Abstract
The platelet receptor for von Willebrand factor (VWF), glycoprotein (GP) Ib-IX, mediates platelet adhesion and activation. The cytoplasmic domains of the GPIb α and β subunits contain binding sites for the phosphorylation-dependent signaling molecule, 14-3-3ζ. Here we show that a novel membrane-permeable inhibitor of 14-3-3ζ-GPIbα interaction, MPαC, potently inhibited VWF binding to platelets and VWF-mediated platelet adhesion under flow conditions. MPαC also inhibited VWF-dependent platelet agglutination induced by ristocetin. Furthermore, activation of the VWF binding function of GPIb-IX induced by GPIbβ dephosphorylation is diminished by mutagenic disruption of the 14-3-3ζ binding site in the C-terminal domain of GPIbα, mimicking MPαC-induced inhibition, indicating that the inhibitory effect of MPαC is likely to be caused by disruption of 14-3-3ζ binding to GPIbα. These data suggest a novel 14-3-3ζ-dependent regulatory mechanism that controls the VWF binding function of GPIb-IX, and also suggest a new type of antiplatelet agent that may be potentially useful in preventing or treating thrombosis.
Introduction
Platelet adhesion plays an important role in hemostasis and thrombosis. Under high shear rate flow conditions such as in arteries and capillaries, platelet adhesion to the subendothelium is dependent on interaction between subendothelial-bound von Willebrand factor (VWF) and its receptor, the platelet glycoprotein Ib-IX-V complex (GPIb-IX-V).1 VWF-GPIb-IX-V interaction also mediates the formation of platelet microaggregates in patients with abnormally large VWF multimers,2 resulting in thrombotic thrombocytopenic purpura (TTP). Interaction of VWF with GPIb-IX-V triggers intracellular signaling events such as elevation of intracellular calcium3 and cyclic guanosine monophosphate (cGMP) levels,4 activation of phosphoinositide 3-kinase,5 and activation of multiple protein kinase pathways.4,6-8 These events lead to the activation of the ligand binding function of the integrin αIIbβ3,9-11 , which mediates platelet spreading and aggregation.
GPIb-IX-V consists of 4 different transmembrane subunits. GPIb, which consists of disulfide-linked GPIbα and GPIbβ subunits, forms a 1:1 complex with GPIX. The GPIb-IX complex (GPIb-IX) is sufficient for ligand binding and signaling function.11,12 GPIb-IX forms a 2:1 complex with GPV.12 The N-terminal region of GPIbα contains binding sites for VWF and thrombin. Binding of VWF to GPIb-IX is tightly controlled, and normally occurs only at sites of vascular injury or under pathologically high shear stress. Shear stress may induce changes in both VWF and platelets. In vitro, ristocetin and botrocetin are used to induce VWF binding to GPIb-IX by mimicking the effects of the subendothelial matrix and shear stress on VWF and/or GPIb-IX. Increasing evidence indicates that VWF binding to GPIb-IX is regulated by intraplatelet signals such as cyclic adenosine monophosphate (cAMP) levels.13-15 Elevation of intracellular cAMP activates the cAMP-dependent protein kinase (PKA), which phosphorylates GPIbβ at Ser166.16,17 We recently showed that PKA-dependent phosphorylation of GPIbβ at Ser166 negatively regulates the VWF binding function of GPIb-IX.15
Several intracellular molecules are associated with the cytoplasmic domain of GPIb-IX. These include filamin, which interacts with the central region of the GPIbα cytoplasmic domain (residues 536-568, 570-590),18-20 calmodulin, which binds to the membrane proximal region of GPIbβ,21 and 14-3-3ζ,22 which is an important signaling molecule modulating intracellular proteins containing specific phosphoserine-centered binding motifs.23 Binding of 14-3-3ζ to GPIb-IX is regulated by phosphorylation at serine residues.24-26 Since we first discovered that 14-3-3ζ binds to GPIb-IX,22 several different binding sites have been reported in the cytoplasmic domain of GPIbα and GPIbβ for dimeric 14-3-3ζ: (1) the GPIbα C-terminal 14-3-3ζ binding site requires the RYSGHpS609L sequence in which phosphorylation of Ser609 plays a key role in high-affinity interaction with 14-3-3ζ26,27 ; (2) a 14-3-3ζ binding site in the cytoplasmic domain of GPIbβ contains an RLpS166LTDP sequence in which phosphoserine-166 plays a key role24,25 ; and (3) the region that is important in filamin binding to GPIbα cytoplasmic domain has also been shown to potentially interact with 14-3-3ζ (R557-G575 and L580-S590).25,28,29 The functional consequences of 14-3-3ζ binding to GPIb-IX, however, have been unclear. There have been apparently controversial data regarding whether deletion of the GPIbα C-terminal 14-3-3ζ binding site reduces GPIb-IX-mediated integrin activation and cell spreading,11,29 or enhances integrin-dependent cell spreading.30,31 In this study, we show that 14-3-3ζ plays an important role in the up-regulation of the VWF binding function of GPIb-IX induced by dephosphorylation of GPIbβ Ser166, and that a membrane-permeable inhibitor of 14-3-3ζ-GPIb-IX interaction, MPαC, diminishes VWF binding to platelets and VWF-mediated platelet adhesion. These data suggest a 14-3-3ζ-dependent regulatory mechanism for GPIb-IX and a novel inhibitor of platelet adhesion.
Materials and methods
Reagents
Monoclonal antibodies against GPIbα, LJ-P3 (provided by Dr Zaverio Ruggeri, The Scripps Research Institute) and WM23, have been previously described.32,33 Monoclonal antibodies, SZ29 against VWF and SZ2 against GPIbα, were generous gifts from Dr Changgeng Ruan (Soochow University, Suzhou, China).34,35 cDNA clones encoding wild-type GPIbα, GPIbβ, and GPIX were generous gifts from Dr Jose Lopez (Baylor College of Medicine, Houston, TX).36,37 The membrane-permeable protein kinase A (PKA) inhibitor, myristoylated PKI, was purchased from Calbiochem (San Diego, CA). Myristoylated peptides MPαC (C13H27CONH-SIRYSGHpSL), MαC (C13H27CONH-SIRYSGHSL), MαCsc (C13H27CONH-LSISYGSHR), MPαCsc (C13H27CONH-LSIpSYGSHR), and nonmyristoylated peptides PαC (SIRYSGHpSL) and αC (SIRYSGHSL) were synthesized by the Protein Research Laboratory, University of Illinois at Chicago. The peptides were more than 95% pure. Myristoylated peptides were dissolved in dimethyl sulfoxide (DMSO).
Recombinant GPIb-IX and mutants
Chinese hamster ovary (CHO) cells expressing recombinant wild-type GPIb-IX, a GPIb-IX mutant with a serine-to-alanine point mutation at Ser166 in GPIbβ (S166A), and GPIb-IX mutants with truncations at residues 591 and 605 in the cytoplasmic domain of GPIbα, have been described previously.15,27 Site-directed mutagenesis replacing Ser609 of GPIbα with alanine (S609A) was performed using a polymerase chain reaction (PCR) method with the forward primer as AGAAGAATTCGCTGCTCTGACCACA and the reverse primer as TAAGTCTAGATCAGAGGGCGTGGCCAGAGT. The PCR product was cloned into wild-type GPIbα in pGEM3Z(+) vector after digestion with the restriction enzymes SmaI and BamHI. The resulting S609A mutant was then subcloned into pCDNA3.1(-) vector after digestion with EcoRI. Correct mutation was verified by DNA sequencing. Transfection of cDNA into CHO cells was performed using LipofectAMINE Plus (Invitrogen, Carlsbad, CA). Stable cell lines were selected using selection media containing 0.5 mg/mL G418 and further selected by cell sorting using the anti-GPIbα monoclonal antibody, SZ2. Expression of GPIb-IX in different cell lines was adjusted by cell sorting to comparable levels before experiments.
Coimmunoprecipitation of GPIb-IX with 14-3-3ζ
CHO cells expressing wild-type and mutant GPIb-IX were resuspended to a concentration of 1.0 × 108 cells/mL, and solubilized in Triton X-100 containing buffer as described previously.15,27 In platelet experiments, MPαC or control peptides were preincubated with 300 μL washed platelets (5 × 108/mL). Platelets were then solubilized as previously described.27 The samples were centrifuged at 100 000g at 4°C for 30 minutes to remove the Triton X-100-insoluble material. The lysates (150 μL) were incubated with the anti-GPIbα monoclonal antibody LJ-P3 for 1 hour, and then with protein G-conjugated Sepharose 4B beads for 1 hour at 4°C. The beads were then washed 3 times. Bound proteins were extracted by the addition of sodium dodecyl sulfate (SDS) sample buffer, analyzed by SDS-polyacrylamide gel electrophoresis (PAGE), and immunoblotted with an anti-14-3-3ζ antibody.38
Platelet preparation and aggregation
For studies involving human subjects, approval was obtained from the University of Illinois at Chicago institutional review board. Informed consent was provided according to the Declaration of Helsinki. Blood collection from healthy donors and preparation of platelet-rich plasma (PRP) and washed platelets were performed as previously described.27 Platelet aggregation was measured using a turbidometric platelet aggregometer at 37°C with a stirring speed of 1000 rpm.
Flow cytometric analysis of VWF binding to GPIb-IX-expressing cells and platelets
Flow cytometric analysis of VWF binding to platelets and CHO cells expressing recombinant GPIb-IX was performed as described previously.14,15 Briefly, ristocetin (Sigma, St Louis, MO; 1.25 mg/mL) and purified VWF (35 μg/mL) were added to CHO cells expressing wild-type (1b9) and mutant GPIb-IX suspended (2.25 × 106 cells/mL) in modified Tyrode buffer,27 and incubated at 22°C for 30 minutes. Washed platelets (1.0 × 107/mL) in phosphate-buffered saline (PBS; 0.01 M NaH2PO4, 0.15 M NaCl, pH 7.4) containing 10 mM EDTA (ethylenediaminetetraacetic acid) and 1% bovine serum albumin (BSA) were preincubated with inhibitors or control peptides at 22°C for 10 minutes, and then incubated with purified VWF (35 μg/mL) and ristocetin (1.0 mg/mL) at 22°C for 30 minutes. After washing once, the cells or platelets were further incubated with 10 μg/mL fluorescein isothiocyanate (FITC)-labeled anti-VWF antibody SZ-29 at 22°C for 30 minutes and then analyzed by flow cytometry. As negative controls, cells or platelets were incubated with ristocetin in the absence of VWF. To examine the effects of myristoylated PKI, cells were preincubated with or without 100 μM PKI for 15 minutes at 22°C, prior to analysis for VWF binding.
Cell adhesion under flow conditions
Purified human VWF was diluted to 30 μg/mL with 0.1 M NaHCO3, pH 8.3, and coated onto glass capillary tubes (inner diameter 0.58 mm; Harvard Apparatus, Holliston, MA) overnight in a humid environment at 4°C. The capillaries were washed with PBS, blocked with 5% BSA in PBS at 22°C for 2 hours, and then installed on the stage of an inverted microscope. Washed platelets (3 × 108/mL) or cells (5 × 106 cells/mL) in modified Tyrode buffer containing 0.5% BSA were perfused by using a syringe pump (PhD; Harvard Apparatus) into the glass capillary at defined shear rates for 2.5 minutes. Shear rate was calculated as described by Slack and Turitto.39 Transient adhesion (rolling) of cells or platelets on the VWF-coated surface was recorded using a video cassette recorder. Rolling cells or platelets were counted in 10 randomly selected fields of 0.25 mm2 within a 10-second time period.
Results
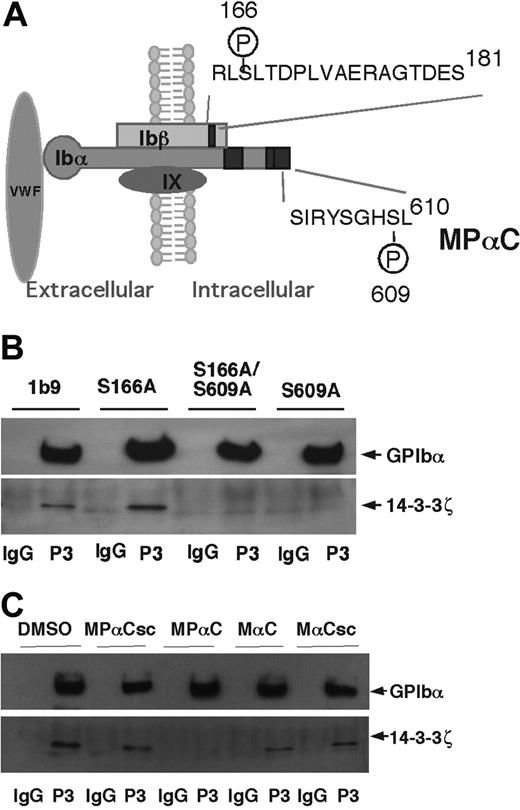
Disruption of 14-3-3ζ interaction with GPIb-IX with a novel membrane-permeable inhibitor and by mutagenesis
Phosphoserine-609 of GPIbα and phosphoserine-166 of GPIbβ are key residues in the 14-3-3ζ binding sites in GPIbα and GPIbβ, respectively (Figure 1A). To investigate the role of 14-3-3ζ in regulating GPIb-IX function, cell lines were established that express the following mutants of GPIb-IX: (1) wild-type GPIbβ and GPIX complexed with a GPIbα mutant bearing a conservative mutation of Ser609 to an alanine (S609A); (2) wild-type GPIbα and GPIX complexed with a mutant of GPIbβ in which Ser166 is mutated to alanine (S166A); and (3) wild-type GPIX complexed with both the GPIbα S609A and GPIbβ S166A mutants (S166A/S609A). By repeated cell sorting using anti-GPIb antibodies, cell lines were obtained in which the expression levels of these different mutants were comparable to a wild-type GPIb-IX cell line (1b9). These cells were analyzed for coimmunoprecipitation of 14-3-3ζ with GPIb-IX. 14-3-3ζ coimmunoprecipiated with wild-type GPIb-IX and also with the S166A mutant (Figure 1B), suggesting that the S166A mutation alone is not sufficient to reduce 14-3-3ζ binding. In contrast, 14-3-3ζ failed to coimmunoprecipitate with either the S609A mutant or the S609A/S166A mutant. Thus, although there are several different 14-3-3ζ binding sites in the cytoplasmic domain of the GPIb-IX complex, the site containing the phosphoserine-609 is required for high-affinity interaction between GPIb-IX and endogenous 14-3-3ζ under these conditions. These data also suggest that the 14-3-3ζ binding site in the cytoplasmic domain of GPIbβ or other potential 14-3-3ζ binding sites in GPIbα, by themselves, are not sufficient to support a high-affinity interaction between GPIb-IX and 14-3-3ζ. Hence, anchoring 14-3-3ζ to the C-terminal domain of GPIbα may facilitate a potential interaction between dimeric 14-3-3ζ and GPIbβ or other sites within the cytoplasmic domains of GPIb-IX. Thus, in order to inhibit 14-3-3ζ binding to GPIb-IX in human platelets, we synthesized a membrane-permeable myristoylated phospho-peptide named MPαC (C13H27CONH-SIRYSGHpSL) based on the sequence of the 14-3-3ζ binding site in the C-terminal region of GPIbα including phosphorylation of Ser609 (Figure 1A). We also synthesized a myristoylated nonphosphorylated peptide with the identical sequence as MPαC (MαC), a myristoylated scrambled peptide (MαCsc), and a myristoylated scrambled phospho-peptide (MPαCsc) as controls. To examine if these peptides interfere with endogenous 14-3-3ζ interaction with GPIb-IX, platelets were preincubated with MPαC or control peptides, and then solubilized. GPIb-IX-14-3-3ζ complex was then coimmunoprecipitated with a monoclonal antibody against GPIbα. MPαC peptide but not the control peptides abrogated coimmunoprecipitation of 14-3-3ζ with GPIb-IX (Figure 1C). Thus, MPαC is a potent inhibitor of 14-3-3ζ binding to GPIb-IX.
Disruption of 14-3-3ζ binding to GPIb-IX by mutagenesis and a novel inhibitor. (A) Schematic depicting GPIb-IX and the reported 14-3-3ζ binding sites (black boxes). Also shown is the GPIbα 14-3-3ζ binding sequence from which a novel membrane-permeable inhibitor of 14-3-3ζ-GPIbα interaction, MPαC, is derived. (B) CHO cells expressing wild-type, GPIb-IX (1b9), or GPIb-IX mutants bearing 14-3-3ζ binding disrupting mutations, S609A (GPIbα Ser609 to alanine mutation), S166A (GPIbβ Ser166 to alanine mutation), and S609A/S166A double mutations, were solubilized in Triton X-100 containing lysis buffer and immunoprecipitated with the anti-GPIbα antibody LJ-P3 (P3), or control IgG. The immunoprecipitates were immunoblotted with anti-GPIbα and anti-14-3-3ζ antibodies. (C) Platelets were preincubated with MPαC, control peptides (MαC, MPαCsc, or MαCsc), or DMSO. Platelets were then solubilized and immunoprecipitated with an anti-GPIbα antibody (P3). Immunoprecipitates were immunoblotted with anti-GPIbα and anti-14-3-3ζ antibodies.
Disruption of 14-3-3ζ binding to GPIb-IX by mutagenesis and a novel inhibitor. (A) Schematic depicting GPIb-IX and the reported 14-3-3ζ binding sites (black boxes). Also shown is the GPIbα 14-3-3ζ binding sequence from which a novel membrane-permeable inhibitor of 14-3-3ζ-GPIbα interaction, MPαC, is derived. (B) CHO cells expressing wild-type, GPIb-IX (1b9), or GPIb-IX mutants bearing 14-3-3ζ binding disrupting mutations, S609A (GPIbα Ser609 to alanine mutation), S166A (GPIbβ Ser166 to alanine mutation), and S609A/S166A double mutations, were solubilized in Triton X-100 containing lysis buffer and immunoprecipitated with the anti-GPIbα antibody LJ-P3 (P3), or control IgG. The immunoprecipitates were immunoblotted with anti-GPIbα and anti-14-3-3ζ antibodies. (C) Platelets were preincubated with MPαC, control peptides (MαC, MPαCsc, or MαCsc), or DMSO. Platelets were then solubilized and immunoprecipitated with an anti-GPIbα antibody (P3). Immunoprecipitates were immunoblotted with anti-GPIbα and anti-14-3-3ζ antibodies.
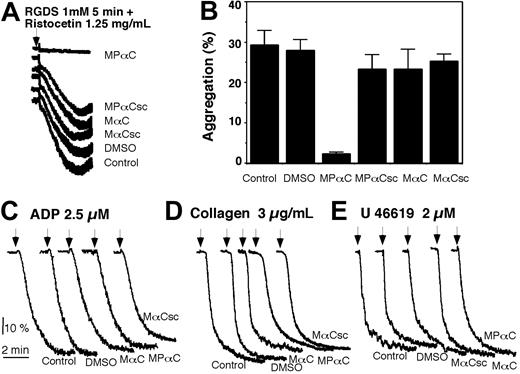
The effect of MPαC on ristocetin-induced platelet aggregation/agglutination
To determine if 14-3-3ζ-GPIb interaction plays a role in the ligand binding function of GPIb-IX in human platelets, we examined the effects of the myristoylated peptide inhibitor of 14-3-3ζ-GPIb-IX interaction, MPαC, on GPIb-IX function in platelets. MPαC dose-dependently inhibited VWF-mediated platelet aggregation induced by ristocetin (Figure 2A). In contrast, the myristoylated control peptides, MαC, MPαCsc, or MαCsc, had no effect (Figure 2B). Similarly, myristic anhydride also had no effect on ristocetin-induced platelet aggregation (Figure 2C), suggesting that myristoylation is not responsible for the effect of MPαC. Conversely, a nonmyristoylated phospho-peptide with an identical peptide sequence to MPαC(PαC) had no effect on ristocetin-induced platelet aggregation (Figure 2C), suggesting that myristoylation is required for the inhibitory effect. Since myristoylation is known to confer membrane permeability to short peptides, these data indicate that the inhibitory effect of MPαC requires membrane permeability of this specific 14-3-3ζ inhibitor. These data also indicate that 14-3-3ζ binding to GPIb-IX is required for ristocetin-induced platelet aggregation.
MPαC specifically inhibits GPIb-IX-dependent platelet agglutination
Ristocetin-induced platelet aggregation involves platelet agglutination (caused directly by VWF binding to GPIb-IX), platelet activation, and subsequent integrin αIIbβ3-mediated platelet aggregation. To determine if the inhibitory effect of MPαC results from inhibition of GPIb-IX-dependent platelet agglutination or GPIb-IX-mediated platelet activation, we examined the effect of MPαC on ristocetin-induced platelet agglutination in the presence of Arg-Gly-Asp-Ser (RGDS) peptide, which blocks integrin-dependent platelet aggregation. MPαC completely inhibited ristocetin-induced platelet agglutination in the presence of RGDS (Figure 3A-B). In contrast, control peptides had no inhibitory effect. MPαC also inhibited botrocetin-induced platelet agglutination (not shown). To exclude the possibility that MPαC may affect general platelet activation processes, we examined the effect of this peptide on platelet aggregation induced by the platelet agonists adenosine diphosphate (ADP), collagen, and the thromboxane A2 analog, U46619. Neither MPαC nor the control peptides had an inhibitory effect on platelet aggregation induced by these agonists (Figure 3C-E). MPαC and control peptides also had no effect on U46619-induced fibrinogen binding to platelets (not shown). Thus the effect of MPαC peptide is specific for GPIb-IX-dependent platelet agglutination.
The effect of an inhibitor of 14-3-3ζ-GPIb-IX interaction, MPαC, on ristocetin-induced platelet aggregation. (A) PRP was preincubated with DMSO as control or with increasing concentrations of the myristoylated 14-3-3ζ inhibitor peptide for 5 minutes, and stimulated with ristocetin to induce platelet aggregation. (B) Ristocetin-induced platelet aggregation in the presence of 100 μM myristoylated 14-3-3ζ inhibitor peptides; MPαC; control peptides MαC, MPαCsc, or MαCsc; or DMSO. (C) Ristocetin-induced platelet aggregation in the presence of nonmyristoylated peptide, PαC (phosphorylated) or αC (nonphosphorylated) with sequence identical to MPαC, or myristic anhydride (MA).
The effect of an inhibitor of 14-3-3ζ-GPIb-IX interaction, MPαC, on ristocetin-induced platelet aggregation. (A) PRP was preincubated with DMSO as control or with increasing concentrations of the myristoylated 14-3-3ζ inhibitor peptide for 5 minutes, and stimulated with ristocetin to induce platelet aggregation. (B) Ristocetin-induced platelet aggregation in the presence of 100 μM myristoylated 14-3-3ζ inhibitor peptides; MPαC; control peptides MαC, MPαCsc, or MαCsc; or DMSO. (C) Ristocetin-induced platelet aggregation in the presence of nonmyristoylated peptide, PαC (phosphorylated) or αC (nonphosphorylated) with sequence identical to MPαC, or myristic anhydride (MA).
MPαC specifically inhibits VWF- and GPIb-IX-dependent platelet agglutination. (A,B) PRP was preincubated with myristoylated peptides MPαC, MαC, MPαCsc, or MαCsc, or vehicle (DMSO) together with 1 mM of the integrin inhibitor RGDS. Ristocetin (1.25 mg/mL) was added to induce GPIb-IX-specific platelet agglutination. Typical agglutination traces are shown in panel A, and quantitative data from 4 experiments are shown in panel B (mean ± SD). (C-E) PRP was preincubated with MPαC; MαC or MαCsc; or DMSO, then stimulated with ADP (C), collagen (D), or the thromboxane A2 analog, U46619 (E) to induce platelet aggregation.
MPαC specifically inhibits VWF- and GPIb-IX-dependent platelet agglutination. (A,B) PRP was preincubated with myristoylated peptides MPαC, MαC, MPαCsc, or MαCsc, or vehicle (DMSO) together with 1 mM of the integrin inhibitor RGDS. Ristocetin (1.25 mg/mL) was added to induce GPIb-IX-specific platelet agglutination. Typical agglutination traces are shown in panel A, and quantitative data from 4 experiments are shown in panel B (mean ± SD). (C-E) PRP was preincubated with MPαC; MαC or MαCsc; or DMSO, then stimulated with ADP (C), collagen (D), or the thromboxane A2 analog, U46619 (E) to induce platelet aggregation.
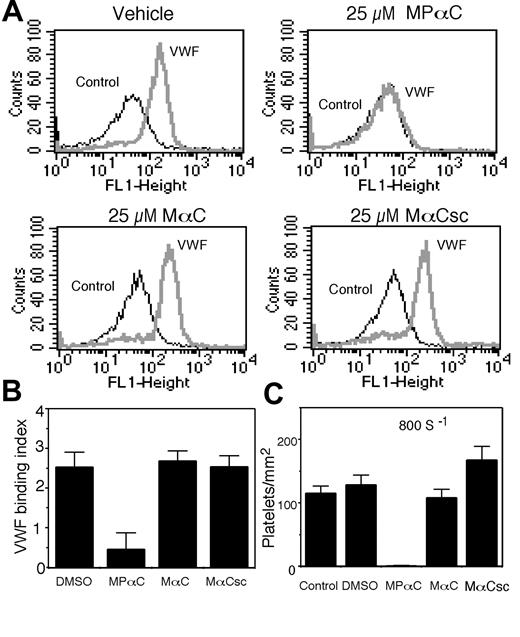
The effect of MPαC on ristocetin-induced VWF binding to platelets
We also directly examined the effect of MPαC on ristocetin-induced VWF binding to platelet GPIb-IX (to exclude the possible role of integrin, binding assays were performed in the presence of RGDS or EDTA). MPαC inhibited ristocetin-induced VWF binding to platelets (Figure 4A-B). In contrast, the control peptides had no inhibitory effect. Thus, a cell-permeable inhibitor of 14-3-3ζ binding to GPIbα specifically inhibits VWF binding to the extracellular ligand binding domain of GPIb-IX, indicating that 14-3-3ζ binding to the C-terminal region of GPIbα is required for the VWF binding function of GPIb-IX in platelets.
A critical role for 14-3-3ζ in platelet adhesion to VWF
The physiologic function of VWF binding to GPIb-IX is to mediate platelet adhesion under flow conditions.40,41 Thus, we examined if MPαC could inhibit platelet adhesion to VWF under flow. To do so, washed platelets were preincubated with peptides or vehicle (DMSO) and then perfused through VWF-coated glass capillaries. To exclude the role of integrins, these experiments were performed in the presence of the integrin inhibitor, RGDS. As expected, platelets preincubated with vehicle or control peptides adhered to the VWF surface. In contrast, there was almost no adhesion of platelets preincubated with MPαC (Figure 4C). This dramatic inhibitory effect of MPαC on platelet adhesion not only indicates that 14-3-3ζ interaction with the cytoplasmic domain of GPIbα is important for platelet adhesive function, but also suggests that MPαC or similar agents that prevent 14-3-3ζ binding to GPIbα may be useful as a new class of antiplatelet agents that specifically inhibit GPIb-IX-dependent platelet adhesion.
The roles of the 14-3-3ζ binding sites in GPIbα and GPIbβ in regulating the VWF binding function of GPIb-IX
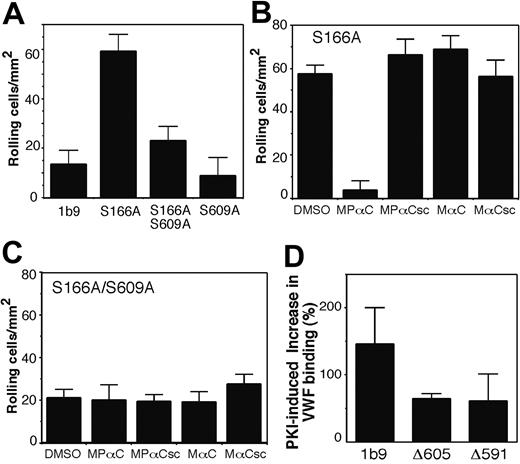
To further exclude the possibility that the peptide inhibitor may affect the VWF binding function of GPIb-IX nonspecifically, we investigated whether disruption of the 14-3-3ζ binding sites in GPIb-IX may also affect the VWF binding function of GPIb-IX. CHO cell lines expressing either wild-type or mutant GPIb-IX were analyzed for ristocetin-induced VWF binding. We detected only a low level of VWF binding to wild-type GPIb-IX expressed in CHO cells (1b9) (Figure 5A). This is because GPIb-IX molecules expressed in CHO cells are fully phosphorylated at Ser166 and thus are in a resting form.15 In comparison, more than 30% of GPIbβ is dephosphorylated at Ser166 in platelets isolated ex vivo,15 which explains why GPIb-IX in isolated platelets appears constitutively active with respect to VWF binding. In contrast to CHO cells expressing wild-type GPIb-IX, VWF binding to cells expressing the S166A mutant lacking the PKA phosphorylation site in GPIbβ was significantly enhanced (Figure 5A-B). The GPIbα S609A mutant by itself was similar to resting wild-type GPIb-IX as indicated by minimal VWF binding. However, the enhancing effect of the GPIbβ S166A mutation on VWF binding was diminished when expressed with the GPIbα S609A mutant (Figure 5A). Thus, 14-3-3ζ binding to the C-terminal site of GPIbα is required for the enhancing effects of the S166A mutation on VWF binding.
We also examined whether the S609A mutation in the GPIbα affected GPIb-IX-dependent cell adhesion to VWF under flow conditions. As shown in Figure 6A, a very small number of CHO cells expressing wild-type GPIb-IX (1b9) were able to adhere and roll on a VWF-coated surface at a shear rate of 150 s-1. In contrast, the S166A mutant cells showed a significantly enhanced adhesion. The enhancing effect of the S166A mutation was diminished in S166A/S609A cells, indicating that the high-affinity 14-3-3ζ binding site in GPIbα is required for the S166A mutation-induced activation of cell adhesion mediated by GPIb-IX. Similar to the effect of S609A mutant coexpression, preincubation of S166A mutant cells with the 14-3-3ζ inhibitor, MPαC, resulted in a significant decrease in the adhesion of S166A mutant cells to VWF under flow conditions (Figure 6B). In contrast, MPαC had no effect on the already reduced adhesion of S166A/S609A cells (Figure 6C) or S609A cells (not shown). These results indicate that the effect of MPαC was similar to the GPIbα S609A mutation, and resulted from inhibition of GPIbα-14-3-3ζ interaction. Therefore, similar mechanisms are responsible for the 14-3-3ζ-mediated regulation of VWF binding function of GPIb-IX both in platelets and in the reconstituted CHO cell model.
Effects of MPαC on VWF binding to GPIb-IX-and VWF-mediated platelet adhesion. (A) Washed human platelets were preincubated with MPαC or control peptides MαC or MαCsc, or DMSO and incubated with ristocetin in the presence (VWF) or absence (control) of VWF. VWF binding was detected using FITC-labeled anti-VWF antibody and flow cytometry. (B) Quantitative data from 3 experiments (mean ± SD). VWF binding index equals total fluorescence/background fluorescence-1. (C) Platelets were preincubated with MPαC or control peptides MαC or MαCsc, or DMSO, and then perfused through VWF-coated capillary tubes. Numbers of adherent platelets were counted at 10 randomly selected time frames and locations (mean ± SD).
Effects of MPαC on VWF binding to GPIb-IX-and VWF-mediated platelet adhesion. (A) Washed human platelets were preincubated with MPαC or control peptides MαC or MαCsc, or DMSO and incubated with ristocetin in the presence (VWF) or absence (control) of VWF. VWF binding was detected using FITC-labeled anti-VWF antibody and flow cytometry. (B) Quantitative data from 3 experiments (mean ± SD). VWF binding index equals total fluorescence/background fluorescence-1. (C) Platelets were preincubated with MPαC or control peptides MαC or MαCsc, or DMSO, and then perfused through VWF-coated capillary tubes. Numbers of adherent platelets were counted at 10 randomly selected time frames and locations (mean ± SD).
The effect of disruption of the 14-3-3ζ binding sites in GPIbα and GPIbβ by mutagenesis on the VWF binding function. (A) CHO cells expressing wild-type GPIb-IX (1b9), S609A, S166A, or S166A/S609A mutants were incubated with human VWF in the presence of ristocetin or with ristocetin alone (controls). VWF binding to cells was analyzed by flow cytometry using FITC-labeled anti-VWF antibody, SZ29. The same cells were also analyzed for surface expression of GPIb-IX by flow cytometry using an anti-GPIbα antibody SZ2 followed by a FITC-labeled goat anti-mouse IgG. Results from a typical experiment are shown in panel A. Quantitative data are shown in panel B, in which fluorescence intensity of VWF binding (mean ± SD, 6 experiments) was corrected for the relative level of GPIb-IX expression. FL indicates fluorescence.
The effect of disruption of the 14-3-3ζ binding sites in GPIbα and GPIbβ by mutagenesis on the VWF binding function. (A) CHO cells expressing wild-type GPIb-IX (1b9), S609A, S166A, or S166A/S609A mutants were incubated with human VWF in the presence of ristocetin or with ristocetin alone (controls). VWF binding to cells was analyzed by flow cytometry using FITC-labeled anti-VWF antibody, SZ29. The same cells were also analyzed for surface expression of GPIb-IX by flow cytometry using an anti-GPIbα antibody SZ2 followed by a FITC-labeled goat anti-mouse IgG. Results from a typical experiment are shown in panel A. Quantitative data are shown in panel B, in which fluorescence intensity of VWF binding (mean ± SD, 6 experiments) was corrected for the relative level of GPIb-IX expression. FL indicates fluorescence.
The role of the 14-3-3ζ binding site in GPIbα in the activation of VWF binding by a PKA inhibitor
Ser166 of wild-type GPIbβ expressed in CHO cells is fully phosphorylated under our experimental conditions. Thus, it is likely that the effect of the S166A mutant in enhancing the VWF binding function of GPIb-IX results from abrogation of PKA-mediated Ser166 phosphorylation. To further confirm if Ser166 dephosphorylation activates the VWF binding function of GPIb-IX in a 14-3-3ζ-dependent manner, cells expressing GPIb-IX were treated with a specific membrane-permeable PKA inhibitor, the myristoylated PKA inhibitor peptide (PKI), and then analyzed for ristocetin-induced VWF binding. We have previously shown that PKI treatment significantly reduced phosphorylation of Ser166 of GPIbβ, but had no significant effect on GPIbα Ser609 phosphorylation.15,26 PKI treatment enhanced VWF binding to wild-type GPIb-IX (1b9 cells), but this effect was significantly reduced in cell lines Δ605 and Δ591 expressing GPIb-IX mutants in which the 14-3-3ζ binding site in the C-terminal domain of GPIbα was deleted (Δ605 lacks the C-terminal 5 residues of GPIbα and Δ591 lacks the C-terminal 19 residues containing the 14-3-3ζ binding site26,27 ; Figure 6D). Similarly, PKI-induced up-regulation of VWF binding was diminished in S609A cells (not shown), suggesting that 14-3-3ζ binding to the C-terminal site in GPIbα is important for the GPIbβ dephosphorylation-induced activation of the VWF binding function of GPIb-IX. Taken together, our data indicate a critical role for 14-3-3ζ in regulating the VWF binding function of GPIb-IX.
The effects of disruption of 14-3-3ζ binding to GPIbα on cell adhesion to VWF and on PKA inhibitor-induced GPIb-IX activation. (A) 1b9, S166A, S166/609A, or S609A cell lines were perfused through VWF-coated capillaries at 150 s-1. Transient adhesion (rolling) of these cells was recorded. The number of rolling cells was counted in 30 randomly selected fields of 0.25 mm2 and at randomly selected time points. The results shown are the mean ± SD of cell number/mm2. (B,C) S166A cells (B) or S166A/S609A cells (C) were preincubated with MPαC; control peptides MαC, MPαCsc, MαCsc; or DMSO, and then perfused into VWF-coated glass capillaries. Numbers of adherent cells were counted at 10 randomly selected time frames and locations (mean ± SD). (D) CHO cells expressing wild-type GPIb-IX (1b9) and mutant GPIb-IX with deletion of the GPIbα C-terminal 14-3-3 binding site (Δ591 and Δ605) were preincubated without or with 100 μM PKI for 15 minutes at 22°C. The cells were then incubated with VWF and ristocetin at 22°C for 30 minutes. VWF binding was detected using the FITC-labeled anti-VWF antibody, SZ29, and flow cytometry. Nonspecific fluorescence was determined by incubating the cells with ristocetin alone. Effects of PKI on enhancing VWF binding in the indicated cell lines were quantified and expressed as the percentage increase in the fluorescence intensity of VWF binding compared with the fluorescence intensity of VWF binding in the absence of PKI. Shown in the figure are the results from 3 separate experiments (mean ± SD).
The effects of disruption of 14-3-3ζ binding to GPIbα on cell adhesion to VWF and on PKA inhibitor-induced GPIb-IX activation. (A) 1b9, S166A, S166/609A, or S609A cell lines were perfused through VWF-coated capillaries at 150 s-1. Transient adhesion (rolling) of these cells was recorded. The number of rolling cells was counted in 30 randomly selected fields of 0.25 mm2 and at randomly selected time points. The results shown are the mean ± SD of cell number/mm2. (B,C) S166A cells (B) or S166A/S609A cells (C) were preincubated with MPαC; control peptides MαC, MPαCsc, MαCsc; or DMSO, and then perfused into VWF-coated glass capillaries. Numbers of adherent cells were counted at 10 randomly selected time frames and locations (mean ± SD). (D) CHO cells expressing wild-type GPIb-IX (1b9) and mutant GPIb-IX with deletion of the GPIbα C-terminal 14-3-3 binding site (Δ591 and Δ605) were preincubated without or with 100 μM PKI for 15 minutes at 22°C. The cells were then incubated with VWF and ristocetin at 22°C for 30 minutes. VWF binding was detected using the FITC-labeled anti-VWF antibody, SZ29, and flow cytometry. Nonspecific fluorescence was determined by incubating the cells with ristocetin alone. Effects of PKI on enhancing VWF binding in the indicated cell lines were quantified and expressed as the percentage increase in the fluorescence intensity of VWF binding compared with the fluorescence intensity of VWF binding in the absence of PKI. Shown in the figure are the results from 3 separate experiments (mean ± SD).
Discussion
The data described in this study indicate that (1) 14-3-3ζ binding to GPIb-IX is required for an active GPIb-IX capable of binding VWF; (2) cAMP-regulated phosphorylation and dephosphorylation of GPIbβ Ser166 via a 14-3-3ζ dependent mechanism controls whether GPIb-IX is in a resting, or an active state; and (3) agents that interfere with 14-3-3ζ-GPIb-IX interaction inhibit VWF binding function of GPIb-IX and thus potentially may be useful as a novel type of antiplatelet drug.
The conclusion that 14-3-3ζ is required for the VWF binding function of GPIb-IX is supported by data that a membrane-permeable inhibitor of 14-3-3ζ-GPIb-IX interaction, MPαC, inhibited GPIb-IX-dependent VWF binding to platelets, platelet agglutination, and adhesion. The effect of MPαC is unlikely to be nonspecific because disruption of the 14-3-3ζ binding site in GPIbα by mutagenesis also abolished activation of the VWF binding function induced by Ser166 dephosphorylation in a CHO cell model. There have been apparently conflicting reports whether GPIb-IX expressed in CHO cells is in an active state or resting state. We showed that wild-type GPIb-IX molecules expressed in CHO cells are mostly in a resting state.14,15 Dephosphorylation or S166A mutation of GPIbβ significantly enhanced the VWF binding function of GPIb-IX.15 Dong and colleagues42,43 reported that wild-type GPIb-IX expressed in CHO cells was in an active VWF binding state. Although the reason for this difference remains to be determined, it is possible that it results from different phosphorylation states of GPIbβ under clearly different experimental conditions. Under our conditions, GPIb-IX molecules expressed in CHO cells are almost fully phosphorylated at Ser166 of GPIbβ,15 which inhibits VWF binding function. However, the findings of Dong et al,42 although showing a partial reduction in VWF binding to GPIbα deletion mutations, are consistent with our finding that disruption of the 14-3-3ζ binding site in GPIbα abolishes activation of VWF binding to GPIb-IX in CHO cells. Our data using the novel membrane-permeable inhibitor thus provide first evidence that, in human platelets, 14-3-3ζ binding to GPIb-IX plays a key physiologic role in regulating VWF binding function of GPIb-IX and VWF-mediated platelet adhesion.
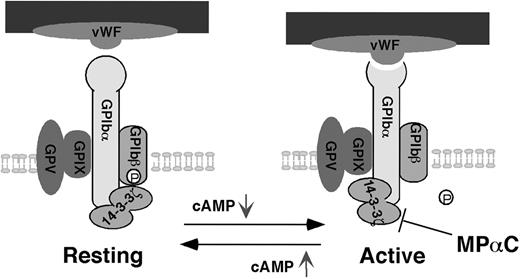
Our data also show that the intracellular signaling messenger, cAMP, by modulating PKA-dependent GPIbβ phosphorylation, mediates 14-3-3ζ-dependent regulation of the VWF binding function of GPIb-IX, thus suggesting a novel regulatory mechanism for GPIb-IX. The 14-3-3ζ protein is dimeric. Each monomer of the 14-3-3ζ dimer has a binding pocket, and thus dimeric 14-3-3ζ is able to simultaneously interact with 2 different sites on a protein or 2 different ligands.23 Therefore, different 14-3-3ζ binding sites in GPIb-IX may interact with a single 14-3-3ζ dimer. Phosphorylation-dependent binding sites for dimeric 14-3-3ζ are present in the cytoplasmic domains of both GPIbα and GPIbβ (Figure 1A). A site critical for 14-3-3ζ binding to GPIbα is located in the C-terminal SIRYSGHpS609L sequence of GPIbα in which Ser609 is constitutively phosphorylated.26,27 The 14-3-3ζ binding site in GPIbβ is located in the RLpS166LTDP sequence,24,25 in which phosphorylation of Ser166 by PKA is dynamically regulated by cAMP levels.15,17 However, GPIbα alone is sufficient to support the binding of 14-3-3ζ without requiring cooperation of the 14-3-3ζ binding site in GPIbβ. On the other hand, deletion of the GPIbα C-terminal binding site abolishes high-affinity binding of 14-3-3ζ to GPIb-IX, suggesting that GPIbβ alone and other sites in GPIbα are not sufficient to support high-affinity binding of 14-3-3ζ without the GPIbα C-terminal site (Figure 1). These data suggest that GPIb-IX may have 2 different 14-3-3ζ interacting modes: (1) 14-3-3ζ dimer is bound to both GPIbα and GPIβ when PKA is activated by elevated cAMP; and (2) 14-3-3ζ dimer is bound only to GPIbα but not GPIbβ when the cAMP level is low. Therefore, we propose a “toggle switch” model as a possible regulatory mechanism for GPIb-IX (Figure 7). In this model, elevation of cAMP induces binding of 14-3-3ζ to both sites in GPIbα and GPIbβ, resulting in a resting GPIb-IX. Decreases in cAMP level lead to GPIbβ-14-3-3ζ dissociation, resulting in the binding of 14-3-3ζ to GPIbα alone and activation of the VWF binding function of GPIb-IX in a 14-3-3ζ-dependent manner (Figure 7). It remains to be determined how disruption of the 14-3-3ζ binding site in GPIbβ causes the 14-3-3ζ-dependent activation of GPIb-IX. However, it is important to note that 14-3-3ζ binding sites have been reported in the cytoplasmic domain of GPIbα in addition to the critical C-terminal SIRYSGHSL sequence. Also, our data suggest that whereas dimeric 14-3-3ζ is required for optimal binding to GPIb-IX,38 disruption of the 14-3-3ζ binding site in GPIbβ does not significantly affect 14-3-3ζ binding to GPIb-IX (Figure 1), suggesting the possibility that the 14-3-3ζ dimer still binds to 2 sites in GPIbα when the binding site in GPIbβ is disrupted. Interestingly, the sequences that may serve as a second binding site for 14-3-3ζ in the cytoplasmic domain of GPIbα overlap with the filamin binding site of GPIbα,18,20,25,29 suggesting the possibility that 14-3-3ζ binding may regulate filamin-mediated GPIb-IX association with the membrane skeleton. We have shown previously that dissociation of GPIb-IX from the membrane skeleton activates the ligand binding function of GPIb-IX.14 Alternatively, it is also possible that dissociation of one of the ligand binding pockets in the 14-3-3ζ dimer from GPIbβ allows 14-3-3ζ to interact with a signaling molecule that in turn regulates GPIb-IX function. In this regard, it has been reported that 14-3-3ζ links GPIb-IX to other intracellular molecules such as phosphoinositide 3-kinase.44 Finally, it is also possible that dimeric 14-3-3ζ may link 2 GPIb molecules into a dimer. It is common that oligomerization of a receptor activates its ligand binding function. Consistent with this, dimerization of GPIb-IX has been shown to activate GPIb-IX-mediated signaling in a CHO cell expression model.45
GPIb-IX as a major platelet adhesion receptor is an excellent target for antithrombosis drug development. Due to the critical roles GPIb-IX plays in platelet adhesion under high shear stress flow conditions, GPIb-IX-specific inhibitors are likely to have selective effects for arterial thrombosis (for example, in stenotic arteries) and microthrombosis (for example, in arterioles and capillaries). Similarly, in patients suffering from thrombotic thrombocytopenic purpura and other types of thrombotic microangiopathy, microthrombosis can be directly induced by the spontaneous binding of circulating VWF to GPIb-IX.2 Thus, development of a GPIb-IX-specific antiplatelet drug would be useful in treating these types of thrombotic diseases. Here we show that pharmacologic blockade of the interaction between 14-3-3ζ and GPIbα with MPαC inhibits the VWF binding function of GPIb-IX and platelet adhesion. Thus, MPαC or similar reagents that block GPIb-IX-14-3-3ζ interaction have the potential to be developed into a new class of antithrombotic agents.
A new toggle switch model for 14-3-3ζ-dependent regulation of VWF binding function of GPIb-IX. Intracellular cAMP levels regulate VWF-dependent platelet adhesion by controlling the 14-3-3ζ binding states in a way similar to a toggle switch. Disruption of 14-3-3ζ interaction with GPIbα inhibits activation of VWF binding function of GPIb-IX and thus platelet adhesion. GP indicates glycoprotein.
A new toggle switch model for 14-3-3ζ-dependent regulation of VWF binding function of GPIb-IX. Intracellular cAMP levels regulate VWF-dependent platelet adhesion by controlling the 14-3-3ζ binding states in a way similar to a toggle switch. Disruption of 14-3-3ζ interaction with GPIbα inhibits activation of VWF binding function of GPIb-IX and thus platelet adhesion. GP indicates glycoprotein.
Prepublished online as Blood First Edition Paper, June 7, 2005; DOI 10.1182/blood-2005-01-0440.
Supported by grants from the National Institutes of Health, HL62 350 and HL68 819 (X.D.), and also RO1 50 744 (M.C.B.). K.D. is a recipient of the Postdoctoral Fellowship Award from the American Heart Association Midwest Affiliate.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Changgeng Ruan, Zaverio Ruggeri, and Jose Lopez for providing reagents.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal