Abstract
Aggregometry is widely used to assess platelet function, but its use in identifying platelet hyperreactivity is poorly defined. We studied platelet aggregation in 359 healthy individuals using the agonists adenosine diphosphate (ADP), epinephrine, collagen, collagen-related peptide, and ristocetin. We also assessed the reproducibility of these assays in 27 subjects by studying them repeatedly on at least 4 separate occasions. Healthy subjects exhibited considerable interindividual variability in aggregation response to agonists, especially at concentrations lower than those typically used in clinical laboratories. For each agonist tested at these submaximal concentrations, a small proportion of individuals demonstrated an unusually robust aggregation response. Subjects who exhibited such in vitro hyperreactivity to one agonist tended to demonstrate a similar response to others, suggesting that hyperreactivity is a global characteristic of platelets. Epinephrine and collagen-related peptide were especially reliable and efficient in detecting hyperreactivity. For epinephrine, excellent reproducibility persisted for up to 3 years, and hyperreactivity was associated with female sex and higher fibrinogen levels (P < .02). We recommend these assays as appropriate candidates for future studies requiring accurate assessment of increased platelet reactivity. These include clinical studies to improve risk assessment for arterial thrombosis, as well as genetic studies to establish determinants of the hyperreactive platelet phenotype.
Introduction
For decades, a standard approach for assessing platelet reactivity in both clinical and research settings has been aggregometry on platelet-rich plasma (PRP).1-3 Due in part to widely held concerns about interindividual variability in response to stimulation, many laboratories use relatively high concentrations of agonists in order to ensure that platelets from most healthy, drug-free subjects show a full or complete aggregation response4 (typically > 60%). This approach has proved quite useful for detecting gross platelet hypofunction, as in uncommon inherited platelet function disorders (eg, Glanzmann thrombasthenia and Bernard-Soulier syndrome)5,6 as well as the more common setting of antiplatelet therapy (eg, aspirin, clopidogrel, and glycoprotein IIb-IIIa [GPIIb-IIIa] blockers).7-10 What is not clear, however, is whether PRP aggregometry can be used to detect an abnormally increased response to agonists, which may be defined as platelet hyperreactivity. Previous work suggests a role for platelet hyperreactivity in the pathophysiology of occlusive arterial thrombi, such as myocardial infarction and stroke.11-15 Platelet hyperreactivity may also modify bleeding risk in hemostatic and other disorders16-18 through augmentation of primary and secondary hemostasis. Aggregation assays that can reliably assess such hyperreactivity could thus fill a great clinical need by providing accurate information on an individual's platelet phenotype and subsequent thrombotic or bleeding risk.
We studied a large group of healthy subjects using a panel of platelet aggregation assays designed to detect platelet hyperreactivity. We have determined that (1) certain apparently healthy individuals demonstrate consistent evidence of relative platelet hyperreactivity and (2) such individuals can be reliably and efficiently identified through use of aggregation assays under conditions not routinely used in clinical laboratory practice. These assays are appropriate candidates for measurement of phenotype when platelet hyperreactivity bears special relevance—for example, in populations of patients at risk for thrombosis and for studies of genetic and environmental influences on platelet function.
Patients, materials, and methods
Subject recruitment and sample collection
Healthy subjects were recruited through flyer postings and local print advertisement. Subjects were excluded if they were taking medications known to affect platelet function, including nonsteroidal anti-inflammatory drugs during the 48 hours prior to phlebotomy and aspirin. Medications not known to affect platelet function were permitted, but for the purpose of studying a relatively healthy population, subjects taking more than one prescription medication were excluded. After informed consent was obtained (in accordance with the Declaration of Helsinki), subjects were asked to fast overnight and to refrain from intensive exercise and tobacco use for 4 hours prior to an early-morning phlebotomy. After resting comfortably for at least 10 minutes, subjects were phlebotomized. Blood was collected through a 19-gauge needle into a syringe containing 3.8% sodium citrate after the first 2 mL was discarded. Demographic information was recorded for each subject based on information obtained via direct interview and a questionnaire (for females, phase of menstrual cycle was classified as described19 ). We conducted 2 distinct studies (each approved by the Baylor College of Medicine institutional review board). In the platelet reactivity (PR) study, 359 subjects were studied once using the assays described in “Plasma assays” (recruitment between July 2001 and September 2004). In the reproducibility study (RS), 27 subjects (a subset of the PR group) were studied on a weekly basis for 4 weeks using a similar panel of assays (recruitment between February and June 2004).
Plasma assays
Reagents. Lyophilized collagen (soluble calf skin) was obtained from Bio/Data (Horsham, PA). Collagen-related peptide (CRP) was synthesized at Baylor College of Medicine and cross-linked with glutaraldehyde. All other reagents were from Sigma-Aldrich (St Louis, MO). We took careful measures to maintain long-term quality control of reagents, instruments, and techniques (eg, purchasing reagents of the same lot number to use throughout the study whenever possible, daily quality control of instruments) so that results could be optimally compared over time.
Platelet aggregation. Blood was centrifuged for 15 minutes at 150g to obtain PRP. PRP platelet count was standardized to between 200 000 and 250 000 platelets/μL with autologous platelet-poor plasma (obtained by centrifuging PRP at 1500g for 10 minutes). Platelet aggregometry was performed by a single operator on a Bio/Data 4-channel platelet aggregometer using techniques based on the method of Born.20 Incubation time was 3 minutes at 37°C, stir bar speed was 1200 rpm, and sample run time was 10 minutes after addition of agonist (20 minutes for spontaneous aggregation). Based on pilot studies directed at detecting platelet hyperreactivity, we tested aggregation response to agonists at various concentrations: no agonist (spontaneous), epinephrine (0.1, 0.2, 0.4, 1.5, 3.0, and 10.0 μM), collagen (2.5, 5, 10, 20, and 50 μg/mL), CRP (0.005, 0.01, 0.02, and 0.05 μg/mL), adenosine diphosphate (ADP; 0.05, 0.5, 1, 4, and 20 μM), and ristocetin (0.1, 0.5, 0.75, 1.0, and 1.5 mg/mL). CRP was not included until 2003, such that fewer PR subjects were studied with this agonist. Collected data included maximal aggregation (%), slope of the aggregation curve, and elapsed time between addition of agonist and onset of aggregation (lag phase). All aggregation studies were completed within 2 hours of phlebotomy.
Fibrinogen assay. Fibrinogen levels were measured using the Clauss method21 on a Dade Behring (New Castle, DE) BCS machine according to the manufacturer's instructions.
Statistical analysis
SPSS version 12.0 was used for all data analyses (SPSS, Chicago, IL). All data were examined using histograms and scatter plots. Within-subject reproducibility of aggregation data over time was assessed visually and through calculation of the intraclass correlation coefficient (ICC).22 An ICC value equal to 1 indicates perfect reproducibility; values of 0.75 or greater are considered to signify excellent reproducibility.23 The chi-squared test and Student t test were used to compare categoric and continuous variables, respectively, between groups. The Spearman rank correlation coefficient24 was calculated from RS data to assess the correlation between different assay results. P values less than .05 were considered significant.
Results
In the PR study, we established platelet phenotypes (based on a single phlebotomy) by studying platelet aggregation in 359 healthy adults under uniform conditions. Demographic characteristics of this study group are listed in Table 1. Since this study was designed to identify determinants of platelet hyperreactivity, we made extensive use of agonist concentrations lower than those typical for PRP aggregometry (“Patients, materials, and methods”). At these “submaximal” agonist concentrations, we sought to identify individuals whose platelets demonstrated an abnormally robust (hyperreactive) response to stimulation.
Demographic information
Subject characteristic . | PR cohort . | RS cohort . |
|---|---|---|
| No. | 359 | 27 |
| Mean age, y, ± SD | 34.2 ± 10.4 | 31.8 ± 7.9 |
| Women, % | 66.4 | 70.4 |
| Race, % | ||
| African-American | 39.8 | 14.8 |
| White | 36.4 | 55.6 |
| Hispanic | 16.0 | 14.8 |
| Asian | 6.7 | 14.8 |
| Other | 1.1 | 0.0 |
| Mean body mass index, kg/m2, ± SD | 26.7 ± 5.8 | 24.4 ± 4.4 |
| Women on oral contraceptives, % | 24.9 | 36.8 |
| Premenopausal women, % | 81.9 | 89.5 |
| Premenopausal women in luteal phase, % | 57.8 | 50.0 |
| Current smokers, % | 11.8 | 18.5 |
| Subjects with hypertension, % | 5.0 | 3.7 |
Subject characteristic . | PR cohort . | RS cohort . |
|---|---|---|
| No. | 359 | 27 |
| Mean age, y, ± SD | 34.2 ± 10.4 | 31.8 ± 7.9 |
| Women, % | 66.4 | 70.4 |
| Race, % | ||
| African-American | 39.8 | 14.8 |
| White | 36.4 | 55.6 |
| Hispanic | 16.0 | 14.8 |
| Asian | 6.7 | 14.8 |
| Other | 1.1 | 0.0 |
| Mean body mass index, kg/m2, ± SD | 26.7 ± 5.8 | 24.4 ± 4.4 |
| Women on oral contraceptives, % | 24.9 | 36.8 |
| Premenopausal women, % | 81.9 | 89.5 |
| Premenopausal women in luteal phase, % | 57.8 | 50.0 |
| Current smokers, % | 11.8 | 18.5 |
| Subjects with hypertension, % | 5.0 | 3.7 |
No subjects had diabetes.
Variability in platelet aggregation—existence of a hyperreactive phenotype
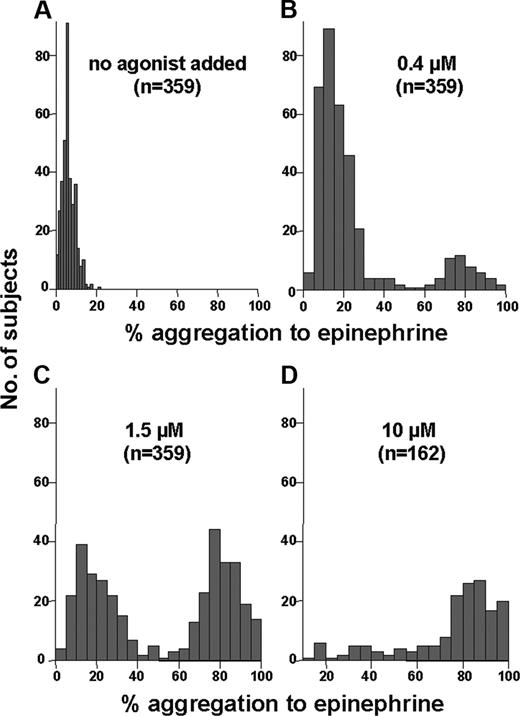
Most subjects exhibited very little spontaneous aggregation (Figure 1A; mean, 6.1%) and only a modest level of interindividual variability was observed (range, 0%-21%). Figure 1B-D show the effects of increasing concentrations of epinephrine on aggregation response. At the low epinephrine concentration of 0.4 μM, although the vast majority of healthy subjects exhibit relatively little platelet aggregation (< 40%), a significant minority (14% of the PR group) demonstrates more than 60% aggregation, consistent with a relatively hyperreactive phenotype (Figure 1B). With increasing epinephrine concentration (Figure 1C-D), the proportion of subjects who showed more than 60% aggregation steadily increased. Considerable interindividual variability was observed among our 359 healthy subjects, with a range of 1% to 100% aggregation at each of the low, intermediate, and high concentrations of epinephrine. Few subjects demonstrated aggregation in the 40% to 60% range at any concentration, suggesting that less than 40% and more than 60% represent appropriate criteria for distinguishing between little or absent aggregation and full or complete aggregation.
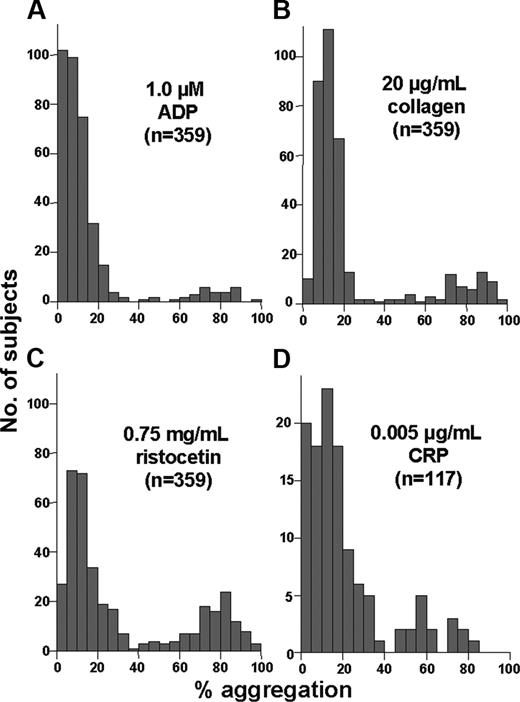
The histograms in Figure 1B-D are similar to those obtained using low, intermediate, and high concentrations respectively, of ADP, collagen, CRP, and ristocetin (data not shown). Figure 2 shows the submaximal concentrations of these other agonists at which a minority of subjects (7%-26%) exhibited atypically robust aggregation (> 60%). At agonist concentrations lower than those shown in Figure 2, very few subjects exhibited more than 60% aggregation (data not shown).
Reproducibility of the hyperreactive phenotype
Given previous widely held concerns about day-to-day variability of PRP aggregometry results, we assessed the reproducibility of these findings by performing these assays repeatedly over 4 consecutive weeks in a subset (n = 27) of the PR study group. Demographic characteristics of this cohort are listed in Table 1. Specifically, we sought to determine whether individuals exist who demonstrate hyperreactivity (ie, the minority depicted in Figures 1B and 2A-D) on a consistent basis and whether our assays could be used to identify such individuals.
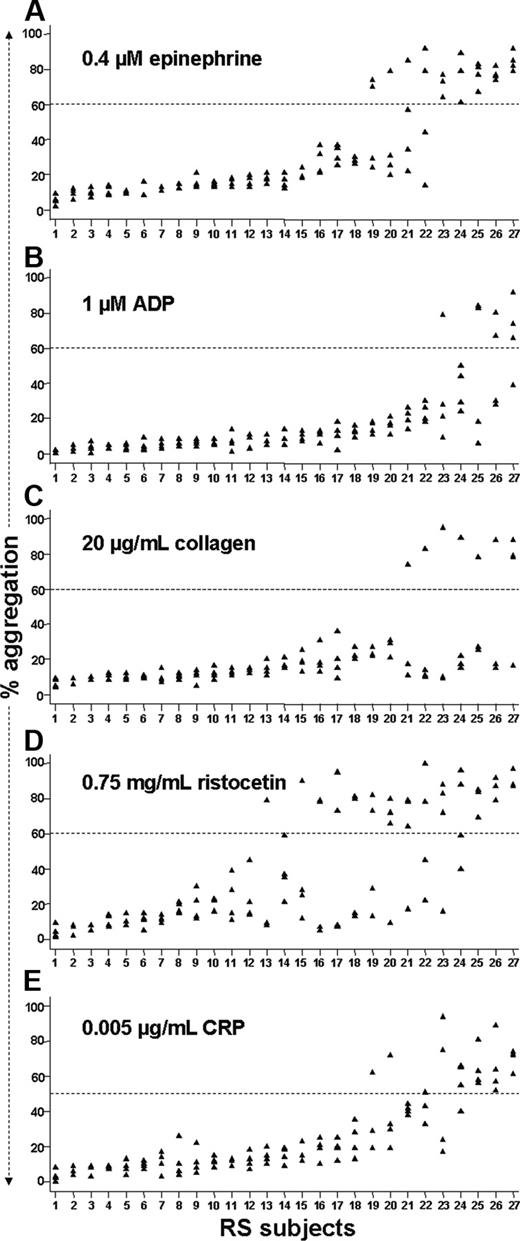
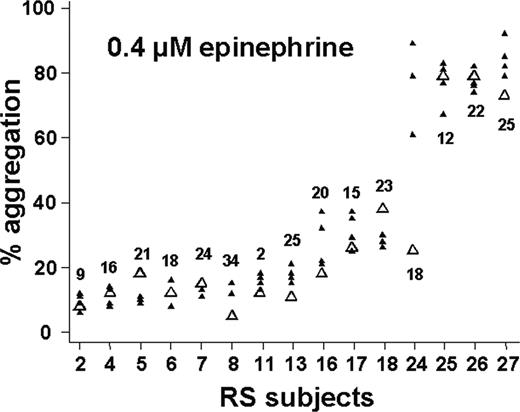
Figure 3 shows the results of our reproducibility study at submaximal agonist concentrations. In response to 0.4 μM epinephrine (Figure 3A), 5 of 27 subjects exhibited more than 60% aggregation on each of 4 occasions; even the minimum aggregation level obtained for each of these individuals allows a clear distinction between these relative hyperresponders and most of the other subjects. The efficiency of this assay for identifying such relative hyperreactivity appears quite good, as only 7 subjects demonstrated greater than 60% aggregation more than once (ie, 5 of 7 subjects who showed > 60% aggregation on at least 2 occasions showed relative hyperreactivity on every occasion). Eighteen of 27 subjects had less than 40% aggregation on all 4 occasions. Therefore, at 0.4 μM epinephrine, 23 (85%) of 27 subjects exhibited extremely consistent results, in keeping with the ICC of 0.81 calculated for this assay. Moreover, when we analyzed available data from the 15 RS subjects who had had aggregometry performed previously as part of the PR study, we found that these results were reproducible over a much longer period of time: 14 of 15 subjects showed results very consistent with prior testing an average of 19 months previously (range, 2-34 months) (Figure 4).
Distribution of aggregation response to increasing epinephrine concentrations among healthy individuals. These histograms depict the number of subjects (y-axis) with a given level of platelet aggregation (x-axis) to no agonist (A) and epinephrine at concentrations of 0.4 μM (B), 1.5 μM (C), and 10 μM (D). Panel D shows fewer subjects because specimens were not tested at higher concentrations if more than 60% aggregation was observed at lower concentrations.
Distribution of aggregation response to increasing epinephrine concentrations among healthy individuals. These histograms depict the number of subjects (y-axis) with a given level of platelet aggregation (x-axis) to no agonist (A) and epinephrine at concentrations of 0.4 μM (B), 1.5 μM (C), and 10 μM (D). Panel D shows fewer subjects because specimens were not tested at higher concentrations if more than 60% aggregation was observed at lower concentrations.
In contrast to epinephrine, no subjects exhibited very consistent evidence of relative platelet hyperreactivity at 1 μM ADP or 20 μg/mL collagen (Figure 3B-C). The reproducibility of aggregation results greater than 60% appeared inadequate as supported by ICC values well below 0.75 for each of these assays. At 0.75 mg/mL ristocetin, 3 of 27 subjects consistently demonstrated agglutination greater than 60% on repeated testing (Figure 3D). However, 9 other subjects exhibited this level of response at least twice, and each of these generated discordant results (ie, < 40% agglutination at least once). The ICC was 0.51. At the next lower ristocetin concentration we studied (0.5 mg/mL), no RS subject attained even 40% agglutination (data not shown). After an interim analysis of these data, we included additional agonist concentrations (eg, 2 μM ADP and 35 mg/mL collagen) for the last 13 subjects in the RS. However, similar to 0.75 mg/mL ristocetin, these concentrations yielded a large proportion of discordant results (data not shown).
Using a slightly lower threshold for classifying relative hyperreactivity to a submaximal concentration of CRP (0.005 μg/mL), we found that 3 of 27 subjects demonstrated more than 50% aggregation on each occasion and a fourth did so 3 of 4 times (Figure 3E). Only one other subject showed greater than 50% aggregation more than once. These results are in accordance with the ICC of 0.75 obtained for these data. Unfortunately (and in contrast to the case for epinephrine), additional historical data are not available for CRP, as we began to use it as an agonist only during the latter part of the PR study (ie, none of the RS subjects who demonstrated > 50% aggregation in response to CRP had been tested previously).
Distribution of aggregation response to submaximal agonist concentrations. These histograms depict the number of subjects (y-axis) with a given level of platelet aggregation (x-axis) to the indicated agonists. Seven percent, 15%, and 26% of subjects exhibited more than 60% aggregation in response to 1 μM ADP (A), 20 μg/mL collagen (B), and 0.75 mg/mL ristocetin (C), respectively. The histogram for 0.005 μg/mL CRP (D) differed slightly, with a small peak of responses just below 60% (13% of subjects showed > 50% aggregation), perhaps due to a smaller number of subjects.
Distribution of aggregation response to submaximal agonist concentrations. These histograms depict the number of subjects (y-axis) with a given level of platelet aggregation (x-axis) to the indicated agonists. Seven percent, 15%, and 26% of subjects exhibited more than 60% aggregation in response to 1 μM ADP (A), 20 μg/mL collagen (B), and 0.75 mg/mL ristocetin (C), respectively. The histogram for 0.005 μg/mL CRP (D) differed slightly, with a small peak of responses just below 60% (13% of subjects showed > 50% aggregation), perhaps due to a smaller number of subjects.
Variables associated with platelet hyperreactivity
Since aggregation response to 0.4 μM epinephrine emerged as a reliable assay, we compared clinical and demographic variables between PR subjects who demonstrated 60% or more aggregation on this assay and those who did not (Table 2). Subjects with hyperreactivity did not differ significantly from others with respect to age, race, body mass index, smoking status, or presence of hypertension. However, the hyperreactive group contained a higher percentage of women (P = .015) and had higher fibrinogen levels (P = .011). We found no significant association between oral contraceptive use, menopausal status, or phase of the menstrual cycle at the time of testing and hyperreactive response among female subjects. In the much smaller group of RS subjects, no variable was found to be significantly associated with hyperreactivity, although 4 of 5 RS subjects who showed consistent hyperreactivity to 0.4 μM epinephrine were women.
Subject demographics by response to 0.4 μM epinephrine, PR study
Subject characteristic . | Less than 60% aggregation . | At least 60% aggregation . | P . |
|---|---|---|---|
| Mean age, y, ± SD | 34.3 ± 10.6 | 33.5 ± 9.1 | .619 |
| % women | 64.0 | 81.6 | .015 |
| Race, % of group | .179 | ||
| African-American | 37.3 | 55.0 | |
| White | 37.3 | 30.6 | |
| Hispanic | 16.9 | 10.2 | |
| Asian | 7.1 | 4.1 | |
| Other | 1.3 | 0.0 | |
| Mean body mass index, kg/m2, ± SD | 26.6 ± 5.8 | 27.3 ± 6.2 | .442 |
| Women using oral contraceptives, % | 25.4 | 22.5 | .701 |
| Premenopausal women, % | 81.2 | 85 | .794 |
| Premenopausal women in luteal phase, % | 54.9 | 71.4 | .107 |
| Current smokers, % | 12.4 | 8.2 | .396 |
| Subjects with hypertension, % | 5.9 | 0 | .081 |
| Mean fibrinogen, mg/dL*, ± SD | 324 ± 91 | 364 ± 110 | .011 |
Subject characteristic . | Less than 60% aggregation . | At least 60% aggregation . | P . |
|---|---|---|---|
| Mean age, y, ± SD | 34.3 ± 10.6 | 33.5 ± 9.1 | .619 |
| % women | 64.0 | 81.6 | .015 |
| Race, % of group | .179 | ||
| African-American | 37.3 | 55.0 | |
| White | 37.3 | 30.6 | |
| Hispanic | 16.9 | 10.2 | |
| Asian | 7.1 | 4.1 | |
| Other | 1.3 | 0.0 | |
| Mean body mass index, kg/m2, ± SD | 26.6 ± 5.8 | 27.3 ± 6.2 | .442 |
| Women using oral contraceptives, % | 25.4 | 22.5 | .701 |
| Premenopausal women, % | 81.2 | 85 | .794 |
| Premenopausal women in luteal phase, % | 54.9 | 71.4 | .107 |
| Current smokers, % | 12.4 | 8.2 | .396 |
| Subjects with hypertension, % | 5.9 | 0 | .081 |
| Mean fibrinogen, mg/dL*, ± SD | 324 ± 91 | 364 ± 110 | .011 |
For subjects with less than 60% aggregation, n = 310; for those with at least 60% aggregation, n = 49. No subjects had diabetes.
For logistical reasons, assays were performed on the first 308 subjects, 266 with less than 60% aggregation and 42 with at least 60% aggregation.
Scatter plots of reproducibility data at submaximal agonist concentrations. For each assay depicted, the extent of aggregation (y-axis) exhibited during each of the 27 subjects' 4 assay runs is shown. Subject numbering (x-axis) in each panel is ordered from the lowest to highest mean percent aggregation for that particular assay and does not correspond with numbering in other panels. The appearance of fewer than 4 data points is due to superimposed values. Dashed lines represent cutoffs for classifying relative platelet hyperreactivity for each assay.
Scatter plots of reproducibility data at submaximal agonist concentrations. For each assay depicted, the extent of aggregation (y-axis) exhibited during each of the 27 subjects' 4 assay runs is shown. Subject numbering (x-axis) in each panel is ordered from the lowest to highest mean percent aggregation for that particular assay and does not correspond with numbering in other panels. The appearance of fewer than 4 data points is due to superimposed values. Dashed lines represent cutoffs for classifying relative platelet hyperreactivity for each assay.
Reproducibility of relative hyperreactivity to epinephrine over months to years. Fifteen subjects in the reproducibility study had had PRP aggregometry at 0.4 μM epinephrine performed in the past. The older data are shown as open triangles and plotted on the corresponding scatter plot from Figure 3A (same subject numbering). Values above/below the data points indicate the number of months (mean = 19 months) elapsed between the first result and subsequent (RS) results.
Reproducibility of relative hyperreactivity to epinephrine over months to years. Fifteen subjects in the reproducibility study had had PRP aggregometry at 0.4 μM epinephrine performed in the past. The older data are shown as open triangles and plotted on the corresponding scatter plot from Figure 3A (same subject numbering). Values above/below the data points indicate the number of months (mean = 19 months) elapsed between the first result and subsequent (RS) results.
Association between hyperreactive phenotypes
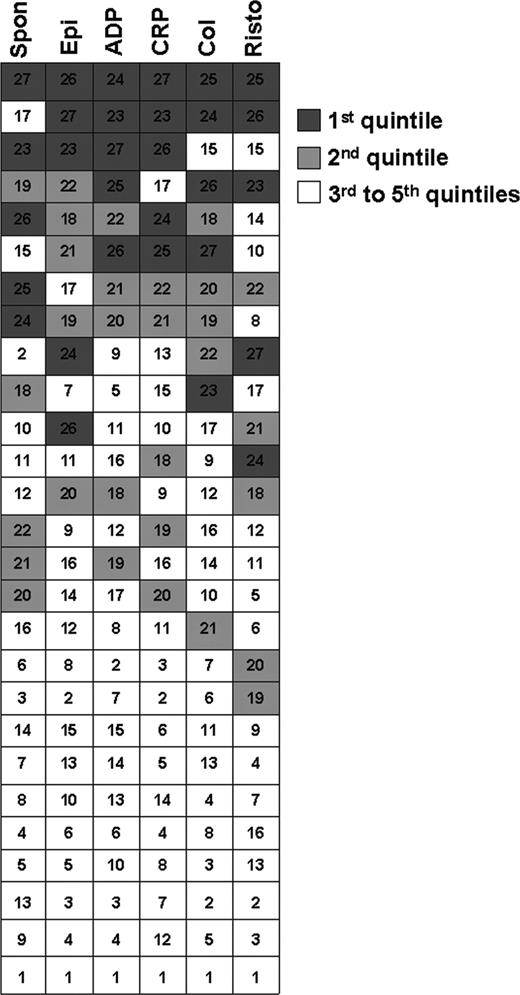
Although this study was not designed to address mechanisms of hyperreactivity, we were interested in whether platelets showing enhanced reactivity to one agonist exhibit similar responses to other agonists. In other words, might hyperreactivity be a “global” (as opposed to agonist-specific) feature of a person's platelets? To address this question, we averaged the 4 values for percent aggregation that each of the RS subjects demonstrated in response to the submaximal agonist concentrations listed in Figure 3, as well as results from spontaneous aggregation. For each assay, we then ranked the 27 subjects in terms of this average percent aggregation. Figure 5 shows that subjects with a high level of aggregation to one agonist tended to exhibit a high level of aggregation to others (note the clustering of filled boxes—particularly the top quintiles—at the top of the figure). Of note, subjects with the greatest extent of spontaneous aggregation also clustered with agonist-induced hyperreactive phenotypes. Although no definitive pattern emerged, these results suggest that platelet hyperreactivity as measured by percent aggregation response to agonists is not agonist specific.
Correlation of extent of platelet reactivity among different agonists. Each of the 27 rows of boxes represents one subject in the reproducibility study. Data from each of 4 runs of each assay were averaged for spontaneous aggregation (Spon) and aggregation to 0.4 μM epinephrine (Epi), 1.0 μM ADP, 0.005 μg/mL CRP, 20 μg/mL collagen (Col), and 0.75 mg/mL ristocetin (Risto). For each assay, subjects were ranked according to average aggregation (1 = lowest, 27 = highest). Boxes are shaded according to ranking by quintiles for each assay (eg, subjects who were ranked 23-27 fell into the top quintile and are marked in dark gray). The number in each box of a given row represents the rank for that subject on a specific assay.
Correlation of extent of platelet reactivity among different agonists. Each of the 27 rows of boxes represents one subject in the reproducibility study. Data from each of 4 runs of each assay were averaged for spontaneous aggregation (Spon) and aggregation to 0.4 μM epinephrine (Epi), 1.0 μM ADP, 0.005 μg/mL CRP, 20 μg/mL collagen (Col), and 0.75 mg/mL ristocetin (Risto). For each assay, subjects were ranked according to average aggregation (1 = lowest, 27 = highest). Boxes are shaded according to ranking by quintiles for each assay (eg, subjects who were ranked 23-27 fell into the top quintile and are marked in dark gray). The number in each box of a given row represents the rank for that subject on a specific assay.
Discussion
Despite widespread clinical use, aggregometry is not routinely used for characterizing an abnormally enhanced platelet response to agonist stimulation. We designed a study to test the feasibility of using this method to identify subjects with in vitro platelet hyperreactivity. We found that submaximal agonist concentrations highlight small populations of individuals with platelets with increased reactivity, that platelets with enhanced reactivity to one agonist generally show enhanced reactivity to others, and that such enhanced reactivity appears to be associated with female sex and higher fibrinogen levels. We have determined agonists and agonist concentrations (0.4 μM epinephrine and 0.005 μg/mL CRP) that identify individuals whose platelets demonstrate reproducible hyperreactivity. These results should permit the use of a commonly available technique to define the platelet hyperreactive phenotype in vitro without the need to study subjects in a severely controlled setting. A well-defined platelet phenotype is essential for assessing an individual's risk for arterial thrombosis and for performing gene-phenotype association studies.
Platelet hyperreactivity
Knowledge of interindividual variation in percent aggregation response is sparse, as previous studies have used limited sample sizes, agonists, and agonist concentrations.3,25 For example, most studies have used only relatively high agonist concentrations intended to detect gross platelet hypofunction. Using 5 different agonists and a broad range of agonist concentrations, we observed substantial interindividual variability in platelet reactivity in our group of healthy subjects (eg, Figure 1B-D). However, for each agonist tested, we have shown that submaximal agonist concentrations (well below those typically used in most clinical and research laboratories) highlight a small proportion of individuals who exhibit a relatively strong response (> 60% aggregation) (Figures 1B, 2). Higher agonist concentrations tend to obscure these hyperresponders, as a greater proportion of subjects demonstrate a full aggregation response at increasing concentrations (eg, Figure 1C-D). Submaximal agonist concentrations thus enable detection of platelet hyperreactivity (as measured by aggregometry); we have identified specific agonists and agonist concentrations that appear useful for this purpose.
Few subjects demonstrated aggregation in the 40% to 60% range at any concentration of any agonist. This imparts a bimodal appearance to the histograms (especially at low and intermediate concentrations) and suggests the existence of a binary, “all or none” type of aggregation response. We speculate that this may be related to quantal platelet activation and subsequent aggregation.26,27
Several comments should be made regarding the use of spontaneous aggregation to identify subjects with hyperreactive platelets. High levels of spontaneous aggregation have been found to predict coronary events and mortality.11 Moreover, our RS subjects with the highest levels of spontaneous aggregation also tended to demonstrate high levels of response to submaximal agonist concentrations (Figure 5). However, from a practical standpoint, the range of spontaneous aggregation exhibited by our subjects was rather narrow (Figure 1A), resulting in substantial overlap between high and low responders. In contrast, the use of submaximal agonist concentrations generates a broad, bimodal distribution of responses (eg, Figure 1B) and allows for clearer identification of individuals with hyperreactive platelets (> 50%-60% aggregation versus all other results).
Reproducibility of platelet hyperreactivity
Because of the marked interindividual variability in aggregation response at submaximal agonist concentrations (eg, 1%-100% at 0.4 μM epinephrine), it is imperative to know whether the hyperreactivity observed in Figures 1B and 2 is reproducible. Assay variability may be caused by (1) technical factors related to assay methodology, (2) environmental factors external to the individual, or (3) intrinsic factors (eg, genetic modifiers). If technical or environmental factors result in unwieldy assay variability for a given subject, the assay will have little clinical or research utility. On the other hand, assay variability due to intrinsic or genetic factors is important to understand and ought to be reasonably reproducible within a given subject.
Using a minimal set of technical and environmental restrictions to study our subjects (“Patients, materials, and methods”), we have shown that low concentrations of epinephrine (0.4 μM) and CRP (0.005 μg/mL) detect relative hyperreactivity quite reliably and efficiently. For both of these aggregation assays, a minority of individuals (3 to 5 of 27 subjects) demonstrated unusually high levels of response on an extremely consistent basis, while most others repeatedly showed a minimal response (Figure 3A,E). In fact, for the epinephrine assay, these results were reproducible over a much longer period of time (Figure 4). At the concentrations used, other agonists (ADP, collagen, and ristocetin) did not demonstrate sufficient reproducibility for reliably detecting a hyperreactive platelet phenotype. These findings have important implications as each of these agonists is used commonly in clinical and research laboratories; future studies of platelet hyperreactivity should either avoid use of these agonists or assess their reproducibility at other concentrations (eg, 1.5 μM ADP, 25-30 μg/mL collagen, and 0.6-0.65 mg/mL ristocetin). Mechanisms underlying the differences in reproducibility between different agonists are unknown, but differences in receptor levels or postreceptor signaling among different subjects may play a role. Perhaps some portion of the poor reproducibility obtained with ADP, collagen, and ristocetin was due to variables such as diet, stress, or other unknown factors affecting activation pathways specific to these agonists.
Day-to-day variability of results from platelet aggregometry testing due to technical and environmental factors has been a source of concern.28,29 However, no reports of the method's reproducibility (or lack thereof) detail measures taken to minimize such within-individual variability as we have in this study.25,30,31 Furthermore, we are unaware of previous reproducibility studies that have used submaximal agonist concentrations as we have. Such differences in study methodology may partially explain why the reproducibility of some of the aggregation assays we performed appears adequate in spite of widely held concerns and previous reports to the contrary.25,30
Association of platelet hyperreactivity with other variables
Platelet hyperreactivity to 0.4 μM epinephrine was significantly associated with female sex and higher fibrinogen levels. Compared with platelets from men, platelets from women have been previously shown to display increased reactivity to standard concentrations of agonists.19,32,33 Our finding that subjects with platelet hyperreactivity exhibit higher plasma fibrinogen levels is also consistent with the observation of Feng et al that higher fibrinogen levels were associated with increased aggregation to epinephrine.34 Higher levels of fibrinogen may modulate some of the increased platelet aggregation. However, other factors must also contribute, since one third of the group hyperreactive to epinephrine had a fibrinogen level less than the mean fibrinogen level for the nonhyperreactive group (data not shown).
Hyperreactivity is often global
Although reproducibility varied between assays, some individuals showed evidence of relative platelet hyperreactivity on each assay that used submaximal agonist concentrations (Figure 3). Of interest, there appears to be some agreement among these assays in identifying subjects with platelet hyperreactivity, as supported by Figure 5 and by the statistical significance of the Spearman rank correlation coefficient between various pairs of these assays (data not shown). Subjects with increased platelet aggregation to 0.4 μM epinephrine also demonstrated higher levels of the activated form of GPIIb-IIIa (as measured by phosphatase of activated cell 1 [PAC-1] binding35 ) after stimulation with ADP (data not shown). These findings suggest that the physiologic determinants of platelet hyperreactivity in response to different agonists are not likely to involve completely independent mechanisms. For many, platelet hyperreactivity is, in some sense, a global phenomenon. Future studies should explore potential mechanisms underlying this observation, such as a final common signaling pathway shared by the agonists we tested.
Clinical implications
From a clinical perspective, the major limitation of our study is its in vitro nature. It will be very important to prospectively evaluate the predictive value of these assays for assessing arterial thrombotic risk. This will require a substantial effort, using either a large cohort of high-risk patients or an even larger cohort of healthy subjects who would be followed for incident events. However, our demonstration of a simple and reproducible measurement of platelet hyperreactivity is a critical first step in planning such studies.
It is possible that the subjects who exhibited hyperreactive responses to epinephrine have a platelet phenotype similar to that described for sticky platelet syndrome,36 a disorder in which clinical thrombosis and hyperaggregability to epinephrine and/or ADP have been reported. The lack of symptoms and history of thrombotic events in our subjects precludes this as a clinical diagnosis; however, these individuals may represent a high-risk group. As sticky platelet syndrome has been described as a relatively common platelet defect,36 its relationship to the reproducible hyperreactivity we observed in healthy individuals requires further study. However, since our work was not designed as a family study (sticky platelet syndrome is thought to be autosomal dominant in its transmission), heritability of the hyperreactive phenotype could not be assessed.
We suggest that submaximal concentrations of epinephrine and CRP can be used to assess in vitro platelet hyperreactivity. Our rigorous demonstration of reproducibility on at least 4 separate occasions bolsters confidence in these assays to a greater degree than other studies that have based conclusions regarding other measures of platelet reactivity on fewer measurements.37,38 Because some subjects had an increased aggregation response that was not reproducible, we recommend that classification of hyperreactivity be restricted to those individuals who demonstrate more than 60% aggregation to epinephrine or more than 50% aggregation to CRP on at least 2 occasions (similar to scenarios such as diagnosis of hypertension, antiphospholipid antibody syndrome, or von Willebrand disease where repeated measurements are accepted practice). We have shown that such a definition results in a high level of specificity, as most subjects who met these criteria demonstrated hyperreactivity on each of multiple testing occasions (Figure 3A,E). Between 10% and 20% of RS subjects met these criteria at 0.4 μM epinephrine and 0.005 μg/mL CRP. The small sample size of the RS limited our ability to confirm more extreme hyperreactivity in a reasonable number of subjects. However, more extreme hyperreactivity appears to exist, as 1 of the 27 RS subjects demonstrated this very consistently (on 5/5 tests) in response to 0.2 μM epinephrine over a 2-year period (data not shown). Finally, since the results of platelet aggregometry are highly dependent on a variety of analytical and preanalytical variables,4,28 the simple precautions we have followed should be taken in other laboratories.
Marked differences in platelet reactivity between healthy individuals raise important questions about their determinants. For instance, what genetic factors underlie the observed differences in platelet reactivity between individuals? An increasing number of studies have identified associations between variations in platelet genes and risk of arterial thrombosis.39-42 Although these findings imply that such genetic variations induce a phenotype of platelet hyperreactivity (as we and others have provided evidence to support43-45 ), there exists neither a standardized definition of platelet hyperreactivity nor a widely accepted assay to test for it. Moreover, with the completion of the sequencing of the human genome, it has become clear that a lack of well-defined phenotypes is a major limitation to establishing genotype-phenotype associations.46-48 Given our findings that platelet hyperreactivity can be reliably identified using straightforward and available methods, investigators will be better equipped to discover new genes responsible for this phenotype and to determine clinical risks associated with it.
Prepublished online as Blood First Edition Paper, June 21, 2005; DOI 10.1182/blood-2005-03-1290.
Supported by National Institutes of Health grants RR17665 (D.L.Y.) and HL65229 (P.F.B.), and Baylor College of Medicine.
D.L.Y. designed parts of this study, analyzed the data, and wrote most of the paper. C.W.S. and A.L.B. performed the research under the direction of J.-f.D. P.F.B. designed much of this study and wrote parts of the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr O'Brian Smith for his provision of statistical support, Jennifer Wood and David Lopez for assistance with data acquisition, and Susana Larrucea for synthesizing CRP.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal