Comment on Rhodes et al, page 1857
In this issue of Blood, Rhodes and colleagues propose that the major effect of Bcl-xL deficiency is ineffective erythropoiesis during the late stages of differentiation.
B-cell lymphoma 2 (Bcl-2) family members comprise a conserved family of proteins that carry out the most important cellular decisions: those involving life and death. The Bcl-2 members coordinate their activities to either prevent or promote mitochondrial release of cytochrome c. The release of cytochrome c triggers programmed cell death cascades. Within the erythroid lineage, the antiapoptotic member of the family named Bcl-xL plays an important effector role in the regulation of growth as the cells differentiate into mature erythrocytes.1 However, the exact stage of erythroid differentiation at which Bcl-xL exerts its effects remains a matter of debate.FIG1
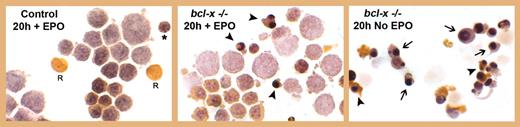
Morphologic appearance of cultured bcl-x–/– and bled control erythroblasts. See the complete figure in the article beginning on page 1857.
Morphologic appearance of cultured bcl-x–/– and bled control erythroblasts. See the complete figure in the article beginning on page 1857.
In this issue of Blood, Rhodes and colleagues show that Bcl-xL is essential for preventing apoptotic death and ineffective erythropoiesis at the terminal stages of erythroblast differentiation. The authors use the same conditional Bcl-xL knockout mouse (MMTV-Cre Bcl-xfl/fl) reported elsewhere.2 In the original report, Wagner et al2 generated mice that delete their Bcl-xL locus as adults to circumvent the developmental lethality associated with Bcl-xL deletion in embryonic stem cells. When examined at 3 months of age, those mice had a phenotype most consistent with severe peripheral hemolysis rather than ineffective erythropoiesis. Rhodes and colleagues examined older mice from the same line. The phenotype in the older mice appears to be more severe, with more severe anemia, increased hyperbilirubinemia, and massive splenomegaly. However, the mice do not maintain appropriate levels of reticulocytosis compared with bled controls, and robust apoptosis at the terminal stages of erythroblast maturation is demonstrated by several assays. Assuming the Bcl-xL deletion is complete by 3 months of age, these data suggest that some effects of Bcl-xL loss may become clearer with advancing age. Unfortunately, the precise cause and extent of peripheral hemolysis among the circulating macrocytes was not studied in the older mice and remains largely unexplained.
Another truly interesting finding is the ability of the mice to support the early stages of committed erythropoiesis in the absence of Bcl-xL. As demonstrated in the figure, nonapoptotic and early-stage erythroblasts (prior to hemoglobinization) are clearly generated in the absence of Bcl-xL. In addition, erythropoietin deprivation resulted in apoptosis among those early erythroblast populations that were able to survive without Bcl-xL (see figure, middle and right panels). Similar results were previously reported using Bcl-xL–/– embryonic stem cells.3 While more quantitative studies would be helpful, the data suggest that a proposed role for Bcl-xL as the primary and direct mediator of antiapoptotic effects of erythropoietin during early stages of erythroblast development requires further consideration.
Understanding the regulation and function of Bcl-xL and other erythroid-regulated members of the Bcl-2 family4 is relevant for translational research focused upon a wide range of diseases including myelodysplasia, erythroleukemia, and Janus kinase 2 (JAK2) V617F–associated myeloproliferative disorders. Rhodes and colleagues have confirmed the critical role of Bcl-xL in preventing erythroblast apoptosis, and their data additionally reveal that Bcl-xL may serve its most important role as an indirect mediator of erythropoietin signaling at the very late stages of erythroblast maturation. Hence, Bcl-xL actions in the context of erythropoiesis are suitably described as better late than never. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal