Abstract
The roles of the 2 major platelet-collagen receptors, glycoprotein VI (GPVI) and integrin α2β1, have been intensely investigated using a variety of methods over the past decade. In the present study, we have used pharmacologic and genetic approaches to study human and mouse platelet adhesion to collagen under flow conditions. Our studies demonstrate that both GPVI and integrin α2β1 play significant roles for platelet adhesion to collagen under flow and that the loss of both receptors completely ablates this response. Intracellular signaling mediated by the cytoplasmic adaptor Src homology 2 domain-containing leukocyte protein of 76 kDa (SLP-76) but not by the transmembrane adaptor linker for activation of T cells (LAT) is critical for platelet adhesion to collagen under flow. In addition, reduced GPVI receptor density results in severe defects in platelet adhesion to collagen under flow. Defective adhesion to collagen under flow is associated with prolonged tail-bleeding times in mice lacking one or both collagen receptors. These studies establish platelet-collagen responses under physiologic flow as the consequence of a close partnership between 2 structurally distinct receptors and suggest that both receptors play significant hemostatic roles in vivo.
Introduction
An initial and critical event in the pathogenesis of human cardiovascular diseases is the adhesion of platelets to the injured vessel wall.1-3 Following vascular injury, platelets in flowing blood are exposed to subendothelial collagen, a matrix protein that stimulates the platelet adhesion and activation required to form arterial thrombi. Although these platelet responses may have evolved to deal with vascular trauma, following plaque rupture in the carotid or coronary arteries platelet-collagen responses are believed to initiate the formation of the intravascular thrombi that result in stroke or myocardial infarction.4-6 A detailed understanding of the molecular basis of platelet-collagen responses in the context of flowing blood may provide new insights into the pathogenesis of arterial vascular diseases and foster the development of new therapeutic approaches.
Circulating platelets adhere to exposed collagen at sites of vessel injury through a series of molecular interactions between platelet receptors that directly bind collagen and circulating von Willebrand factor (VWF) that becomes immobilized on exposed collagen. Significant evidence suggests that the initial interaction between circulating platelets and the vessel wall is mediated by binding of platelet glycoprotein Ib (GPIb) receptors to collagen-bound VWF.7,8 GPIb-VWF interaction permits platelet rolling on exposed collagen but is not sufficient for firm platelet adhesion.9,10 Firm platelet adhesion to collagen is believed to require the participation of 2 structurally distinct platelet-collagen receptors, the immune receptor homolog glycoprotein VI (GPVI) and the integrin α2β1.
The individual roles played by the platelet-collagen receptors GPVI and α2β1 integrin during platelet adhesion to collagen have been extensively debated and undergone frequent revision in recent years.11-18 Prior to the molecular cloning of GPVI, the identification of individuals whose platelets were not responsive to collagen and lacked α2β1 integrin suggested a critical role for this integrin, but these individuals had broader hematologic deficits that subsequently cast doubt on the validity of these findings as an indicator of α2β1 integrin function.19 A critical role for GPVI was also first identified by studies of platelets from GPVI-deficient individuals, and these studies have been supported by analysis of mouse platelets lacking GPVI and its signaling adaptor Fc receptor γ (FcRγ)–chain.1,12,20-23 More recently, the finding that α2β1 integrin requires inside-out activation to engage collagen and reports of near-normal collagen responses in β1-deficient mouse platelets have suggested that α2β1 integrin might play a secondary, nonessential role.12,24 Thus the model of platelet-collagen receptor function has shifted from one in which integrin α2β1 is the critical receptor to one in which GPVI plays the central role.12,17
Differences in the experimental methods used to measure platelet-collagen responses suggest that it may be premature to conclude that they are driven by a single central receptor. Unlike activation of platelets by soluble factors, activation of platelets by collagen in vivo is a process in which signaling and adhesion are intimately and inextricably linked. Thus, ex vivo assays that favor one over the other may overestimate or underestimate the role of individual collagen receptors. This is likely to be the case when platelet-collagen responses are tested by the exposure of stirred platelets to a collagen suspension in the aggregometer, a test that measures the activation of signaling pathways required for fibrinogen binding but fails to measure the role of adhesion during blood flow. Similarly, the more physiologic measurement of platelet adhesion to collagen under flow can be undermined by the use of washed platelets in a buffer that lacks VWF. Finally, the participation of individual platelet-collagen receptors has frequently been deduced from pharmacologic studies using reagents that favor one or another receptor, for example, the use of “soluble” collagens that reduce GPVI participation in favor of integrin α2β1 or reagents with nonspecific effects such as snake venom proteins or differences in efficacy such as different blocking monoclonal antibodies. Even experiments using genetically deficient mouse platelets may be biologically limited by their design (eg, if deficient cells are generated conditionally by Cre-mediated recombination that may not be 100% efficient).
To address the role of the 2 major platelet-collagen receptors physiologically, we have examined platelet adhesion and aggregate formation on type-I fibrillar collagen under flow conditions in whole blood. This assay reproduces the most important known aspect of platelet-collagen responses in vivo—the need to adhere firmly to immobilized collagen in the face of hemodynamic shear—but isolates the role of platelet-collagen receptors by excluding thrombin generation and cellular vessel wall responses. We have compared the responses of mouse and human platelets after pharmacologic receptor inhibition to platelets derived from genetically modified mice that lack GPVI and/or α2β1 integrin, lack the intracellular signaling molecules Src homology 2 domain-containing leukocyte protein of 76 kDa (SLP-76) or linker for activation of T cells (LAT), or express very low levels of GPVI. Our results are concordant and demonstrate that under physiologic flow conditions, both GPVI and α2β1 play important roles for platelet adhesion to collagen and subsequent aggregate formation. We also show that signal transduction is critical for these responses and that reduced GPVI signaling is better tolerated than is reduced GPVI receptor density. These results concur with recent reports demonstrating the role of α2β1 integrin for in vivo thrombotic responses in mice and provide evidence for a more balanced model in which 2 structurally distinct receptors function cooperatively during platelet-collagen responses.25
Materials and methods
Reagents and animals
Type-I fibrillar collagen from equine tendon was purchased from Chronolog (Havertown, PA). Antirat integrin α2 monoclonal antibody, Ha1/29, was obtained from Pharmingen (San Diego, CA). Antihuman integrin α2 antibody, 6F1, was a kind gift from Dr Barry S. Coller. Antihuman GPVI monoclonal antibody, 11A12, was produced as previously described.26 A rat anti–mouse integrin αIIbβ3 monoclonal antibody, Leo.H4, was purchased from Emfret Analytics (Würzburg, Germany). α2β1-Deficient, GPVI-FcRγ– deficient, and low-GPVI transgenic mice were generated as previously described.15,24,26 LAT-deficient mice were a kind gift from Dr Larry Samelson. SLP-76–deficient mice were a gift from Dr Gary Koretzky. BalbC/ByJ and C57Bl/6 mice were used as wild-type mouse controls.
Preparation of collagen-coated glass slides
Glass microscope slides (25 × 75 × 1 mm; Fisher Scientific, Pittsburgh, PA) were prepared as described previously.27 Briefly, a collagen concentration of 300 μg/mL was prepared in phosphate-buffered saline (PBS; 0.008 MNa2HPO4, 0.002 M KH2PO4, 0.14 M NaCl, and 0.01 M KCl) or modified Tyrode buffer (137 mM NaCl, 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 5.6 mM glucose, 1 g/L bovine serum albumin [BSA], 1 mM MgCl2, 2.7 mM KCl, and 3.3 mM NaH2PO4, pH 7.4). This collagen suspension was then placed in a flexiperm well, secured to the glass slide, and incubated overnight at 4°C. Slides were then rinsed with PBS and blocked with 1% denatured BSA in PBS for at least an hour at room temperature prior to use.
Blood collection and preparation
Mouse studies. Whole blood was collected into a 1-mL syringe with 0.1 mL of 150 U/mL heparin from the inferior vena cava of mice anesthetized with pentobarbital. The whole blood was diluted with an equal volume of modified Tyrode buffer. For the inhibition studies, blood was incubated with 30 μg/mL Ha1/29 or Leo.H4 or 5 mM EDTA (ethylenediaminetetraacetic acid) for 30 minutes prior to being diluted and perfused over the immobilized collagen surface.
Human studies. Blood was collected from 5 healthy donors into 20-mL syringes or 5-mL syringes containing 2.0 mL or 0.5 mL of 150 U/mL heparin, respectively. Anticoagulated blood was incubated with 10 μg/mL 11A12, 6F1, both, or 20 μg/mL neutral mouse immunoglobulin G (IgG) for 30 minutes. After antibody incubation, the blood remained undiluted or was diluted with an equal volume of modified Tyrode buffer prior to being perfused over the collagen substrate.
Flow chamber assembly and adhesion under flow experiments
The parallel plate flow chamber was assembled as previously described using the tapered wall flow-channel.27,28 The assembled flow chamber was then placed on the stage of a phase contrast inverted microscope (Diaphot-TMD; Nikon, Tokyo, Japan). Whole anticoagulated blood was perfused over the collagen-coated glass slide at a controlled flow rate of 0.428 mL/min using a syringe pump (Model `11' Plus; Harvard Apparatus, South Natic, MA) at room temperature for 4 minutes. Shear rates were calculated as previously described27,29 and ranged from approximately 400 to 1300 s–1, which are equivalent to shear stresses ranging between 16 and 52 dyne/cm2.7,30 After the perfusion period, the slides were rinsed with modified Tyrode buffer at the same flow rate for at least 10 minutes. Phase-contrast images were recorded, using the CCD camera (model 4912-2000/000; Cohu, San Diego, CA) and Sony VHS recorder (Model SVO-9600MD S-VHS; Sony Medical Systems, Montvale, NJ) at different axial positions that concur with the various shear rates using a 20× Nikon objective lens with a numerical aperture of 0.4.
Bleeding-time experiments
Unanesthetized mice, at least 2 to 3 months old, were restrained, and 2 mm of tail was cut with a razor blade. The tail was immediately immersed in saline at 37°C. Tail bleeding was monitored and determined as the time when bleeding first ceased. The experiment was discontinued after 20 minutes.
Image and statistical analysis
Platelet adhesion was quantified using the phase-contrast images and ImagePro software (Media Cybernetics, Silver Springs, MD) and expressed as the percent of the surface covered by platelets. Aggregate size distribution was also measured using the phase-contrast images and the clusters measurement tool in ImagePro. Both results were reported as the mean plus or minus standard error of the mean (SEM). Statistical relevance was determined using analysis of variance (ANOVA) with P values below .05.
Results
Pharmacologic inhibition of integrin α2β1 severely reduces deposition of mouse platelets on fibrillar collagen under flow
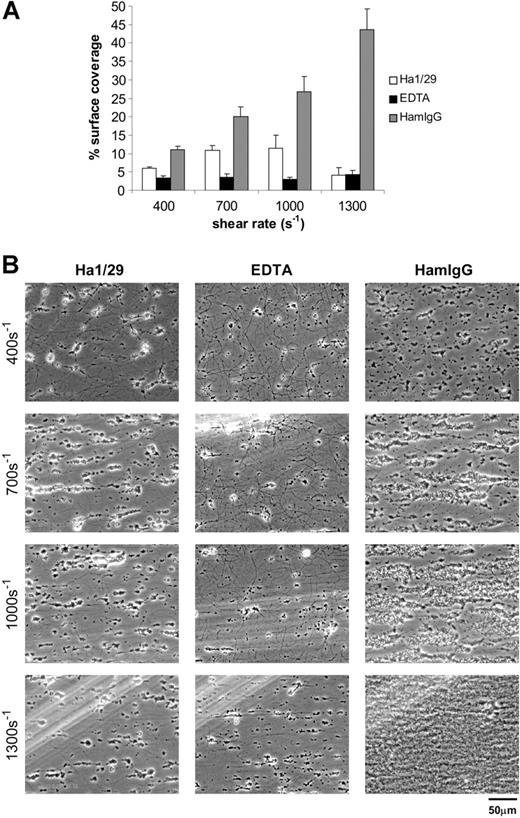
Pharmacologic approaches to testing the requirement for integrin α2β1 during platelet deposition on collagen under flow have yielded variable results.17,31 To assess the role of α2β1 integrin during adhesion of mouse platelets to fibrillar type-I collagen, we used the anti–mouse α2 integrin subunit hamster monoclonal antibody Ha1/29 to block α2β1 integrin interaction with collagen. This monoclonal antibody blocks collagen binding mediated by integrin α2β1 as well as collagen-stimulated tyrosine phosphorylation in GPVI-FcRγ–deficient mouse platelets.31,32 To assess the role of integrin receptors in aggregate, we used EDTA to chelate required cations. Of significance, we have previously found that neither Ha1/29 nor EDTA blocks GPVI-collagen interaction.24 Here we show that treatment of whole mouse blood with 30 μg/mL Ha1/29 reduced the surface coverage on fibrillar collagen after flowing platelets at all specified shear rates. This inhibitory effect was most dramatic at the higher shear rate, with a reduction from 45% coverage observed after addition of 30 μg/mL control IgG to less than 15% following Ha1/29 treatment (P < .05; Figure 1A-B). Addition of EDTA (5 mM) resulted in a more complete inhibition of murine platelet deposition on collagen at the lower shear rates, and a similar level of inhibition at 1300 s–1 (Figure 1A). Thus blockade of α2β1 integrin in particular and platelet integrins in general using these pharmacologic approaches profoundly reduces adhesion of flowing mouse platelets to fibrillar collagen. Of note, although the area of collagen-exposed surface bound by platelets was severely reduced by treatment with anti-α2 integrin subunit antibody, the few adherent platelets were able to form normal platelet aggregates (Figure 1B). In contrast, EDTA blocked both primary platelet adhesion and secondary platelet aggregate formation (Figure 1B), suggesting that α2β1 integrin is required for platelet-collagen adhesion under flow but not for platelet-platelet aggregate formation on adherent platelets.
Pharmacologic inhibition of integrin α2β1 severely reduces deposition of mouse platelets on fibrillar collagen under flow. (A) Percent surface coverage area of platelets after whole mouse blood was treated with 30 μg/mL of the blocking anti-α2 antibody Ha1/29 (□), 5 mM EDTA (▪), or control hamster IgG (HamIgG; ▦). The results shown were determined by image analysis of the phase-contrast images and are the mean ± SEM (n = 5). Statistically significant inhibition was seen at each shear rate for Ha1/29 (P < .05) and EDTA (P < .05). (B) Representative phase-contrast images captured at 400, 700, 1000, and 1300 s–1 after 4 minutes of perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Pharmacologic inhibition of integrin α2β1 severely reduces deposition of mouse platelets on fibrillar collagen under flow. (A) Percent surface coverage area of platelets after whole mouse blood was treated with 30 μg/mL of the blocking anti-α2 antibody Ha1/29 (□), 5 mM EDTA (▪), or control hamster IgG (HamIgG; ▦). The results shown were determined by image analysis of the phase-contrast images and are the mean ± SEM (n = 5). Statistically significant inhibition was seen at each shear rate for Ha1/29 (P < .05) and EDTA (P < .05). (B) Representative phase-contrast images captured at 400, 700, 1000, and 1300 s–1 after 4 minutes of perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Pharmacologic inhibition of integrin α2β1 and/or glycoprotein VI reduces deposition of human platelets on collagen under flow
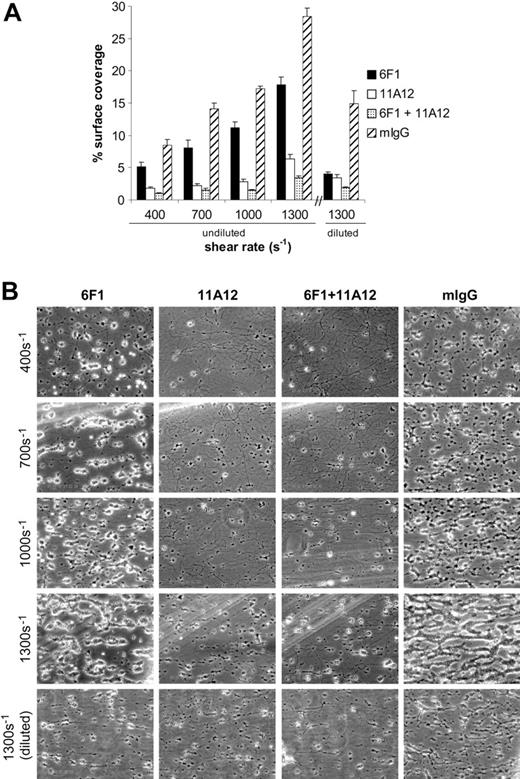
The observation that Ha1/29 anti-α2 integrin subunit antibody profoundly blocks the adhesion of mouse platelets to fibrillar collagen under flow is not supported by previous reports of human platelets treated with anti-α2 agents or reports of genetically β1-deficient mouse platelets (addressed and discussed further in “Discussion”). To assess the effect of pharmacologic inhibition of the α2β1 and GPVI receptors in human platelets exposed to fibrillar collagen in whole blood, we used the monoclonal antibodies 6F1 and 11A12 to block α2 integrin and GPVI collagen interactions, respectively. Since the concentration of circulating mouse platelets is roughly twice that of human circulating platelets, we first assessed the effect of blocking monoclonal antibodies on the adhesion of undiluted human blood to collagen. The ability of 6F1 to block adhesion of human α2β1 to collagen has been well documented, as has the ability of 11A12 to block GPVI-mediated signaling in both human platelets and GPVI-expressing RBL-2H3 cells.24,26,33,34 Treatment of undiluted human blood with 6F1 antibody (10 μg/mL [Figure 2] or 30 μg/mL [Figure S1]) resulted in a modest reduction in the area of collagen-adherent platelets under all shear rates, with the greatest effect seen at 1300 s–1 where surface coverage was reduced from 28.4% ± 1.4% to 17.8% ± 1.2% (Figure 2A). Inhibition of GPVI with the blocking 11A12 antibody (10 μg/mL) had a more profound effect, with the percentage of surface area occupied by adherent platelets dropping from 28.4% ± 1.4% to 6.3% ± 0.7% at 1300 s–1 (Figure 2A). Simultaneous inhibition with both monoclonal antibodies had a greater effect than either agent alone, reducing surface coverage to 3.5% ± 0.3% at 1300 s–1. Since the effect observed with the anti–human α2 antibody 6F1 was less than that seen with the anti–mouse α2 antibody Ha1/29 (Figure 1), we repeated this experiment using whole blood diluted with an equal volume of modified Tyrode buffer (as was done with mouse platelets). The percentage of surface area covered by adherent platelets treated with 6F1 antibody dropped from 14.9% ± 2.0% to 4.0% ± 0.4% at 1300 s–1 with dilution (Figure 2A right panel). These pharmacologic studies support the model that human platelets use both receptors during adhesion to collagen under flow.
The appearance of collagen-adherent human platelets changed markedly with pharmacologic inhibition of either α2β1 integrin or GPVI. Control mouse IgG–treated human platelets adhere to collagen under flow by forming discrete aggregates on the exposed collagen fibers that grow larger and more numerous with increasing shear stress until virtually the entire collagen fiber is coated (Figure 2B right). Inhibition of α2β1 integrin with 6F1 has little effect on the size of aggregates formed but reduces aggregate number (Figure 2B). In contrast, inhibition of GPVI virtually ablates aggregate formation, although it does not completely prevent the firm adhesion of isolated platelet clusters (Figure 2B). The disparate effects of blocking the 2 platelet-collagen receptors in human platelets mirrors that seen with mouse platelets, suggesting a conserved role for the 2 receptors during platelet deposition on collagen under flow.
Pharmacologic inhibition of integrin α2β1 and/or glycoprotein VI reduces deposition of human platelets on collagen under flow. (A) Whole blood was treated with 10 μg/mL of the blocking anti-α2 antibody 6F1 (▪), the blocking anti-GPVI antibody 11A12 (□), both 6F1 and 11A12 (▦), or 20 μg/mL mouse IgG (mIgG; ▨) for at least 30 minutes prior to being perfused over a collagen-coated surface (“undiluted”). To facilitate comparison with the mouse platelet studies shown in Figure 1, this experiment was also performed using human blood diluted with an equal volume of Tyrode buffer (“diluted”). The results shown are the mean ± SEM (n = 4-5). Statistically significant inhibition was seen with all treatments (P < .05) at all shear rates with undiluted blood, and at 1300 s–1 with diluted blood. (B) Representative phase-contrast images after 4 minutes of perfusion of heparinized whole human blood over an immobilized collagen surface. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Pharmacologic inhibition of integrin α2β1 and/or glycoprotein VI reduces deposition of human platelets on collagen under flow. (A) Whole blood was treated with 10 μg/mL of the blocking anti-α2 antibody 6F1 (▪), the blocking anti-GPVI antibody 11A12 (□), both 6F1 and 11A12 (▦), or 20 μg/mL mouse IgG (mIgG; ▨) for at least 30 minutes prior to being perfused over a collagen-coated surface (“undiluted”). To facilitate comparison with the mouse platelet studies shown in Figure 1, this experiment was also performed using human blood diluted with an equal volume of Tyrode buffer (“diluted”). The results shown are the mean ± SEM (n = 4-5). Statistically significant inhibition was seen with all treatments (P < .05) at all shear rates with undiluted blood, and at 1300 s–1 with diluted blood. (B) Representative phase-contrast images after 4 minutes of perfusion of heparinized whole human blood over an immobilized collagen surface. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Platelets derived from mice genetically deficient in α2β1 integrin and/or GPVI-FcRγ demonstrate critical roles for both receptors during platelet deposition on collagen under flow
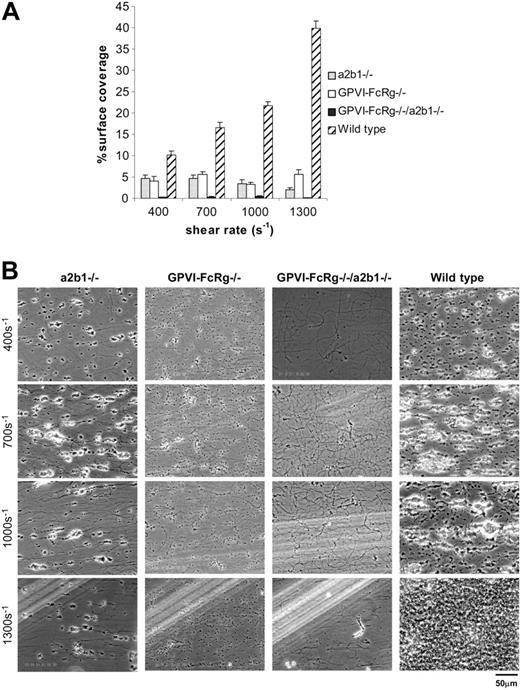
To definitively address the roles of these receptors for platelet deposition on fibrillar collagen, we next studied platelets derived from mice genetically deficient in α2 integrin subunit, GPVI-FcRγ, or both. α2-Deficient platelets lack platelet α2β1 integrin receptors and have been reported to exhibit reduced deposition on collagen under flow in studies performed using washed platelets, but this result has been questioned due to the lack of circulating von Willebrand factor (VWF), a multimeric adhesion molecule known to participate in platelet deposition on collagen under flow (Kroll et al3 ; Chen et al15 ; and discussed further under “Discussion”). To further address the role of α2β1 integrin, we therefore examined the adhesion of α2β1 integrin–deficient platelets to fibrillar collagen in the context of whole blood. The absence of α2β1 integrin severely reduced the adhesion of mouse platelets to fibrillar collagen at all shear rates, decreasing surface coverage to 2.0% ± 0.5% from 39.8% ± 1.8% at 1300 s–1 (Figure 3A). When this experiment was performed with whole blood diluted using only 0.5 × volume of heparinized buffer as in a previously reported study,12 reduced adhesion to collagen was also seen at all shear rates, but the extent of reduction was less than that observed with blood diluted with 1.0 × volume of buffer (Figure S2, available on the Blood website [see the Supplemental Figures link at the top of the online article], vs Figure 3).
FcRγ is an obligate coreceptor for GPVI in platelets, and FcRγ-deficient platelets lack surface GPVI (Nieswandt et al22 ; and Tsuji et al35 ; and data not shown). Consistent with previous reports, we observed a profound reduction in collagen adhesion under flow by GPVI-FcRγ–deficient platelets, with surface coverage reduced to 5.6% ± 1.1% at 1300 s–1. Of interest, there was no statistically significant difference in surface coverage between α2β1 integrin– deficient platelets and GPVI-FcRγ–deficient platelets, except at the shear rate of 1300 s–1 where less surface coverage was observed with α2β1-deficient platelets than with GPVI-FcRγ–deficient platelets (P < .05; Figure 3A). In addition, as observed in experiments using pharmacologic inhibition of these receptors in both mouse and human platelets, genetic loss of integrin α2β1 drastically reduced surface coverage but did not prevent aggregate formation, while genetic loss of GPVI-FcRγ reduced both surface coverage and aggregate formation (Figure 3; Table 1). These results indicate that loss of either integrin α2β1 or GPVI-FcRγ severely reduces platelet deposition on collagen under flow conditions and that α2β1 integrin plays as great a role as GPVI-FcRγ under these physiologic conditions.
Platelet aggregate size distribution at 1300 s–1
Mouse genotype . | Aggregates containing 1 to 2 platelets, % . | Aggregates containing 3 to 10 platelets, % . | Aggregates containing 11 to 30 platelets, % . | Aggregates containing 31 to 50 platelets, % . |
|---|---|---|---|---|
| Wild type | 60.3 ± 2.8 | 21.2 ± 2.3 | 13.7 ± 0.6 | 4.8 ± 0.6 |
| α2β1-/- | 72.8 ± 3.3 | 24.3 ± 2.8 | 2.8 ± 0.9 | 0.1 ± 0.1 |
| GPVI-FcRγ-/- | 97.0 ± 0.4 | 3.0 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| GPVI-FcRγ-/-/α2β1-/- | 96.8 ± 1.5 | 3.2 ± 1.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| SLP-76-/- | 94.7 ± 1.9 | 5.1 ± 1.8 | 0.1 ± 0.1 | 0.1 ± 0.0 |
| LAT-/- | 75.2 ± 4.1 | 19.4 ± 2.8 | 4.8 ± 1.4 | 0.6 ± 0.2 |
| Low-GPVI | 92.7 ± 1.9 | 6.9 ± 1.8 | 0.3 ± 0.1 | 0.0 ± 0.0 |
Mouse genotype . | Aggregates containing 1 to 2 platelets, % . | Aggregates containing 3 to 10 platelets, % . | Aggregates containing 11 to 30 platelets, % . | Aggregates containing 31 to 50 platelets, % . |
|---|---|---|---|---|
| Wild type | 60.3 ± 2.8 | 21.2 ± 2.3 | 13.7 ± 0.6 | 4.8 ± 0.6 |
| α2β1-/- | 72.8 ± 3.3 | 24.3 ± 2.8 | 2.8 ± 0.9 | 0.1 ± 0.1 |
| GPVI-FcRγ-/- | 97.0 ± 0.4 | 3.0 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| GPVI-FcRγ-/-/α2β1-/- | 96.8 ± 1.5 | 3.2 ± 1.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| SLP-76-/- | 94.7 ± 1.9 | 5.1 ± 1.8 | 0.1 ± 0.1 | 0.1 ± 0.0 |
| LAT-/- | 75.2 ± 4.1 | 19.4 ± 2.8 | 4.8 ± 1.4 | 0.6 ± 0.2 |
| Low-GPVI | 92.7 ± 1.9 | 6.9 ± 1.8 | 0.3 ± 0.1 | 0.0 ± 0.0 |
The results shown are the mean ± SEM after analysis of the phase-contrast images (n = 5-10).
Platelets derived from mice genetically deficient in α2β1 integrin and/or GPVI-FcRγ demonstrate critical roles for both receptors during platelet deposition on fibrillar collagen under flow. Whole blood from the specified mice was perfused over an immobilized collagen surface for 4 minutes. (A) Surface coverage area of platelets from α2β1–/– (▦), GPVI-FcRγ–/– (□), GPVI-FcRγ–/–; α2β1–/– (▪), and wild-type (▨) after flow. The results shown are the mean ± SEM (n = 6-11). Statistical significance (P < .01) was seen between the knockouts and the control at and between 400 and 1300 s–1. (B) Representative phase-contrast images after perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Platelets derived from mice genetically deficient in α2β1 integrin and/or GPVI-FcRγ demonstrate critical roles for both receptors during platelet deposition on fibrillar collagen under flow. Whole blood from the specified mice was perfused over an immobilized collagen surface for 4 minutes. (A) Surface coverage area of platelets from α2β1–/– (▦), GPVI-FcRγ–/– (□), GPVI-FcRγ–/–; α2β1–/– (▪), and wild-type (▨) after flow. The results shown are the mean ± SEM (n = 6-11). Statistical significance (P < .01) was seen between the knockouts and the control at and between 400 and 1300 s–1. (B) Representative phase-contrast images after perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Recent models of platelet-collagen receptor function have proposed both independent and interdependent roles for GPVI-FcRγ and α2β1 integrin during adhesion of platelets to exposed collagen.24 To more fully explore the relationship between these 2 receptors, we generated mouse platelets deficient in both α2β1 integrin and GPVI-FcRγ. Platelets lacking both collagen receptors were unresponsive to collagen but had normal dose responses to the G protein–coupled receptor agonists adenosine diphosphate (ADP) and AYPGKF (proteinase-activated receptor 4 [PAR4] activating peptide, Figure S3). Surprisingly, although loss of either GPVI-FcRγ or α2β1 drastically reduces deposition on collagen under flow, loss of both receptors results in a significantly more severe phenotype with virtually complete ablation of all platelet-collagen adhesion at all shear rates (Figure 3A-B). This result indicates that both GPVI-FcRγ and α2β1 integrin mediate some independent platelet-collagen adhesion and that the 2 platelet-collagen receptors are indispensable for adhesion of platelets to collagen under flow.
Platelet deposition on collagen under flow requires signal transduction through the adaptor SLP-76
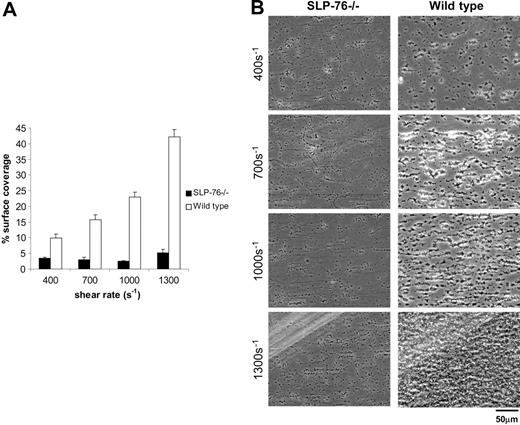
Recent studies have demonstrated that both GPVI-FcRγ and integrin α2β1 can transduce intracellular signals in platelets through the cytoplasmic adaptor protein SLP-76.36-40 However, mutant GPVI receptors unable to transduce such signals can independently mediate collagen adhesion of RBL-2H3 cells under flow.27 It is therefore not clear whether the requirement for GPVI-FcRγ and α2β1 integrin during platelet-collagen adhesion under flow reflects adhesive or signaling roles for these receptors, or both. To distinguish the contribution of collagen-receptor signaling from that of collagen-receptor adhesion, we next studied the ability of mouse platelets lacking SLP-76 to adhere to collagen under flow. SLP-76–deficient platelets express normal levels of GPVI-FcRγ and α2β1 (data not shown), but exhibited severe defects in collagen adhesion under flow at all levels of shear stress, with a surface coverage of 5.2% ± 1.1% compared with 42.4% ± 2.4% for wild-type platelets at 1300 s–1 (P < .01; Figure 4). The magnitude of the defect in collagen adhesion of SLP-76–deficient platelets was similar to that observed for platelets lacking GPVI-FcRγ or α2β1 integrin (Figures 3), suggesting that signal transduction through SLP-76 is an essential part of platelet deposition on collagen under flow. Of significance, like GPVI-FcRγ–deficient platelets, SLP-76–deficient platelets were able to adhere singly to collagen in small numbers, but did not form aggregates (Figure 4B; Table 1), suggesting that aggregate formation is primarily a function of signal transduction through the GPVI-FcRγ receptor. Since SLP-76–deficient mice have platelet counts that are on average two thirds those of wild-type mice, it is possible that reduced adhesion could merely reflect reduced circulating platelet number. To address this possibility, we compared collagen adhesion of wild-type platelets diluted 1-fold with modified Tyrode buffer to those diluted 2-fold, a dilution that approximates the average platelet count of SLP-76–deficient blood. Dilution of wild-type blood to a platelet count similar to that of SLP-76– deficient animals reduced the percentage surface area covered from 40.8 ± 1.0% to 29.7% ± 4.3% at 1300 s–1, a level far above the severe reduction observed with SLP-76 deficiency (Figure S4). Like platelets lacking GPVI-FcRγ or α2β1 integrin, SLP-76– deficient platelets exhibit some residual capacity to bind collagen under flow, suggesting the presence of adhesive events that either do not require signaling or use signals that are SLP-76 independent.
Platelet deposition on collagen under flow requires signal transduction through the adaptor SLP-76. Whole blood from the indicated mice was perfused over collagen-coated glass slides at wall shear rates at and between 400 and 1300 s–1 for 4 minutes. (A) Percent surface coverage of SLP-76–deficient (▪;n = 5) and the wild-type (□;n = 3) platelets after whole blood perfusion over a collagen-coated surface. The results shown are the mean ± SEM. Statistical significance was seen between the SLP-76–deficient platelets and the control (P < .01) at the specified shear rates. (B) Representative phase-contrast images after perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Platelet deposition on collagen under flow requires signal transduction through the adaptor SLP-76. Whole blood from the indicated mice was perfused over collagen-coated glass slides at wall shear rates at and between 400 and 1300 s–1 for 4 minutes. (A) Percent surface coverage of SLP-76–deficient (▪;n = 5) and the wild-type (□;n = 3) platelets after whole blood perfusion over a collagen-coated surface. The results shown are the mean ± SEM. Statistical significance was seen between the SLP-76–deficient platelets and the control (P < .01) at the specified shear rates. (B) Representative phase-contrast images after perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
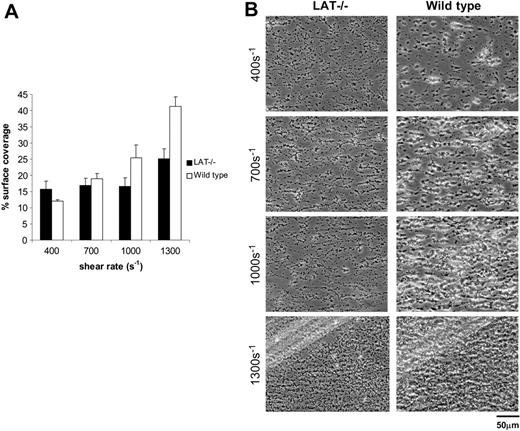
Platelet deposition on collagen under flow does not require signal transduction through the adaptor LAT. Whole blood from the indicated mice was perfused over collagen-coated glass slides at wall shear rates at and between 400 and 1300 s–1 for 4 minutes. (A) Surface coverage of LAT-deficient (▪; n = 6) and wild-type (□; n = 3) platelets. The results shown are the mean ± SEM. There is a statistically significant difference between LAT-deficient and wild-type platelets at 1300 s–1 (P < .05). (B) Representative phase-contrast images after perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Platelet deposition on collagen under flow does not require signal transduction through the adaptor LAT. Whole blood from the indicated mice was perfused over collagen-coated glass slides at wall shear rates at and between 400 and 1300 s–1 for 4 minutes. (A) Surface coverage of LAT-deficient (▪; n = 6) and wild-type (□; n = 3) platelets. The results shown are the mean ± SEM. There is a statistically significant difference between LAT-deficient and wild-type platelets at 1300 s–1 (P < .05). (B) Representative phase-contrast images after perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Platelet deposition on collagen under flow does not require signal transduction through the adaptor LAT
In contrast to SLP-76, the lipid raft transmembrane adaptor LAT is critical for signaling through GPVI-FcRγ but not integrin α2β1.41 Studies of platelet-collagen responses using aggregometry have revealed that SLP-76–deficient platelets fail to respond to collagen while LAT-deficient platelets retain collagen responses despite a severe reduction in GPVI-FcRγ signals.42,43 Whether the ability of LAT-deficient platelets to respond to collagen in the aggregometer indicates a minimal need for GPVI-FcRγ signaling under the more stringent physiologic conditions of flow is unclear. In contrast to mouse platelets lacking SLP-76, LAT-deficient platelets exhibited only mildly reduced surface coverage, 25.2% ± 3.0% compared with 41.3% ± 2.9% for wild-type platelets at 1300 s–1 (P < .05; Figure 5A). Of interest, although surface coverage was relatively preserved, platelet aggregate size was reduced relative to wild-type platelets (Figure 5B; Table 1). These results support those obtained using platelet aggregometry and suggest that GPVI-FcRγ signaling through LAT is not critical for adhesion of platelets to collagen under flow but participates in the formation of platelet aggregates following firm adhesion.
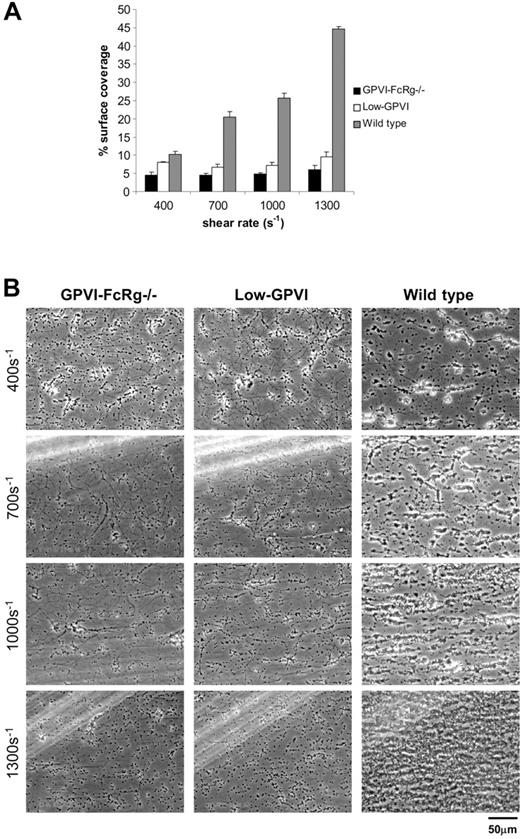
Low levels of GPVI reduce deposition of platelets on collagen under shear stress
Like LAT-deficient platelets, “low-GPVI” platelets (GPVI-FcRγ– deficient platelets rescued by expression of a GPVI-FcγRIIa chimeric receptor at a receptor density 2% that of GPVI in wild-type platelets) exhibit delayed but conserved responses to suspended fibrillar collagen, although they fail to respond to GPVI-specific agonists such as the snake venom convulxin.24,44 Both LAT-deficient and low-GPVI collagen aggregation responses require α2β1 integrin, suggesting that platelet-collagen receptors might function in a cooperative way.24 To define this mechanism further, we tested the ability of low-GPVI platelets to respond to collagen under flow conditions. In contrast to LAT-deficient platelets, low-GPVI platelets exhibited severely reduced deposition on collagen under flow and phenotypically closely resembled GPVI-FcRγ–deficient platelets with a surface coverage of 9.5% ± 1.4% compared with 44.6% ± 0.7% for wild-type platelets at 1300 s–1 (Figure 6A). Similar to both LAT- and GPVI-FcRγ– deficient platelets, low-GPVI platelets failed to form large aggregates (Figure 6B; Table 1). These results indicate that, in contrast to collagen-induced platelet aggregation, platelet deposition on collagen under flow is sensitive to reduced GPVI-FcRγ receptor density.
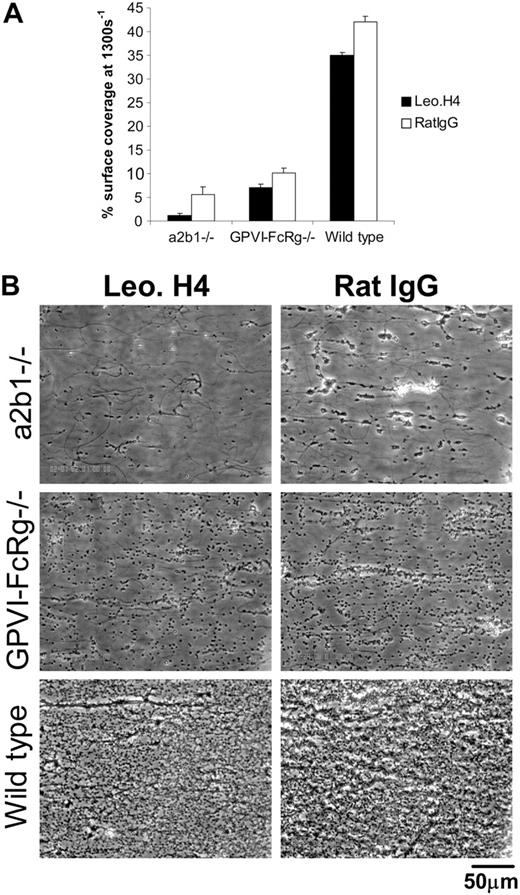
Integrin αIIbβ3 is required for platelet aggregate formation but not for primary adhesion of platelets to collagen under flow ex vivo
In addition to the receptors that directly bind collagen, platelets express receptors that can mediate deposition on collagen indirectly through collagen-bound VWF. Platelet GPIb interaction with collagen-bound VWF is considered requisite for platelet rolling on collagen under high-shear conditions, and the abundant platelet integrin αIIbβ3 is also capable of binding collagen-bound VWF.2 Thus integrin αIIbβ3 might in theory participate in platelet-collagen adhesion under flow in a manner analogous to that of the direct collagen-binding integrin α2β1. To test the role of αIIbβ3, we exposed wild-type, FcRγ-deficient, and α2β1-deficient platelets to the blocking anti–mouse integrin αIIbβ3 monoclonal antibody Leo.H4 or control rat IgG. Doses of 20 and 30 μg/mL Leo.H4 completely blocked platelet aggregation to collagen, although they did not affect shape-change response (data not shown and http://www.emfret.com/Sheets/M021-0%20LeoH4.pdf). Treatment of wild-type mouse blood with Leo.H4 antibody (30 μg/mL) resulted in a small reduction in the area of collagen-adherent platelets under all shear rates, with the greatest effect seen at 1300 s–1 (Figure 7A). Blockade of integrin αIIbβ3 with Leo.H4 antibody had a smaller, nonsignificant effect on the adhesion of FcRγ-deficient mouse platelets to collagen under flow and a more significant effect on the adhesion of α2β1 integrin– deficient platelets (Figure 7A). In all cases, blockade of αIIbβ3 with Leo.H4 antibody eliminated the formation of platelet aggregates and clusters on the exposed collagen surface (Figure 7B). These studies demonstrate that the integrin αIIbβ3 is essential for platelet aggregate formation and contributes to the primary platelet adhesion observed following loss of α2β1, but not GPVI-FcRγ, under these experimental conditions.
Low levels of GPVI reduce deposition of platelets on collagen under shear stress. Whole blood from the indicated mice was perfused over collagen-coated glass slides at wall shear rates at and between 400 and 1300 s–1 for 4 minutes. (A) Percent surface coverage of GPVI-FcRγ–/– (▪), low-GPVI (□), and wild-type (▦) platelets. The results shown are the mean ± SEM after analysis of the phase-contrast images (n = 5). Statistical significance was seen between GPVI-FcRγ–/–, low-GPVI, and wild-type at and between 400 s–1 and 1300 s–1 (P < .01). (B) Representative phase-contrast images after flow. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Low levels of GPVI reduce deposition of platelets on collagen under shear stress. Whole blood from the indicated mice was perfused over collagen-coated glass slides at wall shear rates at and between 400 and 1300 s–1 for 4 minutes. (A) Percent surface coverage of GPVI-FcRγ–/– (▪), low-GPVI (□), and wild-type (▦) platelets. The results shown are the mean ± SEM after analysis of the phase-contrast images (n = 5). Statistical significance was seen between GPVI-FcRγ–/–, low-GPVI, and wild-type at and between 400 s–1 and 1300 s–1 (P < .01). (B) Representative phase-contrast images after flow. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
Mice lacking platelet-collagen receptors exhibit prolonged tail-bleeding times
The finding that mouse platelets lacking either or both platelet-collagen receptors exhibit severe defects in deposition on collagen under physiologic flow conditions suggests that loss of platelet-collagen receptors could compromise hemostasis in vivo. To test this hypothesis, we performed tail-bleeding times on unanesthetized wild-type, FcRγ-deficient, α2-deficient, and double knockout mice lacking both receptors. Since FcRγ-deficient mice were studied in a BalbC background, α2β1 integrin–deficient mice were studied in a mixed C57Bl/6 and SV129 background, and double knock-out mice were studied in a mixture of the 3, we examined tail-bleeding times in both BalbC and C57Bl/6 wild-type control mice. Strain had no detectable influence on tail-bleeding time (Figure 8). It is remarkable that loss of either or both collagen receptors resulted in a prolonged tail-bleeding time (Figure 8). Tail rebleeding was also observed more frequently in single and double knock-out mice than in wild-type controls (data not shown). Thus, deficiencies in platelet deposition on collagen under flow are associated with hemostatic defects in live mice.
Discussion
Investigation of the molecular basis of platelet-collagen responses has increased dramatically over the past several years due to the cloning of the GPVI collagen receptor, the generation and study of gene-targeted mice lacking GPVI-FcRγ and integrin α2β1, and the development of novel pharmacologic inhibitors of these receptors. These studies have drastically altered the original “2-site, 2-step” model, which proposed that integrin α2β1 initiated adhesion to collagen with subsequent activation of GPVI required for platelet signals to foster thrombus growth.13,16,45 More recently, it has been proposed that the GPVI-FcRγ receptor is the central receptor, with α2β1 integrin playing a supportive but unnecessary role.12,17 An important consideration regarding these different models is, however, the extent to which they reflect differences in the methods used to measure collagen-receptor function by different investigators.
Integrin αIIbβ3 is required for platelet aggregate formation but not for primary adhesion of platelets to collagen under flow ex vivo. (A) Percent surface coverage area of platelets from α2β1–/–, GPVI-FcRγ–/–, and wild-type mice after being treated with 30 μg/mL blocking anti-αIIbβ3 antibody Leo.H4 (□) or control rat IgG (RatIgG; ▪) at 1300 s–1. The results shown were determined by image analysis of the phase-contrast images and are the mean ± SEM (n = 4). Statistical significance was seen between treated and untreated wild-type platelets (P < .01). (B) Representative phase-contrast images after flow.
Integrin αIIbβ3 is required for platelet aggregate formation but not for primary adhesion of platelets to collagen under flow ex vivo. (A) Percent surface coverage area of platelets from α2β1–/–, GPVI-FcRγ–/–, and wild-type mice after being treated with 30 μg/mL blocking anti-αIIbβ3 antibody Leo.H4 (□) or control rat IgG (RatIgG; ▪) at 1300 s–1. The results shown were determined by image analysis of the phase-contrast images and are the mean ± SEM (n = 4). Statistical significance was seen between treated and untreated wild-type platelets (P < .01). (B) Representative phase-contrast images after flow.
Mice lacking platelet-collagen receptors exhibit prolonged tail-bleeding times. Tail-bleeding times of wild-type (BalbC and C57Bl/6), α2β1–/–, GPVI-FcRγ–/–, and GPVI-FcRγ–/–;α2β1–/– were determined. Approximately 2 mm of tail was cut from unanesthetized mice, the tail immersed in 37°C saline, and the bleeding time recorded as the moment when bleeding ceased. Each point represents an individual animal (n = 10-17). Mouse strain is indicated in parentheses. WT indicates wild type. The horizontal bars indicate the average tail-bleeding times.
Mice lacking platelet-collagen receptors exhibit prolonged tail-bleeding times. Tail-bleeding times of wild-type (BalbC and C57Bl/6), α2β1–/–, GPVI-FcRγ–/–, and GPVI-FcRγ–/–;α2β1–/– were determined. Approximately 2 mm of tail was cut from unanesthetized mice, the tail immersed in 37°C saline, and the bleeding time recorded as the moment when bleeding ceased. Each point represents an individual animal (n = 10-17). Mouse strain is indicated in parentheses. WT indicates wild type. The horizontal bars indicate the average tail-bleeding times.
In vivo platelet interaction with collagen is restricted to sites of vessel injury where the endothelium is disrupted and collagen exposed. In human disease states such as stroke and myocardial infarction, this occurs intravascularly in flowing blood in the arterial system. In the present work, we set out to examine the role of the platelet-collagen receptors and their signaling pathways when whole blood is flowed over immobilized, fibrillar collagen. This method offers several important advantages for the analysis of platelet-collagen responses: (1) in contrast to aggregation studies, the manner in which platelets are exposed to collagen physiologically reproduces the mechanical forces present during in vivo presentation; (2) the assay uses whole blood and therefore does not exclude collagen-VWF-GPIb interactions; (3) collagen adhesion under flow can be accurately quantified under multiple shear rates that reflect hemodynamic forces in vessels of different size in vivo; and (4) the method can be applied to human and mouse platelets with both pharmacologic and genetic manipulation. Using this approach, we have identified critical roles for both GPVI and α2β1 integrin and selective roles for the intracellular signaling proteins SLP-76 and LAT as well as for GPVI-receptor density.
A fundamental, but still outstanding, question regarding the molecular mechanism of platelet responses to collagen remains: what are the roles played by the 2 major collagen receptors, α2β1 integrin and GPVI-FcRγ, during platelet adhesion to collagen? We have used pharmacologic blockade of human and mouse platelets and analysis of genetically deficient mouse platelets to demonstrate critical roles for both α2β1 integrin and GPVI during this process. The finding that GPVI is required for normal adhesion and aggregate formation of human and mouse platelets on collagen is not surprising and is in agreement with previously published studies using both mouse and human platelets.1,12,46 More surprising is our finding that integrin α2β1 plays a similarly important role. Previous studies using pharmacologic agents to block α2β1 integrin have yielded conflicting results, due at least in part to differences in methodology. These differences include the use of washed platelets versus whole blood (washed platelets lack plasma VWF that would normally bind collagen under shear flow), the use of highly fibrillar versus more soluble forms of collagen (GPVI preferentially binds highly fibrillar collagen and more soluble collagens are therefore more α2β1-integrin specific), and the type of inhibitory agent used (less specific but highly inhibitory snake venoms vs more specific but less inhibitory monoclonal antibodies). Studies using human platelets have reported modest or no effect with blocking anti-α2 monoclonal antibodies and more significant but less readily interpreted effects with blocking snake venoms.2,33,47-49 Our results using 6F1 antibody to block human α2β1 integrin are consistent with this literature—we observed significant inhibition of human platelet adhesion to collagen under all shear rates, but the magnitude of the inhibitory effect varied with experimental conditions (ie, when whole human blood was diluted, the inhibitory effect was magnified to a range similar to that seen with monoclonal inhibition of mouse α2β1 integrin). The effect of α2β1 integrin blockade seen with 6F1 alone is indirectly supported by the finding that 6F1 significantly enhanced inhibition by the 11A12 blocking anti-GPVI antibody, an observation also recently reported by Siljander et al.33 Since a clean human α2-deficiency state has yet to be described, these results support a significant role for α2β1 integrin during the adhesion of human platelets to collagen under flow, but whether this role is smaller than that in mouse platelets or whether its measurement is limited by the ability of 6F1 and other pharmacologic agents to inhibit α2β1 integrin function is not yet clear.
In contrast to human pharmacologic studies, our pharmacologic and genetic studies of mouse platelets clearly define a critical and independent role for α2β1 during platelet adhesion to collagen. Treatment with the anti–mouse α2 antibody Ha1/29 severely reduced adhesion of mouse platelets to collagen, a result only slightly less complete than that observed with α2-deficient platelets. In contrast to GPVI-FcRγ deficiency, α2β1 integrin deficiency reduced initial platelet adhesion but did not prevent aggregate formation, indicating a critical adhesive role but suggesting that α2β1 integrin is not essential for the activation of platelet integrins and release of secondary agonists presumably involved in aggregate formation. These results are consistent with those previously reported using washed α2-deficient platelets, but differ significantly from those reported for β1-deficient platelets.12,15 Two studies of β1-deficient platelets have been reported; in the first, collagen adhesion under low and high shear stress was reported to be normal, while in the second adhesion at 1000 s–1 collagen adhesion was reported to be slightly reduced but was not quantified.12,50 Mouse strain differences are an unlikely explanation for the discrepancy in these studies as we have used the Ha1/29 antibody on C57Bl/6 and BalbC platelets with similar results (data not shown), and a recent study of α2β1 expression in different mouse strains found similar levels in the 2 strains.51 In both previously reported studies, whole blood and type-I fibrillar collagen were used in a manner similar to that reported here. However, small methodologic differences between these studies and ours exist, including differences in the degree of dilution of whole blood prior to flow (addition of 0.5 × volume in the study of Nieswandt et al12 vs 1.0 × volume in our study), the use of PPACK (d-phenylalanyl-l-prolyl-l-arginine chloromethylketone) to inhibit thrombin activity, and the method used to coat coverslips with collagen. To assess the importance of these methodologic differences, we compared the defect of α2-deficient platelets after dilution of whole blood with 0.5 × versus 1.0 × volume of anticoagulated buffer (Figure S2). While α2-deficient platelets exhibited a marked defect in adhesion to collagen under flow even when diluted with 0.5 × volume buffer, the increase in dilution to 1.0 × volume did appear to augment the defect relative to wild-type platelets (Figure S2 vs Figure 3). Thus, methodologic differences are likely to have contributed in both known and unknown ways to the observed differences. A second possible explanation may lie in the manner in which β1-deficient platelets were generated. To generate β1-deficient platelets, Nieswandt et al crossed mice carrying a conditional β1 allele to MX1-Cre transgenic animals to drive inducible hematopoietic excision.12 Since β1 deficiency is dependent on Cre-mediated excision, it is possible that a small fraction of hematopoietic cells (perhaps < 1%) escaped excision. Such a small remnant population of β1-expressing cells might not be detected by Western blot or fluorescence-activated cell sorter (FACS) but could be selected for from among millions of platelets flowed over a collagen surface and thereby rescue collagen adhesion. Given the strong concordance between pharmacologic and genetic loss of α2β1 integrin function in our studies, it seems likely that prior failure to detect the significance of α2β1 integrin for collagen adhesion under flow reflects differences in experimental methods that resulted in assays with different levels of stringency for testing α2β1 function.
It is significant that mouse platelets genetically deficient in both GPVI-FcRγ and α2β1 integrin exhibited measurably less adhesion to collagen than platelets lacking either GPVI-FcRγ or α2β1 integrin alone. It is difficult to explain the adhesion of platelets lacking a single receptor merely by invoking background platelet activation (eg, due to venipuncture), as activated platelets would be expected to bind collagen through collagen-bound VWF interaction with αIIbβ3 integrin receptors, an interaction that would be unperturbed in double knock-out platelets. Instead, it is tempting to speculate that some firm platelet adhesion is possible in the absence of GPVI-FcRγ (perhaps mediated by VWF-GPIb signals) and that GPVI-FcRγ can drive a small degree of firm platelet adhesion in the absence of α2β1 integrin (perhaps by activating αIIbβ3 integrin to bind collagen-bound VWF, as indicated by pharmacologic inhibition of that receptor in Figure 7). Mouse platelets lacking both of these receptors exhibit normal responses to the G protein– coupled receptor agonists ADP and PAR4 agonist peptide AYPGKF (Figure S3), suggesting that a change in secondary G protein– coupled responses is not responsible for the complete loss of adhesion in platelets lacking both collagen receptors.
The profound defect observed in platelets lacking both major collagen receptors suggests that other type-I collagen receptors, if present, do not play major roles in this process and that inhibition of both major platelet-collagen receptors could have profound effects on hemostasis in vivo. The latter is supported by a recent report that tail-bleeding time is prolonged in mice lacking both GPVI and α2β1 integrin but not in animals lacking a single receptor.52 To further address this possibility, we compared tail-bleeding times of mice deficient in one or both major collagen receptors. Unexpectedly, loss of either collagen receptor significantly compromised hemostasis in this model. Our finding of a prolonged tail-bleeding time in mice lacking GPVI-FcRγ or α2β1 integrin differs from previously reported normal bleeding times for such animals.12,52 This difference is likely to reflect differences in the method used to measure tail-bleeding times, as we used immersion of unanesthetized mouse tails in 37°C saline, while previous studies used a dry blotting approach in anesthetized animals.12,52 Nevertheless, the extended bleeding times mirror the observations made in the flow chamber and provide further support for a significant role for both of these collagen receptors for hemostasis in vivo.
A second outstanding question regarding the roles of the defined platelet-collagen receptors is whether they contribute to platelet adhesion and thrombus formation by generating intracellular signals in response to collagen, by forming strong adhesive bonds to collagen, or both. The present model is one in which GPVI functions primarily to generate intracellular signals in response to collagen, while α2β1 integrin operates as an adhesive receptor that requires inside-out activation signals to be engaged.12,17 However, recent studies have obscured this conceptually straightforward division of labor. α2β1 integrin and other platelet integrins can generate outside-in signals through an intracellular pathway containing SLP-76, an adaptor protein that is also required for GPVI-FcRγ signaling.37 Previous studies have demonstrated that when GPVI signaling is reduced (eg, due to pharmacologic blockade loss of the GPVI-specific signaling adaptor LAT or as a result of reduced GPVI receptor density), α2β1 integrin is required for normal platelet aggregation responses to collagen.24 Thus, α2β1 integrin may play a direct or indirect role in collagen-mediated platelet signals in addition to any adhesive role. Conversely, when GPVI is expressed on the surface of RBL-2H3 cells that lack α2β1 integrin, it is sufficient to confer some adhesion under flow even when mutated to block signal transduction.27 Thus collagen signaling and adhesion may not be so easily assigned to individual receptors. Our finding that SLP-76–deficient platelets were significantly but not absolutely deficient in collagen adhesion demonstrates that intracellular signaling through this adaptor is a critical component of the platelet response to collagen under flow and is consistent with a previous report of transgenic mouse platelets expressing mutant FcRγ unable to couple to intracellular signaling pathways.46 The fact that SLP-76–deficient platelets retained some ability to adhere to collagen under flow compared with GPVI/α2β1 integrin double knock-out platelets suggests either that some adhesion is possible in the absence of intracellular signaling or that some intracellular signals can be transduced in the absence of this adaptor.
Our findings support a model of platelet-collagen responses in which the 2 major collagen receptors operate cooperatively and in a highly integrated manner to bind collagen and generate collagen-induced intracellular signals. While it is reasonable to propose that a GPVI signal might be required first to activate and engage α2β1 integrin receptors, GPVI alone is not sufficient, and there is little evidence to conclude that GPVI plays a more central role than α2β1 integrin in this response. The severe deficiencies observed with loss of either receptor alone and the absolute deficiency observed with loss of both collagen receptors appear to leave surprisingly little room for participation by other proposed receptors, such as GPIb signaling after VWF binding or integrin αIIbβ3 adhesion to collagen-bound VWF after GPVI signaling under these experimental conditions. Consistent with the effects of loss of collagen-receptor function, pharmacologic blockade of integrin αIIbβ3 adhesion eliminates aggregate formation as expected but has minimal effect on primary platelet adhesion under flow in wild-type platelets and compensates surprisingly little for the loss of the integrin α2β1. Whether this is the case in vivo or whether these conclusions reflect still-undefined limitations of experimental method (eg, less matrix-bound VWF than may be exposed in vivo) remains to be investigated.
Prepublished online as Blood First Edition Paper, May 10, 2005; DOI 10.1182/blood-2004-11-4434.
Supported by grants HL-67311-01 (M.L.K.) and HL-62550-02 (D.A.H.) from the National Heart, Lung and Blood Institute of the National Institutes of Health.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Barry Coller for 6F1 antibody, Larry Samelson for LAT knock-out mice, Gary Koretzky for SLP-76 knock-out mice, Eric Johnston for technical support, and Bernhard Nieswandt for frank discussion of experimental methods.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal