Abstract
In the new World Health Organization/European Organization for Research and Treatment of Cancer (WHO/EORTC) classification of cutaneous lymphomas, large B-cell lymphomas (LBCLs) are divided into 3 groups: LBCL, leg-type (LBCLLT); follicle center lymphoma, diffuse type (FCLDT); and LBCL, others (LBCLO). We studied a large number of primary cutaneous LBCLs to test the validity of the classification and to identify prognostic factors for these patients. Ninety-three cases of primary cutaneous LBCL were analyzed for clinicopathologic features, expression of several markers including Bcl-2, Bcl-6, MUM-1, and FOX-P1, in situ hybridization for Epstein-Barr virus, and molecular analyses of IGH gene rearrangement and of Borrelia burgdorferi and human herpesvirus 8 DNA. Patients were classified into the following categories: FCLDT, 44 cases; LBCLLT, 40 cases; and LBCLO, 9 cases. Statistical analyses showed that the LBCLLT and FCLDT groups were clearly distinct in terms of clinicopathologic features and survival. The LBCLO group had features in between those of LBCLLT and FCLDT. Our study shows that accurate morphologic and phenotypic analyses allow us to stratify most patients into the prognostically different categories of LBCLLT and FCLDT. The definition of a third category of LBCLO requires further studies to clarify whether these cases indeed show distinct clinicopathologic features. (Blood. 2005;106:2491-2497)
Introduction
In the new World Health Organization/European Organization for Research and Treatment of Cancer (WHO/EORTC) classification of cutaneous lymphomas, large B-cell lymphomas (LBCLs) are divided into 3 groups. Large B-cell lymphoma, leg-type (LBCLLT) is defined as a cutaneous B-cell lymphoma with a predominance of large round cells (centroblasts, immunoblasts) that are positive for Bcl-2.1 Cases of LBCL with a predominant cleaved cell morphology (large centrocytes) are included within the follicle center lymphomas, diffuse type (FCLDT). These cases would be included in the categories of diffuse LBCL in the WHO classification of tumors of hematopoietic and lymphoid tissues2 and of cutaneous follicle center cell lymphomas in the first EORTC classification of primary cutaneous lymphomas, respectively.3 Rare other cases not fitting within these 2 categories are included in a group of large B-cell lymphoma, other (LBCLO). Although the new WHO/EORTC classification is the result of a consensus among representatives of the WHO and EORTC groups, at present there are no clinicopathologic data supporting the inclusions of primary cutaneous LBCLs into the 3 groups. In addition, prognostic factors for these patients are largely unknown.
Recently, we could confirm the clinical value of the classification of cutaneous lymphomas proposed in 1997 by the EORTC.4 In the present study, we analyzed data on a large group of patients with primary cutaneous LBCL, classifying patients according to the new WHO/EORTC classification, and evaluating the prognostic value of several factors including classification, age of patients, anatomic site of onset, number of lesions at presentation, cell morphology, and expression of Bcl-2, multiple myeloma-1 (MUM-1), and Forkhead box-P1 (FOX-P1) proteins.
Patients, materials, and methods
Biopsy specimens from 93 cases of primary cutaneous LBCL were retrieved from the database files of the Division of Dermatopathology, Department of Dermatology, Medical University of Graz. The cases had been registered between 1960 and 2004. Primary cutaneous LBCL was defined as large cells constituting more than 80% of the infiltrate3 and absence of extracutaneous dissemination after staging investigations.1,4 Staging procedures were performed with standard methods available at time of first diagnosis including, when applicable, physical examination, blood-cell count, chest radiograph, computed tomographic scan, abdominal ultrasound sonography, sonography of superficial lymph nodes, and bone marrow biopsy. Unfortunately, we do not have exact data on therapy for all patients. First-line treatment strategies, however, were similar, with radiotherapy generally preferred to chemotherapy, with the exception of cases presenting with disseminated skin involvement.
The histopathologic features were reviewed by at least 3 independent investigators (K.K., C.M., and L.C.). We only included cases characterized by diffuse infiltrates of large cells with morphologic features of large centrocytes, centroblasts, or immunoblasts. Cases with a follicular growth pattern were excluded, as well as cases with the presence of remnants of lymphoid follicles as detected by immunohistologic staining for follicular dendritic cells (CD21). Cases arising in immunosuppressed individuals were excluded as well.
All cases were classified according to the new WHO/EORTC classification of cutaneous lymphomas.1 FCLDT was defined as a tumor with predominance of large cleaved cells, irrespective of Bcl-2 expression. LBCLLT was defined as a tumor with predominance of large round cells and positivity for Bcl-2. LBCLO was defined as a tumor with predominance of large round cells and absence of Bcl-2 expression.
Histology and immunohistochemistry
Biopsy specimens were fixed in 10% buffered formalin and subsequently embedded in paraffin. Sections were stained with hematoxylin and eosin and Giemsa stains for routine histopathologic evaluation. Detailed immunophenotypic analysis was performed on routinely fixed, paraffin-embedded tissue sections according to a standard immunoperoxidase technique, using a broad panel of monoclonal antibodies including CD20, CD21, CD30, CD138, Bcl-2, Bcl-6, MIB-1, MUM-1, and ALK-1 (all from Dakopatts, Glostrup, Denmark), and CD3 and CD10 (Novocastra, Newcastle upon Tyne, United Kingdom). The FOX-P1 antibody was kindly provided by Dr Alison Banham (Nuffield Department of Clinical and Laboratory Sciences, Oxford, United Kingdom). Second and third antibodies were obtained from Dakopatts. Heat-induced antigen retrieval with microwave was used for all of the antibodies.
Staining for CD10, Bcl-2, and Bcl-6 was scored as positive when the majority of the neoplastic cells expressed the protein. Similarly to a previous study,5 staining for FOX-P1 was scored into 3 groups as follows: positive (nearly all tumor cells showed strong, uniform expression of the protein), +/- (weak expression in a variable proportion of tumor cells), and negative (only occasional positive cells observed). The staining for MUM-1 was evaluated in a manner similar to that of FOX-P1.
Histopathologic and immunophenotypic sections were photographed with a Nikon Eclipse E1000M microscope (Nikon, Tokyo, Japan), using a Plan Apo 100×/1.40 NA objective (histologic sections) or a Plan Apo 40×/0.95 NA objective (immunohistologic sections) with the Nikon digital net DN100 camera system. Pictures were edited for contrast only using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Molecular biology
Polymerase chain reaction (PCR) analyses of DNA of Borrelia burgdorferi and human herpes virus 8 (HHV-8), and of immunoglobulin heavy chain (IGH) gene were performed as described previously.6-8
Oligonucleotide primers for B burgdorferi PCR. Oligonucleotide primers for PCR amplification reactions were designed by Wienecke et al9 on the basis of the B burgdorferi-specific gene described by Rosa et al.10 The outer primer pair (Bb1 and Bb2) is flanking a 171-base pair (bp) fragment (nucleotide position/np 143-np313), whereas the inner primer pair (Bb 3 and Bb4) spans a 92-bp amplification product (np160-np251). For internal hybridization control, oligo Bb-hyb, annealing between nucleotides 182 and 217, was used.
PCR amplification. For analysis of B burgdorferi DNA we used a nested PCR technique.7 The 2-step nested PCR procedure substantially increases both sensitivity and specificity of the assay. An aliquot of the first PCR product, produced by the outer primer pair, is amplified by an internal set of primers annealing to B burgdorferi-specific sequences. In this way only B burgdorferi-specific PCR products obtained in the first assay are further amplified, considerably increasing the specificity of the technique. Briefly, 3 μL of each DNA sample served as template for PCR. The reaction cocktail (25 μL) contained 10 mM Tris (tris(hydroxymethyl)aminomethane)-HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 200 μM each of desoxyribonucleotide triphosphate (dATP, dCTP, dGTP, dTTP), 1 U Taq Gold DNA polymerase (Perkin Elmer, Shelton, CT), and 0.2 μM each of the outer primers Bb1 and Bb2. After initial denaturation at 95°C for 12 minutes, samples were subjected to 40 cycles of PCR at 95°C for 1 minute, 50°C for 30 seconds, and 72°C for 30 seconds. Subsequently, 2 μL external PCR product was submitted to nested PCR for 40 cycles using inner primers Bb3 and Bb4 under the same temperature profile as just noted. Inner PCR products were loaded on an ethidium bromide-stained 2.5% agarose gel and visualized by UV light.
For detection of HHV-8 DNA sequences, a nested PCR technique was used as follows. In the first step, a 233-bp product was amplified using the KS330233 primers.11 In the second step, an additional internal 160-bp fragment was amplified using primers NS1 and NS2.12 The β-actin gene was successfully amplified in all cases. Specificity of PCR products was confirmed by P32 Southern blot hybridization using a 25-bp internal oligonucleotide probe as described previously.11
The IGH gene was analyzed using a seminested PCR technique as described by Wan et al13 with minor modifications.7 For the first PCR, 1 μL template DNA was used in a 50-μL PCR containing desoxyribonucleotide triphosphate (dATP, dCTP, dGTP, dTTP; 200 μM each), primers Fr3A and LJH (0.1 μM each), MgCl2 (1.0 mM), KCl (50 mM), and Tris-HCl, pH 8.3 (10 mM). After initial denaturation at 94°C for 5 minutes, 30 cycles of the reaction were carried out (denaturation: 94°C, 60 seconds; annealing: 57°C, 60 seconds; extension: 72°C, 60 seconds) followed by a 7-minute final extension step at 72°C. A second PCR was performed using 1 μL from the first PCR as template in 25 μL of the same buffer except that primer VLJH was used instead of LJH. Twenty-five cycles were carried out using the same temperature profile as in the first PCR.
For visualization, a 10-μL aliquot of the PCR products was applied to a commercially available gel (GeneGel Excel 12.5 cm/24 slots; Amersham Biosciences, Little Chalfont, United Kingdom) and run for 1 hour at 15°C. The running conditions were as follows: voltage 600 V, current 25 mA, power 15 W. Finally, the gels were stained using a DNA silver staining kit (Amersham Biosciences).
The β-actin gene was analyzed as amplification control in all cases.
In situ hybridization for EBV
In situ hybridization for Epstein-Barr virus (EBV) was performed according to standard procedures using the EBV-encoded RNA (EBER-1) in situ hybridization kit by Kreatech Diagnostic (Amsterdam, The Netherlands).
Statistical analyses
Statistical testing with χ2 analysis was used to examine relationships between variables. The paired t test was used to analyze age differences between the groups. Survival time was defined as the time from first diagnosis until death or last follow-up. For evaluation of disease-specific survival death unrelated to lymphoma was not considered an event and was censored. Survival curves were estimated by the Kaplan-Meier method, using log rank to analyze the differences between groups.
Results
Classification of patients
Ninety-three patients were included in the study (42 men, 51 women; median age, 71 years; range, 33-98 years). Cases were classified into the following groups.
FCLDT. Forty-four patients (25 men, 19 women; median age, 66.5 years; range, 33-89 years) were found. In 5 cases, tumors were located solely on the leg, 15 on the back, 9 on the head-neck, 6 on the trunk, 1 on the upper extremities, 1 on the buttock, and 7 at multiple sites (head-neck, upper extremities, and trunk, 4; leg and upper extremities, 1; leg, head-neck, upper extremities, and trunk, 1; leg and buttocks, 1; Figure 1). A single lesion was observed in 12 patients, whereas 32 had multiple lesions.
LBCLLT. Forty patients (15 men, 25 women; median age, 79 years; range, 46-98 years) were classified in this group. In 32 cases, tumors were located on the leg, 2 on the back, 1 on the head-neck, 3 on the trunk, and 2 at multiple sites (trunk and upper extremities, 1; leg, trunk, and upper extremities, 1; Figure 2). Twenty patients presented with a single lesion, the other 20 had multiple lesions.
LBCLO. Nine patients (2 men, 7 women; median age, 70 years; range, 58-86 years) were found in this group. In 5 patients, tumors were located only on the leg, 1 on the head-neck, 1 on the trunk, 1 on the upper extremities, and 1 at multiple sites (leg and head-neck). Lesions were solitary in 7 patients and multiple in 2 patients.
Immunohistology and molecular biology findings
All cases were positive for CD20 and negative for CD3. All tested cases were negative for anaplastic lymphoma kinase 1 (ALK-1) and CD138 (LBCLLT 0 of 29 and 0 of 33, FCLDT 0 of 15 and 0 of 17, LBCLO 0 of 5 and 0 of 7, respectively). CD30 was focally positive only in 2 cases of LBCLLT (LBCLLT 2 of 33, FCLDT 0 of 33, LBCLO 0 of 7). Proliferation rate measured by MIB-1 antibody was high in the majority of cases irrespective of classification (LBCLLT: mean, 71.3%, median, 70%, range 5%-90%; FCLDT: mean, 55.3%, median 60%, range 5%-90%; LBCLO: mean, 62.7%, median, 80%, range, 5%-90%). Expression of CD10, Bcl-2, Bcl-6, MUM-1, and FOX-P1 in the different groups is summarized in Table 1 and shown further in Figures 3, 4, 5.
Comparison of cases classified according to the WHO/EORTC classification
. | LBCLLT . | LBCLO . | FCLDT . |
|---|---|---|---|
| Sex, M:F | 0.6:1 | 0.3:1 | 1.3:1 |
| 5-y survival, % | |||
| Disease-specific | 61.7 | 50.0 | 86.7 |
| Overall | 52.9 | 50.0 | 77.5 |
| 10-y survival, % | |||
| Disease-specific | 0 | 50.0 | 86.7 |
| Overall | 0 | 50.0 | 66.5 |
| Disease-specific survival according to diagnostic category, P | — | .04* | — |
| Overall survival according to diagnostic category, P | — | .03† | — |
| Median age, y | 79 | 70 | 66.5 |
| Disease-specific survival according to age older than 70 y versus age 70 or younger, P | .8 | NA | .03 |
| Solitary/multiple lesions | 20/20 | 7/2 | 12/32 |
| Disease-specific survival according to number of lesions, P | .5 | NA | .6 |
| Site | |||
| Leg/total (%) | 32/40 (80) | 5/9 (55.6) | 5/44 (11.4) |
| Disease-specific survival according to anatomic site (leg vs not the leg), P | .6 | NA | .02 |
| Bcl-2 | |||
| +/total (%) | 40/40 (100) | 0/9 (0) | 10/44 (22.7) |
| Disease-specific survival according to Bcl-2 expression, P | — | — | P>.999 |
| FOX-P1 | |||
| +/total (%) | 23/29 (72.4) | 3/6 (50) | 4/40 (10) |
| Disease-specific survival according to FOX-P1 expression, P | .5 | NA | .6 |
| MUM-1 | |||
| +/total (%) | 22/29 (75.9) | 4/6 (66.7) | 3/43 (7) |
| Disease-specific survival according to MUM-1 expression, P | .9 | NA | .07 |
| Bcl-6/CD10 expression | |||
| Bcl-6, +/total (%) | 30/40 (75) | 7/7 (100) | 41/44 (93.2) |
| CD10, +/total (%) | 2/33 (6.1) | 1/7 (14.3) | 7/39 (17.9) |
| Bcl-6−/CD10− phenotype, +/total (%) | 10/40 (25) | 0/7 (0) | 3/44 (6.8) |
| Disease-specific survival according to CD10/Bcl-6 expression, P | .5 | NA | .2 |
. | LBCLLT . | LBCLO . | FCLDT . |
|---|---|---|---|
| Sex, M:F | 0.6:1 | 0.3:1 | 1.3:1 |
| 5-y survival, % | |||
| Disease-specific | 61.7 | 50.0 | 86.7 |
| Overall | 52.9 | 50.0 | 77.5 |
| 10-y survival, % | |||
| Disease-specific | 0 | 50.0 | 86.7 |
| Overall | 0 | 50.0 | 66.5 |
| Disease-specific survival according to diagnostic category, P | — | .04* | — |
| Overall survival according to diagnostic category, P | — | .03† | — |
| Median age, y | 79 | 70 | 66.5 |
| Disease-specific survival according to age older than 70 y versus age 70 or younger, P | .8 | NA | .03 |
| Solitary/multiple lesions | 20/20 | 7/2 | 12/32 |
| Disease-specific survival according to number of lesions, P | .5 | NA | .6 |
| Site | |||
| Leg/total (%) | 32/40 (80) | 5/9 (55.6) | 5/44 (11.4) |
| Disease-specific survival according to anatomic site (leg vs not the leg), P | .6 | NA | .02 |
| Bcl-2 | |||
| +/total (%) | 40/40 (100) | 0/9 (0) | 10/44 (22.7) |
| Disease-specific survival according to Bcl-2 expression, P | — | — | P>.999 |
| FOX-P1 | |||
| +/total (%) | 23/29 (72.4) | 3/6 (50) | 4/40 (10) |
| Disease-specific survival according to FOX-P1 expression, P | .5 | NA | .6 |
| MUM-1 | |||
| +/total (%) | 22/29 (75.9) | 4/6 (66.7) | 3/43 (7) |
| Disease-specific survival according to MUM-1 expression, P | .9 | NA | .07 |
| Bcl-6/CD10 expression | |||
| Bcl-6, +/total (%) | 30/40 (75) | 7/7 (100) | 41/44 (93.2) |
| CD10, +/total (%) | 2/33 (6.1) | 1/7 (14.3) | 7/39 (17.9) |
| Bcl-6−/CD10− phenotype, +/total (%) | 10/40 (25) | 0/7 (0) | 3/44 (6.8) |
| Disease-specific survival according to CD10/Bcl-6 expression, P | .5 | NA | .2 |
—indicates not applicable; NA, not assessed (group too small for statistical analysis).
LBCLLT versus FCLDT: P = .02; LBCLLT vs LBCLO: P = .6; FCLDT vs LBCLO: P = .7.
LBCLLT vs FCLDT: P = .01; LBCLLT vs LBCLO: P = .5; FCLDT vs LBCLO: P = .7.
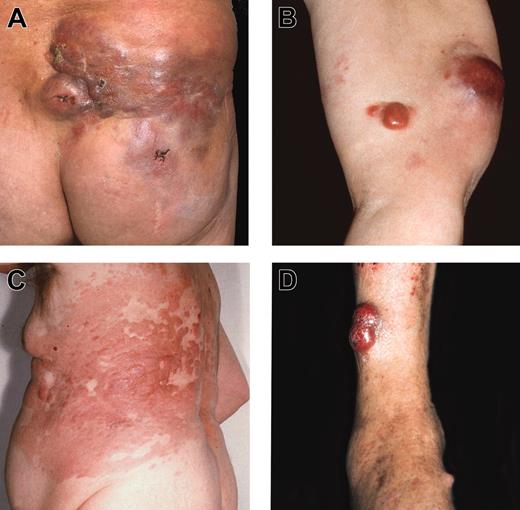
Various clinical features of FCLDT. (A) Multiple red-brown plaques and large tumors on the lower back and buttock; (B) 2 distinct reddish tumors on the upper arm; (C) confluent erythematous plaques and flat tumors involving large part of the trunk (“Crosti lymphoma”); (D) confluent reddish tumors on the lower leg.
Various clinical features of FCLDT. (A) Multiple red-brown plaques and large tumors on the lower back and buttock; (B) 2 distinct reddish tumors on the upper arm; (C) confluent erythematous plaques and flat tumors involving large part of the trunk (“Crosti lymphoma”); (D) confluent reddish tumors on the lower leg.
PCR analysis of IGH gene rearrangement performed in 33 cases revealed a monoclonal band in 23 cases (FCLDT, 9; LBCLLT, 14) and a polyclonal smear in 10 cases (FCLDT, 7; LBCLLT, 3). In situ hybridization for EBV and PCR analysis of B burgdorferi and HHV-8 DNA showed only one case of LBCLLT positive for HHV-8 (EBV: LBCLLT 0 of 36; FCLDT, 0 of 19; LBCLO, 0 of 7; B burgdorferi: LBCLLT 0 of 33, FCLDT 0 of 19, LBCLO, 0 of 4; HHV-8: LBCLLT 1 of 33; FCLDT 0 of 18; LBCLO 0 of 4).
Follow-up
Follow-up data were available for 75 patients (FCLDT: n = 37, median follow-up time, 47 months; LBCLLT: n = 31, median follow-up time, 16 months; LBCLO: n = 7, median follow-up time, 20 months). Fifteen patients died of disease after a period varying between 6 and 93 months (median, 12 months; FCLDT: n = 4, 8-15 months, median: 12.5 months; LBCLLT: n = 10, 6-93 months, median, 12.5 months; LBCLO: n = 1, 36 months). Five died of unrelated causes after 35 to 165 months (median, 48 months; FCLDT: n = 4, 47-165 months, median, 83 months; LBCLLT: n = 1, 35 months). Thirty-four patients are alive with disease after 1 to 241 months (median, 21.5 months; FCLDT: n = 15, 2-241 months, median, 63 months; LBCLLT: n = 14, 2-59 months, median, 15.5 months; LBCLO: n = 5, 1-39 months, median, 20 months). Twenty-one are alive and well after 3 to 166 months (median, 37 months; FCLDT: n = 14, 2-166 months, median, 48.5 months; LBCLLT: n = 6, 10-73 months, median, 31 months; LBCLO: n = 1, 17 months).
Summary of prognostic features in cutaneous LBCLs
Parameter . | 5-y disease-specific survival, % . | 10-y disease-specific survival, % . | P . |
|---|---|---|---|
| Round cells predominate (LBCLLT+LBCLO) | 58.2 | 0 | — |
| Cleaved cells predominate (FCLDT) | 86.7 | 86.7 | .02 |
| Age older than 70 y | 61.4 | 46.0 | — |
| Age 70 y or younger | 88.7 | 88.7 | .007 |
| Site: leg | 52.6 | 0 | — |
| Site: not leg or multiple | 84.9 | 84.9 | .001 |
| No. of lesions | |||
| 1 | 72.3 | 72.3 | — |
| 2 | 75.8 | 68.2 | 1 |
| Bcl-2+ | 66.6 | 44.4 | — |
| Bcl-2− | 84.6 | 84.6 | .05 |
| FOX-P1+ | 57.9 | 43.4 | — |
| FOX-P1− | 85.9 | 85.9 | .03 |
| MUM-1+ | 52.4 | 52.4 | — |
| MUM-1− | 84.8 | 77.8 | .05 |
| CD10 and/or Bcl-6 positive | 77.9 | 70.8 | — |
| CD10 and Bcl-6 both negative | 57.8 | 57.8 | .2 |
Parameter . | 5-y disease-specific survival, % . | 10-y disease-specific survival, % . | P . |
|---|---|---|---|
| Round cells predominate (LBCLLT+LBCLO) | 58.2 | 0 | — |
| Cleaved cells predominate (FCLDT) | 86.7 | 86.7 | .02 |
| Age older than 70 y | 61.4 | 46.0 | — |
| Age 70 y or younger | 88.7 | 88.7 | .007 |
| Site: leg | 52.6 | 0 | — |
| Site: not leg or multiple | 84.9 | 84.9 | .001 |
| No. of lesions | |||
| 1 | 72.3 | 72.3 | — |
| 2 | 75.8 | 68.2 | 1 |
| Bcl-2+ | 66.6 | 44.4 | — |
| Bcl-2− | 84.6 | 84.6 | .05 |
| FOX-P1+ | 57.9 | 43.4 | — |
| FOX-P1− | 85.9 | 85.9 | .03 |
| MUM-1+ | 52.4 | 52.4 | — |
| MUM-1− | 84.8 | 77.8 | .05 |
| CD10 and/or Bcl-6 positive | 77.9 | 70.8 | — |
| CD10 and Bcl-6 both negative | 57.8 | 57.8 | .2 |
— indicates not applicable.
Statistical analyses
Statistical analyses are summarized in Tables 1 and 2. The sex distribution between LBCLLT and FCLDT did not reach statistical significance (χ2 3.135, P = .077). The age distribution was significantly different between these 2 groups (t value 5.510, P < .001), as well as the location on the leg or at other anatomic sites (χ2 40.050, P < .001). Expressions of MUM-1 and FOX-P1 were clearly different in the 2 groups (χ2 36.259 and 33.907, respectively; P < .001). Expression of Bcl-6 was of borderline significance (χ2 3.926; P = .048), and that of CD10 was not significantly different between the 2 groups (χ2 2.310, P = .129). Bcl-2 expression was highly different between the 2 groups (χ2 51.9, P = .001), due also to the definition of LBCLLT as a Bcl-2+ tumor.
Comparison of the LBCLO group with the other 2 revealed differences between LBCLO and LBCLLT concerning the age (t value: 2.622, P = .031) and no statistically significant differences concerning sex distribution, anatomic site, and FOX-P1, MUM-1, Bcl-6, and CD10 expression. Although sex distribution and Bcl-6 and CD10 expression were not statistically different between the groups of LBCLO and FCLDT, the groups differed concerning age (t value 2.808, P = .023), anatomic site (χ2 9.532, P = .002), FOX-P1 expression (χ2 6.470, P = .011), and MUM-1 expression (χ2 15.320, P < .001).
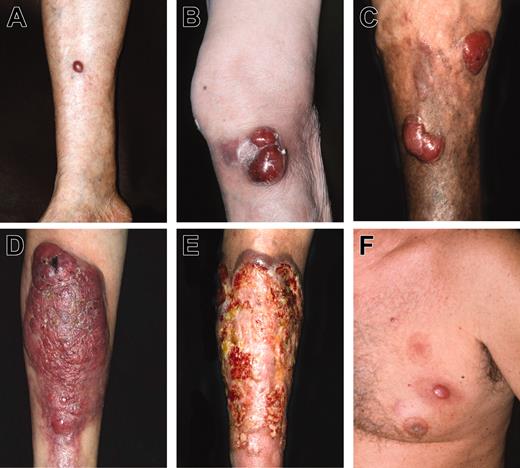
Different morphologic expressions of LBCLLT. (A) Small solitary tumor on the lower leg; (B) confluent large tumors on knee; (C) distinct large tumors on the lower leg on the background of chronic venous insufficiency; (D) large tumor involving almost the entire frontal aspect of the lower leg; (E) large ulcerated lesion involving almost the entire lower leg; (F) erythematous tumor and small plaque on the chest.
Different morphologic expressions of LBCLLT. (A) Small solitary tumor on the lower leg; (B) confluent large tumors on knee; (C) distinct large tumors on the lower leg on the background of chronic venous insufficiency; (D) large tumor involving almost the entire frontal aspect of the lower leg; (E) large ulcerated lesion involving almost the entire lower leg; (F) erythematous tumor and small plaque on the chest.
Discussion
In this study, we clearly demonstrated that the WHO/EORTC classification is applicable to the great majority of patients with cutaneous LBCL. The difference in survival between the groups of LBCLLT and FCLDT is statistically significant (P = .02), confirming the clinical value of the stratification of patients with primary cutaneous LBCL into these 2 groups. Sex distribution between the 2 groups was dissimilar (although did not reach significance); age distribution, anatomic site, and FOX-P1, MUM-1, and Bcl-6 protein expression were significantly different, supporting the concept that the 2 groups represent indeed different entities. In terms of clinicopathologic features, phenotype, and prognosis it seems that LBCLO represents an intermediate group between cases of LBCLLT and those of FCLDT (with more similarities to the first than to the second), and at present it is unclear whether LBCLO represents truly a distinct group of cutaneous LBCLs, or if it should be considered as either a phenotypic (Bcl-2-) variant of the LBCLLT or as a morphologic (round cell) variant of the FCLDT. In fact, it seems likely that the LBCLO group consists of phenotypic or morphologic variations (or both) of both LBCLLT and FCLDT.
Although the vast majority (90.3%) of the cases could be classified as either LBCLLT or FCLDT, it should be mentioned that a few of the patients showed overlapping clinicopathologic features. In fact, 3 patients with FCLDT had lesions located solely on the leg and were Bcl-2+/MUM-1- (2 cases) or Bcl-2-/MUM-1+ (1 case), thus showing similarities to LBCLLT. On the other hand, 3 patients with LBCLLT had lesions located at sites other than the legs and were MUM-1-, thus showing similarities to FCLDT. The existence of such overlapping features underlines the difficulty of classifying accurately some of the cases.
In the past, prognosis of cutaneous LBCLs has been linked to anatomic site (location on the leg versus not on the leg), age at onset, and number of lesions at presentation in one large study,14 and to expression of Bcl-2 in another study.15 In both studies, cases with cleaved and round cell morphology were lumped together. On the other hand, location on the leg and Bcl-2 expression had no prognostic value in another study that lumped together different groups of cutaneous LBCLs.16 In our study, the number of lesions had no prognostic value, neither overall nor in the specific diagnostic groups. Analysis of all patients with primary cutaneous LBCLs showed that prognosis was strongly linked to age at onset, anatomic site, cell morphology, and Bcl-2, MUM-1, and FOX-P1 expression. Older age had a prognostic implication when all LBCLs were analyzed together, consistent with previous results by Grange et al14 and Goodlad et al.17 When age was analyzed separately for the groups of LBCLLT and FCLDT, however, it had no statistically significant prognostic value for the LBCLLTs, although it retained a significant value for FCLDTs. This discrepancy is most likely related to the different median ages of the 2 groups because 82.5% of LBCLLT patients were 71 years or older at first diagnosis.
Lesions of LBCLLT were indeed located solely on the legs in the great majority of cases (80%), thus justifying the term “large B-cell lymphoma of the leg” proposed by Vermeer et al in 199618 and adopted in the EORTC classification of cutaneous lymphomas in 1997,3 and of LBCLLT introduced in the new WHO/EORTC classification.1 The term LBCLLT is better than the old denomination of “large B-cell lymphoma of the leg,” because it reflects more accurately the predominant but not exclusive anatomic location of these tumors. However, tumors confined to the legs only were found also in 55.6% and 11.4% of LBCLOs and FCLDTs, respectively, confirming that the anatomic site alone is not sufficient for classification of the cases. In addition, in 5 further patients lesions were located both on the legs and at other anatomic sites (FCLDT, 6.8%; LBCLLT, 2.5%; LBCLO, 11.1%). Interestingly, location on the legs only had a strong prognostic value when all LBCLs were considered together and had statistical significance for the group of FCLDT as well, suggesting that for cases of FCLDT located on the legs the anatomic site may be more important than morphologic-phenotypic classification when assessing prognosis.
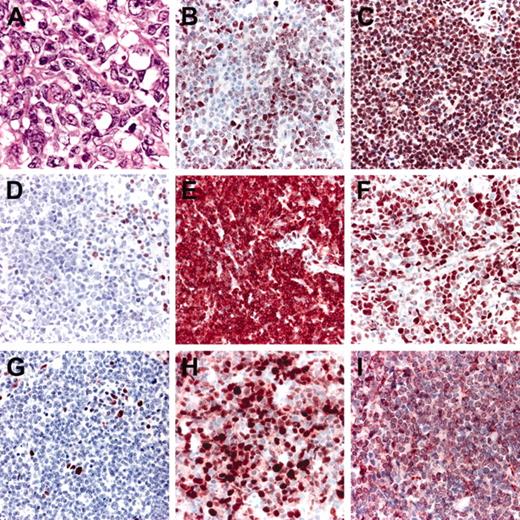
Histopathologic features and phenotypic variations of FCLDT. (A) Large cleaved cells predominate; (B) variable, weak expression of FOX-P1; (C) strong, uniform expression of FOX-P1; (D) negativity for Bcl-2; please note internal positive controls; (E) strong positivity for Bcl-2; (F) positive staining for Bcl-6; (G) staining for MUM-1 showing only scattered positive cells; (H) strong positivity for MUM-1 in the great majority of neoplastic cells; (I) expression of CD10 by the large lymphocytes.
Histopathologic features and phenotypic variations of FCLDT. (A) Large cleaved cells predominate; (B) variable, weak expression of FOX-P1; (C) strong, uniform expression of FOX-P1; (D) negativity for Bcl-2; please note internal positive controls; (E) strong positivity for Bcl-2; (F) positive staining for Bcl-6; (G) staining for MUM-1 showing only scattered positive cells; (H) strong positivity for MUM-1 in the great majority of neoplastic cells; (I) expression of CD10 by the large lymphocytes.
Similar to the study by Grange et al,15 Bcl-2 expression had a bad prognostic implication when cases were not stratified into precise diagnostic categories. However, it did not retain a statistically significant value when the analysis was performed separately for the 2 groups with cleaved (FCLDT) and round cell morphology (including LBCLLTs and LBCLOs). Thus, it does not seem that Bcl-2 expression has any prognostic value in cases that are appropriately classified. Interestingly, although in a previous report Bcl-2 expression was strictly related to the anatomic site (cases on the legs were always positive, whereas those on the head-neck or trunk always negative),19 we did not find such a strict association, because 19% of cases with tumors located only on the legs were Bcl-2-, and 28.9% of those with tumors located only on the head-neck or trunk were Bcl-2+. Thus, it does not seem that expression of this protein is strictly related to any anatomic site.
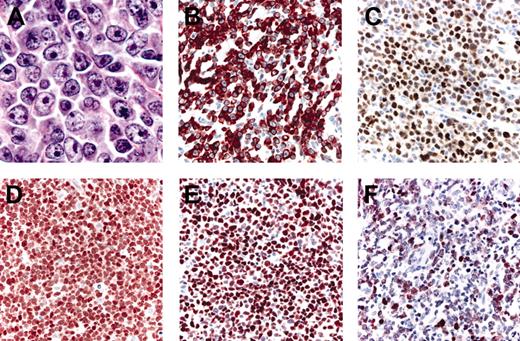
Histopathologic features and phenotypic variations of LBCLLT. (A) Large round cells predominate; (B) strong positivity for Bcl-2; (C) positive staining for Bcl-6; (D) strong, uniform expression of FOX-P1; (E) strong, uniform positivity for MUM-1; (F) staining for MUM-1 showing only a weak expression of the protein.
Histopathologic features and phenotypic variations of LBCLLT. (A) Large round cells predominate; (B) strong positivity for Bcl-2; (C) positive staining for Bcl-6; (D) strong, uniform expression of FOX-P1; (E) strong, uniform positivity for MUM-1; (F) staining for MUM-1 showing only a weak expression of the protein.
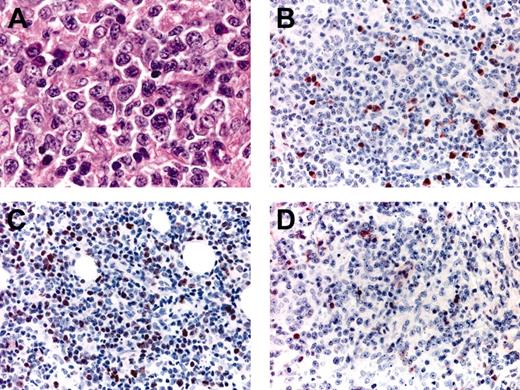
Histopathologic features and phenotypic variations of LBCLO. (A) Large round cells predominate; (B) only a proportion of the large cells are positive for MUM-1; (C) variable expression of Bcl-6 in the majority of neoplastic cells; (D) negative staining for Bcl-2; please note positive internal controls (small lymphocytes).
Histopathologic features and phenotypic variations of LBCLO. (A) Large round cells predominate; (B) only a proportion of the large cells are positive for MUM-1; (C) variable expression of Bcl-6 in the majority of neoplastic cells; (D) negative staining for Bcl-2; please note positive internal controls (small lymphocytes).
MUM-1/IRF4 is a transcription factor that is considered to play a crucial role in lymphoid differentiation and development. In normal lymphoid tissue, it is mainly expressed by plasma cells and a subset of germinal center B lymphocytes. MUM-1 expression was found in all cases of cutaneous LBCL of the leg classified according to the first EORTC classification in one study,20 and in the new WHO/EORTC classification of cutaneous lymphomas it is suggested that MUM-1 expression is found constantly in LBCLLT and is absent in FCLDT.1 In our study, specificity and sensitivity of MUM-1 expression for diagnosis of LBCLLT were 85.7% and 75.9%, respectively. Strong MUM-1 positivity was found in 7% of cases of FCLDT, showing that expression of this marker is not constantly absent in this diagnostic group. In addition, 7 cases of LBCLLT (24.1%) showed a lower degree of positivity for MUM-1, similar to that found in 13 cases of FCLDT (30.2%). Thus, although strong positivity for MUM-1 is mostly consistent with a diagnosis of LBCLLT or LBCLO, a weaker expression can be observed in all diagnostic groups. Strong MUM-1 expression was linked to a worse prognosis when all cases of LBCL were analyzed together, most likely due to the predominant expression of this marker in cases with round cell morphology. Interestingly, a worse prognosis (though not statistically significant) was found also for MUM-1+ cases of FCLDT. In FCLDT, strong MUM-1 expression, like location on the leg, may point at a more aggressive behavior of the disease. The prognostic implications of these 2 parameters in patients with FCLDT underline once again the overlapping features of some cases of cutaneous LBCL.
FOX-P1 is a member of the FOX-P subfamily of transcription factors, and it has been demonstrated predominantly in the nongerminal center type of nodal diffuse LBCL. Previous studies in nodal lymphomas showed that FOX-P1 is expressed predominantly in cases with nongerminal center phenotype.21,22 Although expression of FOX-P1 had no prognostic value in one study,22 2 other reports found an association with a worse outcome.5,23 We found a strong FOX-P1 expression in 22 of our cases (FCLDT, 10%; LBCLLT, 72.4%; LBCLO, 50%). When all primary cutaneous LBCLs were analyzed together, strong expression of this protein was clearly linked to a worse prognosis. However, when data were analyzed separately for each diagnostic group, expression of the protein failed to reach a statistically significant prognostic value. Thus, it seems likely that in cutaneous LBCLs the prognostic value of FOX-P1 is mainly linked to the predominant expression of this protein in cases with round cell morphology.
Bcl-6 and CD10 are markers of germinal center B cells24,25 and have been used for characterization of LBCLs of germinal center origin in the appropriate context.26-29 We could demonstrate Bcl-6 positivity in the vast majority of cases, irrespective of anatomic site, cell morphology, and classification (FCLDT, 93.2%; LBCLLT, 75%; LBCLO, 100%). By contrast, CD10 was expressed only in a minority of cases and slightly more frequently in FCLDTs (FCLDT, 17.9%; LBCLLT, 6.1%; LBCLO, 14.3%). A similar phenotype was observed in a recent study by Hoefnagel et al.30 Thirteen of our cases were negative for both markers (FCLDT, 6.8%; LBCLLT, 25%). Interestingly, 2 of 3 cases of FCLDT that were CD10-/Bcl-6- were positive for MUM-1, suggesting an activated rather than germinal center B-cell phenotype, and confirming again that a few cases of FCLDT reveal overlapping phenotypic features with LBCLLT. A recent study on 14 patients with primary cutaneous LBCL suggested a favorable prognostic significance of Bcl-6 expression.31 In our study expression of Bcl-6 had no impact on prognosis overall and in the 2 groups of LBCLLT and FCLDT (data not shown), and the Bcl-6/CD10 double-negative phenotype had no prognostic implications as well, but especially for cases of FCLDT this may be due also to the small number of such cases.
The etiology of cutaneous LBCLs is unknown. We analyzed our cases for presence of infectious agents including HHV-8, EBV, and B burgdorferi, but could find only one case of LBCLLT positive for HHV-8. HHV-8 is well known as the causative agent of Kaposi sarcoma and it is detected in 95% to 100% of cases in this condition.32 Recently, HHV-8 has also been identified in cases of primary effusion lymphoma, which is a subtype of diffuse LBCL.33,34 B burgdorferi was demonstrated in 15% to 20% of cases of primary cutaneous BCL in 3 independent studies in the past,6,35,36 and EBV is a virus with a well-known association with some human lymphomas. However, our results suggest that these 3 microorganisms are not implicated in the pathogenesis of primary cutaneous LBCLs, irrespective of classification.
At extracutaneous sites, some authors showed the presence of a subgroup of diffuse LBCL positive for ALK-1 and negative for CD30.37 In our study we could find positivity for CD30 in 2 of 72 cases only (both of them were cases of LBCLLT showing focal positivity), and negativity for ALK-1 in all tested cases (49 of 49). Thus, these 2 antibodies have no relevance in diagnosis and classification of primary cutaneous LBCLs.
Recently, microarray studies suggested that nodal diffuse LBCLs can be subclassified according to the molecular signature of neoplastic cells.38,39 Studies of cases of cutaneous BCL showed that subdivision of primary cutaneous LBCLs into the groups of FCLDT and LBCLLT was supported by the molecular profile of neoplastic cells.40,41 Our findings clearly show that a clinically relevant classification of primary cutaneous LBCLs into 2 main groups (FCLDT, LBCLLT) can be achieved in most cases with morphologic and phenotypic analyses. Although prognosis is mainly related to specific classification, cases of FCLDT arising on the legs or showing an activated B-cell phenotype (strong MUM-1 expression or double negativity for CD10 and Bcl-6 or both) should be approached carefully because they may have a worse prognosis than other cases of this group. The definition of a third category of cutaneous LBCL characterized by predominance of round cells and absence of Bcl-2 expression, currently included into the group of LBCLO in the WHO/EORTC classification, requires further studies to clarify whether these cases show indeed peculiar clinicopathologic features or, more likely, represent only a morphologic or phenotypic variant of FCLDTs and LBCLLTs.
Prepublished online as Blood First Edition Paper, June 9, 2005; DOI 10.1182/blood-2005-03-1175.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to express our thank to Uli Schmidbauer, Ulrike Michelitsch, and Barbara Bäck for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal