopenic purpura (TTP), characterized by the systemic occlusion of the microcirculation that frequently affects the central nervous system.1-4
von Willebrand factor (VWF) plays an important mechanistic role in TTP. Upon stimulation of endothelial cells, this adhesive glycoprotein is released from storage organelles (Weibel-Palade bodies) as unusually large multimers (ULVWF) that are physiologically cleaved by the plasma metalloprotease ADAMTS13 and degraded into smaller multimers ranging in size from 500 kDa to approximately 20 000 kDa.5 On the basis of in vitro studies using artificial flow systems, the following pathogenetic mechanisms are postulated in TTP. Upon release, ULVWF multimers remain anchored to the endothelial-cell surface in a P-selectin–dependent manner, and form extraordinary long strings.6,7 ADAMTS13 docks to the endothelial cells through binding to both the A1 and A3 domains of the mature VWF subunit6,7 and cleaves VWF within the A2 domain at the tyrosine 1605–methionine 1606 bond. Failure of this physiologic processing of ULVWF is thought to favor the adhesion of platelets to these highly thrombogenic multimers, resulting in platelet aggregation and the occlusion of the microcirculation by platelet-rich thrombi, the pathophysiologic hallmark of TTP.
A severe deficiency of the VWF-cleaving protease, ADAMTS13, was reported to be a specific finding of acute TTP.8-10 Two main forms of TTP are distinguished. Hereditary TTP (Upshaw-Schulman syndrome) is the result of a constitutional deficiency of ADAMTS13 (a disintegrin-like and metalloproteinase with thrombospondin type 1 motif) due to compound heterozygous or homozygous mutations in the ADAMTS13 gene,11-18 whereas the acquired form is often caused by circulating autoantibodies (inhibitors) that neutralize ADAMTS13 activity.8,9,19 Observation of a patient with TTP and severe acquired ADAMTS13 deficiency who lacked neutralizing antibodies but had nonneutralizing IgG and IgM antibodies provided evidence for an additional mechanism for acquired ADAMTS13 deficiency.20 Recently, we developed a new enzyme-linked immunosorbent assay (ELISA) using immobilized recombinant ADAMTS13 to detect anti-ADAMTS13 autoantibodies.20 Using this assay, we investigated the prevalence of these antibodies in patients with acute TMA with or without severe ADAMTS13 deficiency. Furthermore, we established their frequency in healthy donors and in various immunologic diseases.
Patients, materials, and methods
Anti-His tag antibody was purchased from Qiagen (Hilden, Germany), alkaline phosphatase–conjugated goat anti–human IgG (Fc specific), alkaline phosphatase–conjugated goat anti–human IgM (μ chain specific), and p-nitrophenyl phosphate (PNPP) were from Sigma (St Louis, MO). Recombinant C-terminally His-tagged ADAMTS13 was constructed by the in-frame fusion of 6 histidine and 3 glycine residues as a linker with the C-terminal threonine residue (Thr-1427) of full-length ADAMTS13, and expressed in human embryonic kidney cells (HEK293) as described.21
Diagnostic criteria and patient features
The 4 participating centers (center 1: Bern, Switzerland; center 2: Frankfurt, Germany; center 3: Bergamo, Italy; and center 4: Milano, Italy) provided frozen plasma samples of patients diagnosed with acquired or hereditary TMA. Acquired TMA was either idiopathic or associated with such clinical conditions as cancer, bone marrow transplantation, and drug intake. Diagnostic criteria and results obtained in some of these patients have been previously reported.22-25 In brief, all patients given the comprehensive diagnosis of TMA (n = 59) had thrombocytopenia and microangiopathic hemolytic anemia with no apparent alternative etiology. A tentative diagnosis of TTP was made when symptoms of focal neurologic ischemia dominated the clinic picture (n = 51). A tentative diagnosis of HUS was made in patients with prevailing symptoms of acute renal failure (n = 4). Enterohemorrhagic E coli–associated, diarrhea-positive HUS was diagnosed in only one of those patients. In another 4 cases it was not possible to make a diagnosis of TTP or HUS, so those patients were referred to as having TMA.
Patients with TMA were assigned to 3 groups according to the clinical and laboratory information provided by the participating centers: (1) acute untreated TMA with severe ADAMTS13 deficiency (conventionally established at < 10% of normal plasma; n = 36); (2) acute untreated TMA without severe ADAMTS13 deficiency (ADAMTS13 activity > 10%; n = 15); and (3) hereditary forms of TMA (n = 8). Informed consent was provided according to the Declaration of Helsinki. Plasma samples from 111 blood donors and from 50 patients with thrombocytopenia associated with various underlying diseases other than TMA, whose clinical characteristics were previously described,10 were provided by center 1. Samples (n = 36) from patients with systemic lupus erythematosus (SLE), whose clinical and laboratory characteristics have been described,26 were provided by center 4. A subset of 4 additional SLE plasma samples was provided by center 2. Plasma samples from patients with the antiphospholipid antibody syndrome (APS) complicated by thromboembolic events (venous thromboembolism, ischemic stroke, myocardial infarction, or peripheral arterial thrombosis), positive or negative for lupus anticoagulant, were provided by center 2 (n = 10) and center 4 (n = 45).27 Center 4, in addition, provided 15 plasma samples from patients with severe hemophilia A complicated by anti-FVIII alloantibodies.
Determination of ADAMTS13 activity and anti-ADAMTS13 inhibitory activity
ADAMTS13 activity and anti-ADAMTS13 inhibitory activity were assessed using 3 different assays: quantitative immunoblotting of purified, protease-free VWF degraded by BaCl2-activated diluted plasma ADAMTS138,28 in center 1, residual ristocetin cofactor activity of degraded VWF29 in center 2, and residual collagen-binding activity of degraded VWF30 in centers 3 and 4.
Anti-ADAMTS13 antibody detection by ELISA
Anti-His tag antibody (1 μg/mL) was coated onto the surface of ELISA plates (Maxisorp; Nunc, Rochester, NY) in a volume of 100 μL/well for 3 hours at room temperature. Free binding sites were blocked for 2 hours at room temperature with phosphate-buffered saline (PBS), containing 2% (wt/vol) bovine serum albumin (PBS-BSA). Recombinant His-tagged ADAMTS13 was added at a concentration of 2 μg/mL to a final volume of 100 μL. After 3 hours of incubation at room temperature wells were washed 5 times with Tris {tris(hydroxymethyl)aminomethane}-buffered saline (TBS) containing 0.1% (vol/vol) Tween 20 (TBST). Patient plasma samples were initially diluted 1:20, 1:50, and 1:100 in PBS-BSA; for accurate antibody titration, positive samples were further diluted up to 1:3200. The volume of diluted plasma was 100 μL and incubation time was overnight at 4°C. Wells were washed 5 times with PBS and secondary detection antibodies were added. Anti-IgG antibody was diluted 1:70 000 and anti-IgM antibody was diluted 1:10 000 in PBS-BSA, respectively. Antibodies were incubated for 2 hours at room temperature and unbound antibodies were removed by 5 washing steps using PBS. Bound antibodies were detected by enzymatic reaction using PNPP as substrate (1 mg/mL) in an alkaline phosphatase buffer (50 mM Tris, pH 10.0, 150 mM NaCl, 2 mM MgCl2). Color reaction was measured at 405 nm versus 620 nm as a reference after 45 minutes of incubation.
Inter-assay precision was determined by evaluating a single normal human plasma (NHP) pool and a reference TTP plasma (positive for anti-ADAMTS13 IgG and IgM antibody titers) in 27 and 20 consecutive assay runs for IgG and for IgM detection, respectively, on different days. The results were expressed as the interassay percent CV (coefficient of variation) according to the standard formula: % CV = SD /mean × 100. The inter-assay percent CV for both assays was found to be less than 20% and therefore in the range considered to be acceptable for immunoassays.31
Data analysis and statistical calculations
The ELISA read out (optical density [OD]) from 111 healthy donors tested for IgG and IgM antibodies against ADAMTS13 was used to calculate the ratio of sample OD to background OD for each sample on each plate. As background OD, we used the signal derived from an NHP pool of at least 25 donors. To achieve an approximate normal distribution, the OD ratio values of the 111 control plasma samples were transformed by the function y = log (1 + log (x)). Normal distribution was assessed by quantile-quantile plots and Shapiro-Francia tests.32 Then the means and standard deviations of the resulting 111 ratios of several ELISA plates were calculated. The thresholds were obtained by back-transformation of the means plus 2 standard deviations of the transformed data, resulting in a cut-off level of 1.5 for IgG and 3.3 for IgM. Samples with a ratio below 1.5 and 3.3 for IgG and IgM autoantibodies, respectively, were judged negative. When a series of sample dilutions was compared with the cut-off value, the last dilution above cut-off value was taken as positive.
Results
Anti-ADAMTS13 antibodies in healthy donors
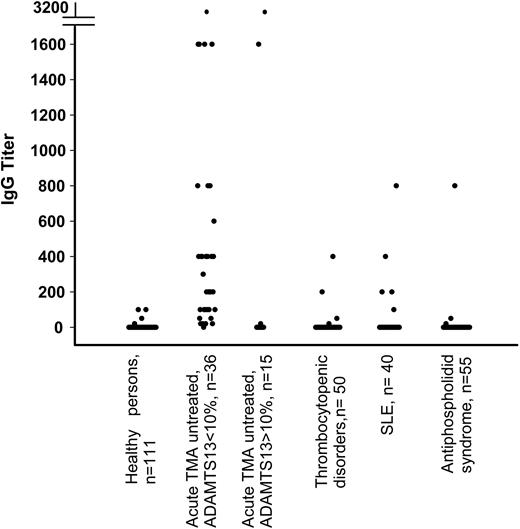
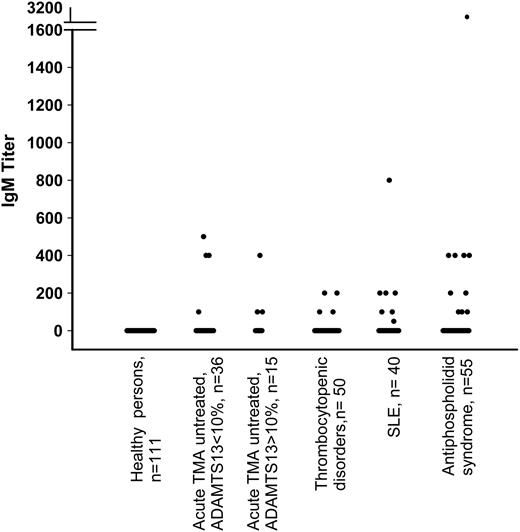
Plasma samples from 111 healthy control donors were investigated for the presence of anti-ADAMTS13 antibodies. Low titers of IgG antibodies ranging between 20 and 100 were detected in 4 of 111 healthy control donors (3.6%) (Figure 1) who lacked anti-ADAMTS13 neutralizing activity by inhibitor assays. The presence of anti-ADAMTS13 IgG antibodies was confirmed by radioimmune precipitation experiments (data not shown). The ADAMTS13 activity levels in the 4 healthy donors with IgG antibodies were within the normal range (between 60% and 92% of normal). No healthy donor plasma contained IgM antibodies (Figure 2).
Anti-ADAMTS13 IgG antibody titers in healthy donors, patients with TMA, thrombocytopenic disorders, and autoimmune diseases other than TMA. Truncation of y-axis is indicated by horizontal bars.
Anti-ADAMTS13 IgG antibody titers in healthy donors, patients with TMA, thrombocytopenic disorders, and autoimmune diseases other than TMA. Truncation of y-axis is indicated by horizontal bars.
Anti-ADAMTS13 antibodies in patients with acute TMA
Patients with acute untreated TMA and severe to borderline-severe ADAMTS13 deficiency (<10%). Anti-ADAMTS13 antibodies were measured in 36 patients with acute TMA in plasma samples obtained before starting any treatment. Patients had acute idiopathic TTP (n = 33) or drug-related (n = 2) TMA and one patient had TMA associated with metastatic lung cancer. Platelet counts ranged from 5 × 109/L to 92 × 109/L. Anti-ADAMTS13 IgG antibodies were detected in 35 of 36 patients (97%), with titers ranging from 20 to 3200 (Figure 1). There were 4 anti-ADAMTS13 IgG–positive patients who also tested positive for anti-ADAMTS13 IgM (Figure 2). Table 1 gives details of 7 patients who had divergent results for assays of ADAMTS13 neutralizing antibodies (inhibitors) and ELISA. Six of these patients were negative in the inhibitor assay, but anti-ADAMTS13 IgG antibodies, with titers ranging from 200 to 3200 (Table 1, P1-P6), were detected by ELISA. Conversely, one patient was positive in the inhibitor assay (1.5 BU/mL) but the ELISA detected neither IgG nor IgM antibodies (Table 1, P7).
Clinical presentation and laboratory findings in untreated patients with acute TMA
Patient ID . | Diagnosis . | Age . | Sex . | Platelets, × 109/L . | ADAMTS13 activity, % . | Inhibitor, BU/mL . | IgG titer . | IgM titer . |
|---|---|---|---|---|---|---|---|---|
| P1 | Post-ticlopidine TMA | 68 | M | 14 | < 5 | Neg | 3200 | 400 |
| P2 | Cancer-associated TMA | 39 | M | NA | 8 | Neg | 1600 | 100 |
| P3 | Idiopathic TTP | 70 | F | 9 | < 3 | Neg | 600 | 400 |
| P4 | Idiopathic TTP | 35 | F | 9 | < 6 | Neg | 300 | Neg |
| P5 | Idiopathic TTP | 62 | F | NA | < 3 | Neg | 400 | Neg |
| P6 | Idiopathic TTP | 56 | M | 57 | < 6 | Neg | 200 | Neg |
| P7 | Idiopathic TTP | 58 | F | 92 | < 6 | 1.5 | Neg | Neg |
| P8 | Idiopathic TTP | 36 | F | 13 | 14 | 2 | 20 | Neg |
| P9 | Idiopathic TTP | 32 | F | 10 | 16 | ND | 1600 | Neg |
| P10 | Idiopathic TTP | 17 | F | 59 | 11 | 1.5 | 3200 | Neg |
| P11 | Unspecified TMA | 34 | M | NA | 35 | ND | Neg | 100 |
| P12 | HUS | 23 | M | 36 | 79 | Neg | Neg | 400 |
| P13 | HUS | 0.5 | M | NA | 40 | ND | Neg | 100 |
Patient ID . | Diagnosis . | Age . | Sex . | Platelets, × 109/L . | ADAMTS13 activity, % . | Inhibitor, BU/mL . | IgG titer . | IgM titer . |
|---|---|---|---|---|---|---|---|---|
| P1 | Post-ticlopidine TMA | 68 | M | 14 | < 5 | Neg | 3200 | 400 |
| P2 | Cancer-associated TMA | 39 | M | NA | 8 | Neg | 1600 | 100 |
| P3 | Idiopathic TTP | 70 | F | 9 | < 3 | Neg | 600 | 400 |
| P4 | Idiopathic TTP | 35 | F | 9 | < 6 | Neg | 300 | Neg |
| P5 | Idiopathic TTP | 62 | F | NA | < 3 | Neg | 400 | Neg |
| P6 | Idiopathic TTP | 56 | M | 57 | < 6 | Neg | 200 | Neg |
| P7 | Idiopathic TTP | 58 | F | 92 | < 6 | 1.5 | Neg | Neg |
| P8 | Idiopathic TTP | 36 | F | 13 | 14 | 2 | 20 | Neg |
| P9 | Idiopathic TTP | 32 | F | 10 | 16 | ND | 1600 | Neg |
| P10 | Idiopathic TTP | 17 | F | 59 | 11 | 1.5 | 3200 | Neg |
| P11 | Unspecified TMA | 34 | M | NA | 35 | ND | Neg | 100 |
| P12 | HUS | 23 | M | 36 | 79 | Neg | Neg | 400 |
| P13 | HUS | 0.5 | M | NA | 40 | ND | Neg | 100 |
Patients 1 to 7 presented with severe ADAMTS13 deficiency (< 10% of normal); discrepant results between the ADAMTS13 inhibitor assay and ELISA results for anti-ADAMTS13 IgG and IgM antibodies are shown. Patients 8 to 13 presented without severe ADAMTS13 deficiency (> 10% of normal); anti-ADAMTS13 IgG or IgM antibodies detected by ELISA are shown.
M indicates male; Neg, negative; NA, not available; F, female; and ND, not determined.
Patients with acute untreated TMA without severe ADAMTS13 deficiency (>10%). Of 15 patients in this group, 8 were diagnosed with acute idiopathic TTP, 1 with TMA after bone marrow transplantation, 4 with HUS (in 1, diarrhea-associated), and 2 with unspecified TMA. Among them, 12 (80%) were negative for anti-ADAMTS13 IgG (Figure 1). The remaining 3 (all diagnosed with TTP) had IgG antibody titers of 20, 1600, and 3200, together with moderately reduced ADAMTS13 activities (14%, 16%, and 11%, respectively) and low platelet counts (13 × 109/L, 10 × 109/L, and 59 × 109/L, respectively) (Table 1, P8, P9, P10). Two of these patients were positive using a standard inhibitor assay. The third patient was not screened for inhibitors. None of the 4 patients diagnosed with HUS had IgG antibodies. There were 3 patients (P11, P12, P13), 2 of them diagnosed with HUS, who had IgM antibody titers ranging from 100 to 400 (Figure 2, Table 1). ADAMTS13 activities were 35% (IgM titer 100) for P11, 79% (IgM titer 400) for P12, and 40% (IgM titer 100) for P13. The only patient with diarrhea-associated HUS was negative for anti-ADAMTS13 antibodies (ADAMTS13 activity 40%).
Patients with hereditary TMA. There were 8 patients who had a clinical and family history suggesting a diagnosis of hereditary TMA, with ADAMTS13 activity levels less than 5% in the absence of an ADAMTS13 inhibitor. No anti-ADAMTS13 IgG or IgM was detected by ELISA in these patients (data not shown).
Anti-ADAMTS13 antibodies in patients with disorders other than TMA
Thrombocytopenia unrelated to TMA. In 50 patients with thrombocytopenia (range: 7 × 109/L to 120 × 109/L), low platelet counts were due to severe sepsis (n = 17), disseminated intravascular coagulation (n = 1), immune thrombocytopenia (n = 8), Evans syndrome (n = 1), myelofibrosis (n = 3), acute leukemia (n = 7), myelodysplastic syndrome (n = 3), aplastic anemia (n = 2), and other disorders including paroxysmal nocturnal hemoglobinuria and the hemophagocytic syndrome (n = 8). ADAMTS13 activity ranged from 20% to 100%. Both anti-ADAMTS13 IgG (n = 4) and IgM (n = 4) were detected in these 50 patients, with only one patient having both immunoglobin classes at the same time (Figure 1 and Figure 2). Inhibitor assays were used with 2 IgG-positive plasma samples and found to be negative. There was no association between the presence of anti-ADAMTS13 antibodies, ADAMTS13 activity, and the degree of thrombocytopenia (data not shown).
Immunomediated diseases. To evaluate the presence of anti-ADAMTS13 antibodies in chronic immunologic disorders, a group of 40 patients with SLE and 55 patients diagnosed with APS complicated by thromboembolic events were investigated. In patients with SLE, ADAMTS13 activities varied from 22% to 172%. There was no relation between the ELISA results and platelet count in patients with SLE. Of 40 patients with SLE, 5 (13%) had anti-ADAMTS13 antibodies of the G isotype, associated with normal ADAMTS13 activity (Figure 1). Antibody titers and ADAMTS13 activity were 200/137%, 400/90%, 200/102%, 100/96%, and 800/102%, respectively. There were 7 (18%) patients from this group who had an anti-ADAMTS13 antibody of the M isotype (Figure 2), with titers ranging between 50 and 800 and corresponding ADAMTS13 activities ranging between 54% and 172%. Among 55 patients with APS, 3 (5%) were positive for anti-ADAMTS13 IgG, whereas as many as 10 (18%) had detectable anti-ADAMTS13 IgM. There was no correlation between anti-ADAMTS13 IgM titers and plasma levels of ADAMTS13 activity in patients (ADAMTS13 activity varied between 32% and 114%.)
Anti-ADAMTS13 IgM antibody titers in healthy donors, patients with TMA, thrombocytopenic disorders, and autoimmune diseases other than TMA. Truncation of y-axis is indicated by horizontal bars.
Anti-ADAMTS13 IgM antibody titers in healthy donors, patients with TMA, thrombocytopenic disorders, and autoimmune diseases other than TMA. Truncation of y-axis is indicated by horizontal bars.
Patients with hemophilia A and factor VIII inhibitors. In plasma samples from 15 patients with hemophilia with factor VIII inhibitors, no anti-ADAMTS13 IgG antibodies were found. However, 5 (33%) tested positive for anti-ADAMTS13 IgM antibodies with titers ranging from 20 to 100 (Table 2).
Summary of findings for ADAMTS13 activity, ADAMTS13 neutralizing inhibitor, and anti-ADAMTS13 IgG and IgM antibodies
. | . | . | Presence of anti-ADAMTS13† . | . | . | ||
|---|---|---|---|---|---|---|---|
| Diagnosis . | Patients, n . | ADAMTS13 activity, range, % . | Inhibitor* . | IgG, n (%) . | IgM, n (%) . | ||
| Patients with acute acquired TMA | |||||||
| Untreated TMA, ADAMTS13 < 10% | 36 | < 3-10 | 30/36 (83%) | 35 (97) | 4 (11) | ||
| Untreated TMA, ADAMTS13 > 10% | 15 | 11-100 | 2/5 | 3 (20) | 3 (20) | ||
| Patients with thrombocytopenia, of various causes other than acute TMA or immunologic disorders | |||||||
| Thrombocytopenia | 50 | 20-100 | 0/0 | 4 (8) | 4 (8) | ||
| Systemic lupus erythematosus | 40 | 22-172 | 0/5 | 5 (13) | 7 (18) | ||
| Antiphospholipid antibody syndrome with thromboembolic complications | 55 | 32-114 | 0/10 | 3 (5) | 10 (18) | ||
| Hemophilia A with anti—factor FVIII alloantibodies | 15 | 10-91 | 0/0 | 0 | 5 (33) | ||
. | . | . | Presence of anti-ADAMTS13† . | . | . | ||
|---|---|---|---|---|---|---|---|
| Diagnosis . | Patients, n . | ADAMTS13 activity, range, % . | Inhibitor* . | IgG, n (%) . | IgM, n (%) . | ||
| Patients with acute acquired TMA | |||||||
| Untreated TMA, ADAMTS13 < 10% | 36 | < 3-10 | 30/36 (83%) | 35 (97) | 4 (11) | ||
| Untreated TMA, ADAMTS13 > 10% | 15 | 11-100 | 2/5 | 3 (20) | 3 (20) | ||
| Patients with thrombocytopenia, of various causes other than acute TMA or immunologic disorders | |||||||
| Thrombocytopenia | 50 | 20-100 | 0/0 | 4 (8) | 4 (8) | ||
| Systemic lupus erythematosus | 40 | 22-172 | 0/5 | 5 (13) | 7 (18) | ||
| Antiphospholipid antibody syndrome with thromboembolic complications | 55 | 32-114 | 0/10 | 3 (5) | 10 (18) | ||
| Hemophilia A with anti—factor FVIII alloantibodies | 15 | 10-91 | 0/0 | 0 | 5 (33) | ||
Not all patients were tested for the presence of ADAMTS13 inhibitors, therefore indicated as n positive/n tested.
All patients were investigated for the presence of anti-ADAMTS13 IgG and IgM antibodies.
A summary of the results of anti-ADAMTS13 IgG and IgM in relation to clinical presentation, the ADAMTS13 activity levels, and inhibitor assay data are given in Table 2.
Discussion
Even though the majority of cases of acquired TTP are currently thought to result from the development of autoantibodies against ADAMTS13, standard inhibitor assays cannot formally establish that the measured neutralizing activity is truly directed against the protease rather than against an accessory protein or factor that interferes with the enzymatic reaction of VWF cleavage by the protease. To evaluate the prevalence of antibodies against ADAMTS13 more directly, we developed a new ELISA, based on the binding of antibodies of G and M isotypes to immobilized recombinant ADAMTS13, followed by their visualization by means of secondary enzyme-labeled antibodies. We surveyed a series of plasma samples collected from patients suffering from thrombocytopenia due to TMA or to various other reasons and from patients with a variety of immunomediated diseases. The ELISA assay, which is easy to perform, detected anti-ADAMTS13 IgG antibodies in nearly all patients with acute acquired TTP associated with severe ADAMTS13 deficiency (35 of 36; 97%), but no IgG antibody was found in 4 patients diagnosed with HUS. The search for neutralizing anti-ADAMTS antibodies (inhibitors) gave negative results in 6 (17%) of the IgG-positive patients with severe ADAMTS13 deficiency. These findings extend our previous observation made in a patient with severe ADAMTS13 deficiency who had anti-ADAMTS13 antibodies by ELISA contrasting with no measurable neutralizing activity (inhibitor).20 On the other hand, a single patient included in this study, although positive for an inhibitor (1.5 BU/mL), had no detectable anti-ADAMTS13 antibodies by ELISA. Because high levels of plasma hemoglobin as a potential inhibitor33 were excluded, the nature of the inhibitory activity in this patient is still unclear.
Anti-ADAMTS13 antibodies were detected less frequently in the subgroup of patients who had acute TMA without severe ADAMTS13 deficiency (in 6 of 15, 3 with IgG and 3 with IgM isotype). IgG antibodies were associated with reduced but measurable ADAMTS13 activity (11% to 16%), with the presence of an inhibitor (in 2 of 2 cases tested) and with a diagnosis of TTP, whereas the presence of IgM antibodies was associated with higher ADAMTS13 activities (35% to 79%) and the diagnosis of unspecified TMA or HUS. The presence of anti-ADAMTS13 antibodies in the plasma of these patients despite that they are not severely deficient in ADAMTS13 activity opens up the possibility that their disease phenotypes are mechanistically related to immunologic mechanisms directed toward ADAMTS13. In a recent study we have shown that all plasmas from patients with acute TTP with low to undetectable ADAMTS13 activity contain anti-ADAMTS13 antibodies reactive with the cysteine-rich spacer domain.34 It will be interesting to determine if antibodies associated with moderately decreased ADAMTS13 activity do recognize epitopes on ADAMTS13 different from antibodies associated with severe deficiency. Alternatively, some anti-ADAMTS13 antibodies might have type II kinetic inactivation properties, as has been shown for anti-FVIII antibodies. Heterogeneity of the kinetic behavior of anti-ADAMTS13 antibodies could be responsible for either total blockage of ADAMTS13 enzymatic activity or severely reduced half-life because of rapid clearance, or it could, in certain cases, allow for some residual activity of the circulating ADAMTS13-antibody complex.
The anti-ADAMTS13 ELISA evaluated in this study may have clinical applications. Of clinical relevance is the possibility of using it for the rapid causal attribution of acute acquired TMA to anti-ADAMTS13 antibodies, because the documented presence of these antibodies would perhaps help to provide a rationale for the prescription of immunosuppressant drugs in addition to plasma exchange (PE) treatment. Moreover, immunoadsorption on protein A–sepharose columns might be preferred over PE if IgG antibodies are detected. The anti-ADAMTS13 ELISA might also be of value to monitor treatment because the results can be made available within a few hours, and the test has apparently a much greater sensitivity than the currently available inhibitor assays.
A mechanistic role and a clinical significance is difficult to attribute for the anti-ADAMTS13 IgM antibodies detected in approximately 20% of patients with such immunomediated diseases such as SLE and APS, contrasting with the much lower prevalence of these antibodies in healthy donors and patients with acute TMA. Acute TMA is sometimes reported in SLE and other autoimmune disorders,22,35-37 and although antibodies may be detected many years before the onset of overt autoimmune disorders,38 it seems unlikely that as many as 20% of the patients with SLE and APS will develop acute TMA in the future. Both SLE and APS are immunomediated diseases that are frequently associated with recurrent thromboembolic complications. Whether anti-ADAMTS13 IgM antibodies are mechanistically involved in these processes remains to be determined. It is also interesting to note that 5 of 40 (13%) patients with SLE had substantial IgG titers (range: 100-800) with essentially normal ADAMTS13 activity (range: 90%-137%). Epitope mapping studies might be able to shed some light on the characteristics of these antibodies, too. On the other hand, it cannot be ruled out that the relatively high titers obtained in some SLE plasma samples might have been caused by large immune complexes binding to ADAMTS13, thereby leading to an amplification of the ELISA signal.
To sum up, this study shows that in patients with acute acquired TMA associated with severe ADAMTS13 deficiency, autoantibodies against ADAMTS13 are detected more frequently by ELISA than by inhibitor assay. The ELISA has the additional advantage of establishing the isotype of the autoantibody, which may help to tailor treatment. Anti-ADAMTS13 antibodies of the G isotype were found more frequently in TMA than in other immunomediated diseases as exemplified by SLE and APS. The significance of the moderately frequent detection of the M isotype in the latter group of patients remains to be established.
Prepublished online as Blood First Edition Paper, May 17, 2005; DOI 10.1182/blood-2004-11-4490.
Supported in part by a grant from the Swiss National Science Foundation (grant no. 32-66 756.01 to B.L.); a grant from Fondazione Italo Monzino (P.M.M. and F.P.); and a grant from Telethon (grant no. GGPO2162 to M.G. and G.R.).
M.R., A.H., G.G., C.K., K.Z., B.P., and F.S. are employed by Baxter BioScience, whose potential product was studied in this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Elise Langdon-Neuner for expert editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal