Abstract
Triptolide (TPT) is a chemically defined, potent immunosuppressive compound isolated from an anti-inflammatory Chinese herbal medicine. TPT has been reported to inhibit autoimmunity, allograft rejection, and graft-versus-host disease (GVHD), and its efficacy was previously attributed to the suppression of T cells. Since dendritic cells (DCs) play a major role in the initiation of T-cell–mediated immunity, we studied the effects of TPT on the phenotype, function, and migration of human monocyte–derived DCs. TPT treatment, over a pharmacologic concentration range, inhibited the lipopolysaccharide (LPS)–induced phenotypic changes, characteristic of mature DCs and the production of interleukin-12p70 (IL-12p70). Consequently, the allostimulatory functions of DCs were impaired by TPT treatment. Furthermore, the calcium mobilization and chemotactic responses of LPS-stimulated DCs to secondary lymphoid tissue chemokine (SLC)/CC chemokine ligand 21 (CCL21) were significantly lower in TPT-treated than untreated DCs, in association with lower chemokine receptor 7 (CCR7) and higher CCR5 expression. Egress of Langerhans cells (LCs) from explanted mouse skin in response to macrophage inflammatory protein-3β (MIP-3β)/CCL19 was arrested by TPT. In vivo administration of TPT markedly inhibited hapten (fluorescein isothiocyanate [FITC])–stimulated migration of mouse skin LCs to the draining lymph nodes. These data provide new insight into the mechanism of action of TPT and indicate that the inhibition of maturation and trafficking of DCs by TPT contributes to its immunosuppressive effects.
Introduction
The Chinese herb Tripterygium wilfordii Hook F (TWHF, known in China as Lei-Gong-Teng, which translates into the “thunder god” vine, a vinelike member of the Celastraceae plant family), has been used in traditional Chinese medicine (TCM) for the treatment of autoimmune diseases including rheumatoid arthritis (RA),1,2 systemic lupus erythematosus (SLE),3,4 discoid lupus erythematosus,5 and pyoderma gangrenosum.6 One major active component isolated from TWHF is triptolide (TPT, also designated as PG-490), a diterpenoid triepoxide.7 TPT and its derivatives have been shown to inhibit collagen induced arthritis8 and experimental autoimmune uveoretinitis,9 to prolong allograft survival,10 prevent graft-versus-host disease (GVHD),11 and suppress rheumatoid arthritis in human patients.10,12 A recent study demonstrated that TPT combined with a subtherapeutic dose of tacrolimus produced a synergistic effect that prolonged rat cardiac allograft survival.13 The clinical application for TPT to improve transplantation outcomes is under development in the United States.14
The immunosuppressive action of TPT has been generally attributed to its suppression of T-lymphocyte activation,15,16 including inhibition of lymphocyte proliferation, interleukin-2 (IL-2) receptor expression and IL-2 production,15,17 interferon-γ (IFN-γ) production18 and induction of T-cell apoptosis.19 A recent study indicated that TPT effectively prevented lethal GVHD in a bone marrow transplantation model and induced the engrafted cells to develop host-specific tolerance. In this model, the engrafted T cells were not deleted by TPT treatment, but were tolerized. Although the donor T cells failed to respond to the host antigen, they responded normally to T-cell receptor (TCR) cross-linking. The allograft unresponsiveness was not overcome by supplementation with exogenous IL-2.11 This observation suggests that since TPT induces specific peripheral tolerance, TPT may also have inhibitory effects on the maturation, antigen processing and presentation by dendritic cells (DCs).
DCs represent a heterogeneous population of professional antigen-presenting cells (APCs) that initiate primary immune responses.20 DCs initiate immunity by activating naive T cells and subsequently the effector cells of the adaptive immune system. Besides linking innate and adaptive immunity, DCs also control immunity based on their ability to induce T-regulatory cells to promote antigen-specific unresponsiveness of lymphocytes in primary and secondary lymphoid tissues.20 Upon maturation, DCs acquire the ability to produce IL-12, a cytokine necessary for the development of T helper 1 (Th1) cells and cell-mediated immunity.20 It has been shown that in vivo administration of TPT suppresses the development of experimental autoimmune uveitis (EAU) by down-regulating Th1-type response.9
In the present study, we demonstrate that in a pharmacologic concentration range (0.5-10 nM), TPT inhibited the differentiation, maturation, and allostimulation of human monocyte–derived DCs (MoDCs). After TPT treatment, the chemotactic and calcium mobilization responses of LPS-stimulated DCs to secondary lymphoid tissue chemokine (SLC)/CC chemokine ligand 21 (CCL21) were reduced, while responses to RANTES (regulated on activation, normal T expressed and secreted)/CCL5 were increased, which is characteristic of low expression of CCR7 and elevated expression of CCR5 of immature DCs. Furthermore, TPT arrested Langerhans cell emigration in response to mMIP-3β from ex vivo cultured skin explants and inhibited in vivo hapten (fluorescein isothiocyanate [FITC])–bearing Langerhans cell migration to draining lymph nodes (LNs). Therefore, our data clearly establish that TPT suppresses DC maturation and migration, which may be an important contributor to its immunosuppressive effects.
Materials and methods
Mice and reagents
BALB/c females (6-8 weeks old) were obtained from the Animal Production Area of the National Cancer Institute-Frederick (Frederick, MD). Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals.
Granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 4 (IL-4), MIP-3β, tumor necrosis factor (TNF), and RANTES were purchased from PeproTech (Rocky Hill, NJ). Triptolide (TPT, C20H24O6, molecular weight [MW] 360, = 98%), FITC isomer I and LPS (lot no. 121H4026, from Salmonella Minnesta RE 595) were purchased from Sigma (Saint Louis, MO). Soluble CD40 ligand (sCD40L) and ligand enhancer was obtained from Alexis (San Diego, CA). Poly (I:C) were purchased from InvivoGen (San Diego, CA). The structure of TPT was confirmed by mass spectrography and nuclear NMR was provided by SAIC-NMR services (Frederick, MD). TPT was reconstituted in dimethyl sulfoxide (DMSO) at concentration of 10 mM and was stored in -20°C under argon. The working solution of TPT was freshly prepared by dilution of TPT in the culture medium. Antihuman antibodies were from BD Pharmingen (San Diego, CA), including anti-CD1a (mouse IgG1, phycoerythrin [PE]–Cy5 [BD Cy-Chrome; BD Pharmingen], HI149), anti-CD14 (mouse IgG2a, PE, M5E2), anti-CD40 (mouse IgG1, FITC, 5C3), anti-CD80 (mouse IgG1, FITC, L307.4), anti-CD83 (mouse IgG1, FITC, HB15e), anti-CD86 (mouse IgG1, PE-Cy5, 2331 [FUN-1]), anti–human leukocyte antigen (HLA)–DR (mouse IgG2a, PE, G46-6 [L243]) and isotype control antibodies: mouse IgG1 (PE-Cy5, MOPC-21 [clone of Ab]), mouse IgG2a (PE, G155-178), mouse IgG1 (FITC, MOPC-21). Anti–mouse CD11c (Armenian hamster IgG1, PE, HL3), anti–mouse I-A/I-E (rat IgG2a, FITC, 2G9), isotype control antibodies Armenian hamster IgG1 (PE, A19-3), rat IgG2a (FITC, R35-95), and purified anti-CD16/CD32 (2.4G2) were purchased from BD Pharmingen. The following antihuman antibodies were purchased from R&D Systems (Minneapolis, MN): anti-CCR5 (mouse IgG2b, FITC, 45531), anti-CCR7 (mouse IgG2a, PE, 150503) and isotype control mouse IgG2b (133303, FITC).
Cell isolation and purification
Human peripheral blood enriched in mononuclear cells was obtained from healthy donors by leukapheresis (Transfusion Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD, with an approved human subjects agreement). The blood was centrifuged through Ficoll-Hypaque (Sigma), and peripheral blood mononuclear cells (PBMCs) collected at the interface were washed with PBS and centrifuged through isoosmotic Percoll (Pharmacia, Uppsala, Sweden) gradient. The enriched monocyte populations were obtained at the very top of the gradient (top fraction). CD4+ T cells were purified from PBMCs by the use of Human CD4 T-Cell Enrichment Columns (R&D Systems).
Allogeneic and anti-CD3/CD28–induced CD4+ T-cell activation
For the bidirectional mixed lymphocyte reaction (MLR), the same number (3 × 105) of allogeneic PBMCs from 2 different donors were cocultured in 96-well flat-bottom plates. After 5 days, proliferation was measured. Purified CD4+ T cells (105/well) were cultured in 96-well flat-bottom plates with CD3/CD28 T-cell expansion Dynabeads (5 × 103/well; Dynal ASA, Oslo, Norway). After 72 hours, proliferation was measured. TPT (0-10 nM) was added to the cell suspension from the beginning of culture. The cells were pulsed with 0.037 MBq (1 μCi) [3H]thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) per well for the last 15 hours of culture period. Cells were then harvested onto filter membranes using an Inotech harvester (Inotech Biosystems, Rockville, MD), and the amount of incorporated [3H]thymidine was measured with a Wallac Microbeta counter (PerkinElmer Life and Analytical Sciences, Boston, MA).
DC culture and TPT treatment
DCs were generated as described previously.21 In brief, monocyte-derived immature DCs (iDCs) were generated by incubating purified monocytes at 1 × 106/mL in G4 medium (RPMI 1640 containing 10% fetal bovine serum (FBS), 2 mM glutamine, 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 100 U/mL penicillin, 100 μg/mL streptomycin, 50 ng/mL recombinant human (rh) GM-CSF, and 50 ng/mL rhIL-4) at 37°C in a CO2 (5%) incubator for 7 days. The cultures were fed with the same cytokine-containing medium every 2 to 3 days. To induce DC maturation, iDCs were cultured in the same cytokine cocktails with the addition of LPS (10 ng/mL) for 2 days. In some experiments, soluble CD40 ligand (sCD40L; 300 ng/mL) and ligand enhancer (1 μg/mL), or TNFα (10 ng/mL) or poly (I:C) (12.5 μg/mL) was used to induce iDC maturation. For the TPT treatment group, TPT was added to the cell suspension at day 2 of culture and washed thoroughly at day 7 (D2-7 treatment), and cells were cultured with G4 medium with LPS but without TPT at day 8 and day 9. In some experiments, monocytes were cultured in the G4 medium (free of TPT) for 7 days, TPT was added to the cell suspension at day 8, together with the addition of LPS (D8-9 treatment) or other stimuli [sCD40L, TNFα, and poly (I:C)], and TPT treatment was added last for the maintaining period of culture.
Flow cytometric analysis
Cells (5 × 105) were first blocked with 10% of normal AB serum (for human cells) or anti-CD16/CD32 (for mouse cells). Cell staining was performed using FITC-, PE-, or Cychrome-conjugated monoclonal antibodies (mAbs) in 4°C for 30 minutes. Flow cytometric analysis was performed on a FACScan (BD Biosciences, Mountain View, CA) using CellQuest software (BDIS, San Jose, CA). Results are expressed as the percentage of positive cells or as mean fluorescence intensity (MFI).
Detection of cytokine
DCs were cultured with G4 medium and treated with 0.5 to 10 nM of TPT from day 2 of culture. At day 7 the cells were thoroughly washed, resuspended, and cultured with 10 ng/mL of LPS, but free from TPT. Forty-eight hours later, the supernatants were collected and cytokine measurement was performed by analysis of supernatant with SearchLight Mouse Cytokine Array (Pierce Biotechnology, Woburn, MA).
MLR assay
To assess DCs stimulatory activity, 7-day (iDCs) or 9-day (mDCs) cultured DCs were extensively washed, irradiated, 30 Gy (3000 rad), and added in graded doses to wells containing 1 × 105 purified allogeneic responder T cells in 96-well flat-bottom plates. After 5 days of culture, proliferation was measured. For proliferation assay, cells were pulsed with 0.037 MBq (1 μCi) [3H]thymidine (Amersham Pharmacia Biotech) per well for the last 15 hours of incubation. Cells were then harvested onto filter membranes using an Inotech harvester (Inotech Biosystems) and the amount of incorporated [3H] thymidine was measured with a Wallac Microbeta counter (PerkinElmer Life and Analytical Sciences).
Apoptotic assay
ApoAlert Annexin V–PE Apoptosis Kit (BD Pharmingen) was used to measure the potential cytotoxic effect of TPT on the cultured DCs. Briefly, monocyte-derived DCs were treated with 0.5 to 10 nM of TPT from day 2 to day 7 (D2-7) of culture. Then the cells were harvested and doubly labeled with PE-conjugated annexin V and 7-amino-actinomycin (7-AAD) according to the manufacturer's instructions. Annexin V and 7-AAD uptake were detected using FACScan, and data were analyzed using CellQuest software.
Chemotactic assay
Migration of human monocyte–derived DCs was assessed using a 48-well microchemotaxis chamber technique. RANTES or SLC (1-1000 ng/mL; PeproTech) were diluted in chemotaxis medium (CM) (RPMI 1640, 1% bovine serum albumin [BSA, A4301; Sigma], 25 mM HEPES) and placed in the lower wells. A 50 μL cell suspension (106 cells/mL in chemotaxis medium) was placed in the upper wells. A polycarbonate filter was used to separate the 2 compartments (5-μm pore size; Neuroprobe, Gaithersburg, MD). After incubation at 37°C in humidified air with 5% CO2 (1.5 hours), the filter was removed, fixed, and stained with Diff-Quik (Harlew, Gibbstown, NJ). The cells on the underside of the membrane were counted at × 200 magnification. The data were expressed as the migrating cell number/HPF (high-power field).
Calcium mobilization
Day-7 cultured immature DCs and day-9 cultured mature DCs were resuspended at 107 cells/mL in loading buffer (138 mM NaCl, 6 mM KCl, 1 mM CaCl2, 10 mM HEPES, 5 mM glucose, and 0.1% BSA, pH 7.4) and were incubated with 5 μM Fura-2 (Sigma) at 37°C for 30 minutes. The dye-loaded cells were washed twice with 10% FBS Dulbecco modified Eagle medium (DMEM), washed once with loading buffer, and were then resuspended in fresh loading buffer at a concentration of 5 × 105cells/mL. Cell suspension (2 mL) was pipetted into quartz cuvettes in a luminescence spectrometer LS50 B (Perkin-Elmer Life and Analytical Sciences). Stimulants (500 ng/mL of RANTES or SLC) were added in a volume of 20 μLto the cuvettes at indicated time points. The ratio of fluorescence at 340- and 380-nm wavelengths was calculated using FL WinLab (Perkin-Elmer Life and Analytical Sciences).
Epidermal-sheet preparation and immunolabeling
The density of epidermal LCs was examined by FITC–anti–I-A/I-E Ab in situ immunolabeling. Mouse ears were excised, split, and floated dermal side down in 0.5 M ammonium thiocyanate for 20 minutes at 37°C. Epidermal sheets were separated from the dermis, and fixed with acetone for 5 minutes at -20°C. FITC-labeled anti–I-A/I-E (1/100 in 5% BSA/PBS) was applied to stain LCs in epidermal sheets, using FITC-labeled isotype-matched nonspecific Abs as control.22 The sheets were washed and mounted on microscope slides. The images of epidermal sheet were captured with a SPOT RT digital camera (Diagnostic Instruments, Sterling Heights, MI) connected to an Olympus BX60 fluorescence microscope (Olympus Optical, Tokyo, Japan) equipped with an Olympus UPlanF1 40 ×/0.75 objective lens.
Skin-explant culture
The epidermal LCs egress procedure was modified from that of Kellerman et al.23 The mouse ears were surgically removed, briefly washed in 70% ethanol, and split into dorsal and ventral halves. The single ear half was placed split-side down in 1 mL of RPMI 1640 containing 10% FCS for 30 minutes at 37°C in a humidified 10% CO2 incubator to eliminate the many non-DCs initially released into the culture. The skin then was transferred onto another 1-mL culture medium with or without of 100 ng/mL of murine MIP-3β to enhance DC migration. After 24 hours of further incubation at 37°C, the cells that had migrated into the culture medium were harvested and kept in cold medium. The skin was transferred onto fresh warm medium with or without of MIP-3β and then incubated a further 24 hours at 37°C. The cells that migrated out of the skin over the first and second 24-hour incubations then were pooled. The cells were stained for PE–anti-CD11c and suspended in 0.3 mL of PBS, analyzed by fluorescence-activated cell sorting (FACS) for 50 seconds. The data were expressed as number of CD11c+ cells present during 50-second analysis.
FITC-bearing LC migration assay
The approach was modified from that of Fukunaga et al.24 FITC was dissolved in acetone/dibutylphthalate (1:1) before application. Mice were painted on the dorsal ear half with 10 μL of 1% FITC solution. TPT (0.1 mg/kg/d, intraperitoneally) is injected for 5 days before the FITC application. Twenty-four hours after FITC painting, draining auricular lymph nodes were collected, placed in RPMI 1640, and the single-cell suspension was prepared and the cell number was determined. The cells were incubated on ice for 30 minutes with PE-conjugated anti–mouse CD11c mAb or isotype control. Flow cytometry analysis was performed on a FACScan using CellQuest software.
Statistical analysis
All experiments were performed at least 3 times and the results of a representative experiment presented. The data are expressed as means ± SD where an error bar appears. The significance of the difference between TPT treatment group and control groups was analyzed with a Student t test.
Results
TPT inhibits DC maturation in a dose-dependent manner
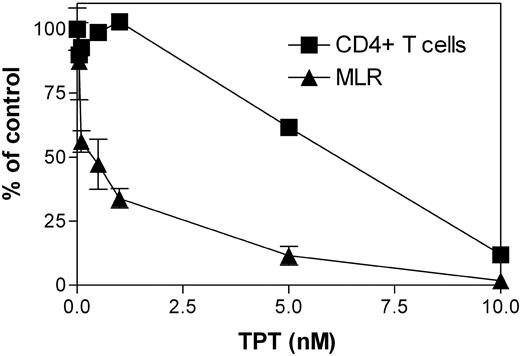
To test our hypothesis that TPT may also target DCs, we compared the effects of TPT (0.1-10 nM) on the MLR of allogeneic PBMCs and the proliferative response of purified CD4+ T cells to anti-CD3/CD28 antibodies. As shown in Figure 1, the MLR was more substantially inhibited by TPT than the proliferation of purified CD4+ T cells, especially when TPT was used in lower concentrations. Therefore, we hypothesized that, in addition to inhibiting T cells, TPT may also target APCs and consequently inhibit APC-initiated T-cell response.
We next used GM-CSF– and IL-4–treated human MoDCs to test the impact of TPT on the differentiation and maturation of DCs. To avoid interference with the adherence of cells to the plastic wall of the plate, which may influence the differentiation process of DCs, we treated human monocytes with TPT from day 2 of culture. At day 7 the cell suspension was thoroughly washed and resuspended in G4 medium supplemented with 10 ng/mL of LPS, without TPT, for another 48-hour culture to induce maturation of DCs. CD83 is a marker of mature DCs.25 In our experiment system, stimulation with LPS resulted in 70% to 80% of DCs expressing surface CD83. By using this marker, we determined the doses of TPT that inhibit DC maturation. CD83 expression was inhibited by a pharmacologic concentration range of TPT (0.5-10 nM)26 in a dose-dependent manner with an inhibition at 50% concentration (IC50) of 2.5 nM (Figure 2A). The expression of CD1a and CD86 were similarly reduced (data not shown). Furthermore, TPT (D2-7) treatment dose-dependently inhibited the capacity of LPS-stimulated DCs to produce IL-12p70 (Figure 2B). Thus, TPT is a potent inhibitor of DC maturation based on suppression of maturation markers and cytokine production.
It has been reported that TPT at 10 ng/mL (or 27.8 nM) had the capacity to induce apoptosis of mouse bone marrow–derived DCs.27 We therefore evaluated the effect of 0.5 to 10 nM TPT on the apoptosis of DCs. Results, shown in Figure 2C, indicated that TPT treatment did induce DC apoptosis at high concentration (5 nM and 10 nM). However, at a concentration of 2.5 nM, the capacity of TPT to induce DC apoptosis is very limited (annexin V+7-AAD- cells was 0.35% in the medium control group and 0.95% in the TPT-treated group). Furthermore, the cell viability based on trypan blue assay and recovery of cultured DCs also were not affected significantly by 2.5 nM of TPT (D2-7) treatment (data not shown). Therefore, we used 2.5 nM of TPT for our subsequent experiments.
Effect of TPT on the proliferative response of bidirectional MLR and anti-CD3/CD28–stimulated CD4+T cells. Allogeneic human PBMCs from 2 different donors (3 × 105 cells/well each) were cocultured in 96-well flat-bottom plates in the presence of the indicated concentration of TPT. After 5 days, proliferation was measured. Purified CD4+ T cells (105 cells/well) were cultured in the presence of indicated concentration of TPT and stimulated with CD3/CD28 T-cell expansion Dynabeads (5 × 103/well). After 72 hours, proliferation was measured. The data shown are the percent of control group proliferation (without TPT), which are from a representative experiment out of 3 performed. Error bars indicate standard deviation.
Effect of TPT on the proliferative response of bidirectional MLR and anti-CD3/CD28–stimulated CD4+T cells. Allogeneic human PBMCs from 2 different donors (3 × 105 cells/well each) were cocultured in 96-well flat-bottom plates in the presence of the indicated concentration of TPT. After 5 days, proliferation was measured. Purified CD4+ T cells (105 cells/well) were cultured in the presence of indicated concentration of TPT and stimulated with CD3/CD28 T-cell expansion Dynabeads (5 × 103/well). After 72 hours, proliferation was measured. The data shown are the percent of control group proliferation (without TPT), which are from a representative experiment out of 3 performed. Error bars indicate standard deviation.
Effects of TPT on human monocyte–derived DCs. (A-B) Human monocytes were cultured with 50 ng/mL of GM-CSF and 50 ng/mL of IL-4 (G4 medium). After 24 hours, the medium was replaced with fresh G4 medium in the presence or absence of desired concentrations of TPT (0.5 nM-10 nM). At day 7 of incubation, cells were thoroughly washed, and the medium was supplemented with 10 ng/mL LPS. Forty-eight hours later, supernatants were collected for cytokine measurement and cells were labeled with the FITC–anti-CD83 mAb and analyzed by FACS. Data shown are summarized from 3 separate experiments (means ± SD). (A) Percent inhibition of CD83 expression and (B) IL-12p70 production by LPS-stimulated DCs. (C) Effect of TPT on apoptosis of DCs. Human monocytes were cultured for 7 days with G4 medium in the presence of TPT (D2-7, 0.5-10 nM) or medium alone. The percentage of apoptotic cells was determined by FACS analysis using Annexin V/7-AAD staining. The data are representative of at least 5 separate experiments with cells derived from different donors. Numbers indicate percentage of cells present in corresponding quadrants.
Effects of TPT on human monocyte–derived DCs. (A-B) Human monocytes were cultured with 50 ng/mL of GM-CSF and 50 ng/mL of IL-4 (G4 medium). After 24 hours, the medium was replaced with fresh G4 medium in the presence or absence of desired concentrations of TPT (0.5 nM-10 nM). At day 7 of incubation, cells were thoroughly washed, and the medium was supplemented with 10 ng/mL LPS. Forty-eight hours later, supernatants were collected for cytokine measurement and cells were labeled with the FITC–anti-CD83 mAb and analyzed by FACS. Data shown are summarized from 3 separate experiments (means ± SD). (A) Percent inhibition of CD83 expression and (B) IL-12p70 production by LPS-stimulated DCs. (C) Effect of TPT on apoptosis of DCs. Human monocytes were cultured for 7 days with G4 medium in the presence of TPT (D2-7, 0.5-10 nM) or medium alone. The percentage of apoptotic cells was determined by FACS analysis using Annexin V/7-AAD staining. The data are representative of at least 5 separate experiments with cells derived from different donors. Numbers indicate percentage of cells present in corresponding quadrants.
Effects of TPT on differentiation and maturation of DCs
Human monocytes, when cultured with GM-CSF plus IL-4, differentiate into immature DCs.28 During this process, they down-regulate the monocyte/macrophage markers CD14 and CD16 but express DC marker CD1a as well as modest levels of HLA-DR, CD40, CD80, and CD86, but not CD83. TPT (2.5 nM) treatment from day 2 to day 7 (D2-7) of culture did not alter the differentiation of monocytes into immature DCs, since the cells no longer expressed CD14 and CD16. However, TPT-treated cells expressed even lower levels of CD1a, HLA-DR, CD40, CD80, and CD86 than untreated DCs (Figure 3A).
Immature DCs obtained by a 7-day culture with GM-CSF and IL-4 can be induced to mature by stimulation with LPS.29 DC maturation is accompanied by induction of the maturation marker CD83, and marked up-regulation of HLA-DR, CD40, CD80, and CD86 (Figure 3B). We investigated whether TPT-treated DCs at differentiation stage (D2-7) could still undergo maturation. Therefore, TPT-treated (D2-7) DCs were washed thoroughly and stimulated another 48 hours with LPS (without TPT). These cells expressed markedly lower levels of presentation molecules (CD1a and HLA-DR) and costimulatory molecules (CD40, CD80, and CD86) as well as less of the maturation antigen (CD83), compared with LPS-stimulated DCs that had not been exposed to TPT (Figure 3B). Therefore, the capacity of LPS-stimulated DCs to undergo maturation was suppressed by prior exposure to TPT.
We next tested the effect of TPT treatment during the maturation period. DCs that had been cultured with GM-CSF and IL-4, without TPT, for 7 days, were then stimulated with LPS in the presence of IC50 concentration of TPT (2.5 nM) or medium alone and cultured for an additional 48 hours. TPT treatment at this stage (D8-9) also suppressed DC maturation to the same extent as treatment during the period of differentiation (D2-7; Figure 3B).
We also examined the effects of TPT on DC maturation induced by other stimuli (eg, CD40L, TNFα, and poly (I:C)). In these experiments, TPT was added to DC cultures during days 8 and 9. Results showed that TPT treatment inhibited the expression of CD40, CD80, CD83, and CD86 induced by CD40L, TNFα, and poly (I:C) (Figure 3C). Therefore, TPT suppressed DC maturation triggered by a broad spectrum of proinflammatory environmental stimuli.
Inhibition of DC allostimulatory capacity
We then investigated the functional properties of TPT-treated DCs. The most characteristic functional feature that discriminates DCs from other APCs is their ability to induce T-cell proliferative responses, as determined by allostimulatory assays. Figure 4 shows that untreated DCs and even more so LPS-stimulated DCs strongly stimulated allogeneic T-cell proliferation. The allostimulatory capacity of DCs and of LPS-stimulated DCs was markedly inhibited by TPT treatment at D2-7 (and D8-9 for LPS-stimulated DCs). Therefore, TPT treatment not only inhibited the phenotypic maturation of DCs, but also impaired their function.
TPT inhibits the chemotactic response of LPS-stimulated DCs to SLC/CCL21
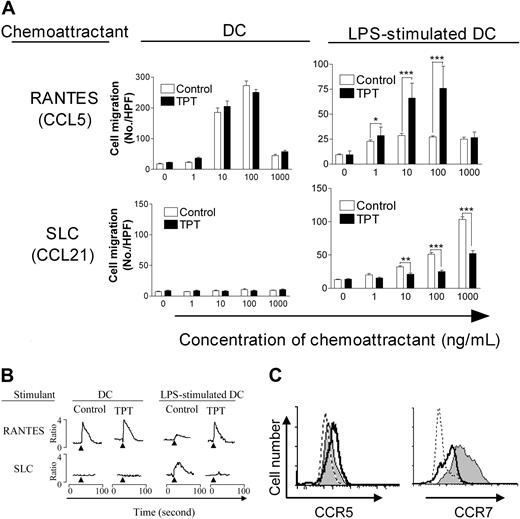
We determined if TPT also altered the chemotactic response of DCs. As shown in Figure 5A, untreated DCs migrated in response to RANTES (CCL5) but not to SLC (CCL21), while LPS-stimulated DCs showed reduced capacity to respond to RANTES, and acquired the capacity to response to SLC. Although TPT-treated (D2-7) DCs, after LPS stimulation, maintained their chemotactic response to RANTES, they had markedly reduced capacity to migrate in response to SLC (P < .01-.001). Furthermore, TPT-treatment of LPS-stimulated DCs also retained RANTES-mediated calcium mobilization, but resulted in diminished SLC-induced calcium flux (Figure 5B). As expected, FACS analysis showed that following treatment with TPT (D2-7), LPS-stimulated DCs expressed persistent levels of CCR5 and failed to express elevated levels of CCR7, compared with LPS-stimulated mDCs (Figure 5C). Therefore, TPT treatment not only phenotypically and functionally suppressed DC maturation, but also altered their chemokine receptor expression and chemotactic response.
TPT impairs epidermal Langerhans cell migration ex vivo and in vivo
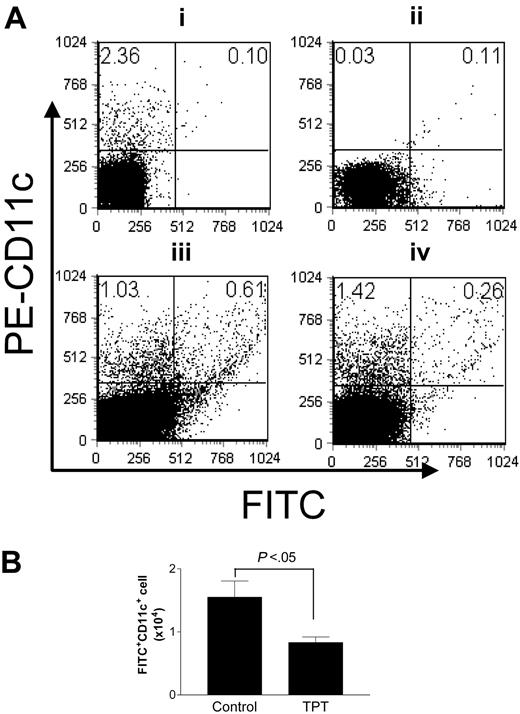
We next investigated the possibility that TPT treatment impaired the capacity of DCs to emigrate out of epidermis and immigrate into secondary lymphoid organs. SLC/CCL21 and MIP-3β/CCL19 have been shown to induce egress of LCs from explanted skin.23 We confirmed that murine MIP-3β/CCL19 was a potent chemoattractant for LCs in skin explant culture, as MIP-3β/CCL19 induced 7-fold more CD11c+ LCs to appear in the culture medium than in medium without CCL19. There was a concurrent reduction in the LC density in the epidermal sheet (data not shown). The addition of TPT to the skin explant culture resulted in retention of higher number of LCs in the epidermal sheet, while fewer LCs appeared in the culture medium (P < .05) than in the absence of TPT (Figure 6A-B), suggesting that TPT arrested LC emigration out of the skin. We then examined whether this also occurred in the in vivo setting. For this purpose we painted FITC on mouse ears, as FITC is a fluorescent marker for migratory LCs.30 TPT treatment did not reduce the frequency of LCs in the skin (data not shown). By 24 hours after treatment with FITC, the proportion of FITC+CD11c+ cells in the draining lymph nodes of untreated mice (0.61%) was markedly higher than in those of TPT-treated mice (0.26%; Figure 7A). The draining LNs of untreated mice contained more FITC+CD11c+ cells (1.55 ± 0.58 × 104) than TPT-treated mice (0.83 ± 0.20 × 104, P < .05; Figure 7B). Therefore, treatment with TPT reduced DC migration to the draining LNs.
Inhibition of maturation of DCs by TPT. (A) Human monocytes were cultured for 7 days with 50 ng/mL GM-CSF and 50 ng/mL IL-4 in the presence of TPT (D2-7, 2.5 nM; TPT-DC) or medium alone (Control-DC). Cells were stained with the designated mAb and analyzed by FACS. A typical experiment from at least 5 independent experiments with similar result is shown. Open profiles show staining with an isotype control, and black profiles show staining pattern with mAbs of the indicated specificity. (B) Human monocytes were cultured for 7 days with 50 ng/mL GM-CSF and 50 ng/mL IL-4 in the presence of TPT (TPT[D2-7], 2.5 nM) or medium alone (control). After thorough washing, the medium was restored in the presence of 10 ng/mL of LPS without TPT (columns 1 and 2). In another setting, control DCs were suspended in medium containing 10 ng/mL LPS supplemented with 2.5 nM of TPT (TPT (D8-9); column 3). After 48 hours of incubation, cells were labeled with the designated mAb and analyzed by FACS. Data shown are representative of at least 5 experiments with similar results. Open profiles show staining of LPS-stimulated DCs with an isotype control, black profiles show staining patterns with mAbs of the indicated specificity. For comparison, the staining pattern for day-7 cultured DCs was shown by dashed profiles. (C) Human monocytes were cultured with GM-CSF and IL-4 for 7 days. Then, the cells were simulated with sCD40L (300 ng/mL) or TNF (10 ng/mL), or poly (I:C) (12.5 μg/mL), in the presence of medium alone or TPT (2.5 nM). After 48 hours of incubation, cells were labeled with the designated mAb and analyzed by FACS. Data shown are representative of 3 experiments with similar results. Gray profiles show staining pattern of untreated DCs with mAbs of the indicated specificity, open profiles show staining pattern of TPT(D8-9)–treated DCs with indicated mAbs, and dashed profiles show isotype control.
Inhibition of maturation of DCs by TPT. (A) Human monocytes were cultured for 7 days with 50 ng/mL GM-CSF and 50 ng/mL IL-4 in the presence of TPT (D2-7, 2.5 nM; TPT-DC) or medium alone (Control-DC). Cells were stained with the designated mAb and analyzed by FACS. A typical experiment from at least 5 independent experiments with similar result is shown. Open profiles show staining with an isotype control, and black profiles show staining pattern with mAbs of the indicated specificity. (B) Human monocytes were cultured for 7 days with 50 ng/mL GM-CSF and 50 ng/mL IL-4 in the presence of TPT (TPT[D2-7], 2.5 nM) or medium alone (control). After thorough washing, the medium was restored in the presence of 10 ng/mL of LPS without TPT (columns 1 and 2). In another setting, control DCs were suspended in medium containing 10 ng/mL LPS supplemented with 2.5 nM of TPT (TPT (D8-9); column 3). After 48 hours of incubation, cells were labeled with the designated mAb and analyzed by FACS. Data shown are representative of at least 5 experiments with similar results. Open profiles show staining of LPS-stimulated DCs with an isotype control, black profiles show staining patterns with mAbs of the indicated specificity. For comparison, the staining pattern for day-7 cultured DCs was shown by dashed profiles. (C) Human monocytes were cultured with GM-CSF and IL-4 for 7 days. Then, the cells were simulated with sCD40L (300 ng/mL) or TNF (10 ng/mL), or poly (I:C) (12.5 μg/mL), in the presence of medium alone or TPT (2.5 nM). After 48 hours of incubation, cells were labeled with the designated mAb and analyzed by FACS. Data shown are representative of 3 experiments with similar results. Gray profiles show staining pattern of untreated DCs with mAbs of the indicated specificity, open profiles show staining pattern of TPT(D8-9)–treated DCs with indicated mAbs, and dashed profiles show isotype control.
Discussion
TPT is a potent immunosuppressive compound isolated from a traditional Chinese medicine. Mechanistic studies on its immunosuppressive action have generally focused on its inhibition of T cells. This study was aimed at defining a possible new cellular target of this immunosuppressive compound by examining whether TPT exerts its effects on DCs. The present study, for the first time, clearly demonstrates that TPT inhibited the phenotypic and functional maturation, cytokine production, and chemotactic response of DCs in vitro and in vivo.
Chemokine receptors are important in regulating DC localization and homing.31 Immature DCs express CXC chemokine receptor 1 (CXCR1), CCR1, CCR2, and CCR5, which confer responsiveness to inflammatory chemokines and direct the migration of DCs toward peripheral inflammatory sites.32,33 Upon exposure to maturation-inducing stimuli, such as inflammatory cytokines and products of pathogens, DCs down-regulate CCR1 and CCR5, up-regulate CCR7 expression, and acquire responsiveness to CCL19/MIP-3β and CCL21/SLC, thus enabling emigration of DCs out of the epidermis into secondary lymphoid organs.34,35 The interaction of DCs with naive T cells in secondary lymphoid organs is pivotal to the activation of T cells and initiation of adaptive immune response against exogenous antigens.31,36,37 Deficiencies or alterations in chemokine receptor expression have been shown to profoundly affect immunity.38 TPT-treated DCs exhibited normal chemotactic response to RANTES (Figure 5A-B), suggesting that DC infiltration into tissues may be unaffected. This is supported by our observation that TPT-treated mice had a similar frequency of LCs in the epidermis of the skin compared with normal control mice (data not shown). The chemotactic response of LPS-stimulated DCs to SLC was impaired after TPT treatment (Figure 5A-B), as a consequence of reduced expression of CCR7 (Figure 5C). Furthermore, egress of LCs from cultured skin explant and hapten-stimulated migration of LCs to the draining lymph nodes were each inhibited by TPT treatment (Figures 6, 7). This suggests that TPT treatment reduces interactions between DCs and T cells in secondary lymphoid organs, resulting in suppression of T-cell–mediated immune response.
Inhibition of TPT on the allostimulatory activity of DCs and LPS-stimulated DCs. (A) Human monocytes were cultured with 50 ng/mL GM-CSF and 50 ng/mL IL-4 in the presence of TPT (TPT[D2-7], 2.5 nM) or medium alone (control). After 7 days, DCs were extensively washed, irradiated (30 Gy [3000 rad]), and added at different concentrations to 1 × 105/well purified allogeneic responder T cells in a 96-well flat-bottom plates. (B) In a second type of experiment, TPT2-treated DCs (TPT[D2-7], 2.5 nM) and control DCs were washed and cultured for another 48 hours with LPS (10 ng/mL). Aliquots of control DCs were cultured with LPS in the presence of TPT (TPT[D8-9], 2.5 nM). After 48 hours, DCs were thoroughly washed and irradiated (30 Gy [3000 rad]), and added in graded doses to 1 × 105/well purified allogeneic responder T cells in a 96-well flat-bottom plates. Each group was tested in triplicate, and thymidine incorporation was measured on day 5 by a 15-hour pulse with [3H] thymidine. The data shown are representative of 3 independent experiments with similar results. Error bars indicate SD.
Inhibition of TPT on the allostimulatory activity of DCs and LPS-stimulated DCs. (A) Human monocytes were cultured with 50 ng/mL GM-CSF and 50 ng/mL IL-4 in the presence of TPT (TPT[D2-7], 2.5 nM) or medium alone (control). After 7 days, DCs were extensively washed, irradiated (30 Gy [3000 rad]), and added at different concentrations to 1 × 105/well purified allogeneic responder T cells in a 96-well flat-bottom plates. (B) In a second type of experiment, TPT2-treated DCs (TPT[D2-7], 2.5 nM) and control DCs were washed and cultured for another 48 hours with LPS (10 ng/mL). Aliquots of control DCs were cultured with LPS in the presence of TPT (TPT[D8-9], 2.5 nM). After 48 hours, DCs were thoroughly washed and irradiated (30 Gy [3000 rad]), and added in graded doses to 1 × 105/well purified allogeneic responder T cells in a 96-well flat-bottom plates. Each group was tested in triplicate, and thymidine incorporation was measured on day 5 by a 15-hour pulse with [3H] thymidine. The data shown are representative of 3 independent experiments with similar results. Error bars indicate SD.
Alteration of LPS-stimulated DC chemotaxis, calcium mobilization, and chemokine receptor expression by TPT treatment. (A) Effects of TPT treatment on DCs and LPS-stimulated DC chemotactic response to RANTES and SLC. Chemokine (RANTES and SLC) was placed in the lower well of chemotaxis chamber. DC suspension was placed in the upper well of the chemotaxis chamber. Polycarbonate filters separated the upper and lower wells. After incubation, the cells that migrated across the filters were stained and counted. The open bars show data from untreated control group; black bars, data from the TPT (D2-7)–treated group. Comparison with untreated control: *P < .05; **P < .01; ***P < .001. Error bars indicate SD. (B) Effects of TPT (D2-7) treatment on RANTES- or SLC-mediated calcium mobilization by DCs and LPS-stimulated DCs. The cultured DCs and LPS-stimulated DCs were loaded with Furo-2 and stimulated with 500 ng/mL of RANTES or SLC. The ratio of fluorescence at 340- and 380-nm wavelengths was recorded and calculated using FL Win Lab program (Perkin-Elmer Life and Analytical Sciences). Arrows indicate addition of stimuli. (C) Effects of TPT (D2-7) treatment on CCR5 and CCR7 expression by Day 9 LPS-stimulated DCs. For cells labeled with mAb or isotype IgG, the CCR5 or CCR7 expression was analyzed by FACS. Dashed-line histogram shows isotype control; gray histogram shows untreated control of LPS-stimulated DCs, and solid-line histogram shows LPS-stimulated DCs which were pretreated with TPT(D2-7). The data are representative of 3 separate experiments with similar results.
Alteration of LPS-stimulated DC chemotaxis, calcium mobilization, and chemokine receptor expression by TPT treatment. (A) Effects of TPT treatment on DCs and LPS-stimulated DC chemotactic response to RANTES and SLC. Chemokine (RANTES and SLC) was placed in the lower well of chemotaxis chamber. DC suspension was placed in the upper well of the chemotaxis chamber. Polycarbonate filters separated the upper and lower wells. After incubation, the cells that migrated across the filters were stained and counted. The open bars show data from untreated control group; black bars, data from the TPT (D2-7)–treated group. Comparison with untreated control: *P < .05; **P < .01; ***P < .001. Error bars indicate SD. (B) Effects of TPT (D2-7) treatment on RANTES- or SLC-mediated calcium mobilization by DCs and LPS-stimulated DCs. The cultured DCs and LPS-stimulated DCs were loaded with Furo-2 and stimulated with 500 ng/mL of RANTES or SLC. The ratio of fluorescence at 340- and 380-nm wavelengths was recorded and calculated using FL Win Lab program (Perkin-Elmer Life and Analytical Sciences). Arrows indicate addition of stimuli. (C) Effects of TPT (D2-7) treatment on CCR5 and CCR7 expression by Day 9 LPS-stimulated DCs. For cells labeled with mAb or isotype IgG, the CCR5 or CCR7 expression was analyzed by FACS. Dashed-line histogram shows isotype control; gray histogram shows untreated control of LPS-stimulated DCs, and solid-line histogram shows LPS-stimulated DCs which were pretreated with TPT(D2-7). The data are representative of 3 separate experiments with similar results.
The inhibition by TPT of costimulatory molecules expressed by DCs provides a basis for the immunosuppressive effects of TPT, given the critical role of costimulatory signals delivered by CD40, CD80, and CD86 for optimal T-cell activation.39,40 Disrupting the CD40-CD154 pathway40 and/or blocking the CD80/CD86-CD28 pathway39 has been shown to be effective in several models of autoimmune diseases and allograft rejection. This suggested that the immunosuppressive activity exerted by TPT in autoimmune diseases and allograft rejection models9,10 may at least in part be due to down-regulation of costimulatory molecule expression. Interestingly, TPT inhibited LPS-mediated up-regulation of costimulatory molecules when given during the induction stage (D2-7) and in the stimulation stage (D8-9; Figure 3B). TPT has been shown to inhibit nuclear factor–κB (NF-κB) activation in T and B lymphocytes,41 gastric cancer cells,42 and human bronchial epithelial cells.43 The inhibitory action of immunosuppressive drugs on DC function has been associated with Janus kinase 2/signal transducer and transcriptional activator 4 (Jak2/Stat4) signaling pathway,44 NF-κB, and mitogen-activated protein kinase (MAPK) pathway signaling,45 suggesting that TPT's ability to inhibit the NF-κB pathway could contribute to its inhibition of DC function. This notion is supported by our observation that TPT inhibited DC maturation induced by a number of stimuli (Figure 3C). Since TPT (D2-7) treatment also inhibits LPS-mediated DC maturation, the pretreatment of TPT might result in a profound inhibition of NF-κB that cannot be reversed by LPS stimulation. Certain cytokines, such as transforming growth factor β (TGFβ)46 and IL-10,47 have the capacity to suppress DC maturation. However, those immunosuppressive cytokines could not play a role in TPT-mediated suppression of DC maturation, since the production of TGFβ and IL-10 was also inhibited by the treatment of TPT (data not shown).
TPT inhibits epidermal Langerhans cell migration from skin explants. The ear halves from groups of 5 mice were cultured in 24-well plates as described in “Materials and methods” (with 100 ng/mL murine MIP-3β) and contained 2.5 nM TPT or medium alone. (A) 48 hours later, epidermal sheets were prepared from the ear halves and immunolabeled with FITC–anti–I-A/I-E antibody. Epidermal sheets were observed and photographed under a fluorescence microscope (magnification, × 40). (B) FACS analysis of LC egress from cultured ear halves. Cells in culture medium of the ear halves were collected and stained with PE–anti-CD11c and resuspended in 0.3 mL PBS and analyzed on FACScan for 50 seconds. The data are representative of 3 independent experiments with similar results. Error bars indicate standard deviation.
TPT inhibits epidermal Langerhans cell migration from skin explants. The ear halves from groups of 5 mice were cultured in 24-well plates as described in “Materials and methods” (with 100 ng/mL murine MIP-3β) and contained 2.5 nM TPT or medium alone. (A) 48 hours later, epidermal sheets were prepared from the ear halves and immunolabeled with FITC–anti–I-A/I-E antibody. Epidermal sheets were observed and photographed under a fluorescence microscope (magnification, × 40). (B) FACS analysis of LC egress from cultured ear halves. Cells in culture medium of the ear halves were collected and stained with PE–anti-CD11c and resuspended in 0.3 mL PBS and analyzed on FACScan for 50 seconds. The data are representative of 3 independent experiments with similar results. Error bars indicate standard deviation.
Dendritic cells with an immature DC phenotype, as assessed by low expression of major histocompatibility complex (MHC) class II, CD40, CD80, CD86, and IL-12 are tolerogenic.48 Tolerogenic DCs have been generated by immunosuppressive drugs such as LF 15-0195 (in combination with anti-CD45RB),49 vitamin D3 in combination with mycophenolate mofetil,49 and dexamethasone.50 These drugs also have the potential to generate CD4+CD25+ T-regulatory cells.49 We therefore examined the effects of TPT on the generation of CD4+CD25+ T-regulatory cells. The result showed that CD4 T cells coincubated with TPT-treated DCs for 5 days failed to express an elevated level of forkhead box P3 (FoxP3; data not shown). Furthermore, in vivo treatment of TPT (1 mg/kg/d intraperitoneally for 1-5 days) did not increase the proportion of CD4+CD25+ T cells in mouse peripheral lymphoid organs (data not shown). Therefore, the tolerogenic effect of TPT-treated DCs was not based on the induction of CD4+CD25+ T-regulatory cells.
TPT inhibits epidermal Langerhans cell migration in vivo. (A) A lower frequency of hapten-bearing cells were detected in the draining LNs of TPT-treated mice. The mice were treated with TPT (0.1 mg/kg/d, intraperitoneally) or vehicle alone for 5 days. On the last day of TPT treatment, all mice were painted on the dorsum of their ears with 10 μL 1% FITC in acetone/dibutylphalate (1:1). 24 hours later, auricular LNs (ALNs) were taken, and single-cell suspensions were prepared, stained with PE–anti-CD11c, and then analyzed on FACScan. (Ai) ALN cells from naive mice, staining with PE-CD11c. (Aii) ALN cells from FITC-painting mice. (Aiii) ALN cells from untreated mice stained with PE-CD11c. (Aiv) ALN cells from TPT-treated mice stained with PE-CD11c. Numbers indicate percentage of cells present in quadrants. (B) TPT-treatment reduced the FITC-bearing cell number in the draining LNs. The FITC-bearing cell number was calculated from the percentage of FITC+ cells and total ALN cell number. The data are representative of 3 independent experiments with similar results. Error bars indicate SD.
TPT inhibits epidermal Langerhans cell migration in vivo. (A) A lower frequency of hapten-bearing cells were detected in the draining LNs of TPT-treated mice. The mice were treated with TPT (0.1 mg/kg/d, intraperitoneally) or vehicle alone for 5 days. On the last day of TPT treatment, all mice were painted on the dorsum of their ears with 10 μL 1% FITC in acetone/dibutylphalate (1:1). 24 hours later, auricular LNs (ALNs) were taken, and single-cell suspensions were prepared, stained with PE–anti-CD11c, and then analyzed on FACScan. (Ai) ALN cells from naive mice, staining with PE-CD11c. (Aii) ALN cells from FITC-painting mice. (Aiii) ALN cells from untreated mice stained with PE-CD11c. (Aiv) ALN cells from TPT-treated mice stained with PE-CD11c. Numbers indicate percentage of cells present in quadrants. (B) TPT-treatment reduced the FITC-bearing cell number in the draining LNs. The FITC-bearing cell number was calculated from the percentage of FITC+ cells and total ALN cell number. The data are representative of 3 independent experiments with similar results. Error bars indicate SD.
A safe and effective dose of TPT to treat rheumatoid arthritis, according to the Bureau of Chinese Medicine Administration, is in the range of 0.5 to 5 μg/kg/d. This dose range is roughly equivalent to 1.5 to 15 nM in vitro.26 Therefore, the concentrations (ie, 0.5-10 nM) we used in the present study match clinically effective doses. TPT inhibits multiple key functions of DCs including differentiation, maturation, allostimulation, cytokine production, and migration.
While we were completing this report, Liu and colleagues reported that up to 10 ng/mL (27.8 nM) TPT failed to affect the phenotype differentiation and LPS-induced maturation of murine bone marrow–derived DCs, but dramatically reduced cell recovery by inducing apoptosis of DCs.27 This differs from our observation, perhaps because we tested the effect of TPT on human monocyte–derived DCs rather than mouse bone marrow–derived DCs, since the sensitivity to cytotoxic effects of immunosuppressive agents of DCs has been reported to be precursor dependent.51 However, in Liu et al's paper, 1 ng/mL (2.8 nM) of TPT was also not cytotoxic,27 which agrees with our finding that 2.5 nM of TPT had no significant cytotoxic effects on DCs.
In conclusion, our data clearly show that nontoxic doses of TPT suppresses DC maturation and trafficking. These data provide new insight into the immunopharmacology of TPT and suggest a novel approach to the manipulation of DCs for therapeutic and experimental application.
Prepublished online as Blood First Edition Paper, June 14, 2005; DOI 10.1182/blood-2005-03-0854.
Supported in part with federal funds from the National Cancer Institute under contract no. N01-CO-012400, and by the Intramural Research Program of the National Institutes of Health (NIH) National Cancer Center for Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the US government.


![Figure 3. Inhibition of maturation of DCs by TPT. (A) Human monocytes were cultured for 7 days with 50 ng/mL GM-CSF and 50 ng/mL IL-4 in the presence of TPT (D2-7, 2.5 nM; TPT-DC) or medium alone (Control-DC). Cells were stained with the designated mAb and analyzed by FACS. A typical experiment from at least 5 independent experiments with similar result is shown. Open profiles show staining with an isotype control, and black profiles show staining pattern with mAbs of the indicated specificity. (B) Human monocytes were cultured for 7 days with 50 ng/mL GM-CSF and 50 ng/mL IL-4 in the presence of TPT (TPT[D2-7], 2.5 nM) or medium alone (control). After thorough washing, the medium was restored in the presence of 10 ng/mL of LPS without TPT (columns 1 and 2). In another setting, control DCs were suspended in medium containing 10 ng/mL LPS supplemented with 2.5 nM of TPT (TPT (D8-9); column 3). After 48 hours of incubation, cells were labeled with the designated mAb and analyzed by FACS. Data shown are representative of at least 5 experiments with similar results. Open profiles show staining of LPS-stimulated DCs with an isotype control, black profiles show staining patterns with mAbs of the indicated specificity. For comparison, the staining pattern for day-7 cultured DCs was shown by dashed profiles. (C) Human monocytes were cultured with GM-CSF and IL-4 for 7 days. Then, the cells were simulated with sCD40L (300 ng/mL) or TNF (10 ng/mL), or poly (I:C) (12.5 μg/mL), in the presence of medium alone or TPT (2.5 nM). After 48 hours of incubation, cells were labeled with the designated mAb and analyzed by FACS. Data shown are representative of 3 experiments with similar results. Gray profiles show staining pattern of untreated DCs with mAbs of the indicated specificity, open profiles show staining pattern of TPT(D8-9)–treated DCs with indicated mAbs, and dashed profiles show isotype control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2005-03-0854/6/m_zh80190584800003.jpeg?Expires=1769080694&Signature=CpuppP4AaLK0yf2L95wS0~Z1TC~OAKkTXXZzr5pFO7LfLslPUN-3rF6LdeeDnbB1xbBWmT7AyKZxFg2TOXUkCtgnmcQckrxyOQ8SDoxrl1m-54nxwWWroUyCSMiAozYS1lWW6vN79-Rj0eZWHd8KQj08acVvVLyd9-LUW1Ua7Oy~wu-qNfQQSHe8etY-jr~uWe0vcgWUMh9H2n3B2YKRQbUqngyx0OkTZdwFORUC~6ZzZw72EhtPghHY7J9~L~JLQQbELWtZ-DPsdEevfsPjuSB42kwS4TSidYg9fiWCmJO-in0fTKS~ErzoO4fUqq5uM4CcA2myyRwaE4ao0-V1hA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Inhibition of TPT on the allostimulatory activity of DCs and LPS-stimulated DCs. (A) Human monocytes were cultured with 50 ng/mL GM-CSF and 50 ng/mL IL-4 in the presence of TPT (TPT[D2-7], 2.5 nM) or medium alone (control). After 7 days, DCs were extensively washed, irradiated (30 Gy [3000 rad]), and added at different concentrations to 1 × 105/well purified allogeneic responder T cells in a 96-well flat-bottom plates. (B) In a second type of experiment, TPT2-treated DCs (TPT[D2-7], 2.5 nM) and control DCs were washed and cultured for another 48 hours with LPS (10 ng/mL). Aliquots of control DCs were cultured with LPS in the presence of TPT (TPT[D8-9], 2.5 nM). After 48 hours, DCs were thoroughly washed and irradiated (30 Gy [3000 rad]), and added in graded doses to 1 × 105/well purified allogeneic responder T cells in a 96-well flat-bottom plates. Each group was tested in triplicate, and thymidine incorporation was measured on day 5 by a 15-hour pulse with [3H] thymidine. The data shown are representative of 3 independent experiments with similar results. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2005-03-0854/6/m_zh80190584800004.jpeg?Expires=1769080694&Signature=gTsh0SdPuXmWVN0Dh3kxZDQz6yfNf6qS4l25eF9cki3iWDw4LD6T6bvvM7np12H~kP2XzsI93SJa~pv44xYQxXBpSQi8~VCNVadk38mWBKWnCdh4bDO18e~q9jp83dRgr2GYR1Ajnzybjzw-HhLVV6D1IAZMFqQpXB2cmvi75V~pMUUFvQu~NMu5s2CXrZGMrWq-BiHpRgIL2BEGEMPptJOvZSUhlFFwtNBfnr934NDAhjXe~mGofhBg0vZ9xqjxJkRPYjI7Qi-k~yfWxa9Omp5aUm55wOj3STU~b8QvtTegmZFxNOP~73Hm5DijnlUCc7vo9VvE7OqU80BBqYGAVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal