Abstract
Investigations of natural killer (NK) cells in simian models of disease have been hampered by a lack of appropriate phenotypic markers and by an inadequate understanding of the regulation of NK cell activities. In the present study, a panel of monoclonal antibodies (mAbs) specific for various human NK receptors was screened for cross-reactivity with NK cells from rhesus macaques and pigtailed macaques. Flow cytometric analyses using anti-human NKG2A and anti-human NKp80 mAbs individually, and particularly in combination with anti-CD16 mAb, allowed for the identification of the entire NK cell population in both species. NK cells in monkeys were generally identified by negative selection of peripheral blood mononuclear cells (PBMCs) for the absence of T-cell, B-cell, and monocyte markers. mAb-mediated ligation of NKp80 induced NK cell cytotoxicity, while in the case of NKG2A it displayed a clear capability to inhibit the lysis of target cells by NK cells from macaques, as well as from humans. This new phenotypic and functional characterization of NKG2A and NKp80 in rhesus and pigtailed macaque NK cells provides a new approach in the analysis of their innate immune system. (Blood. 2005;106:1718-1725)
Introduction
Nonhuman primate animal models have greatly expanded our understanding of numerous pathogenic conditions, as well as the role of various immune cell subsets in the effective control or the exacerbation of these conditions.1-4 Natural killer (NK) cells play an important role in the innate immune system and are the body's first line of defense against certain viral infections and neoplasms by targeting virus-infected and tumor cells.5 However, the ability to investigate the role of NK cells in simian models of disease has been greatly hampered by the lack of accurate phenotypic markers for this cellular subset in most nonhuman primates. In addition, little is known about the regulation of NK cell function in simians.
In humans, NK cells express CD56, a surface antigen that has also been found on a subset of T lymphocytes and on cells derived from neural, muscle, and embryonic tissues.6 Another common marker on human NK cells is CD16, an FcγRIII receptor involved in antibody-dependent cellular cytotoxicity (ADCC). CD16 is also expressed on a subset of monocytes/macrophages, mast cells, and neutrophil granulocytes.6 In humans, NK cells can be identified as CD3neg, CD56dim-bright, and CD16pos-dim/neg; in particular, the majority of NK cells are CD56dim/CD16pos (approximately 85%-90%), whereas a minority (approximately 10%-15%) are CD56bright/CD16dim/neg.7,8 Previous studies suggested that NK cells in primates can be defined by the expression of CD56.9 However, in rhesus macaques (RMs) it has been demonstrated that monocytes, rather than lymphocytes, express CD56,10 while NK cells of cynomologous monkeys only partially cross-reacted with anti-human CD56 monoclonal antibodies (mAbs).11 Therefore, CD56 is not an appropriate surface antigen for the identification of NK cells in most simians. Similarly, CD16 alone cannot be used as a marker of NK cells in simian species given that a subset of monocytes also express this antigen12-14 and, in general, the NK cell population in nonhuman primates is identified by negative selection of peripheral blood mononuclear cells (PBMCs) for the absence of T-cell, B-cell, and monocyte markers.13-16
In humans, NK cell-mediated cytolytic activity is determined by a delicate balance of signals generated by both inhibiting and activating receptors, some of which are exclusively expressed on NK cells.17 Inhibitory NK receptors (iNKRs) can be divided into 2 categories: killer immunoglobulin-like receptors (KIRs) and C-type lectin receptors.18 These receptors interact with specific allelic forms of class I major histocompatibility complex (MHC-I) molecules and deliver inhibitory signals to NK cells. If signaling via inhibitory NK receptors is weak or lacking, NK cell-mediated lytic activity can be triggered by the engagement of activating receptors, such as natural cytotoxicity receptors (NCRs) NKp44, NKp46, NKp30, or NKG2D by cell surface ligand(s) on allogeneic and pathogen-infected or tumor cells. NCRs are specifically expressed on resting and/or activated NK cells. In humans, the signals generated through iNKRs are typically dominant over those induced by NCRs and NKG2D under physiologic conditions.18-20 In addition, a panel of activating coreceptors expressed on both NK and T cells have been identified that play a role in NK cell-mediated cytotoxicity in synergy with NCRs.17,20
Several studies have investigated the expression, primarily at a molecular level, of inhibitory and activating NK cell receptors in nonhuman primates.21-23 KIRs have been found to be expressed by NK cells in chimpanzees, RMs, orangutans, and baboons, although there was extensive diversity in gene sequence and structure when compared with those of humans.21-24 In addition, several members of the CD94/NKG2 family, a C-type lectin receptor, have been found in chimpanzees, RMs, rats, and mice. Of interest, CD94/NKG2A and NKG2D, an inhibitory and an activating NK receptor respectively, from RMs and humans exhibit a high degree of homology.25 Very little is known about NCRs on NK cells of nonhuman primates, although one study found that NKp46 and NKp30 from humans and cynomologous monkeys are highly homologous.11 While these studies are very informative, to date no surface markers have been reported to fully and accurately identify the NK cell population in most nonhuman primates.
In the present study, we have attempted to identify markers on macaque PBMCs that would positively delineate the NK cell population that we isolated by negative selection. We screened several mAbs directed against numerous human activating and inhibitory NK receptors for their ability to bind PBMC subsets of RMs and pigtailed macaques (PTMs). We show here that anti-human NKp80 and NKG2A mAbs were also able to cross-react at high affinity with similar receptors on NK cells of RMs and PTMs. Moreover, anti-human NKG2A and anti-human NKp80 mAbs individually or in combination with anti-human CD16 mAb discriminated the entire NK cell population within the lymphocyte gate of the cytofluorometric analysis of PBMCs of both macaque species. In addition, the respective activating and inhibitory function of NKp80 and NKG2A was verified in RM and PTM NK cells by an antibody redirected killing assay. Finally, we have delineated the appropriate conditions for the amplification of NKp46, NKp30, NKG2D, NKp80, NKG2A, and CD94 gene products in PBMCs from RMs and PTMs that could prove useful in future studies.
Materials and methods
Study subjects
We studied 17 RMs (Macaca mulatta) and 10 PTMs (Macaca nemestrina). Dr Guido Silvestri (Emory University, Atlanta, GA) kindly provided 3 RMs and Dr Genoveffa Franchini (National Cancer Institute, NIH, Bethesda, MD) provided 6 RMs. The specimens from the other 8 RMs and all PTMs were a generous gift of Dr Vanessa Hirsch (National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD). Animals were housed in accordance with the Guide for the Care and Use of Laboratory Animals26 and the U.S. Department of Agriculture through the Animal Welfare Act (Public Law 91-579). Blood from 13 healthy human donors was provided by the Transfusion Medicine Department of the Warren Grant Magnuson Clinical Center, National Institutes of Health, Bethesda, MD.
Isolation and culture of PBMCs and NK cells
PBMCs were isolated from whole blood over Ficoll-Hypaque gradients (ICN Biomedicals, Aurora, OH). NK cells from RMs and PTMs were isolated from PBMCs by using a column-based cell separation technique (StemCell Technology, Vancouver, BC, Canada) according to the protocol provided by the manufacturer. The enrichment cocktail used for simian NK cell isolation contained antihuman mAbs specific to CD3, CD14, CD20, and CD66e and is known to cross-react with cells from RMs. The purity of CD16pos NK cells in both species was equal to 85%. Human NK cells were isolated by negative depletion using a column-based cell separation technique (StemCell Technology), as previously described.27 Purified human NK cells contained 3% contamination with other PBMC subsets (as determined by expression of CD3, CD4, TCR α/β, TCR γ/δ, CD19, or CD14). To assess cytolytic activity, freshly isolated NK cells were cultured for 6 days in 96-well round-bottom plates at a concentration of 106 cells/mL with RPMI 1640 medium supplemented with penicillin-streptomycin, l-glutamine (GIBCO, Grand Island, NY), 10% fetal calf serum (FCS; Hyclone, Logan, UT) and recombinant interleukin 2 (rIL-2) at 200 IU/mL (Roche Molecular Biochemicals, Indianapolis, IN).
Monoclonal antibodies
The following panel of antihuman mAbs was provided by A.M., D. Pende (Istituto Nazionale per le Ricerca sul Cancro, Genova, Italy), and S. Parolini (University of Brescia, Italy): 289 (IgG2a anti-CD3), C218 and FS280 (IgG1 and IgG2a anti-CD56, respectively), KD1 (IgG2a anti-CD16), Z270 and Z199 (IgG1 and IgG2b anti-NKG2A, respectively), Xa185 and Y9 (IgG1 and IgM anti-CD94, respectively), Bab 281 (IgG1 anti-NKp46), Az20 and Z25 (IgG1 anti-NKp30), Z231 (IgG1 anti-NKp44), On72 and Bat221 (IgG1 anti-NKG2D), Ma152 and Lapi (IgG1 anti-NKp80), Cer1 (IgGM anti-NKp80), Ma127 (IgG1 anti-NTB-A), pp35 (IgG1 anti-2B4), Gl183 (IgG1 anti-p58.2/KIR2DL2), 11pb6 (IgG1 anti-p58.1/KIR2DL1), Z27 (IgG1 anti-p70/KIR3DL1), Q66 (IgM anti-p140/KIR3DL2), Qa79 (IgG1 anti-p75/AIRM1), and F278 (IgG1 anti-LIR1/ILT2). D1-12 (IgG2a anti-human leukocyte antigen-DRpos [HLA-DR]) was kindly provided by Dr R. S. Accolla (University of Insubria, Varese, Italy). Fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-labeled anti-CD3, anti-CD4, anti-CD14, anti-CD16, anti-CD20 mAbs reactive against nonhuman primate cells and PE-labeled anti-human CD94 mAb were purchased from BD-PharMingen (San Jose, CA) and anti-CD8 mAb (IgG2a) from Serotec (Oxford, United Kingdom).
Flow cytometric analysis and cytolytic assay
For 1- or 2-color flow cytometric analysis (FACS Calibur, Becton Dickinson, San Diego, CA), PBMCs and NK cells were stained with the appropriate mAbs followed by PE- or FITC-conjugated isotype-specific goat anti-mouse second reagent (Southern Biotechnology Associates, Birmingham, AL, and IDLabs, London, ON, Canada). Data were analyzed using Cell Quest software. After 6 days of activation with rIL2, NK cells from monkeys were tested for cytolytic activity in a 4-hour Cr51 release assay, as described previously.27 The effector-to-target (E/T) ratio was 20:1. The saturating concentrations of the various mAbs added were 0.5 μg/mL for redirected killing assay performed with the FcγR + P815 target cell line.
RNA and cDNA preparation
Briefly, total RNA was extracted from fresh or frozen monkey or human PBMCs by TRIZOL (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Total RNA was used for cDNA preparation. For first-strand cDNA synthesis, 1.5 μg total RNA was reverse-transcribed using 50 μg/mL oligo (dT)12-18 primer in a final volume of 20 μL, in the presence of 200 U SuperScript II reverse transcriptase (Invitrogen). The reaction was carried out in 2 steps: first, the RNA was denaturated at 65°C for 5 minutes; second, all products were incubated at 42°C for 45 minutes.
RT-PCR
Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed in a total volume of 100 μL, containing 10 μL cDNA, 200 nmol of each upstream and downstream primer, and 2 U AmpliTaq Gold (Applied Biosystems, Foster City, CA). The cycle program for human NKp80 primers consisted of 40 cycles of denaturation at 95°C for 20 seconds, annealing at 64°C for 1 minute and elongation at 72°C for 1 minute. For human and monkey NKG2A primers, the program consisted of 40 cycles of denaturation at 95°C for 30 seconds, annealing at 56°C for 1 minute and elongation at 72°C for 1 minute. For human and monkey CD94 primers, the program consisted of 25 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 1 minute, and elongation at 72°C for 1 minute. For human NKG2D primers, the program consisted of 30 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 1 minute, and elongation at 72°C for 1 minute. For monkey NKG2D primers, the program consisted of 35 cycles of denaturation at 95°C for 30 seconds, annealing at 58°C for 1 minute, and elongation at 72°C for 1 minute. For human and monkey NKp46 primers, the program consisted of 35 cycles of denaturation at 95°C for 30 seconds, annealing at 62°C for 1 minute, and elongation at 72°C for 1 minute. For human NKp30 primers, the program consisted of 40 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and elongation at 72°C for 1 minute. For monkey NKp30 primers, the program consisted of 40 cycles of denaturation at 95°C for 30 seconds, annealing at 58°C for 30 seconds, and elongation at 72°C for 30 seconds. The cycle program was preceded by an initial denaturation at 95°C for 5 minutes and followed by a final extension at 72°C for 7 minutes. PCR products were analyzed by 1.0% agarose gel electrophoresis and visualized with ethidium bromide. The following RNA transcripts were detected via amplification of the corresponding cDNAs: NKp80, using a primer pair composed of the sense primer 5′-CTTACATTTAAAGATTATTCAGTGACGTTGCACTGG-3′ and the antisense primer 5′-GGCAGCACAGCTGTTTTCTTTAGCTGGTCCC-3′; human NKG2A, using a primer pair composed of the sense 5′-GCTTACAATGATATATTATTGAAGATCCACAC-3′ and the antisense 5′-CAAGGAGTAATCTACTCAGACCTGAATCTGC-3′; monkey NKG2A, using the primer set composed of the sense 5′-AAGAGGCAGCAGCAAAAACC-3′ and antisense 5′-CGAAACACATCAATCCATGAGG-3′; human NKG2D, using a primer pair composed of the sense 5′-GAAGGCTTTTATCCACAA-3′ and the antisense 5′-CCCCAGCCCATCCACTCT-3′; monkey NKG2D, using a primer pair composed of the sense 5′-TCCTCTCTGCGGTAGACG-3′ and the antisense 5′-TCTGTTCCTGGCTTTTATTGAG-3′; human NKp46, using a primer pair composed of the sense 5′-GAATCTGAGCGATGTCTTCC-3′ and the antisense 5′-TCCGTGGGTCCAACACAG-3′; monkey NKp46, using a primer pair composed of the sense 5′-CTGAGCGATGTCTTCCACAC-3′ and the antisense 5′-CCGCCCAGGCTCAACACC-3′; human NKp30, using a primer pair composed of the sense 5′-ACCCAGACCTCACTGCT-3′ and the antisense 5′-GATTTATTGGGGTCTTTTGAAG-3′; monkey NKp30, using a primer pair composed of the sense 5′-GACATCTTCCGACATGGC-3′ and the antisense 5′-CTTGGCTGGCATTGAAGG-3′; CD94, using a primer pair composed of the sense 5′-CTTCTCTACTTCGCTCTTGG-3′ and the antisense 5′-GGTCTTTACTCTCCACCTTC-3′.
A 557-base pair (bp) NKp80 sequence for both human (accession number AJ305 370) and monkeys (accession number AJ426 430) and a 681-bp sequence for the human NKG2A sequence (accession number AF461 812) were identified using MacVector version 7.2 (Accelrys, San Diego, CA). The complementarity between human and monkey sequences was obtained by basic local alignment search tool (BLASTN) search algorithm. For both RMs and PTMs, NKG2A was identified by a 484-bp fragment. NKG2D was identified by a fragment of 1030 bp for human28 and 723 bp for both monkey species29 ; NKp46 by a fragment of 1014 bp for human (accession number NM004 829) and 986 bp for RMs and PTMs11 ; NKp30 by a fragment of 649 bp for human (kindly provided by Dr C. Cantoni, University of Genova, Italy) and 149 bp for both monkey species11 ; CD94 by a fragment of 610 bp for human and both simian species.29
PCR fragments were cloned using the TOPO TA Cloning system with TOP 10 F' cells (Invitrogen). Plasmid DNA was recovered using the QIAprep Spin Miniprep kit (Qiagen, Valencia, CA) and sequenced using the Applied Biosystems 3100 system. Sequences were analyzed using Sequencher version 4.1 (Gene Code, Ann Arbor, MI) and used to translate nucleotide sequences to a protein sequences by MacVector version 7.2.
Transient transfections and binding experiments
Simian full-length NKp80 cDNA sequences were cloned into pcDNA3.1/V5-His (Invitrogen). NKp80 was amplified in PTMs with sense 5′-ATGCAAGATGAAGAAAGATAC and the antisense 5′-CTAATATTAATCTCCAGGTGT; in RMs NKp80 was amplified performing a nested PCR with sense 5′-ACACATTCACTCACATTGAAG and antisense 5′-GATTTTAAAGTGCAATTAAAT. For binding experiments COS-7 cells were transfected using lipofectamine (Invitrogen). At 48 hours after transfection, cells were stained with 3 different anti-human NKp80 mAbs (Ma152, Lapi, and Cer1) followed by PE-conjugated isotype-specific second reagent, and analyzed by flow cytometry.
Statistical analysis
Each RM and PTM immune response variable in the Cr51 release cytolytic assay was evaluated by using the paired t test. All P values are 2-sided and unadjusted. Statistical associations between simian lymphocyte subsets were determined by the Spearman rank test for correlation.
Results
Detection of NK cell receptors on PBMC subsets of RMs and PTMs using anti-human mAbs
A large panel of anti-human NK cell receptor mAbs was screened for cross-reactivity with macaque PBMC subsets. Within the lymphocyte gate, based on its characteristic of forward-scatter versus side-scatter patterns, RM and PTM NK cells were identified by their lack of the T-lymphocyte marker CD3, and of HLA-DR, which was expressed at high levels on all CD20pos B cells (data not shown). As previously reported,10 we also found that in RMs CD56 was expressed on monocytes rather than lymphocytes (data not shown). The CD3neg/HLA-DRneg NK cells were largely negative for CD56 whereas the majority expressed CD16. A similar pattern of CD56 and CD16 expression was observed in the CD3neg/HLA-DRneg NK cell population from PTMs (data not shown). Given that so little is known about the phenotypic characteristics of leukocytes in monkeys, our definition of RM and PTM NK cells as CD3neg/HLA-DRneg is likely, but remains an assumption that needs to be verified with different approaches.
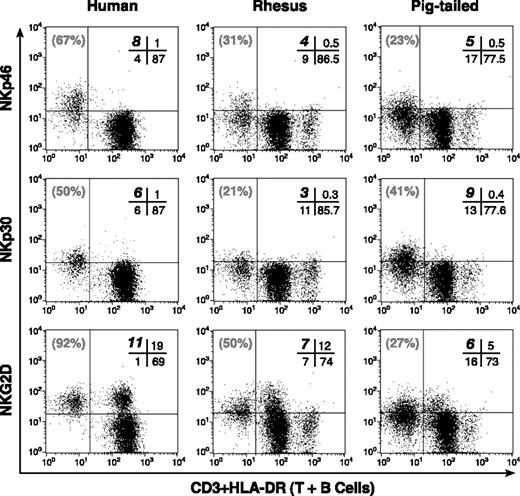
Phenotypic analysis of NKp46, NKp30, and NKG2D. Representative examples of double-color flow cytometric analysis within the lymphocyte compartment of PBMCs. Cross-reactivity of mAbs specific to human NKp46 (top row), NKp30 (middle row), and NKG2D (bottom row) along with anti-CD3 and anti-HLA-DR mAbs in a human donor (left column), and in rhesus (middle column), and pigtailed (right column) macaques. The cells with the brightest mean fluorescence intensity in the lower right quadrant of each rhesus and pigtailed monkey dot plot corresponded to the HLA-DRpos (or CD20pos) lymphocyte subset that identified the B-cell population. The absolute percentage of CD3neg/HLA-DRneg (NK) lymphocytes positive for the relevant receptor is highlighted in bold in the upper left quadrant of the cross bar in the upper right quadrant of each dot plot. The relative percentage of total CD3neg/HLA-DRneg (NK) population positive for NKp46, NKp30, and NKG2D is indicated in gray in the upper left quadrant of each dot plot.
Phenotypic analysis of NKp46, NKp30, and NKG2D. Representative examples of double-color flow cytometric analysis within the lymphocyte compartment of PBMCs. Cross-reactivity of mAbs specific to human NKp46 (top row), NKp30 (middle row), and NKG2D (bottom row) along with anti-CD3 and anti-HLA-DR mAbs in a human donor (left column), and in rhesus (middle column), and pigtailed (right column) macaques. The cells with the brightest mean fluorescence intensity in the lower right quadrant of each rhesus and pigtailed monkey dot plot corresponded to the HLA-DRpos (or CD20pos) lymphocyte subset that identified the B-cell population. The absolute percentage of CD3neg/HLA-DRneg (NK) lymphocytes positive for the relevant receptor is highlighted in bold in the upper left quadrant of the cross bar in the upper right quadrant of each dot plot. The relative percentage of total CD3neg/HLA-DRneg (NK) population positive for NKp46, NKp30, and NKG2D is indicated in gray in the upper left quadrant of each dot plot.
Lymphocyte subsets from both species of macaques were also analyzed for reactivity with anti-human NKp46, NKp30, and NKG2D mAbs and variable frequencies of monkey CD3neg/HLA-DRneg NK cells stained positively with these anti-human mAbs (Figure 1). As in humans,20 the expression of NKp46 and NKp30 was specifically confined to the NK cell population in both nonhuman primates whereas NKG2D was also expressed on a subset of CD3pos/HLA-DRneg cells. However, a large fraction of CD3neg/HLA-DRneg NK cells in both RMs and PTMs remained negative for NKp46, NKp30, and NKG2D (Tables 1, 2, 3).
Cross-reactivity of NKp46 (Bab281 mAb) on human and simian lymphocytes
. | Mean (range), % . | . | . | ||
|---|---|---|---|---|---|
| Donors . | CD3neg/HLA-DRneg NKp46pos . | CD3neg/HLA-DRneg NKp46neg . | CD3pos/HLA-DRpos NKp46pos . | ||
| Human, n = 13 | 56 (31-73) | 44 (27-69) | 0.4 (0.2-1.3) | ||
| Rhesus, n = 17 | 29 (13-45) | 71 (55-87) | 0.3 (0.3-0.9) | ||
| Pigtailed, n = 10 | 21 (12-32) | 78 (68-88) | 0.4 (0.2-1) | ||
. | Mean (range), % . | . | . | ||
|---|---|---|---|---|---|
| Donors . | CD3neg/HLA-DRneg NKp46pos . | CD3neg/HLA-DRneg NKp46neg . | CD3pos/HLA-DRpos NKp46pos . | ||
| Human, n = 13 | 56 (31-73) | 44 (27-69) | 0.4 (0.2-1.3) | ||
| Rhesus, n = 17 | 29 (13-45) | 71 (55-87) | 0.3 (0.3-0.9) | ||
| Pigtailed, n = 10 | 21 (12-32) | 78 (68-88) | 0.4 (0.2-1) | ||
Cross-reactivity of NKp30 (Az20 mAb, Z25 mAb) on human and simian lymphocytes
. | Mean (range), % . | . | . | ||
|---|---|---|---|---|---|
| Donors . | CD3neg/HLA-DRneg NKp30pos . | CD3neg/HLA-DRneg NKp30neg . | CD3pos/HLA-DRpos NKp30pos . | ||
| Human, n = 13 | 41 (26-61) | 59 (39-74) | 0.4 (0.1-1.2) | ||
| Rhesus, n = 17 | 23 (15-53) | 77 (47-85) | 0.6 (0.2-0.9) | ||
| Pigtailed, n = 10 | 45 (14-59) | 55 (41-86) | 0.4 (0.3-0.8) | ||
. | Mean (range), % . | . | . | ||
|---|---|---|---|---|---|
| Donors . | CD3neg/HLA-DRneg NKp30pos . | CD3neg/HLA-DRneg NKp30neg . | CD3pos/HLA-DRpos NKp30pos . | ||
| Human, n = 13 | 41 (26-61) | 59 (39-74) | 0.4 (0.1-1.2) | ||
| Rhesus, n = 17 | 23 (15-53) | 77 (47-85) | 0.6 (0.2-0.9) | ||
| Pigtailed, n = 10 | 45 (14-59) | 55 (41-86) | 0.4 (0.3-0.8) | ||
The anti-human NKp30 mAbs Az20 and Z25 cross-reacted with the same affinity with human and simian lymphocytes.
Cross-reactivity of NKG2D (Bat221 mAb, On72 mAb) on human and simian lymphocytes
. | Mean (range), % . | . | . | ||
|---|---|---|---|---|---|
| Donors . | CD3neg/HLA-DRneg NKG2Dpos . | CD3neg/HLA-DRneg NKG2Dneg . | CD3pos/HLA-DRpos NKG2Dpos . | ||
| Human, n = 13 | 93 (86-97) | 7 (3-14) | 23 (16-49) | ||
| Rhesus, n = 17 | 52 (28-70) | 48 (30-72) | 15 (12-23) | ||
| Pigtailed, n = 10 | 30 (15-42) | 70 (58-85) | 9 (5-21) | ||
. | Mean (range), % . | . | . | ||
|---|---|---|---|---|---|
| Donors . | CD3neg/HLA-DRneg NKG2Dpos . | CD3neg/HLA-DRneg NKG2Dneg . | CD3pos/HLA-DRpos NKG2Dpos . | ||
| Human, n = 13 | 93 (86-97) | 7 (3-14) | 23 (16-49) | ||
| Rhesus, n = 17 | 52 (28-70) | 48 (30-72) | 15 (12-23) | ||
| Pigtailed, n = 10 | 30 (15-42) | 70 (58-85) | 9 (5-21) | ||
The anti-human NKG2D mAbs Bat221 and On72 cross-reacted with the same affinity with human and simian lymphocytes.
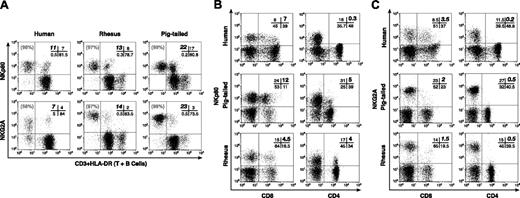
In contrast, virtually all NK cells from RMs and PTMs stained strongly with mAbs directed against human NK cell activating coreceptor NKp80 and the inhibiting NK receptor NKG2A. In human lymphocytes a considerable proportion of CD3neg/HLA-DRneg NK cells were negative for NKG2A expression, but not for NKp80. In RMs and PTMs NKp80 was also positive on a large percentage of CD3pos/HLA-DRpos cells, whereas a very small fraction of CD3pos/HLA-DRpos cells coexpressed NKG2A (Figure 2A; Table 4; Table 5). NKG2A and NKp80 were never detected on CD20pos or HLA-DRpos B cells, nor were they detected on CD14pos, CD56pos, or CD16pos monocytes from either RMs or PTMs (data not shown). To better understand whether NKp80 and NKG2A were expressed on CD3pos cells, we performed further flow cytometric analysis on T-cell subsets. Antihuman NKp80 mAbs were also found to bind to a subset of CD3pos/CD8pos T cells (range: 4%-29% in RMs and 3%-31% in PTMs) and, unlike the situation with human cells,30 the antibodies also bound to CD3pos/CD4pos T cells (range: 0.5%-6% in RMs and 2%-7% in PTMs). However, only a small fraction of simian CD3pos/CD8pos T cells (range: 0.5%-6% in RMs and 2%-7% in PTMs) and virtually none of the CD3pos/CD4pos T cells were positive for NKG2A (Figure 2B-C).
Cross-reactivity of NKG2A (Z199 mAb) on human and simian lymphocytes
. | Mean (range), % . | . | . | ||
|---|---|---|---|---|---|
| Donors . | CD3neg/HLA-DRnegNKG2Apos . | CD3neg/HLA-DRnegNKG2Aneg . | CD3pos/HLA-DRposNKG2Apos . | ||
| Human, n = 13 | 67.4 (58-75) | 32.6 (25-42) | 22 (6-36) | ||
| Rhesus, n = 17 | 97.4 (97-98) | 2.6 (1-3) | 2.9 (0.5-7) | ||
| Pigtailed, n = 10 | 97.4 (97-99) | 2.6 (1-3) | 3.2 (1-8) | ||
. | Mean (range), % . | . | . | ||
|---|---|---|---|---|---|
| Donors . | CD3neg/HLA-DRnegNKG2Apos . | CD3neg/HLA-DRnegNKG2Aneg . | CD3pos/HLA-DRposNKG2Apos . | ||
| Human, n = 13 | 67.4 (58-75) | 32.6 (25-42) | 22 (6-36) | ||
| Rhesus, n = 17 | 97.4 (97-98) | 2.6 (1-3) | 2.9 (0.5-7) | ||
| Pigtailed, n = 10 | 97.4 (97-99) | 2.6 (1-3) | 3.2 (1-8) | ||
Z270 anti-human NKG2A mAb also cross-reacted with RM and PTM lymphocytes, but its lower mean fluorescence intensity did not allow us to exactly discriminate the CD3neg/HLA-DRneg/NKG2Apos from CD3neg/HLA-DRneg/NKG2Aneg (NK) cells.
Cross-reactivity of NKp80 (Ma152 mAb, Lapi mAb, Cer1 mAb) on human and simian lymphocytes
. | Mean (range), % . | . | . | ||
|---|---|---|---|---|---|
| Donors . | CD3neg/HLA-DRnegNKp80pos . | CD3neg/HLA-DRnegNKp80neg . | CD3pos/HLA-DRposNKp80pos . | ||
| Human, n = 13 | 96.7 (95-98) | 2.3 (2-5) | 29 (7-42) | ||
| Rhesus, n = 17 | 97.5 (97-99) | 2.5 (1-3) | 21 (7-36) | ||
| Pigtailed, n = 10 | 97.8 (97-99) | 2.2 (1-3) | 24 (8-37) | ||
. | Mean (range), % . | . | . | ||
|---|---|---|---|---|---|
| Donors . | CD3neg/HLA-DRnegNKp80pos . | CD3neg/HLA-DRnegNKp80neg . | CD3pos/HLA-DRposNKp80pos . | ||
| Human, n = 13 | 96.7 (95-98) | 2.3 (2-5) | 29 (7-42) | ||
| Rhesus, n = 17 | 97.5 (97-99) | 2.5 (1-3) | 21 (7-36) | ||
| Pigtailed, n = 10 | 97.8 (97-99) | 2.2 (1-3) | 24 (8-37) | ||
The anti-human NKp80 mAbs Ma152, Lapi, and Cer1 cross-reacted with the same affinity with human and simian lymphocytes.
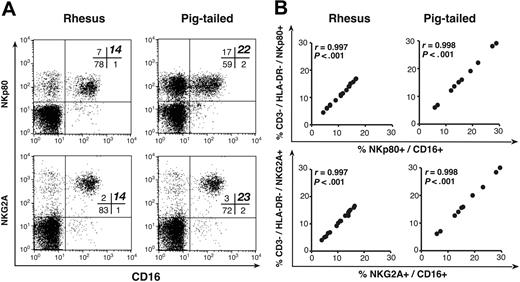
In most simian species, CD16 is expressed by all NK cells8-10 as well as on a subset of monocytes. Combining mAbs directed against CD16 and either NKG2A or NKp80 allowed the clear positive identification of the entire NK cell population (CD16pos/NKp80pos or NKG2Apos/CD16pos) that we had isolated in both RMs and PTMs for the absence of T-cell and B-cell markers within the lymphocyte gate of PBMCs. The frequencies of NK cells detected in both monkey lymphocytes using these double stains correlated exactly with those obtained by analyzing NKp80 or NKG2A expression alone in the CD3neg/HLA-DRneg NK population. The ratios of the reactivity of the anti-human NKp80 and NKG2A mAbs on simian CD3neg/HLA-DRneg versus CD16pos lymphocytes were extremely high (r = 0.997) and statistically significant (P < .001; Figure 3). We were unable to detect any reactivity on RM and PTM PBMCs with mAbs directed against human iNKRS and KIRs, such as p58.2/KIR2DL2, p58.1/KIR2DL1, p70/KIR3DL1, p140/KIR3DL2, p75/AIRM1, and LIR1/LT2. Furthermore, human mAbs directed against human 2B4 and NTB-A, 2 other activating NK coreceptors, failed to recognize the same molecules in either simian species.
Phenotypic and functional analysis of NKp80 and NKG2A on purified NK cells isolated from rhesus and pigtailed macaques
To confirm the phenotypic analysis performed on gated non-B, non-T, and nonmonocyte PBMCs, we purified NK cells from the peripheral blood of macaques by negative selection using a cocktail of mAbs directed at all other mononuclear cell subsets.
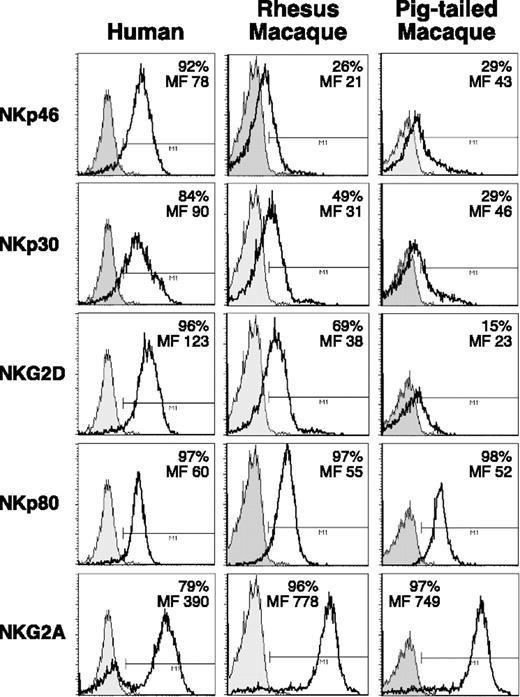
The resulting cell population was more than or equal to 85% CD16pos. The same RM and PTM purified NK cells in most instances displayed low reactivity with NKp46, NKp30, and NKG2D mAbs, whereas NKp80 and NKG2A were highly expressed on 96% of the cells (Figure 4). Similar findings were made using purified CD16pos simian NK cells that had been activated with rIL2. No cross-reactivity with an anti-human NKp44 mAb was detected on activated NK cells isolated from either macaque species (data not shown).
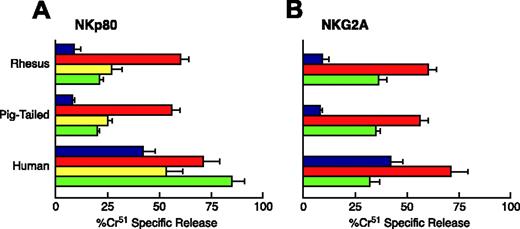
Having verified that anti-human NKp80 and NKG2A mAbs also cross-reacted at high affinity with macaque NK cells, we tested these and other NK receptors for their ability to modulate simian NK cell cytolytic activity. In humans NKp80 is an activating coreceptor that normally works in synergy with NCRs,20,30 whereas NKG2A is an inhibitory receptor that belongs to the CD94/NKG2 C-type lectin molecules.18 NK cells were purified from both RMs and PTMs, cultured in the presence of rIL-2 for 6 days, and analyzed in a redirected killing assay against FcγR + P815 target cells either in the absence or in the presence of different mAbs specific for triggering NK cell receptors. Upon activation of NK cells with rIL-2, anti-NKp80 mAb induced in both macaque species a statistically significant increase in the NK cell cytolytic response when compared with the baseline lysis (P < .05), although not to the levels achieved by anti-CD16. Unlike the situation with humans,20,30 mAb-mediated ligation of NKp46 induced weaker NK cytolytic activity in both RMs and PTMs when compared with that of NKp80, reflecting the NKp46dim phenotype shown by NK cells from both species (Figure 5A). To determine whether NKG2A acted as an inhibitory receptor in simian NK cells, anti-NKG2A was tested for its ability to suppress anti-CD16-induced cytotoxic activity. As shown in Figure 5B, NKG2A was able to significantly inhibit cytotoxicity in both simian species (P < .05).
RT-PCR analysis and binding experiments
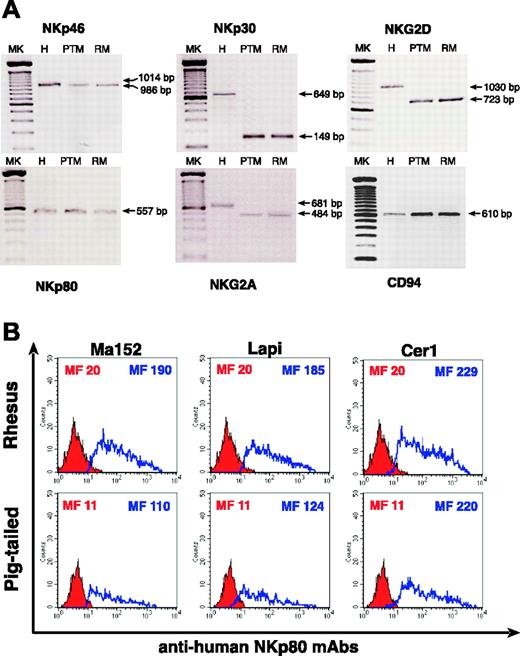
We performed RT-PCR analysis of several activating NK receptors, such as NKp30, NKp46, and NKG2D as well as NKp80 and NKG2A (Figure 6A). Using available primers we were able to amplify NKp46 (1014-bp product for human and 986-bp product for macaques), NKp30 (649-bp product for human and 149-bp product for macaques), and NKG2D (1030-bp product for human and 723-bp product for macaques). The 557-bp product of NKp80 was amplified using primers designed from human NKp80 sequences. Despite the high degree of NKG2A sequence homology shared between RMs and humans, the primers designed for human NKG2A did not amplify the same 681-bp product in PTM and in RM PBMCs. Therefore, we designed primers based on sequencing of a 662-bp NKG2A fragment obtained from RM PBMCs and were then able to amplify a specific 484-bp product for both species of macaques. Although we did not find any cross-reactivity with anti-human CD94 mAb on simian NK cells, we amplified a 610-bp CD94 product in both RMs and PTMs using primers derived from human CD94 sequences.
Phenotypic analysis of NKp80 and NKG2A. Representative examples of double-color flow cytometric analysis within the lymphocyte compartment of PBMCs. (A) Cross-reactivity of mAbs specific to human NKp80 (top row) and NKG2A (bottom row) along with anti-CD3 and anti-HLA-DR mAbs in a human donor (left column), and in rhesus (middle column) and pigtailed (right column) macaques. The cells with the brightest mean fluorescence intensity in the lower right quadrant of each rhesus and pigtailed monkey dot plot corresponded to the HLA-DRpos (or CD20pos) lymphocyte subset that identified the B-cell population. The absolute percentage of CD3neg/HLA-DRneg (NK) lymphocytes positive for the relevant receptor is highlighted in bold in the upper left quadrant of the cross bar in the upper right quadrant of each dot plot. The relative percentage of total CD3neg/HLA-DRneg (NK) population positive for NKp80 and NKG2A is indicated in gray in the upper left quadrant of each dot plot. (B-C) Percentage (highlighted in bold in upper right quadrant of the cross bar in the upper right quadrant of each dot plot) of FITC-labeled CD8+ T cells (left column) and FITC-labeled CD4+ T cells (right column) positive for NKp80 (B) and NKG2A (C) in humans (top row), and in pigtailed (middle row) and rhesus (bottom row) macaques.
Phenotypic analysis of NKp80 and NKG2A. Representative examples of double-color flow cytometric analysis within the lymphocyte compartment of PBMCs. (A) Cross-reactivity of mAbs specific to human NKp80 (top row) and NKG2A (bottom row) along with anti-CD3 and anti-HLA-DR mAbs in a human donor (left column), and in rhesus (middle column) and pigtailed (right column) macaques. The cells with the brightest mean fluorescence intensity in the lower right quadrant of each rhesus and pigtailed monkey dot plot corresponded to the HLA-DRpos (or CD20pos) lymphocyte subset that identified the B-cell population. The absolute percentage of CD3neg/HLA-DRneg (NK) lymphocytes positive for the relevant receptor is highlighted in bold in the upper left quadrant of the cross bar in the upper right quadrant of each dot plot. The relative percentage of total CD3neg/HLA-DRneg (NK) population positive for NKp80 and NKG2A is indicated in gray in the upper left quadrant of each dot plot. (B-C) Percentage (highlighted in bold in upper right quadrant of the cross bar in the upper right quadrant of each dot plot) of FITC-labeled CD8+ T cells (left column) and FITC-labeled CD4+ T cells (right column) positive for NKp80 (B) and NKG2A (C) in humans (top row), and in pigtailed (middle row) and rhesus (bottom row) macaques.
To confirm the specificity of anti-human NKp80 mAbs in binding similar receptors in simians, we transfected a COS-7 cell line with a construct encoding RM or PTM cDNA NKp80 sequences. At 48 hours after transfection, the 3 anti-human NKp80 mAbs (Ma152, Lapi, and Cer1) that cross-reacted with simian lymphocytes were also able to bind with high affinity a similar molecule expressed on the cells transfected with RM and PTM pNKp80 (Figure 6B).
Discussion
The ability to investigate the role of NK cells in nonhuman primate models of disease has been greatly limited by the lack of appropriate phenotypic markers for this cellular population in simian species. In the present study, we screened mAbs directed against human activating or inhibiting NK receptors or coreceptors with the goal of improving the identification of NK populations in nonhuman primates. We demonstrate that anti-human NKp80 and NKG2A mAbs recognize similar receptors on NK cells of RMs and PTMs within the lymphocyte compartment of PBMCs. Furthermore, either of these mAbs alone, but in particular in combination with anti-CD16, allow for the positive identification of the entire NK cell population determined by negative selection of PBMCs for the absence of T cells, B cells, and monocyte markers in both macaque species. In addition, the function of NKp80 and NKG2A, cotriggering and inhibiting NK receptors, respectively, on simian NK cells was demonstrated to be similar to that described in human systems. Finally, we have delineated appropriate conditions and primer/probe sets for the amplification of numerous NK cell receptor gene products in simian PBMCs.
Classic phenotypic markers used for the identification of human NK cells (CD56 and CD16) fail to completely and specifically identify this population in most nonhuman primates.10-14 We demonstrate that both anti-human NKp80 and NKG2A mAbs bind to the entire CD3neg/HLA-DRneg NK cell population in RMs and PTMs. While anti-NKp80 recognized virtually all NK cells in humans and macaques, anti-NKG2A proved to be significantly more specific than anti-NKp80 for simian NK cells. Nevertheless, the same specific recognition of NK cells could be achieved with anti-NKp80 if used in combination with anti-CD16. Interestingly, while NKG2A was expressed on all simian NK cells, this was not the case for the human NK cell population. However, these differences in the frequency of NKG2Apos NK cells in the human versus simian NK cell populations were not reflected in the ability of this inhibitory NK coreceptor to suppress CD16-mediated NK cell cytolytic activity as determined by a redirected killing assay. The human CD94/NKG2 receptor family is composed of 5 different members (NKG2A, B, C, E, and H) that are expressed on the cell surface as heterodimers together with an invariant CD94 chain. Functionally, NKG2A and NKG2B appear to transduce inhibitory signals due to the presence of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic tail. In contrast, NKG2C, E, and H, which lack ITIMs and are associated with immunoreceptor tyrosine-based activation motif (ITAM)-containing polypeptides, appear to act as activating receptors.25,31 We could not identify any anti-human CD94 mAbs capable of cross-reacting with RM and PTM NK cells. Nevertheless, previous molecular studies based on a PCR-based approach have demonstrated the existence of homologs of human NKG2A, B, C, and CD94 in monkeys and chimpanzees,25,29 and we were able to amplify a 610-bp CD94 product in both RM and PTM lymphocytes even though such a product was not antigenically recognized by anti-human mAbs. In RMs, the NKG2A molecule contains 2 ITIMs in their cytoplasmic domain.25 The conservation of the 2 ITIM motifs, essential for the inhibitory signaling upon engagement of NKG2A, is in line with the inhibitory function in monkey NKG2A that we detected in redirected killing assays.
Phenotypic analysis of NKp80 and NKG2A. (A) Representative examples of double-color flow cytometric analysis within the lymphocyte compartment of PBMCs. mAbs specific to human NKp80 (top row) and NKG2A (bottom row) along with anti-CD16 mAb in rhesus (left column) and pigtailed (right column) macaques. The percentage of NKp80pos/CD16pos and NKG2Apos/CD16pos cell subsets are highlighted in bold in the upper right quadrant of the cross bar in the upper right quadrant of each dot plot. Of note, in each rhesus and pigtailed macaque whose flow cytometric analyses were chosen as representative examples of all animals studied, the percentage of total CD3neg/HLA-DRneg (NK) cells shown in Figure 1 and Figure 2A exactly matched with the NKp80pos/CD16pos (top row), NKG2Apos/CD16pos (bottom row) lymphocyte subsets shown in Figure 3. (B) Graphs showing the ratios of the reactivity of anti-human NKp80 (top row) and NKG2A (bottom row) mAbs that cross-reacted with CD3neg/HLA-DRneg versus CD16pos (NK) lymphocytes in rhesus (left column) and pigtailed (right column) macaques.
Phenotypic analysis of NKp80 and NKG2A. (A) Representative examples of double-color flow cytometric analysis within the lymphocyte compartment of PBMCs. mAbs specific to human NKp80 (top row) and NKG2A (bottom row) along with anti-CD16 mAb in rhesus (left column) and pigtailed (right column) macaques. The percentage of NKp80pos/CD16pos and NKG2Apos/CD16pos cell subsets are highlighted in bold in the upper right quadrant of the cross bar in the upper right quadrant of each dot plot. Of note, in each rhesus and pigtailed macaque whose flow cytometric analyses were chosen as representative examples of all animals studied, the percentage of total CD3neg/HLA-DRneg (NK) cells shown in Figure 1 and Figure 2A exactly matched with the NKp80pos/CD16pos (top row), NKG2Apos/CD16pos (bottom row) lymphocyte subsets shown in Figure 3. (B) Graphs showing the ratios of the reactivity of anti-human NKp80 (top row) and NKG2A (bottom row) mAbs that cross-reacted with CD3neg/HLA-DRneg versus CD16pos (NK) lymphocytes in rhesus (left column) and pigtailed (right column) macaques.
Expression of NKp46, NKp30, NKG2D, NKp80, and NKG2A on purified NK cells from macaques. Cross-reactivity of mAbs specific to human NKp46, NKp30, NKG2D, NKp80, and NKG2D on CD16pos freshly purified NK cells (open histograms) from a representative example of a human donor (left column), and of rhesus (middle column) and pigtailed (right column) macaques. Gray shaded histograms represent the negative controls stained with the second reagent alone. The level of surface expression of the relevant receptors is indicated as absolute percentage and as mean fluorescence intensity (MF) in each histogram plot.
Expression of NKp46, NKp30, NKG2D, NKp80, and NKG2A on purified NK cells from macaques. Cross-reactivity of mAbs specific to human NKp46, NKp30, NKG2D, NKp80, and NKG2D on CD16pos freshly purified NK cells (open histograms) from a representative example of a human donor (left column), and of rhesus (middle column) and pigtailed (right column) macaques. Gray shaded histograms represent the negative controls stained with the second reagent alone. The level of surface expression of the relevant receptors is indicated as absolute percentage and as mean fluorescence intensity (MF) in each histogram plot.
Functional evaluation of NKp80 and NKG2A on activated NK cells from macaque. Effect of anti-human NKp80 (A) and NKG2A (B) mAbs on NK-mediated cytotoxicity against FcγR+ P815 target cells. Data are from a representative example of a human donor, and a pigtailed and a rhesus macaque. E/T ratio is 20:1. Each graph shows the baseline lysis (blue bars), the maximal lysis triggered by anti-CD16 mAb (red bars), the killing driven by anti-NKp80 and anti-NKp46 mAb (yellow and green bars, respectively, in panel A), and the inhibition of killing by NKG2A cotriggered with anti-CD16 mAb (green bars in panel B). Data are presented as an average (± SD) of experiments conducted on 5 human donors and 5 rhesus and pigtailed macaques.
Functional evaluation of NKp80 and NKG2A on activated NK cells from macaque. Effect of anti-human NKp80 (A) and NKG2A (B) mAbs on NK-mediated cytotoxicity against FcγR+ P815 target cells. Data are from a representative example of a human donor, and a pigtailed and a rhesus macaque. E/T ratio is 20:1. Each graph shows the baseline lysis (blue bars), the maximal lysis triggered by anti-CD16 mAb (red bars), the killing driven by anti-NKp80 and anti-NKp46 mAb (yellow and green bars, respectively, in panel A), and the inhibition of killing by NKG2A cotriggered with anti-CD16 mAb (green bars in panel B). Data are presented as an average (± SD) of experiments conducted on 5 human donors and 5 rhesus and pigtailed macaques.
We then confirmed the specificity of anti-human NKp80 mAbs in binding a similar simian receptor by transfecting a COS-7 cell line with constructs encoding RM or PTM NKp80 cDNA sequences. LaBonte et al performed similar experiments in RMs by transfecting the 293T cell line with cDNA encoding for RM CD94 and NKG2A.32,33 The anti-human mAb that was used to detect the NKG2A surface expression on transfected cells was the same commercially available mAb used in our study (Z199). The same experiments were not repeated in PTMs. However, we amplified the RM and PTM NKG2A cDNA products with the same primer pairs and the 2 sequences showed 98% nucleotide homology. This high similarity in cDNA sequences between RMs and PTMs was also reflected in their NKG2A proteins, which were 98% identical (data not shown). These sets of experiments confirmed that the anti-human NKp80 and NKG2A mAbs cross-reacted and recognized similar molecules in simians.
RT-PCR and NKp80 binding experiments. (A) RT-PCR analysis of NKp46, NKp30, NKG2D, NKp80, NKG2A, and CD94 in a human, and in a pigtailed and a rhesus macaque. Lane 1: 100-bp ladder (MK); lane 2: human (H) PBMCs; lane 3: pigtailed macaque (PTM) PBMCs; lane 4: rhesus macaque (RM) PBMCs. (B) Histograms show the detection of rhesus (top row) and pigtailed (bottom row) NKp80 receptors on the cell surface of transfected COS-7 cells using anti-human NKp80 mAbs (blue line). Red shaded histograms represent the negative controls stained with the second reagent alone. The cell surface level of simian NKp80 was detected with 3 different anti-human NKp80 mAbs (Lapi, Ma152, and Cer 1) in both macaques, as mean fluorescence intensity (MF) indicates in each histogram plot.
RT-PCR and NKp80 binding experiments. (A) RT-PCR analysis of NKp46, NKp30, NKG2D, NKp80, NKG2A, and CD94 in a human, and in a pigtailed and a rhesus macaque. Lane 1: 100-bp ladder (MK); lane 2: human (H) PBMCs; lane 3: pigtailed macaque (PTM) PBMCs; lane 4: rhesus macaque (RM) PBMCs. (B) Histograms show the detection of rhesus (top row) and pigtailed (bottom row) NKp80 receptors on the cell surface of transfected COS-7 cells using anti-human NKp80 mAbs (blue line). Red shaded histograms represent the negative controls stained with the second reagent alone. The cell surface level of simian NKp80 was detected with 3 different anti-human NKp80 mAbs (Lapi, Ma152, and Cer 1) in both macaques, as mean fluorescence intensity (MF) indicates in each histogram plot.
The panel of anti-human NK receptor mAbs tested in the present study that cross-reacted with monkey PBMCs included anti-NKp46, anti-NKp30, and anti-NKG2D, as well as NKp80 and NKG2A. Anti-NKp46 and anti-NKp30 failed to bind to the entire monkey NK cell population and frequencies of NK cells positive for these markers were generally lower on monkey than on human NK cells. Nevertheless, cytofluorometric analyses confirmed that these receptors are NK cell-specific in both monkey and human systems. Anti-NKG2D, on the other hand, recognized the majority of human, but not monkey, NK cells and was found to be expressed on other PBMC subsets in both humans and macaques. Also, the RT-PCR techniques developed in this study to amplify the specific gene products of NKp46, NKp30, NKG2D, NKG2A, CD94, and NKp80 might be important tools to investigate the transcriptional regulation of NK cell receptors in nonhuman primates.
Previous studies have attempted to evaluate the role of NK cells in the pathogenesis of several diseases or in the control of invading pathogens in nonhuman primates.2-4,34-36 However, it should be pointed out that the majority of these studies was conducted using unfractionated PBMCs and gating their analysis on CD16pos or CD56pos cells, which have been described as not specific enough to clearly identify the entire NK cell population in this species. In this regard, some studies have focused their attention on simian immunodeficiency virus (SIV) infection in monkeys to better understand the involvement of NK cells in disease pathogenesis.9,13,14,16 Again, the lack of specific markers that identify the entire simian NK cell population have significantly limited our understanding of the role of this population in disease states for which only nonhuman primates offer an appropriate animal model. In particular, the SIV-infected macaque is the only practical animal model for pathogenic HIV infection/immunization and it allows for the investigation of events immediately following infection,37-39 an opportunity rarely possible in humans infected with HIV. Large gaps exist in our understanding of the role of NK cells in the elimination of SIV-infected cells, before or after the establishment of the adaptive immune response. Furthermore, little is known regarding the importance of NK cells in generating the appropriate cytokine and chemokine milieu necessary for initiating and/or recruiting an effective adaptive immune response. Because NKG2A appears to be highly specific for simian NK cells, anti-NKG2A may allow for specific NK cell depletion in vivo, and thus definitive experiments elucidating the role of NK cells in various pathogenic conditions may be conducted. It has become clear that the innate immune response greatly influences establishment of the adaptive immune response; therefore, such information may be useful in the design of effective vaccine strategies. The phenotypic analyses of simian NK cells using anti-human NKp80 and NKG2A should allow investigators to begin studies directed toward addressing these questions.
Prepublished online as Blood First Edition Paper, May 17, 2005; DOI 10.1182/blood-2004-12-4762.
D.M. and J.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Tae Wook Chun for his invaluable assistance in reviewing the manuscript, Dean Follman for reviewing the statistical analysis, and Ivana Matera for her helpful suggestions and assistance with molecular cloning and transfections. This work is dedicated to the memory of Michelino Colombara.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal