Abstract
We investigated whether HIV-1 antigen-specific CD4+ T cells expressed the viral coreceptor CCR5 during primary HIV-1 infection (PHI). In the peripheral blood of subjects with very early PHI (< 22 days after onset of symptoms), there was a 10- to 20-fold increase in the proportion of highly activated (CD38+++) and proliferating (Ki-67+) CD4+ T cells that expressed CCR5+, and were mostly T-cell intracellular antigen-1 (TIA-1)+ perforin+ granzyme B+. Inthe same patient samples, CD4+ T cells producing interferon (IFN)–γ in response to HIV group-specific antigen (Gag) peptides were readily detected (median, 0.58%) by intracellular cytokine assay—these cells were again predominantly CD38+++, Ki-67+, and TIA-++, as well as Bcl-2low. On average, 20% of the Gag-specific CD4+ T cells also expressed interleukin-2 (IL-2) and were CD127 (IL-7R)+. Taken together, these results suggest that Gag-specific T-helper 1 (Th1) effector cells express CCR5 during the primary response and may include precursors of long-term self-renewing memory cells. However, in PHI subjects with later presentation, antigen-specific CD4+ T cells could not be readily detected (median, 0.08%), coinciding with a 5-fold lower level of the CCR5+CD38+++ CD4+ T cells. These results suggest that the antiviral response to HIV-1 infection includes highly activated CCR5+CD4+ cytotoxic effector cells, which are susceptible to both apoptosis and cytopathic infection with HIV-1, and rapidly decline.
Introduction
Antigen-specific memory CD4+ T cells are not often found in untreated chronic HIV-1 infection, using the standard in vitro proliferation assay.1 It remains unknown whether the scarcity of proliferative HIV-specific CD4+ T cells is due to dysfunction,2,3 inappropriate apoptosis,4 or is a result of cytopathic infection of these cells.5 This deficit of antigen-specific CD4+ T cells may represent a major impediment to immune control of HIV-1 infection. In most, but not all, animal models of adaptive immune responses to viral infection, optimal clearance of virus depends on synergistic interactions between antigen-specific populations of helper CD4+ T cells, antibody-producing B cells, and cytotoxic CD8+ T cells.6,7 In particular, it is believed that effective CD8+ T-cell function in HIV-1 infection is reliant on CD4+ T-cell function.8
Previous studies of primary immune responses to viral infection in mice have shown that antigen-specific T-helper 1 (Th1) CD4 responses can be readily detected in the early stages of the infection, but rapidly decline as antigen is cleared.9,10 Similarly, human CD4+ T-cell immune responses to primary herpesvirus infections exhibit a peak response in the first few weeks,11,12 with markedly reduced responses at follow-up. These results suggest that antigen-specific CD4+ T cells should be generated at a relatively high level during primary HIV-1 infection.
Interferon (IFN)–γ producing antigen-specific CD4+ T cells have been demonstrated in primary HIV-1 infection, despite high levels of viremia.13-17 Furthermore, proliferative responses were maintained if antiretroviral therapy was instituted during acute HIV-1 infection.13,14,18 Further evidence for the presence of antigen-specific CD4 T cells is the production of high-affinity, isotype-switched antibodies to HIV-1, which presumably requires the provision of help for B-cell responses by CXCR5+ CD4+ follicular helper T cells.19,20
Although CD4+ T cells that proliferate in vitro in response to HIV-1 antigens are mostly absent in untreated chronically infected subjects, an average of approximately 0.1% of peripheral blood CD4+ T cells capable of producing IFN-γ can be detected in most HIV-infected individuals by enzyme-linked immunospot (ELISPOT) assay or by intracellular cytokine assay.21,22 It is an absence of those HIV-specific CD4+ T cells that synthesize IL-223-27 that probably causes the proliferative defect in viremic patients.26,28 However, IL-2–producing CD4+ memory cells typically belong to the CCR7+, CCR5– central memory subset,29 and therefore are not directly susceptible to infection by CCR5-tropic HIV-1 strains in early infection. Furthermore, 2 recent studies of HIV-specific IFN-γ–producing CD4+ T cells during primary HIV-1 infection (PHI) has shown that the vast majority were CCR7– cells which did not produce IL-2.16,30 In the latter study, IL-2–producing antigen-specific CD4+ T cells were readily detected in control subjects with Epstein-Barr virus (EBV) or herpes simplex virus (HSV) infections.30
A possible explanation may come from studies of murine models, which suggest that resting memory CD4+ T cells arise directly from effector cells.31,32 Theoretically, then, IL-2–producing HIV-specific memory cells may be derived from Th1 effector cells, which reportedly express CCR5.33 We have previously found that approximately 50%, on average, of CD4+ T cells proliferating in vivo during primary HIV-1 infection express CCR5.34 If the proliferating CD4+ T cells included antigen-specific effector cells, then it is likely that many of these activated effector cells were CCR5+. Recently we confirmed the existence of CCR5+ antigen-specific CD4+ T cells in a HIV+ long-term nonprogressor infected with an attenuated strain of HIV-1.35 These CCR5+ cells produced IL-2 and proliferated strongly in response to HIV-1 p24. Furthermore, these cells were also cytotoxic T lymphocytes (CTLs), similar to cloned CD4+ T cells from patients treated early in acute HIV-1 infection36 and consistent with our previous finding that perforin+ CD4+ T cells were elevated in HIV-1 infection.37 A similar population of cytomegalovirus (CMV)–specific CCR5+ cytotoxic CD4+ T cells was also found in healthy adults, at surprisingly high frequencies.35
We hypothesized that there would be CCR5+ antigen-specific CD4+ T cells produced at the earliest stages of primary HIV-1 infection. We first examined the phenotype of activated, proliferating effector CD4+ T cells in PHI, and then used the flow cytometric intracellular cytokine assay to determine the cell surface phenotype of antigen-specific CD4+ T cells, which subsequently allowed us to further define these cells in samples of fresh whole blood.
Patients, materials, and methods
Patients
A total of 33 patients, diagnosed with primary HIV-1 infection,34 and who then enrolled in the PHAEDRA observational cohort, were included in this study. All patients were males whose risk group was sex with males. Symptoms associated with primary HIV-1 infection were recorded as previously described.38
Patients were subdivided into 2 groups, early PHI (n = 19) and late PHI (n = 14), based on serology at presentation and clinical history of onset of symptoms (Table 1). A Western blot intensity score (Ramacciotti T, Cunningham P, Grey P, et al, manuscript in preparation) was calculated as the sum of each band, multiplied by its intensity on a scale of 1+ to 3+ (for example, a subject with no bands will have a score of 0, while a subject with 3 bands of 1+ intensity and 1 band of 2+ intensity will have a score of 5). Early PHI was defined as having a Western blot intensity score of 5 or less, and in our subject group, this corresponded to presentation within 22 days following onset of symptoms. Late PHI was defined as an intensity score greater than 5, and all these subjects in our cohort had a score of 12 or higher, and presented 28 days or more since onset of symptoms. Two subjects in the late PHI group did not record any symptoms of PHI, and therefore the midpoint between their last negative and first positive HIV antibody tests was used as an estimate of infection date.
Demographics of primary HIV-1 infection (PHI) patients
. | Early PHI n = 19 . | Late PHI n = 14 . |
|---|---|---|
| Age, median y | 38 (33-43) | 35 (30-39) |
| Time since onset of symptoms, d | 15 (11-16) | 42 (32-76) |
| Symptom count | 5 (3-7) | 4 (1-7) |
| Western blot intensity score | 1 (0-3) | 13 (12-20) |
| CD4 count, cells/μL | 396 (239-554) | 608 (566-684) |
| Plasma HIV RNA, log10 copies/mL | 5.9 (5.5-6.7) | 4.5 (3.5-4.9) |
| Time from diagnostic visit to study sample, d | 7 (0-11) | 12 (8-25) |
. | Early PHI n = 19 . | Late PHI n = 14 . |
|---|---|---|
| Age, median y | 38 (33-43) | 35 (30-39) |
| Time since onset of symptoms, d | 15 (11-16) | 42 (32-76) |
| Symptom count | 5 (3-7) | 4 (1-7) |
| Western blot intensity score | 1 (0-3) | 13 (12-20) |
| CD4 count, cells/μL | 396 (239-554) | 608 (566-684) |
| Plasma HIV RNA, log10 copies/mL | 5.9 (5.5-6.7) | 4.5 (3.5-4.9) |
| Time from diagnostic visit to study sample, d | 7 (0-11) | 12 (8-25) |
Numbers in parentheses indicate interquartile ranges for all parameters.
One subject, no. 9400101, was originally diagnosed with early PHI, but was concurrently diagnosed with primary CMV infection, exhibiting a new CMV immunoglobulin M (IgM)+ reactivity, together with detectable CMV viral load, 1 week after PHI diagnosis. Therefore, this individual was excluded from the cohort analyses, and was considered separately. CMV serology was performed by routine diagnostic assay (Vidas; BioMerieux, Marcy-l'Etoile, France).
Healthy HIV-negative university and hospital staff members were recruited as controls for this study. The PHAEDRA study was approved by the local institutional ethics committee, and all subjects gave informed consent.
Cell-surface phenotyping of peripheral blood CD4+ T cells
Staining of T lymphocyte subsets in fresh peripheral blood and 6-color flow cytometric analysis on a dual-laser LSR II flow cytometer (Becton Dickinson, San Jose, CA), was performed as previously described.35 Monoclonal antibodies used were CD3–peridinin chlorophyll protein (PerCP)–Cy5.5, CD4-phycoerythrin (PE)–Cy7, CD8-allophycocyanin (APC)–Cy7, CD11a–fluoroscein isothiocyanate (FITC), CD27-FITC, CD28-PE, CD38-APC, CD38-PE, and CD38-FITC, human leukocyte antigen (HLA)–DR–FITC, CD57-FITC, CD62L-FITC, CD95-PE, CD154 (CD40L)–PE, and IL-2Rα (CD25)–PE and IL-2Rα-FITC (from Becton Dickinson); CXCR4-PE, CCR5-APC, CCR5-PE, and CCR5-FITC, CCR7-PE, CD45RA-APC, CD45RO-FITC, tumor necrosis factor receptor 2 (TNFR2) (CD120b)–PE, Ki-67–FITC, Bcl-2–PE and Bcl-2–FITC, IL-12Rβ1–PE, perforin-FITC, and granzyme A–FITC (Pharmingen, San Diego, CA); TIA-1/GMP-17–PE, IL-2Rβ (CD122)–PE, and IL-7R (CD127)–PE (Beckman Coulter, Hialeah, FL); IL-18R–PE (R&D Systems, Minneapolis, MN); and granzyme B–APC (Caltag, Burlingame, CA).
For CCR5 analysis, whole blood was processed an average of 1.75 hours after venepuncture, to minimize spontaneous loss of this marker, as previously described.35 Intracellular staining was performed using FACSlyse and FACSPermeabilizing Reagents (Becton Dickinson) according to the manufacturer's directions, and analyzed as previously described.34
Intracellular cytokine assay
A subset of 14 consecutive patients (7 early PHI and 7 late PHI) were studied for the presence of HIV group-specific antigen (Gag)–specific CD4+ T cells using a whole-blood intracellular cytokine (ICC) assay35 using 6-color flow cytometry. Overlapping HIV-1 Gag 15-mer peptides, from the sequence of strain HXB2, were obtained from the NIH AIDS reference reagents program. Gag peptides were used as a pool of 122 peptides, at an individual concentration of 2 μg/mL each. For analysis, 300 000 events were collected, T lymphocytes were first gated on CD3-PerCP-Cy5.5 versus side scatter, then on CD4-PE-Cy7+/CD8-APC-Cy7– cells, and finally IFN-γ–APC+ cells were analyzed for the various FITC and PE antibodies. This method has a validated cut-off for positive results of 0.08% of CD4+ T cells, based on background results plus 3 times the standard deviation, from study of 16 HIV– controls (Munier M, Ip S, Zaunders J, et al, manuscript in preparation).
Statistics
Lymphocyte phenotyping results were expressed as a percentage of CD4+ T lymphocytes. Results for each cohort were expressed as medians and interquartile ranges. The Mann-Whitney U test was performed to compare early and late subgroups of primary HIV-1 infection patients with each other and with the HIV– controls, using Statview v5.0 for Macintosh (Abacus Concepts, Berkeley, CA). A 2-sided P value less than .05 was considered statistically significant. The relationship between different phenotypes was determined by Spearman rank correlation (Statview). Graphs of longitudinal data were plotted with Lowess curve-fitting, with tension set to 66% (Statview).
Results
Changes in CD4+ T-cell subsets during early PHI
Several subsets of CD4+ T cells were present only as very minor subsets in HIV– controls, but were found to be prominent in patients with early PHI. In particular, CCR5+CD38+++ CD4+ T cells (Figure 1A) were highly elevated during early PHI, compared with late PHI and healthy adult controls (medians: 5.3% vs 1.0% vs 0.3% of CD4+ T cells, respectively; Figure 1E).
CCR5+TIA+Ki-67+ (Figure 1B) and perforin+granzyme B+ (Figure 1C) CD4+ T cells were also elevated during early PHI, compared with late PHI and healthy controls (3.6% vs 1.0% vs 0.2% and 11.6% vs 5.% vs 1.6%, respectively; Figure 1E).
Similarly, there was an elevation in CD127 (IL-7R)– CD57– (Figure 1D) CD4+ T cells during early PHI, compared with late PHI and healthy controls (medians: 13.7% vs 6.9% vs 5.2%, respectively; Figure 1E). These CD127– CD4+ T cells were also CD45RA– (not shown).
Changes in cell-surface phenotypes of CD4+ T cells during primary HIV-1 infection. Flow cytometry histograms, gated on CD3+CD4+ T cells, show increases in (A) CCR5+CD38+++; (B) CCR5+TIA-1+Ki-67+; (C) perforin+granzyme B+; and (D) CD127– CD57– T cells from representative early PHI patients and HIV– controls. Subpopulations in quadrants are shown as percentages of CD3+CD4+ (A, C, D) or CD3+CD4+Ki-67+ (B) cells. Results from all patients and controls are summarized in panel E. Box plots show 10th, 25th, median, 75th, and 90th percentiles for each marker for each cohort.
Changes in cell-surface phenotypes of CD4+ T cells during primary HIV-1 infection. Flow cytometry histograms, gated on CD3+CD4+ T cells, show increases in (A) CCR5+CD38+++; (B) CCR5+TIA-1+Ki-67+; (C) perforin+granzyme B+; and (D) CD127– CD57– T cells from representative early PHI patients and HIV– controls. Subpopulations in quadrants are shown as percentages of CD3+CD4+ (A, C, D) or CD3+CD4+Ki-67+ (B) cells. Results from all patients and controls are summarized in panel E. Box plots show 10th, 25th, median, 75th, and 90th percentiles for each marker for each cohort.
All the subset changes in early PHI were statistically significant when compared with healthy adult controls (P < .001), and also when compared with late PHI (P < .01). Differences between late PHI patients and HIV– controls were also statistically significant (P < .01; except for CD127(IL-7R)– CD57– CD4+ T cells, P = .05).
Intracellular cytokine assay of HIV-1 Gag-specific CD4+ T cells during PHI
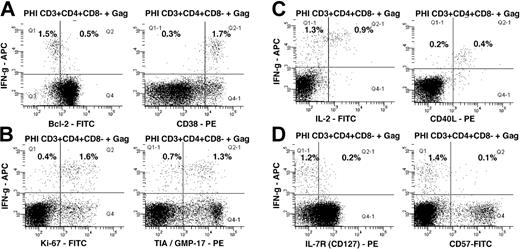
In 6 out of 7 subjects studied during early PHI, Gag-specific CD4+ T cells were detected by IFN-γ production in the ICC assay, ranging from 0.3% to 1.6% of CD4+ T cells. Furthermore, HIV-1 Gag-specific IFN-γ+ CD4+ T cells were consistently CD38+++ and Bcl-2dim (Figure 2A), mostly Ki-67+ and TIA-1/GMP-17+ (Figure 2B), CD40L+ (Figure 2C) and CD57– (Figure 2D). A small, but consistent, proportion of IFN-γ+ Gag-specific CD4+ T cells also produced IL-2 (Figure 2C) and expressed cell-surface CD127 (Figure 2D).
Gag-specific IFN-γ+ CD4+ T cells were found in only 1 patient with late PHI and were too low to obtain reliable phenotype data (not shown).
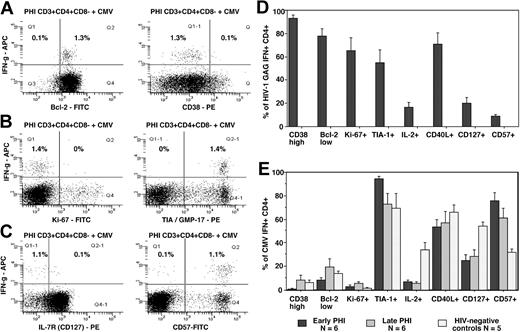
In contrast, CMV-specific IFN-γ+ CD4+ T cells in 5 of the same early PHI individuals who were also CMV IgG seropositive, at the same time points, were predominantly CD38low and Bcl-2high (Figure 3A), Ki-67– (Figure 3B), and CD57+ (Figure 3C). CMV-specific CD4+ T cells were similar to Gag-specific CD4+ T cells in their expression of TIA-1/GMP-17 (Figure 3B), CD127 (Figure 3C), CD40L, and IL-2 (not shown). The phenotyping results for HIV-specific and CMV-specific CD4+ T cells from all early PHI subjects studied are summarized in Figure 3D and E, respectively.
Of interest, patient no. 90400101, undergoing concurrent primary HIV-1 and CMV infections, had 4.0% CMV-specific CD4+ T cells which were phenotypically very similar to his HIV-specific CD4+ T cells, being CD38+++ and Bcl-2dim, CD57–, Ki-67+, TIA-1/GMP-17+, and CD40L+ (data not shown).
In subjects with late PHI and in HIV– adults (who were CMV seropositive), CMV-specific IFN-γ+ CD4+ T cells were also CD38low and Bcl-2high, Ki-67– and CD57+, TIA-1/GMP-17+, and CD40L+ (summarized in Figure 3E). However, in HIV– adult controls, there was an increased proportion of CMV-specific IFN-γ+ CD4+ T cells which produced IL-2 and were CD127+ and CD57– compared with both early (P < .01) and late PHI subjects (P < .02; Figure 3E). It is important to note that the low expression of CD38 and Ki-67 by CMV-specific CD4+ T cells suggests that their expression is not acutely up-regulated by the 6 hours exposure to antigen in vitro.
Close relationship of circulating CCR5+, CD38+++, and Gag-specific CD4+ T cells
When we compared the CD38+++ phenotype of Gag-specific CD4+ T cells, obtained from the ICC assays (Figure 2A), with the coexpression of CCR5 on CD38+++ CD4+ T cells in whole blood samples from the same patients at the same time points (Figure 1A), the results infer that antigen-specific CD4+ T cells were CCR5+ immediately ex vivo. Direct examination of CCR5 expression in the ICC assay was precluded by down-regulation of CCR5 both spontaneously and in response to antigen.35
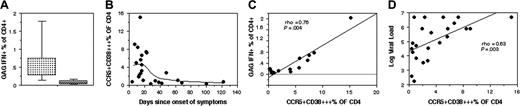
Overall, Gag-specific CD4+ T cells detected by IFN-γ production, in subjects studied during early PHI, represented a median of 0.58% of CD4+ T cells, whereas subjects with late PHI had a significantly lower median, 0.08% (P < .01; Figure 4A), very close to the detection limit of the ICC. Again, this is consistent with the relative levels of CCR5+CD38+++ CD4+ T cells in these 2 subject subgroups (Figure 1E).
Moreover, we observed a very close correlation between the proportion of CCR5+CD38+++ CD4+ T cells and the proportion of IFN-γ+ Gag-specific CD4+ T cells (rho = 0.76; P = .004; Figure 4B). The results show, however, that the proportion of Gag-specific CD4+ T cells consistently represented only one-tenth of the proportion of CCR5+CD38+++ CD4+ T cells (Figure 4B).
Intracellular cytokine responses to HIV-1 Gag peptide pool. Flow cytometry histograms, gated on CD3+CD4+CD8– T cells, show the phenotype of IFN-γ+ cells: (A) Bcl-2 and CD38; (B) Ki-67 and TIA-1; (C) IL-2 and CD154 (CD40L); and (D) CD127 (IL-7R) and CD57. Subpopulations in quadrants are shown as percentages of CD3+CD4+CD8– cells. Histograms are representative of 6 different subjects, all showing similar results.
Intracellular cytokine responses to HIV-1 Gag peptide pool. Flow cytometry histograms, gated on CD3+CD4+CD8– T cells, show the phenotype of IFN-γ+ cells: (A) Bcl-2 and CD38; (B) Ki-67 and TIA-1; (C) IL-2 and CD154 (CD40L); and (D) CD127 (IL-7R) and CD57. Subpopulations in quadrants are shown as percentages of CD3+CD4+CD8– cells. Histograms are representative of 6 different subjects, all showing similar results.
Patient no. 90400101, who was undergoing both primary HIV-1 and CMV infection, had 55% of CD4+ T cells with a CCR5+CD38+++ phenotype, and had 1.1% HIV-specific and 4.0% CMV-specific CD4+ T cells. Therefore, in this individual, the combined proportions of HIV- and CMV-specific CD4+ T cells were also close to one-tenth of the CCR5+CD38+++ CD4+ T cells. The much larger primary CD4+ T cell response to CMV, compared with the response to HIV-1 Gag, has previously been reported in 4 similarly coinfected patients.16
In this cross-sectional study of PHI patients at presentation, the level of CCR5+CD38+++ CD4+ T cells peaked within the first 22 days following onset of symptoms, and appeared to decline rapidly after that time (Figure 4C). A similar peak was also observed when plotted against Western blot (WB) intensity score (not shown). The level of CCR5+CD38+++ CD4+ T cells was highly variable and not all early PHI subjects exhibited a large population of CCR5+CD38+++ CD4+ T cells. Nearly all of the early PHI patients in the current study commenced treatment, so it has not been possible so far to conclusively define the natural history of the CCR5+CD38+++ CD4+ T cells in individual patients.
The level of CCR5+CD38+++ CD4+ T cells appeared to have a complex relationship with viral load, but there was an association between the 2 variables (rho = 0.63; P = .003; Figure 4D).
Intracellular cytokine responses to CMV lysate and comparison with responses to HIV-1 Gag peptide pool. Flow cytometry histograms, gated on CD3+CD4+CD8– T cells, show the phenotype of IFN-γ+ cells: (A) Bcl-2 and CD38; (B) Ki-67 and TIA-1; and (C) CD127 (IL-7R) and CD57. Subpopulations in quadrants are shown as percentages of CD3+CD4+CD8– cells. Histograms shown are representative of results from 6 different subjects, all showing similar results. Results from all patients and controls are summarized in panels D-E. Bars represent means ± SE for each group of patients.
Intracellular cytokine responses to CMV lysate and comparison with responses to HIV-1 Gag peptide pool. Flow cytometry histograms, gated on CD3+CD4+CD8– T cells, show the phenotype of IFN-γ+ cells: (A) Bcl-2 and CD38; (B) Ki-67 and TIA-1; and (C) CD127 (IL-7R) and CD57. Subpopulations in quadrants are shown as percentages of CD3+CD4+CD8– cells. Histograms shown are representative of results from 6 different subjects, all showing similar results. Results from all patients and controls are summarized in panels D-E. Bars represent means ± SE for each group of patients.
Further phenotyping of CCR5+CD38+++ CD4+ T cells in fresh whole blood
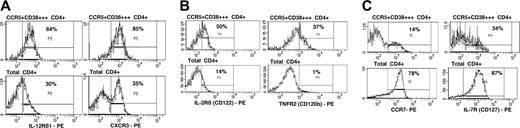
We investigated the expression of trafficking markers, adhesion molecules, costimulatory molecules, and cytokine receptors on the CCR5+CD38+++ CD4+ T cells in samples of fresh whole blood, from 4 subjects with early PHI. Representative results are shown in Figure 5.
The CCR5+CD38+++ CD4+ T cells exhibited relative up-regulation of the chemokine receptor CXCR3 and the cytokine receptor IL-12Rβ1 (Figure 5A), consistent with the phenotype of IFN-γ and IL-2 producing Th1 CD4+ T cells.33
The CCR5+CD38+++ CD4+ T cells slightly up-regulated CD122 (Figure 5B) and CD132 (not shown) but not CD25 (not shown), representing IL-2R β-, γ-, and α-chains, respectively. Expression of TNFR2 (CD120b) was also increased (Figure 5B).
The cell-surface expression of IL-7R (CD127) was mostly down-regulated, although one-third of cells retained this receptor (Figure 5C). Also, there was a consistently observed minority of CCR5+CD38+++ that coexpressed CCR7 (Figure 5C), but these cells had completely down-regulated CD45RA (not shown). These cells also maintained expression of CD62L (not shown) and had increased expression of CD49d, integrin β7, and LFA-1 (not shown), relative to other CD4+ T cells.
The CCR5+CD38+++ CD4+ T cells also maintained expression of the costimulation molecules, CD28 and CD27 (not shown).
Relationship of Gag-specific CD4+ T cells to time since infection, subset changes, and viral load. (A) Comparison of levels of Gag-specific CD4+ T cells in early (▦;N=7) versus late (▨;N=7) PHI subjects. Box plots show 10th, 25th, median, 75th, and 90th percentiles for each cohort. (B) Plot of CCR5+CD38+++ CD4+ T cells in all PHI patients versus time since onset of symptoms. The regression line is a Lowess curve. (C) Correlation of Gag-specific CD4+ T cells with proportion of CCR5+CD38+++ CD4+ T cells in all PHI patients. Dotted horizontal line indicates zero on the y-axis. (D) Plot of CCR5+CD38+++ CD4+ T cells in all PHI patients versus plasma HIV-1 RNA viral load. The linear regression curve is shown.
Relationship of Gag-specific CD4+ T cells to time since infection, subset changes, and viral load. (A) Comparison of levels of Gag-specific CD4+ T cells in early (▦;N=7) versus late (▨;N=7) PHI subjects. Box plots show 10th, 25th, median, 75th, and 90th percentiles for each cohort. (B) Plot of CCR5+CD38+++ CD4+ T cells in all PHI patients versus time since onset of symptoms. The regression line is a Lowess curve. (C) Correlation of Gag-specific CD4+ T cells with proportion of CCR5+CD38+++ CD4+ T cells in all PHI patients. Dotted horizontal line indicates zero on the y-axis. (D) Plot of CCR5+CD38+++ CD4+ T cells in all PHI patients versus plasma HIV-1 RNA viral load. The linear regression curve is shown.
Discussion
The current study of the phenotype of HIV-specific CD4+ T cells, in fresh whole-blood samples obtained during primary HIV-1 infection, demonstrated that these cells were highly activated CD38+++ cells. Parallel analysis of the same blood samples showed that the CD38+++ CD4+ T cells coexpressed the HIV-1 coreceptor, CCR5. Together, these results establish that circulating HIV-1 antigen-specific CD4+ T cells were CCR5+ during primary HIV-1 infection. Expression of CCR5 on these highly activated antigen-specific CD4+ T cells suggests that they are likely to be particularly susceptible to HIV-1 infection, and possibly lost as a result of either direct cytopathic effect by HIV-1, or lysis by CD8+ CTLs.
Consistent with this possibility, our cross-sectional study of patients at presentation found that there was an apparent rapid decline in antigen-specific CD4 T cells over time, as defined both functionally (by ICC assay) and phenotypically (CCR5+CD38+++). In order to establish whether loss of antigen-specific CD4+ T cells is due to HIV-1 infection, it will be important to confirm the kinetics in more detailed longitudinal studies of individual subjects. It will also be necessary to perform cell sorting of CD38+++ CD4+ T cells during early primary infection and determine whether they preferentially contain HIV-1 DNA. Previous studies of CD4+ T cell subsets containing HIV-1 DNA have shown relatively increased infection of antigen-specific CD4+ T cells5 but the cell surface expression of CCR5 in that analysis was not examined.
In the present study, HIV-1 Gag-specific CD4+ T cells were also predominantly Ki-67+, indicating proliferative expansion. It has been shown during acute simian immunodeficiency virus (SIV) infection that Ki-67+ CD4+ T cells in lymphoid tissue increase dramatically about 10 days following inoculation, and are the main producers of SIV during acute infection,39 although again in that study, expression of CCR5 was not reported. Our results would suggest that proliferation of antigen-specific CD4+ T cells contributes to an increase in target cells in lymphoid tissue. However, cell-cell transmission of SIV to adjacent Ki-67– cells in lymphoid tissue was also observed during acute infection,39 suggesting that highly productively infected Ki-67+ CD4+ T cells may act as a focus of spreading infection.
Cell-surface expression of chemokine and cytokine receptors on CCR5+CD38+++ CD4+ T cells. (A) Expression of IL-12Rβ1 and CXCR3 on CCR5+CD38+++ CD4+ T cells (top row) versus expression on all other CD4+ T cells (bottom row). (B) Expression of IL-2Rβ (CD122) and TNFR2 (CD120b) on CCR5+CD38+++ CD4+ T cells (top row) versus expression on all other CD4+ T cells (bottom row). (C) Expression of CCR7 and IL-7R (CD127) on CCR5+CD38+++ CD4+ T cells (top row) versus expression on all other CD4+ T cells (bottom row). Subpopulations in regions are shown as percentages of CCR5+CD38++CD4+ cells (top row) or of CD4+ cells (bottom row). Histograms shown are representative of results from at least 3 different subjects, all showing similar results.
Cell-surface expression of chemokine and cytokine receptors on CCR5+CD38+++ CD4+ T cells. (A) Expression of IL-12Rβ1 and CXCR3 on CCR5+CD38+++ CD4+ T cells (top row) versus expression on all other CD4+ T cells (bottom row). (B) Expression of IL-2Rβ (CD122) and TNFR2 (CD120b) on CCR5+CD38+++ CD4+ T cells (top row) versus expression on all other CD4+ T cells (bottom row). (C) Expression of CCR7 and IL-7R (CD127) on CCR5+CD38+++ CD4+ T cells (top row) versus expression on all other CD4+ T cells (bottom row). Subpopulations in regions are shown as percentages of CCR5+CD38++CD4+ cells (top row) or of CD4+ cells (bottom row). Histograms shown are representative of results from at least 3 different subjects, all showing similar results.
An important question is whether all the observed CCR5+ CD38+++ CD4+ T cells were HIV specific. Our findings agree with earlier descriptions of CD38+, Ki-67+ CMV- and EBV-specific CD4+ T cells during primary infection with these respective viruses.11,12 In the present study, 1 early PHI patient concurrently underwent primary CMV infection and had high levels of both CMV- and HIV-specific CD4+ T cells that were similarly CCR5+CD38+++ and Ki-67+. Conversely, in the other PHI patients (without evidence of primary CMV infection), our results showed that “bystander” CMV-specific effector memory CD4+ T cells, at the same time points, were neither activated, nor proliferating. It should be noted, however, that a large fraction of these resting CMV-specific memory CD4+ T cells were CD57+ and did not produce IL-2, suggesting that they were terminally differentiated effector cells, which previous in vitro studies would predict were unlikely to proliferate.40
There are several possible reasons why the level of HIV-specific CD4+ T cells measured in our assay may not match the level of CD38+++ CD4+ T cells in the blood samples, even if these cells were largely antigen specific. We have not completely investigated the extent of CD4+ T-cell responses to all HIV-1 proteins. Most studies do suggest that CD4+ T-cell responses to HIV-1 Gag are highly immunodominant,1 particularly in patients during acute infection16 as well as patients treated during acute infection who underwent treatment interruption.41,42 However, glycoprotein (gp) 160–specific CD4+ T-cell responses were also commonly found in very early PHI in 1 report.41 Additional responses might also be detected to variant autologous sequences,43 to frame-shifted peptides,44 or to peptides from recombinant variants,45 but these were not tested in the present study. Moreover, there may be a sublineage of antigen-specific Th1 effector cells which do not express IFN-γ, and may be precursors of memory cells.46 It has also been reported that major histocompatibility complex (MHC) class I tetramer+ HIV antigen-specific CD8+ T cells do not uniformly produce IFN-γ in response to their cognate antigen during periods of high viremia following treatment interruptions.47
Increased activation of CD4+ T cells is prominent in PHI48 and this activation is dramatically reduced following initiation of antiretroviral therapy.34,49 Increased expression of CD38 on T cells, particularly on CD8+ T cells, is highly correlated with disease progression during chronic HIV-1 infection.50 Elevated CD38 expression has also been reported on HIV-specific CD8+ T cells during acute HIV-1, although at the same time it was observed that CMV- and EBV-specific CD8+ T cells similarly expressed relatively high levels of CD38.51,52 Therefore the question of whether elevated activation is limited to HIV-specific T cells remains unclear, but overall the present results indicate that CD38 up-regulation on CD4+ T cells, early in infection, is related to antigen-specific activation. It will also be important to determine whether CCR5 expression is coupled to elevated CD38 expression throughout HIV-1 infection.
However, HIV-1 infection of CCR5+CD38+++ CD4+ T cells is probably not the main reason why these cells decline in the circulation as the primary infection resolves. Studies in murine models have shown that CD4+ T cell responses to viral infection show a peak response in the range of 0.5% to 10% of CD4+ T cells, followed by a decrease of 10- to 20-fold.9,10
Also, a similar peak and rapid decline in CMV-specific CD4+ T cells has been observed in primary CMV infection.11 Possible explanations for such a normal decline in viral antigen-specific CD4+ T cells include apoptosis and trafficking. Firstly, we have now directly shown that the antigen-specific CD38+++ CD4+ T cells also contained decreased levels of Bcl-2. We had previously shown that CCR5+ and CD38+ T cells during acute HIV-1 infection contained low levels of Bcl-2 and spontaneously underwent apoptosis in vitro,53 but these cells could be rescued from apoptosis by incubation with IL-15 or IL-2, which increased intracellular levels of Bcl-2.53 Th1 effector cells are particularly susceptible to apoptosis,54 and interestingly, the products of these cells, including IL-2, IFN-γ, and perforin, have all been shown to be involved in feedback regulation of T-cell responses in vivo.55-58 Comparison of primary HIV-1 infection with acute EBV infection, where we have also shown an elevation of CD38++ CD4+ T cells, as well as proliferating CCR5+ CD4+ T cells with low Bcl-2 and increased spontaneous apoptosis,34,53 may allow study of a normal contraction of these CD4+ effector cells.
Another possible reason for the observed decline of CCR5+CD38+++ CD4+ T cells may be trafficking out of the circulation to sites of inflammation, consistent with the cell-surface phenotype of CXCR3+ and CCR5+,59 as well as high expression of LFA-1 and CD62L.60 Antiviral CD8+ effector T cells proliferate in lymph nodes and the spleen, exit into the circulation, and then approximately half of these cells traffic to organs such as the bone marrow, lung, liver, and gut (reviewed in Masopust and Lefrancois61 ). In particular, it is believed that CCR5+ CD4+ T cells in gut-associated lymphoid tissue represent a main target of HIV-1 in acute infection.62,63 We found that the majority of CCR5+ CD38+++ CD4+ T cells expressed the gut-homing marker, integrin α4β7. Interestingly, a previous study showed that there was a selective loss of the integrin α4β7+ subset of CCR5+ CD4+ T cells from the circulation during acute HIV-1 infection.64
However, the apparent decline in antigen-specific T cells may alternatively simply reflect anergy of these cells, resulting in an inability to detect them by functional assays in vitro. Exhaustion of antigen-specific CD4+ T cells has been observed in persistent viral infections in mice.65 Similarly, exposure to persistent antigen in vivo led to a dramatic reduction in cytokine production by recently proliferated CD4+ T cells in a mouse model of anergy.66 It is important to note that a histologic feature of acute HIV-1infection is the accumulation of large numbers of virions retained by the follicular dendritic cell network within lymphoid tissue.67 Constant exposure to HIV-1 antigens, therefore, may prevent the transition to resting memory cells, or induce exhaustion. One study has already demonstrated that CD4+ T cells specific for HIV-1 envelope, which had been present at a detectable level during primary HIV-1 infection, only persisted at a greatly reduced, functionally undetectable level following resolution of the acute phase of infection41 and required prolonged incubation in vitro in IL-2 containing medium for detection.
Finally, it is possible that the antigen-specific CD4+ T cells switch to production of alternative cytokines not measured in the current study, such as IL-10, rather than IFN-γ.68 A more comprehensive analysis of cytokines and effector molecules produced by the Gag-specific CD4+ T cells in PHI is now feasible and warranted. Similarly, development of CD25+ suppressor cells69 may occur after resolution of the primary infection, blocking in vitro responsiveness and studies of the effect of depletion of such CD25+ CD4+ T cells may be informative.
Approximately half of the activated, proliferating CD4+ T cells also expressed markers of cytotoxic T lymphocytes, including TIA-1/GMP-17/NKG-7, as well as granzyme B and perforin. The results of the current study are in agreement with previous findings that immune responses to HIV-1 and CMV include CD4+ T cells with a CTL phenotype and function.35-37,70 These consistent findings suggest that the normal role of CD4+ T cells is not just to help B cells and CD8+ T cells, but also indicates a direct antiviral effect. It had been reported that MHC class II+ cells in vivo can present endogenously produced viral antigenic peptides to CD4+ T cells71 and a recent paper has now shown that CD4+ CTLs specifically clear viral peptide-loaded target cells in vivo.72
Most CCR5+CD38+++ CD4+ T cells exhibited an up-regulation of the IL-2Rβ chain, but not the IL-2Rα chain, consistent with a role for IL-15 in survival of these cells.73 We also found an up-regulation of the IL-12Rβ1 chain on these cells, at least during the acute phase. Previous studies have shown that incubation with IL-1574,75 or IL-1276 increased antigen-specific responses in vitro. The presence of these receptors is consistent with the ability of IL-15 to induce CCR5 expression on resting T cells77 and IL-12 to regulate CCR5 expression following T-cell activation.78 We also found an up-regulation of the TNFR2 on the CCR5+ CD38+++ CD4+ T cells, which may contribute to apoptosis in combination with low Bcl-2,79 but may also be involved in enhancement of HIV-1 replication.80
Studies of effector CD4+ cells in murine models suggest that as viral antigen is cleared, a subset of effector cells will convert to resting memory cells.31,32 In particular, those antigen-specific CD4+ T cells which retain expression of the IL-7R are precursors of memory cells.81 In our earlier study,35 IL-7R– CD38++ T cells from subjects during primary HIV-1 infection underwent spontaneous apoptosis in vitro, but we did not study whether there was a subset of activated T cells which retained IL-7R and could be induced to become resting memory cells by incubation in vitro with IL-7, as suggested in the murine studies.81
We had also previously studied the T-cell receptor variable beta chain (TCRVB) repertoire of Ki-67+, CD38++, and CCR5+ CD4+ T cells and found that it was surprisingly broad.53 Taken together with the current results, it is likely that many clones are involved in the initial antigen-specific response to HIV. Patients treated during acute infection have the broadest responses to HIV proteins,42 which also suggests a broad base of responses during acute infection.
Our results showing expression of CCR5 on activated CD4+ T cells may help to explain the effects of immunosuppressive treatment with hydroxyurea82 and cyclosporine A83 in acute HIV-1 infection. The presence of CCR5 on antigen-specific CD4+ T cells predicts that blockade of this receptor will be particularly beneficial, not only during acute infection, but possibly also during therapeutic immunization or treatment interruptions. It will also be important to confirm that expression of IL-7R is an early marker of long-term self-renewing memory cells, and may represent a useful guide to vaccine efficacy.
Appendix
Members of the PHAEDRA Study Team are P. Grey, J. Kaldor, D. A. Cooper, T. Ramacciotti, K. Petoumenos, D. Smith, M. Bloch, N. Medland, R. Finlayson, A. McFarlane, N. J. Roth, C. Workman, A. Carr, A. D. Kelleher, J. Zaunders, and P. Cunningham.
Prepublished online as Blood First Edition Paper, May 19, 2005; DOI 10.1182/blood-2005-01-0206.
Supported by the Commonwealth Department of Health and Ageing through the Australian National Council on AIDS, Hepatitis C and Related Diseases (The National Centre in HIV Epidemiology and Clinical Research). This project was funded by an AIEDRP grant through the National Institutes of Health Division of AIDS, and a program grant from the Australian National Health and Medical Research Council.
A complete list of members of the Primary HIV and Early Disease Research: Australian Cohort (PHAEDRA) Study Team appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank the patients and their physicians for their participation, Kate McGhie, Ciara McGinley, and Palanee Ammaranond for help with specimen organization and logistics, and the NIH Reference Reagents Program for provision of HIV-1 overlapping Gag peptides.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal