Abstract
Gene therapy holds a major promise. However, until now, this promise was fulfilled only in few cases, in rare genetic diseases. One very common clinical condition is anemia. Patients with anemia of chronic renal failure are treated with erythropoietin. The objective of this study was to develop a therapeutic platform for serum-secreted proteins like erythropoietin. We developed a tissue protein factory based on dermal cores (Biopump) harvested and implanted autologously. In this study, an adenovector was designed to express the human erythropoietin under the control of the cytomegalovirus (CMV) promoter. This vector transduced the harvested dermal cores ex vivo. The transduced cores were implanted, and erythropoietin and reticulocyte counts were measured. Dermal cores were harvested from 13 patients with chronic renal failure, and implantation was performed in 10. There were no significant drug-related side effects to this procedure. Erythropoietin serum levels increased significantly to therapeutic levels from day 1 after implantation reaching a peak during the first week of follow-up. The expression period was transient for up to 14 days. The rise of erythropoietin was followed by a transient significant increase in reticulocyte counts. The decrease of erythropoietin expression coincided with a significant dermal infiltrate of CD8 cytotoxic T cells. Antierythropoietin antibodies were not detected until day 90 following implantation. Implantation of dermal cores ex vivo transduced with human genes could eventually be used in the clinical setting to express therapeutic serum proteins. However, nonimmunogenic delivery system should be tested as gene vehicles.
Introduction
Although therapeutic serum proteins are powerful tools used in the treatment of a wide range of diseases, major challenges are encountered in their production and delivery to the patient. Chronic monogenic diseases, including coagulopathy as well as lysosomal storage diseases, are currently being treated with plasma-derived or recombinant proteins. During the past few years, the gene therapy platform, although applied mostly for nonmetabolic maladies, has also been assessed as a therapeutic approach for the treatment of monogenetic diseases, using nonviral or viral delivery systems, with various degrees of clinical benefits.1,2 Currently, most of the gene therapy targets in the monogenic disease group have been limited to disease affecting small groups of patients.3
One very common condition associated with hemoglobinopathies, chronic infections, and malignancies is chronic anemia. One specific example is anemia in patients with chronic renal failure, which is primarily caused by a lack of adequate production of erythropoietin (EPO) by the damaged kidneys. The treatment of the anemia of kidney disease with recombinant human EPO (hEPO) has been successfully carried out for more than a decade. However, the short half-life of the currently available recombinant products, their potential to cause antibodies against hEPO, and their high cost provide an incentive to significantly improve care in this area with the use of a genetic therapy approach.
To provide the most efficient erythropoiesis, hEPO should be kept at a steady level of approximately 40 to 200 mU/mL above baseline levels.4 Higher levels lead to hEPO wastage, and lower levels cause inefficient erythropoiesis. Current injectable EPO dosing regimens are affected by their being intermittent and by the short half-life of the preparations (3-48 hours), leading to serum EPO levels that are either too low or too high for a significant fraction of the treatment cycle. However, a continuous secretion approach could maintain a relatively steady-state level of hEPO within the desired range, optimizing efficiency of erythropoiesis. In addition, intermittent bolus injections of hEPO are associated with frequent overshoot or undershoot of target hemoglobin levels.4 All therapeutically used recombinant hEPO is currently produced in nonhuman mammalian cells in culture and are different either in amino acid content, glycosylation pattern, and formulation from the naturally produced protein.4 Formation of antibodies to recombinant hEPO has now been reported in individuals receiving recombinant hEPO α and β, resulting in the development of pure red-cell aplasia (PRCA), a devastating and life-threatening disease, which is currently being treated with immunosuppression and transplantation.5,6 In contrast, a gene therapy-based system that would produce hEPO from human cells, directly secreting the protein without any additional formulation or manufacturing, is anticipated to substantially reduce the risk of antibody formation. Moreover, the high cost of recombinant hEPO has a significant effect on practice patterns, with payers reluctant to permit administration of this protein until anemia is severe, and with upper limits on levels of hemoglobin leaving patients with significant anemia and related symptoms.7 Continuous secretion of hEPO could significantly lower the cost of therapy and justly return therapeutic decisions to doctors and patients rather than to payers. In an effort to meet these needs, we have recently developed a novel system in which the dermal tissue is converted into a cell/tissue factory through ex vivo transduction and autologous transplantation. We call this delivery method Biopump. Preclinical studies with severe combined immunodeficient mice, and human dermal tissue demonstrated that this technologic approach enabled a sustained secretion of therapeutic quantities of hEPO.
Here, we report our results with the use of the Biopump transduced with the adenovector expressing hEPO and transplanted into patients with anemia associated with chronic renal failure. The patients enrolled in this study had no significant adverse effects associated with the experimental drug other than local mild-to-moderate symptoms at the implantation site. Following implantation, we observed a clinically significant increase in hEPO serum levels followed by an increase in reticulocyte counts.
Patients, materials, and methods
Overview of the study
Patients who were screened and enrolled in the study had previously been diagnosed by a nephrologist with chronic kidney disease (CKD). The severity of the disease was of stages III and IV. Chronic renal failure diagnosis was determined by calculated glomerular filtration rate (GFR) between 15 and 60 cc/min. The study was approved by the national review board of Israel, and also by the institutional review boards of Rambam Medical Center (Haifa, Israel), Hadassah Hebrew University Hospital (Jerusalem, Israel), and Sourasky Medical Center (Tel Aviv, Israel). All patients had signed a written informed consent following an in-depth explanation of the study protocol and potential side effects, and they were also informed not to expect any clinical benefit from this experimental procedure. After the patient screening visit (visit 0), the first step in preparing a Biopump was the removal of several small dermal cores from the patient using a standardized procedure with local anesthesia as shown in Figure 1 (visit 1, day 0). On visit 1, subcutaneous dermal core samples (in the order of 1.5- to 2.5-mm diameter, 3- to 3.5-cm long) were removed in an ambulatory operating room.
The dermal samples were transferred in a sterile manner to the Good Manufacturing Production (GMP) facility at Hadassah-Hebrew University Hospital where they were maintained as dermal micro-organ structures in a viable condition outside the body (Figure 1). The adenovector (Ad-MG/EPO-1; see details in “Clinical-grade adenovector”) was used to transiently transduce the dermal core (Biopump) ex vivo, enabling the tissue to secret hEPO. Following several days in culture, hEPO production rate was determined and used for preimplantation dosing. To determine the effect of the immune response against the transduced Biopump, on selected patients one implanted Biopump was not transduced (a negative control dermal core) with the Adeno-5 viral vector and was maintained ex vivo as the other Biopumps were implanted separately. Each Biopump that passed the strict release criteria (detailed in “Biopump production”) was considered approved for implantation at visit 2 (V2).
On visit 2, 9 to 10 days after tissue harvest, the Biopump-hEPO implantation procedure was performed in an ambulatory operating room. Implantation of the desired number of Biopumps-hEPO was performed by implanting the Biopump-hEPO under the skin (Figure 1). The implantation site healing process was observed and documented on visit 3 (V3) taking place 6 to 8 days after Biopump-hEPO grafting.
Harvesting, ex vivo culturing, implantation, and after implantation steps of the Biopump in patients. (A) The harvesting step. (B) The Biopump ex vivo at the transduction step. (C). Implantation of the Biopump. (D) Implantation area 1 week after implantation. (E) Implantation area 4 weeks after implantation. (F) Implantation area 18 weeks after implantation.
Harvesting, ex vivo culturing, implantation, and after implantation steps of the Biopump in patients. (A) The harvesting step. (B) The Biopump ex vivo at the transduction step. (C). Implantation of the Biopump. (D) Implantation area 1 week after implantation. (E) Implantation area 4 weeks after implantation. (F) Implantation area 18 weeks after implantation.
Visit 4 (V4), termination visit, was performed 20 to 22 days after Biopump-hEPO implantation. On visit 4, a small 3-mm punch biopsy or incisional biopsy was taken from the Biopump-hEPO implantation site for histologic analysis. The treatment site healing process was observed and documented on the follow-up visit (V5), taking place 9 to 11 days after visit 4.
In addition to the patient visits to the treatment center, a qualified study nurse performed home visits during and following the trial to observe the site healing process and to obtain vital signs and blood for laboratory tests. After harvesting, the treatment site was photographed at each visit as depicted in Figure 1.
Patients
Twenty-two patients referred from 3 different medical centers were screened. Patient characteristics of those found eligible for the study are summarized in Table 1 as collected at the screening visit (V0). Seven men and 6 women were found eligible to participate in the study. Their average age was 51.7 ± 17.6 years (range, 39-69 years), and their average weight was 78.2 ± 17.5 kg (range, 63-121 kg). All patients were diagnosed with moderate chronic renal failure for at least 3 years before entering the study. Seven patients had diabetes mellitus and hypertensive nephropathy, 4 had polycystic kidney disease, 1 had nephrolithiasis, and 1 had focal segmental glomerulosclerosis and hyalinosis. Twenty-four-hour creatinine clearance of patients was between 15 and 59 mL/min. All had normal transferrin saturation, low reticulocyte count, and no antierythropoietin antibodies. Blood pressure was well controlled in all participants. Hemoglobin levels are depicted in Table 1.
Patient clinical characteristics
Patient ID no. . | Diagnosis . | CrCl, mL/min . | Epo, mU/mL at V2, t0 . | Ret, %, at V2, t0 . | Hb, g/dL at V2, t0 . | Weight, kg . | Age, y . | Gender, F/M . |
|---|---|---|---|---|---|---|---|---|
| H001 | PCKD | 21 | 8.9 | 0.2 | 9.8 | 84 | 44 | M |
| H002 | Nephrolithiasis | 21 | 8.0 | 0.2 | 11.7 | 80 | 62 | F |
| H003 | DM, HTN | 31 | NR | NR | 10.7 | 64 | 44 | F |
| H004 | DM, HTN | 43 | NR | NR | 10.3 | 121 | 68 | M |
| H005 | DM, HTN | 24 | NR | NR | 9.8 | 71 | 66 | M |
| H006 | FSGS | 38 | 11.8 | 1.0 | 10.3 | 67 | 27 | M |
| H007 | PCKD | 15 | 4.2 | 1.0 | 10.7 | 63 | 60 | F |
| H008 | DM, HTN | 26 | 9.5 | 2.0 | 10.0 | 76 | 65 | M |
| H009 | DM, HTN | 33 | 14.3 | 2.0 | 10.1 | 74 | 69 | F |
| H010 | PCKD | 28 | 8.9 | 0.6 | 10.8 | 72 | 57 | F |
| H011 | DM, HTN | 34 | 11.8 | 1.4 | 8.9 | 63 | 65 | M |
| H012 | PCKD | 59 | 3.2 | 0.9 | 11.4 | 108 | 40 | M |
| H013 | DM, HTN | 24 | 5.6 | 1.5 | 13.0 | 74 | 60 | F |
Patient ID no. . | Diagnosis . | CrCl, mL/min . | Epo, mU/mL at V2, t0 . | Ret, %, at V2, t0 . | Hb, g/dL at V2, t0 . | Weight, kg . | Age, y . | Gender, F/M . |
|---|---|---|---|---|---|---|---|---|
| H001 | PCKD | 21 | 8.9 | 0.2 | 9.8 | 84 | 44 | M |
| H002 | Nephrolithiasis | 21 | 8.0 | 0.2 | 11.7 | 80 | 62 | F |
| H003 | DM, HTN | 31 | NR | NR | 10.7 | 64 | 44 | F |
| H004 | DM, HTN | 43 | NR | NR | 10.3 | 121 | 68 | M |
| H005 | DM, HTN | 24 | NR | NR | 9.8 | 71 | 66 | M |
| H006 | FSGS | 38 | 11.8 | 1.0 | 10.3 | 67 | 27 | M |
| H007 | PCKD | 15 | 4.2 | 1.0 | 10.7 | 63 | 60 | F |
| H008 | DM, HTN | 26 | 9.5 | 2.0 | 10.0 | 76 | 65 | M |
| H009 | DM, HTN | 33 | 14.3 | 2.0 | 10.1 | 74 | 69 | F |
| H010 | PCKD | 28 | 8.9 | 0.6 | 10.8 | 72 | 57 | F |
| H011 | DM, HTN | 34 | 11.8 | 1.4 | 8.9 | 63 | 65 | M |
| H012 | PCKD | 59 | 3.2 | 0.9 | 11.4 | 108 | 40 | M |
| H013 | DM, HTN | 24 | 5.6 | 1.5 | 13.0 | 74 | 60 | F |
Creatinine clearance (CrCl) was calculated according to Cockcroft-Gault formula.
Ret indicates reticulocyte count; Hb, hemoglobin; F, female; M, male; PCKD, polycystic kidney disease; DM, diabetic nephropathy; HTN, hypertensive nephropathy; NR, not relevant (These patients did not receive an implant, so this result is not relevant.); FSGS, focal segmental glomerulosclerosis and hyalinosis.
Harvesting and implantation of dermal structures (Biopump)
On visit 1, we performed the harvesting of the dermal core. The procedure was performed in an outpatient ambulatory surgical room. The donor site area was shaved upon need, and prophylactic antibiotics, 1 g cephalosporin (Monocef), was administered intravenously. The donor area was cleaned and locally anesthetized. A plastic surgeon removed subcutaneous dermal samples (in the order of 1.5- to 2.5-mm diameter, 3- to 3.5-cm long) through a tiny skin cut by a specially designed dermal core harvester. The wound was photographed by a digital camera.
The implantation of the Biopump was performed on visit 2, between days 7 and 10. The number of Biopump-hEPO to be implanted per patient was decided on the day of implantation according to the ex vivo secretion rate of each Biopump (Table 2). One Biopump that was not transduced was implanted as a control to compare the histologic results. The implantation procedure was performed in the same location as the harvesting procedure. The implantation area was shaved upon need and cleaned. A sterile field was created, and the entire procedure was performed under strict sterile regulations. The implantation area was marked with a sterile marker according to the number of Biopumps to be implanted. The implantation area was locally anesthetized. The dermal Biopumps were loaded into an injection needle and were injected approximately 2 cm apart immediately under the skin. A digital camera photographed the implantation site. The harvesting and implantation sites were followed up by clinical examinations and clinical photography for local adverse effects for 6 weeks.
hEPO Biopump production and number of implanted dermal cores
Patient ID no. . | Dose† . | Treatment group . | Patient weight (kg) . | Glucose consumption, μg/day/BP . | No. of Biopumps implanted . |
|---|---|---|---|---|---|
| H001 | 16.3/1369 | LD | 84.0 | 360.41 ± 131.34 | 7 |
| H002 | 17.5/1407 | LD | 80.5 | 336.49 ± 169.82 | 2 |
| H006 | 19.9/1331 | LD | 67.0 | 780.49 ± 87.66 | 1 |
| H007 | 19.7/1242 | LD | 63.0 | 320.18 ± 193.97 | 2 |
| H008 | 63.3/4843 | HD | 76.5 | 478.38 ± 145.00 | 6 |
| H009 | 62.8/4644 | HD | 74.0 | 463.10 ± 107.65 | 3 |
| H010 | 64.3/4631 | HD | 72.0 | 640.41 ± 173.01 | 3 |
| H011 | 19.6/1234 | LD | 63.0 | 482.42 ± 51.46 | 4 |
| H012 | 64.2/6928 | HD | 108.0 | 791.64 ± 74.14 | 5 |
| H013 | 64.0/4545 | HD | 71.0 | 626.70 ± 128.59 | 3 |
Patient ID no. . | Dose† . | Treatment group . | Patient weight (kg) . | Glucose consumption, μg/day/BP . | No. of Biopumps implanted . |
|---|---|---|---|---|---|
| H001 | 16.3/1369 | LD | 84.0 | 360.41 ± 131.34 | 7 |
| H002 | 17.5/1407 | LD | 80.5 | 336.49 ± 169.82 | 2 |
| H006 | 19.9/1331 | LD | 67.0 | 780.49 ± 87.66 | 1 |
| H007 | 19.7/1242 | LD | 63.0 | 320.18 ± 193.97 | 2 |
| H008 | 63.3/4843 | HD | 76.5 | 478.38 ± 145.00 | 6 |
| H009 | 62.8/4644 | HD | 74.0 | 463.10 ± 107.65 | 3 |
| H010 | 64.3/4631 | HD | 72.0 | 640.41 ± 173.01 | 3 |
| H011 | 19.6/1234 | LD | 63.0 | 482.42 ± 51.46 | 4 |
| H012 | 64.2/6928 | HD | 108.0 | 791.64 ± 74.14 | 5 |
| H013 | 64.0/4545 | HD | 71.0 | 626.70 ± 128.59 | 3 |
BP indicates Biopump.
*Patients were assigned to a low-dose (LD) and high-dose (HD) treatment group.
Dose is presented as IU per kg per day/IU total
Biopump production
Following harvesting, each individual dermal core sample was sterilely transported in a commercial incubator in a well plate containing medium to the GMP facility at the Hadassah-Hebrew University Hospital. Transduction was performed following a 1-day equilibration period after harvesting. An aliquot of the GMP-produced viral vector (Ad-MG/EPO-1) was thawed at room temperature (up to 15 minutes or until thawed) and diluted to the final working concentration of 1010 ip/mL by adding production medium (Dulbecco minimal essential medium-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [DMEM-HEPES] medium with high glucose 4500 mg/L and 25 mM HEPES) to the viral stock. Transduction was performed in a 24-well multiplate. The medium was carefully removed from each well without disturbing the dermal core inside. Each well was filled with 250 μL viral solution. The plate was returned to the incubator and was agitated on a digital microtiter shaker at 300 rpm for a period of 2 hours. Twenty-four hours later, the solution containing the viral vector was removed and discarded, and the dermal core was washed with 1 mL fresh production medium. The dermal core were washed a total of 6 times as described in the previous step.
After performing an in vitro assay to determine the Biopump's hEPO secretion capacity, the exact number of individual Biopumps to be implanted on visit 2 was determined according to the desired dose. In addition, 72 hours after the removal of the viral vector, the production medium was replaced with fresh medium, and aliquots of the spent medium were assayed for releasing criteria, including viability and sterility assays. All Biopumps were found to be sterile, Mycoplasmas negative, endotoxins below 0.59 EU/mL, and bioburden below 5. Tissue glucose consumption determining that all tissues were valid for implantation was also conducted at this point. The amount of residual adenovector in the medium following the transduction step and before implantation is depicted in Table 3. On day 9 after skin harvest (day of implantation), nonimplanted Biopumps were extracted in DMEM using a Teflon manual homogenizer, and the supernatant was titrated for infectious particles (IPs). The residual viral vector was titrated according to the protocol of the Williamsburg Foundation for titration of Adeno.8 Assay results were validated using the Adenovirus Reference Material (ARM-American Type Culture Collection [ATCC] No. VR-1516; Manassas, VA). As can be seen in Table 2, approximately 0.06% of the original viral vector was retained in the tissue as residual vector only.
Amount of viral vector retained as residual vector ex vivo in the Biopump culture
Patient ID no. . | No. of Biopumps tested . | Infection titer, ip/BP . | Average of residual infectious titer of virus/Biopump . |
|---|---|---|---|
| H002 | 2 | 2.5 × 109 | 1.15 × 106ip/Biopump |
| H006 | 2 | 2.5 × 109 | 0.4 × 106ip/Biopump |
| H007 | 2 | 2.5 × 109 | 0.18 × 106ip/Biopump |
Patient ID no. . | No. of Biopumps tested . | Infection titer, ip/BP . | Average of residual infectious titer of virus/Biopump . |
|---|---|---|---|
| H002 | 2 | 2.5 × 109 | 1.15 × 106ip/Biopump |
| H006 | 2 | 2.5 × 109 | 0.4 × 106ip/Biopump |
| H007 | 2 | 2.5 × 109 | 0.18 × 106ip/Biopump |
Clinical-grade adenovector
To construct and produce the clinical-grade Ad-MG/EPO-1 (Ad5 E1/E3 deleted), the human EPO cDNA was inserted into the pAd-lox shuttle vector9 containing the cytomegalovirus (CMV) promoter and simian virus-40 (SV40) polyA site (the vector was kindly provided by Dr Paul Robbins, University of Pittsburgh, PA). The Ad-MG/EPO-1 used to manufacture the clinical-grade material was produced by Molecular Medicine BioServices, San Diego, CA. Ad-MG/EPO-1 production incorporates the use of a Molecular Medicine's characterized 293 subclone designated AC-2, and the Ad-MG/EPO-1 Master Viral Bank. Cells were thawed and expanded to seed the final production vessel (Cell Cube; Corning Enterprises, Corning, NY). Once the Cell Cube reached 80% confluence, the infection was initiated with a predetermined multiplicity of infection, infection, and harvest times using the Ad-MG/EPO-1 Master Viral Bank. Upon crude harvest, the contents (cells and supernatant) were collected and macrofluidized (cellular disruption). The material was then further concentrated and benzonased under optimal time and temperature conditions for the purpose of digesting extracellular components and followed by Anion Exchange Column (AEX) chromatography. The virus was collected using an elution step process, concentrated by diafiltration/ultrafiltration, formulated using a standard glycerol-based formulation, and filled at target concentrations. The particle count of the production lot, by OD260 was 1.45 × 1012 particles/mL, the viral titer was 1.6 × 109 plaque-forming units (pfu)/mL. The clinical-grade material passed numerous tests among them for Mycoplasma that was negative; it passed the sterility testing, and the level of endotoxins was less than 0.5 EU/mL.
DNA sequencing of the Ad-MG/EPO-1 expression cassette was performed and confirmed to contain the sequence of the human EPO cDNA without mutations. Moreover, the full-length DNA sequence of the entire viral vector genome was completed and submitted to the committee by the GMP Vector Production Facility as part of the Food and Drug Administration (FDA) requirements for clinically used viral vectors. The level of replication-competent adenovirus (RCA) in the clinical stock was performed by AppTec Laboratory Services (Camden, NJ). Detection of RCAs was performed with multiple screening levels. No RCAs were detected in any of the screening levels tested.
As part of GMP vector acceptance criteria, each vector shipment was titrated according to the Williamsburg Foundation's protocol for titration of adenovirus.8 The titration assay results were validated by using the Adenovirus Reference Material (ARM-ATCC No. VR-1516). Titration of the clinical vector was performed every 1 to 2 months to examine the stability of the clinical Adeno-hEPO vector as a function of time.
Determination of the infectious titer of Adeno viral particles was done on HEK-293 cell line according to the Williamsburg Foundation's protocol using 96-well plates, in duplicates. Cytopathic effect was examined 10 days after infection with the various dilutions of the adenovirus samples, and the amount of infectious particles was calculated. The viral stocks were stable over a period of more than 15 months during the clinical study.
Toxicologic Good Laboratory Practice preclinical assessment was conducted on rodents and was accepted by the national institutional review board (IRB) as acceptable for phase 1 gene therapy clinical study.
Histology and immunohistochemical studies
To analyze the efficiency of transduction and whether there is an immune response following autologous implantation, Biopumps and Biopump biopsies were fixed in 10% buffered paraformaldehyde, and 4- to 5-mm paraffin-embedded sections were prepared for staining with hematoxylin and eosin, and the following antibodies: anti-hEPO (R&D Systems, Minneapolis, MN; using 1:1000 dilution following pretreatment with citrate buffer pH 6), Vimentin monoclonal antibodies (Dako, Carpinteria, CA; fibroblasts specific using 1:1000 dilution following pretreatment with citrate buffer pH 6), anti-CD4 (Zymed Laboratories, San Francisco, CA; ready to use, following pretreatment with EDTA [ethylenediaminetetraacetic acid], pH 8.5, T helper [Th]), anti-CD8 (Zymed Laboratories; ready to use, following pretreatment with EDTA pH 8.5, cytotoxic T lymphocyte [CTL]), anti-CD20 (Dako; B-cell specific using 1:1000 dilution following pretreatment with citrate buffer pH 6), and myeloperoxidase (MPO) staining (Dako; using 1:100 dilution, neutrophils).
EPO measurements and reticulocyte counting
hEPO concentration and secretion levels were assayed using an enzyme-linked immunosorbent assay (ELISA) kit (Quantikine human erythropoietin; R&D Systems), according to the manufacturer's instructions. Reticulocyte counting was performed by an independent lab (American Medical Laboratories, Herzeliah, Israel). Two independent individuals in the lab counted twice the number of reticulocytes per 1000 red blood cells (RBCs; approximately 100 RBCs per field).
Anti-EPO and antiadenovirus serum antibodies
For anti-hEpo a radioimmunoprecipitation assay was used as described in a previous publication on the development of epoetin-induced PRCA10 and reported in detail elsewhere.9 Briefly, the serum samples are incubated first with 125I-radiolabeled epoetin and then with protein G, which binds immunoglobulin G (IgG). The antibody/protein G complexes are captured by centrifugation, and the radioactivity of the pellet is measured. This test is very sensitive because it can detect the presence of high-affinity anti-hEpo IgG when their concentration is as low as 10 ng/mL (S. Swanson; personal communication). To confirm these results we have adopted an additional assay for selected samples. This confirmation assay is an enzyme immunoassay used to evaluate the presence of antierythropoietin antibodies in plasma samples (MDS, Montreal, Canada; proprietary technology). Basically, the assay consists of a radioimmunoprecipitation where radioactively labeled antigen (EPO) of a known concentration is allowed to react with any anti-EPO present in a sample. The complex is then precipitated out of solution and centrifuged into a pellet, and any excess antigen not bound is washed out in the supernatant. The pellet is then counted on a gamma counter. For the detection of antiadenovirus antibodies we used the Ridascreen EIA kit (R-Biopharme AG, Darmstadt, Germany). Antiadenovirus IgG serum titers were measured at V0, V1, V4, and V5.
Results
Biopump ex vivo hEPO production
The aim of this study was to determine the safety and potential efficacy of implanting autologous human dermal cores transduced with adenovector encoding the hEPO gene to produce and secrete hEPO into the circulation. To fulfill these objectives, we conducted a preimplantation secretion analysis step to determine the level of hEPO produced by each Biopump per day on harvested dermal cores. The preimplantation testing for viability (glucose consumption), sterility, and hEPO secretion were both performed on day 8 of ex vivo culturing. As can be depicted in Table 2, all implants were viable and sterile at this time and secreted hEPO. To select a dose escalation scheme for the Biopump technology, a dosing model was formulated by Dr Anatole Besarab (Henry Ford Hospital, Detroit, MI), assuming 100% bioavailability of hEPO from the dermal implant. Clinical data collected from the first cohort of 4 patients (see Figure 2) treated with the Biopump receiving the first dose (mean of 18 IU/kg/d) indicates that the original calculated conversion factor, between ex vivo preimplantation daily secretion and the rise of hEPO levels in the serum following implantation, should be modified and reduced. The new conversion factor that is based on the actual patient pharmacokinetics predicts that to reach a 100 mU/mL increase; the patient should receive implants with a set of Biopumps secreting approximately 120 IU/kg/d. From these data, the patients in the second cohort received implants with Biopumps secreting a mean of 60 IU/kg/d. With the new conversion factor, the serum EPO level peaks were expected to be approximately 50 mU/mL over baseline following implantation. According to these calculations, we decided on the number of dermal cores to be implanted. It should be indicated that in 3 patients, H003, H004, and H005, the ex vivo production capacity did not reach the expected hEPO secretion, 54, 122, and 52 IU/d/Biopump, respectively, and in these cases the dermal cores were not implanted. Interestingly, according to our calculation, we should have been able to reach 100 mU/mL by implanting the appropriate number of dermal cores from patients H006, H007, H009, H010, H012, and H013.
EPO secretion and effect in patients
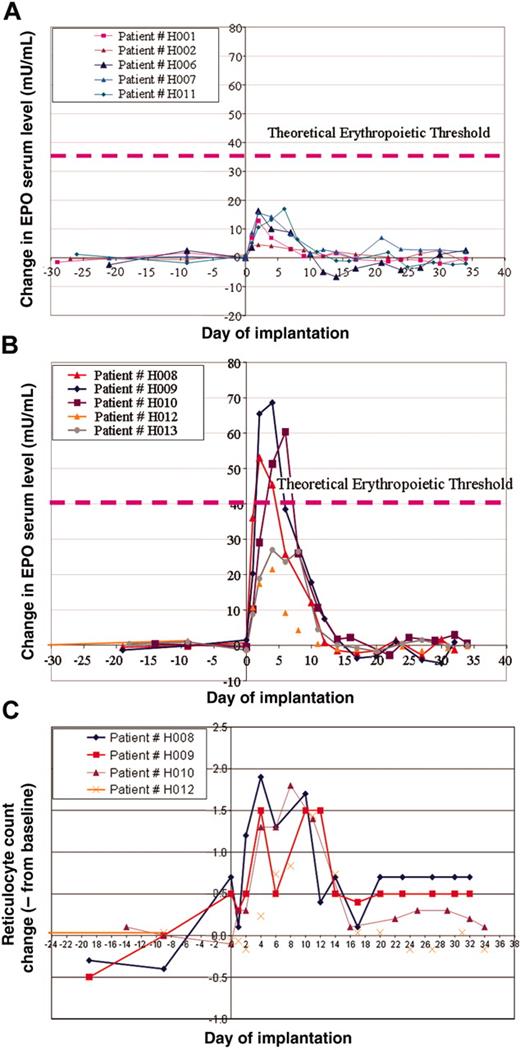
In this study, we grouped patients into 2 cohorts. The first cohort with a predicted low dose of hEPO secretion (16-18 IU/d/Kg) and a second group with a predicted high-dose secretion (55-65 IU/d/kg). As described in previous sections, on the basis of the preimplantation hEPO secreted levels ex vivo, we were able to determine the number of dermal cores to be implanted into patients' abdominal dermis to reach the target dose. We initiated the study with the low-dose group that included patients H001, H002, H006, H007, H008, and patient H011, who was projected to be included in the high-dose group; however, because of low preimplantation hEPO levels, he was grouped in the low-dose cohort (Figure 2A). The high-dose cohort included patients H009, H010, H012, and H013 (Figure 2B).
Patients were planned to be assigned to a specific cohort according to the expected level of hEPO secretion. However, for safety and efficacy analysis as depicted in this report, we had grouped patients according to actual hEPO secretion; see figures and Table 2. In the first cohort of patients, we depicted an increase of hEPO serum levels in those patients initiating immediately following transplantation on day 1. This level peaked in most cases on day 3 and gradually declined until 10 days after implantation (Figure 2A). On day 10 after implantation, hEPO serum levels reached baseline levels in most patients. In this cohort, 2 patients (H001 and H002) had relatively low peak hEPO serum levels of 4 mIU/mL and 13 mIU/mL, respectively. Patients H006, H007, and H011 had a higher peak of about 15 mIU/mL. Interestingly, although we intended to include patient H008 in the low-dose group, because his hEPO reached a higher than expected serum level, peaking on day 4 to greater than 20 mIU/mL, we included this patient in the high-dose cohort (Figure 2B). In the high-dose group of patients, we observed a significant high rise of serum hEPO above 50 mIU/mL in 3 patients. In one additional patient, we also depicted a significant increase, but of a lesser degree (Δ > 25 mIU/mL). The rise in hEPO levels, although transient, was associated with an increase of reticulocyte count as shown in Figure 2C. The hemoglobin levels were not significantly changed following the implantation.
hEPO serum levels and reticulocyte counts in patients who received implants with the adenovector Biopump. (A) Measurements of differences in hEPO levels before and after Biopump implantations for patients in the low-dose cohort. (B) Measurements of differences in hEPO levels before and after Biopump implantations for patients in the high-dose cohort. (C) Measurements of the reticulocyte count of 4 patients from the high-dose cohort.
hEPO serum levels and reticulocyte counts in patients who received implants with the adenovector Biopump. (A) Measurements of differences in hEPO levels before and after Biopump implantations for patients in the low-dose cohort. (B) Measurements of differences in hEPO levels before and after Biopump implantations for patients in the high-dose cohort. (C) Measurements of the reticulocyte count of 4 patients from the high-dose cohort.
Adverse events
In this study, we recruited 13 patients. Ten patients completed all visits and stages of the protocol (visits 0 to 5). We harvested the dermal cores from 3 additional patients; however, Biopumps were not implanted in these patients (visit 0 and visit 1). These 3 patients did not experience any adverse events (AEs). None of the patients had any severe adverse events with a casual relationship to the experimental drug. To our surprise, the patients' need for analgesics following the harvesting and implantation steps was negligible. No patients have experienced wound-healing problems such as bleeding, infection, or scaring. Only one patient (H006) had AEs with relationship to the experimental drug, including skin redness because of sensitivity to an unremoved band-aid. One additional patient (H013) suffered 4 AEs, including one severe (mild cerebrovascular accident [CVA]), 2 moderate (pain and weakness in right arm and leg edema), and one mild (hypertension). This patient had an old CVA as apparent from the brain computed tomography examination. None of the AEs for patient H013 had a casual relationship with the experimental drug (this statement was approved by the hospital's IRB).
Anti-hEPO antiadenovirus antibodies
The transient effect of the implanted Biopump transduced with an adenovector expressing hEPO could be related to numerous factors. The development of anti-hEPO is one possibility. In addition, the generation of an immune response against the transgene is a safety concern associated with this study. The humoral response against hEPO was measured for each patient before implantation (for those who were treated before the study with recombinant EPO) and on 2 consecutive visits (V3 and V4-V6 to 8 days and 20 to 22 days after grafting, respectively). We had in addition performed tests to determine whether anti-hEPO antibodies had developed at long-term follow-up. No anti-hEPO antidotes were detected up to 90 days following the implantation of the Biopump, indicating that the immune response against hEPO was neither a threat nor the reason for the decline of hEPO levels.
Antiadenovirus IgG levels did not change significantly in sera of patients between before Biopump implantation and at the 2 last visits (day 32 to 33) of the study (data not shown).
Cellular immune response against the autologous implants
The cellular immune response against the adenovector-transduced dermal core implanted in patients of this study could be the cause for the decline of hEPO serum levels. Following our initial observation (in patients H001 and H002) that the increase in serum levels hEPO was transient, we conducted histologic analysis of transduced dermal cores for patients H006 and H007. These preliminary studies suggested that the implanted dermal cores were infiltrated with a dense inflammatory infiltrate. In an effort to determine the cellular nature of this infiltrate and the potential mechanism responsible for the decline of hEPO levels in sera of patients participating in this study, we were allowed by the IRB to conduct a skin biopsy of implanted tissues, which were transduced as well as in implanted nontransduced dermal cores. On visit 4 (days 20-22) of the study, we performed a 3-mm standard skin punch biopsy in 5 patients both with transduced and nontransduced implants on 2 opposite sides of the abdominal skin (patients H008, H009, H010, H011, and H012). Prior to implantation, we performed immunohistologic analysis on the ex vivo samples of the Biopump not planned for implantation from the same patients. In those Biopumps that were not implanted, we were able to see positive staining both for hEPO and Vimentin, on the day of implantation (day 9 after transduction), indicating that hEPO was expressed in the dermal cores out of the fibroblasts. In addition, we did not detect any sign of cellular immune infiltrate in these dermal cores prior to implantation (staining for CD4, CD8, CD20, and MPO were all negative), as depicted in Table 4. Multiple serial sections of each dermal core of the transduced and the nontransduced Biopumps were performed on implants as depicted in Table 4. In the nontransduced Biopumps, we detected almost no inflammatory infiltrate. In the transduced dermal cores, we detected neither a neutrophilic infiltrate nor B-cell-type lymphocytes (negative CD20 staining). However, there was a massive infiltrate of cytotoxic T cells (positive CD8 staining), and also a moderate positive CD4 staining. Altogether, this information is suggestive of a cellular immune response against the transduced cells. However, based on these data, it is hard to predict against which expressed genes was this inflammatory reaction. This could include the hEPO or viral expressed genes. In addition, the silencing of the CMV promoter could also contribute to the transient expression of the hEPO. An additional possibility could be the effect of the host innate immune response. This effect should also be assessed in future studies.
Inflammatory infiltrate in Biopumps in patients with CKD
Biopump . | αCD4 . | αCD8 (CTL) . | αCD20 (B cell) . | αMPO (neutrophils) . | αVimentin (fibroblasts) . | αEPO . |
|---|---|---|---|---|---|---|
| Transduced, n = 4 | + | ++++ | +/– | + | ++++ | – |
| Nontransduced, n = 2 | – | +/– | – | +/– | ++ | – |
| In vitro | – | – | – | – | ++++ | +++ |
Biopump . | αCD4 . | αCD8 (CTL) . | αCD20 (B cell) . | αMPO (neutrophils) . | αVimentin (fibroblasts) . | αEPO . |
|---|---|---|---|---|---|---|
| Transduced, n = 4 | + | ++++ | +/– | + | ++++ | – |
| Nontransduced, n = 2 | – | +/– | – | +/– | ++ | – |
| In vitro | – | – | – | – | ++++ | +++ |
– indicates 0 cells stained; +/–, 1-3 cells stained; +, 3-8 cells stained; ++, 8-15 cells stained; +++, 15-25 cells stained; and ++++, more than 25 cells stained.
Discussion
The results of this study represent proof of principle that the implantation of an autologous genetically modified tissue into human dermis could significantly and safely increase the level of secreted proteins in the serum of patients. Furthermore, the secreted protein induced a physiologic effect by increasing the level of the reticulocyte count. The implantation and physiologic effects were not associated with any significant side effects associated with the experimental drug. However, the transient effect was not associated with a clinically significant increase in hemoglobin levels. This is probably related to the fact that the hEPO increase was short lived.
Numerous previous studies have suggested applying somewhat different or similar approaches to induce hEPO production and secretion.11 The reported methods included genetically transduced encapsulated myoblasts.12 In this case, the investigators used the transgenic mouse strain 134.3 LC, Epo-TAgH, which has chronic anemia resulting from EPO deficiency. Mice received a transplant with mice myoblasts, which were ex vivo transduced. The increase of EPO levels in these mice was transient because of an immune response against EPO. A different group used the DBA/2FG-pcy mouse model of polycystic kidney disease that is associated with chronic anemia. To assess the therapeutic effect of EPO in this model, they used an adenovector expressing hEPO directly injected into the peritoneal cavity to transduce mesothelial cells.13 They were able to detect a transient increase in reticulocyte counts with a later increase in hematocrit. Retroviruses were also used to transduce mesothelial cells to express EPO.14 The strategy of that group was to collect cells ex vivo and to transduce them in culture. Following intraperitoneal transplantation of cells, they were able to see a transient increase in EPO serum levels. All of these examples assessed the potential use of EPO therapy for chronic anemia associated with renal failure.
Treatment with EPO is currently indicated not only with chronic renal failure but also in many clinical conditions associated with anemia. These include anemia associated with cancer or chronic infections. Other investigators were interested in addressing the question of whether EPO therapy could also alleviate inherited hemoglobinopathies. One specific very prevalent example is thalassemia. This is an inherited malady that is a major health problem all over the world and could probably worsen in the coming years. Patients affected with this disease consume a significant portion of blood supplies in blood banks mainly in the Mediterranean basin and Southeast Asia. Bohl et al,15 from the Pasteur Institute, addressed the issue of using gene therapy in a mice model with β-thalassemia. The idea of treating this group of patients with EPO was previously suggested by Rachmilewitz et al.16 They were able to show that transduced mouse muscle cells with an adenovirus-associated virus (AAV) expressing the mouse EPO produces and secretes EPO into the serum, induces a sustained stimulation of β-minor globin (the mouse equivalent to human γ-globin) synthesis, and improves erythropoiesis in this mouse model. Furthermore, they were able to control EPO production in the same system with the doxycycline-controlled system.17 Similar results were also generated by Dalle et al18 and Sommer et al.19 Those studies referred to in this discussion have provided us with the groundwork in defining the rationale for the clinical study we have conducted. Our results clearly suggest that it is possible to convert human normal tissue into a protein factory. This is also associated with minimal morbidity to patients in the time frame described in this report.
Two recent reports had raised concerns related to the expression of Epo following recombinant AAV20,21 mediated gene transfer with the Epo gene. The groups of Chenuaud et al20 and Gao et al21 transduced muscle and lung tissues of nonhuman primates, with different serotypes of AAV2, harboring the homologous Epo gene. In some of the treated animals severe autoimmune anemia developed coinciding with the detection anti-Epo antibodies in sera of monkeys. In most cases the animals had supraphysiologic levels of Epo. However, the specific mechanism explaining the generation of anti-Epo antibodies is not clear. In our study we had moderate levels of hEpo in serum of patients, and we did not detect anti-hEpo during the first 3 months following therapy. However, future hEpo gene therapy programs should carefully monitor the development of anti-hEpo levels.
The results of this study suggest that the immunologic response against the transduced dermal cores could be the reason for the decrease in production of hEPO. The inflammatory infiltrate, predominantly composed of CTLs in the dermal tissue implanted, was probably targeting the proteins expressed from the first generation adenovirus (ΔE1 and ΔE3) that we had used for this study. However, other potential mechanisms could have contributed to the short-lived expression of hEPO such as the silencing of expression from the CMV promoter. Further studies in humans using improved delivery methods are needed to further assess the clinical benefit of long-term hEPO secretion from genetically modified cells. Several potential methods are currently available that could be used to improve the delivery and expression of hEPO in human tissue. This could include nonimmunogenic or low immunogenic viral vectors like AAV or adeno-gutless vectors,22 or other nonviral methods recently developed.23
Prepublished online as Blood First Edition Paper, March 29, 2005; DOI 10.1182/blood-2004-11-4174.
Supported by the Israeli Ministry of Science through its National Knowledge Center in Gene Therapy at the Hadassah-Hebrew University Hospital in Jerusalem (E.G.) and by grants from the Grinspoon, Blum, and the Horwitz Foundations (E.G.).
At the time of the study, E.G., Y.L., and M.D-E. served as consultants for Biogenics Ltd, which provided support for the study.
Several of the authors (E.B.A., C.M.-Z., and N.S.) have declared a financial interest in Medgenics whose potential product, the Biopump, was studied in the present work. In addition, these 3 authors were also employed by Medgenics while conducting the present work. However, all 3 are no longer employed by Medgenics, and the company was dissolved.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mery Clausen for her editorial assistance. This paper is dedicated to the memory of Dr Leonard (Lenny) Garfinkel, our colleague and friend who was part of the team to initiate this project, and who died during the course of the preparation of this report.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal