Abstract
Agonistic monoclonal antibodies to CD40 (CD40 mAbs) have a puzzling dual therapeutic effect in experimental animal models. CD40 mAbs induce tumor regression by potentiating antitumoral T-cell responses, yet they also have immunosuppressive activity in chronic autoimmune inflammatory processes. CD40 mAbs are thought to act on antigen presentation by dendritic cells (DCs) to T cells. DCs can be distinguished as either immature or mature by their phenotype and their ability to generate an effective T-cell response. Here we found that, on human cells, although anti-CD40 led immature DCs to mature and became immunogenic, it also reduced the capacity of lipopolysaccharide (LPS) and tumor necrosis factor α (TNF-α)-matured DCs to generate a specific CD4 T-cell response. This inhibitory effect was related to rapid and selective apoptosis of mature DCs. Anti-CD40-mediated apoptosis was due to an indirect mechanism involving cooperation with the death domain-associated receptor Fas, leading to activation of Fas-associated death domain protein (FADD) and caspase-8. On human cells, CD40 activation by such agonists could, therefore, trigger immune responses to antigens presented by immature DCs, which are otherwise nonimmunogenic, by inducing maturation. On the other hand, anti-CD40 mAbs, by rapidly inducing apoptosis, may reduce the capacity of inflammatory signal-matured immunogenic DCs to generate an effective T-cell response. These results call for caution in CD40 mAb-based immunotherapy strategies. (Blood. 2005;106:2806-2814)
Introduction
CD40 is a 48-kDa transmembrane glycoprotein cell surface receptor that shares homology with the tumor necrosis factor α (TNF-α) receptor family. CD40 is expressed by dendritic cells (DCs), macrophages, epithelial cells, hematopoietic progenitors, and activated CD8 T cells.1 The CD40 ligand is a 34- to 39-kDa type II integral membrane protein expressed on activated but not resting CD4 T cells and also on activated B cells and activated platelets.1 CD40 ligation plays a critical role in CD4+ T cell-dependent humoral immune responses.1 CD40 ligation is also crucial for CD4 help to mouse and human CD8 T cells.2-4 In these latter studies, injection of agonistic anti-CD40 in mice led to optimal activation of CD8 T cells that, in quiescent conditions, become tolerant. It was inferred from these results that the CD40 signal delivers CD4 help indirectly to CD8 T cells via DCs.
Agonistic anti-CD40 has therapeutic potential in a range of models of preclinical solid tumors and lymphomas.5-11 The observed tumor regression may be induced by potentiation of antitumoral T-cell responses or apoptosis of CD40+ malignant cells.5-10,12
Unexpectedly, agonistic anti-CD40 monoclonal antibodies (mAbs) and CD40 ligand also exerted an immunosuppressive action in models of collagen-induced arthritis,13 melanoma,14 and type 1 diabetes,15 pointing to a dual function of CD40 agonists.16
CD40 agonists may act on T-cell responses by modulating antigen presentation by DCs.2-4 DCs can be distinguished as immature or mature according to their phenotype and their ability to generate a T-cell response.17 DCs can be induced to mature by a broad spectrum of exogenous and endogenous factors, including Toll-like receptor (TLR) agonists, cytokines, heat shock proteins, and CD40 ligation.17 On encountering such signals, immature DCs located in peripheral tissue undergo a dual process of maturation/activation and migrate toward draining lymph nodes, where they can efficiently activate T cells.17,18 However, immature DCs are also present in lymphoid tissues, where they may play a role in maintaining peripheral self-tolerance.1,19,20
Materials and methods
Cells
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors (buffy coat provided by Laboratoire de cytaphérèse, Hôpital Saint-Louis, Paris, France) following Ficoll gradient centrifugation. Highly purified monocytes were isolated by positive selection with anti-CD14-coated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Monocyte preparations were more than 97% pure on the basis of CD11b staining. To obtain DCs, monocytes were cultured for 7 days with 100 ng/mL granulocyte-macrophage colony-stimulating factor and interleukin 4 (IL-4; Peprotech, Rocky Hill, NJ). To induce maturation, 1 μg/mL lipopolysaccharide (LPS; Sigma, St Louis, MO), or 50 ng/mL TNF-α (R&D Systems, Abingdon, United Kingdom) was added to immature DCs for 24 hours. Purified CD4 T cells and CD8 T cells were obtained from CD14-depleted PBMCs by positive selection with anti-CD8- and anti-CD4-coated magnetic beads (Miltenyi Biotec).
Antibodies for flow cytometry
Phycoerythrin-cyanin 5 (PECy5)-anti-CD1a and phycoerythrin (PE)-anti-Fas were from BD PharMingen (San Diego, CA); fluorescein isothiocyanate (FITC)-anti-TNF-R1 and PE-anti-TNF-R2 were from R&D Systems (Minneapolis, MN); and PE-conjugated anti-FasL was from Caltag (Burlingame, CA). PE-anti-CD95 was from BD PharMingen. PE-anti-CD40, FITC-anti-CD83, PE-anti-HLA-DR, and PE-anti-CD86 were from Immunotech (Marseille, France). Anti-TNF-related apoptosis-inducing ligand (TRAIL)-PE, anti-DR4-PE, and anti-DR5-PE were from eBioscience (San Diego, CA). Flow cytometry was performed with an EPICS XL device (Coulter, Brea, CA). Labeled isotype controls were from Immunotech, and unlabeled isotype controls were from R&D Systems.
Apoptosis experiments
The following 3 agonistic anti-CD40 mAbs were used: clone B-B20 (mouse IgG1; Diaclone, Besançon, France), clone Mab89 (mouse IgG1, Immunotech), and clone G28-5 (mouse IgG1, American Type Culture Collection, Manassas, VA). Isotype controls were from R&D Systems. For apoptosis experiments, 5 × 105 DCs/well were activated for various times with 5 μg/mL antibody, then stained with FITC-conjugated annexin V (Beckman Coulter, Marseille, France) and 7-amino-actinomycin D (7-ADD; Molecular Probes, Eugene, OR), according to the manufacturer's instructions, before flow cytometry.
For flow cytometric analysis of caspase activation, DCs were activated with 5 μg/mL of the indicated mAb for various times, and caspase-specific fluorescent peptide was added to the medium 1 hour before the end of incubation (Caspatag activity kit; Intergen, Purchase, NY). DCs were then washed and analyzed by flow cytometry according to the manufacturer's instructions.
For apoptosis inhibition assays, DCs were pretreated for 30 minutes with 25 μM z-LETD-FMK (caspase-8 inhibitor) and z-LEHD-FMK (caspase-9 inhibitor), both from BD PharMingen, then activated with anti-CD40 for 6 hours. DCs were then stained with annexin V and 7-AAD and analyzed by flow cytometry.
To block ligands of death domain-associated receptors, DCs were pretreated for 1 hour with 10 μg/mL of the following neutralizing antibodies: anti-TNF-α (Mab1 clone), anti-Fas ligand (clone NOK-1, referred to as clone 1 in Figure 4G), and anti-TRAIL (clone RIK-2; all mouse IgG1; BD PharMingen). Another anti-Fas ligand (clone 100419, mouse IgG1; R&D Systems, referred to as clone 2 in Figure 4G) was also tested. DCs were then activated with anti-CD40 and apoptosis was examined as described.
To inhibit Fas expression at the surface of mature DCs, we used specific siRNA (Fas Smart Pool siRNA, and the corresponding negative control; Dharmacon, Lafayette, CO). DCs were matured with 50 ng/mL TNF-α (according to the instructions of the manufacturer) for 24 hours, then transfected with 300 pmol siRNA by using the Dendritic Cell Nucleofector kit (Amaxa, Cologne, Germany). After 18 hours, DCs were tested for Fas expression and activated with anti-CD40 B-B20 (2 μg/mL) or the isotype control for 6 hours, then stained with annexin V FITC and 7-AAD, as previously described. Results were expressed as the percentage inhibition of anti-CD40-induced apoptosis in DCs transfected with Fas-specific siRNA or with nonrelevant siRNA, as compared to mock-transfected cells:
DC/T-cell coculture
Purified monocytes, CD4 T cells, and CD8 T cells were isolated as described (see “Cells”) from the same donor. Monocytes were differentiated into DCs for 6 days, while T cells were maintained in complete medium containing 5 U/mL IL-2. On day 6, DCs were incubated with 1 μg/mL tetanus toxoid and tuberculin for 24 hours. On day 7, DCs were matured with LPS or TNF-α for 24 hours and T cells were washed with complete medium, then cultured for an additional 24 hours without IL-2. On day 8, T cells and DCs were cocultured in 200 μL complete medium at a T-cell/DC ratio of 5:1. Anti-CD40 or isotype control (5 μg/mL) was added to the medium as indicated in the figure legends. On day 13, 1 μCi (0.037 MBq)/well radioactive thymidine was added overnight. Cells were harvested and thymidine incorporation was counted in a Microbeta counter (Wallac, Turku, Finland). For apoptosis experiments, T cells and DCs were cocultured as described and stained for 6 hours after the beginning of coculture with CD40-PE, CD3-PECy5, and annexin V FITC. DC apoptosis was examined by flow cytometry.
Western blotting
We used the following antibodies: anti-caspase-3 (mouse IgG1, clone 10C1.C9) and anti-caspase-8 (mouse IgG1, clone 1-3) from Oncogene Research Products (Boston, MA); anti-caspase-9 (mouse IgG1, clone 2-22), anti-FADD (mouse IgG1, clone A66-2), and anti-cytochrome c (mouse IgG1, clone 7H8.2C12) from BD PharMingen; and anti-CD40 (rabbit IgG) and anti-Fas (rabbit IgG) from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase (HRP)-labeled secondary antibodies were from Santa Cruz Biotechnology. DCs were treated with 5 μg/mL anti-CD40 or isotype control for various times, then washed with cold phosphate-buffered saline (PBS) containing 1 mM Na3VO4 and resuspended in lysis buffer with a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Cell lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Hybond-C Extra nitrocellulose membranes (Amersham Life Sciences, Buckinghamshire, United Kingdom). The membranes were blocked with Tris (tris(hydroxymethyl)aminomethane)-buffered saline (TBS) 0.1% Tween-20 (TTBS) containing 3% nonfat milk and probed with 1 μg/mL specific antibodies. The membranes were washed and then incubated with HRP-conjugated secondary antibody. After washing, bands were visualized by adding Luminol reagent (Santa Cruz Biotechnology).
For cytoplasmic extracts for cytochrome c Western blotting, DCs were resuspended at a density of 5 × 106 cells/mL in buffer containing 10 mM Tris-HCl, pH 7.5, 0.3 M sucrose, and 1 × protease inhibitor cocktail. The suspension was homogenized with a needle and centrifuged for 60 minutes at 10 000g at 4°C. The supernatant and pellet were then processed for Western blotting.
For coimmunoprecipitation assays, DCs were activated and lysed in Western blot lysis buffer. Lysates were incubated with 2 μg antibody and 50 μL protein A microbeads (Miltenyi Biotec) for 1 hour on ice. Complexes were isolated on a magnetic μMACS column (Miltenyi Biotec) and eluted with preheated Laemmli buffer before SDS-PAGE and Western blotting. Membranes were probed with anti-FADD antibody, revealed, stripped (Restore Western-Blotting Stripping Buffer; Pierce, Rockford, IL) and reprobed with anti-Fas antibody.
Confocal microscopy
For CD40-FADD colocalization analysis, mature DCs were activated with 5 μg/mL anti-CD40 (clone B-B20) or 5 μg/mL isotype control at 37°C, then washed. Cells were then stained at 4°C for 30 minutes with anti-CD40 (clone B-B20) or isotype control (R&D Systems), followed by PE-conjugated rabbit anti-mouse IgG. DCs were washed, fixed, permeabilized (Cytofix/Cytoperm; BD PharMingen) and stained at 4°C with 10 μg/mL anti-FADD or isotype control (goat IgG; Santa Cruz Biotechnology) for 30 minutes, followed by 10 μg/mL Alexa 488-labeled secondary IgG (Molecular Probes), and were then cytospun on glass slides.
For Fas internalization experiments, immature or mature DCs were activated at 4°C or 37°C with 5 μg/mL anti-CD40 clone B-B20 or 5 μg/mL isotype control, then washed, stained for 30 minutes at 4°C with PE-labeled anti-Fas (clone DX2) or PE-labeled anti-HLA-DR, fixed (Cytofix Buffer), and cytospun on glass slides.
For Fas and lipid raft staining, immature or LPS-matured DCs (0.5 × 106) were activated for 1 hour with 5 μg/mL anti-CD40 (BB20) or the isotype control, then washed and stained with anti-Fas-PE for 30 minutes at 4°C. After washing, cells were stained with 10 μg/mL Alexa 488-conjugated cholera toxin subunit B (Molecular Probes), a marker of GM1 lipids, for 30 minutes at 4°C. Cells were then washed, fixed, and cytospun on glass sides. Immunofluorescence imaging was carried out with an LSM510-Meta confocal system (Carl Zeiss, Jena, Germany) and a Zeiss Axiovert 200M microscope, using a 63×/1.4 NA oil-immersion Plan-Apochromat lens. Laser excitation line at 488 nm (Ar-ion laser), with BP505-550 filter and 543-nm (He-Ne laser) excitation ray, with LP 560 filter, were used to visualize Alexa-488 and phycoerythrin fluorescences, respectively. Images were acquired using Zeiss AIM software, version 3.2.
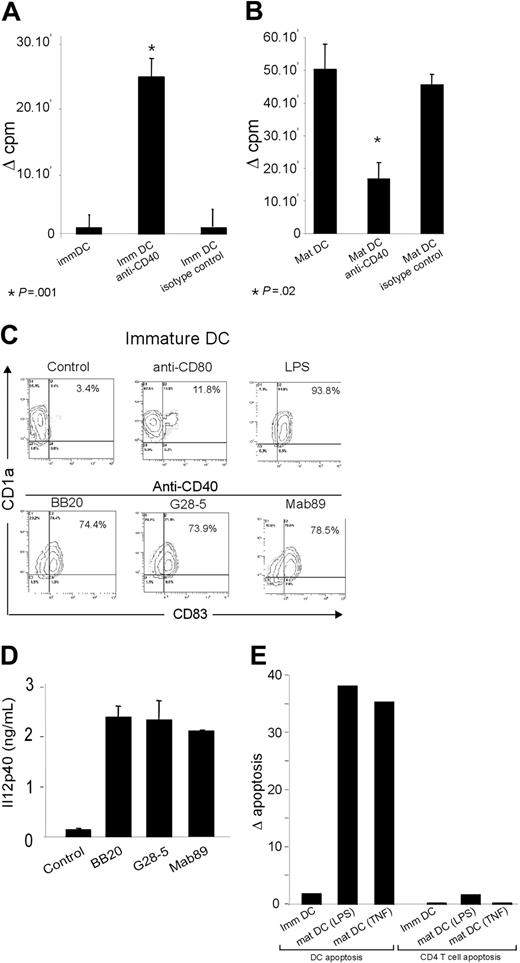
Effect of anti-CD40 on the capacity of mature DCs to generate a specific memory T-cell response. (A-B) Purified CD4+ T cells were cocultured for 5 days with tetanus toxoid and tuberculin-loaded autologous immature (A) or LPS-matured (B) DCs (see “Materials and methods”), in the presence of anti-CD40 mAb or an isotype control. Proliferation was assayed by measuring thymidine incorporation. Specific proliferation is expressed as Δ cpm after subtracting T-cell proliferation in cocultures without antigen. The results are the means ± SEM of 3 independent experiments. (C) Immature DCs were activated for 24 hours with 5 μg/mL of the indicated antibody and stained with anti-CD1a-PECy5 and anti-CD83-FITC before flow cytometry. Numbers in the quadrants indicate the percentages of double-positive cells. These results are representative of 5 independent experiments. (D) Supernatants of immature DCs activated for 24 hours with 5 μg/mL anti-CD40 were tested by enzyme-linked immunosorbent assay (ELISA) for their IL-12p40 content. The results are the means ± SEM of 2 independent experiments. (E) Antigen-loaded DC/CD4 T cells were cocultured in the presence of anti-CD40 mAb or an isotype control as in panels A and B. Six hours after the beginning of coculture, cells were stained with anti-CD40-PE, anti-CD3-PECy5, and annexin V FITC. For DC apoptosis analysis, cells were gated according to their forward/side scatter (FS/SS) properties, and annexin V staining was analyzed in the CD40+CD3- population. For T-cell apoptosis, annexin V staining was analyzed in the CD3+CD40- population. Results are expressed as Δ apoptosis = (percentage of annexin V+ cells in coculture with anti-CD40) - (percentage of annexin V+ in coculture with the isotype control).
Effect of anti-CD40 on the capacity of mature DCs to generate a specific memory T-cell response. (A-B) Purified CD4+ T cells were cocultured for 5 days with tetanus toxoid and tuberculin-loaded autologous immature (A) or LPS-matured (B) DCs (see “Materials and methods”), in the presence of anti-CD40 mAb or an isotype control. Proliferation was assayed by measuring thymidine incorporation. Specific proliferation is expressed as Δ cpm after subtracting T-cell proliferation in cocultures without antigen. The results are the means ± SEM of 3 independent experiments. (C) Immature DCs were activated for 24 hours with 5 μg/mL of the indicated antibody and stained with anti-CD1a-PECy5 and anti-CD83-FITC before flow cytometry. Numbers in the quadrants indicate the percentages of double-positive cells. These results are representative of 5 independent experiments. (D) Supernatants of immature DCs activated for 24 hours with 5 μg/mL anti-CD40 were tested by enzyme-linked immunosorbent assay (ELISA) for their IL-12p40 content. The results are the means ± SEM of 2 independent experiments. (E) Antigen-loaded DC/CD4 T cells were cocultured in the presence of anti-CD40 mAb or an isotype control as in panels A and B. Six hours after the beginning of coculture, cells were stained with anti-CD40-PE, anti-CD3-PECy5, and annexin V FITC. For DC apoptosis analysis, cells were gated according to their forward/side scatter (FS/SS) properties, and annexin V staining was analyzed in the CD40+CD3- population. For T-cell apoptosis, annexin V staining was analyzed in the CD3+CD40- population. Results are expressed as Δ apoptosis = (percentage of annexin V+ cells in coculture with anti-CD40) - (percentage of annexin V+ in coculture with the isotype control).
Results
Anti-CD40 reduces the capacity of mature DCs to generate a specific T-cell response
Immature human DCs were obtained from highly purified monocytes as previously described.23,24 CD1a+CD83- immature DCs were further treated with LPS, a potent trigger of DC maturation. LPS-treated immature DCs expressed the mature DC marker CD83+ and up-regulated the expression of several surface molecules involved in antigen presentation to T cells (CD40high, CD80high, CD86high, HLA-DRhigh; not shown). To examine the ability of DCs to induce a T-cell response, we used a coculture system in which immature or mature DCs presented peptides derived from common recall antigens (tuberculin and tetanus toxoid) to autologous purified CD4 T cells. Antigen-presenting mature DCs triggered memory CD4 T-cell responses, whereas immature DCs did not (Figure 1A-B). Activation with 3 agonistic monoclonal anti-CD40 antibodies led immature DCs to mature (Figure 1C) and to produce large amounts of IL-12p40 (Figure 1D). Treatment of immature DCs with anti-CD40 enabled them to activate specific memory CD4 T cells (Figure 1A). Surprisingly, anti-CD40 reduced the capacity of mature DCs to efficiently activate CD4 T cells (Figure 1B). Cell survival analysis after 24 hours of coculture revealed a high level of mature DC apoptosis in the presence of CD40 mAbs (Figure 1E). This was not observed in cocultures of immature DCs (Figure 1E). No significant apoptosis of CD4 T cells was found in CD40 mAb-treated cocultures (Figure 1E). We then examined whether anti-CD40 could directly trigger apoptosis of mature DCs.
Anti-CD40 induces apoptosis of mature DCs
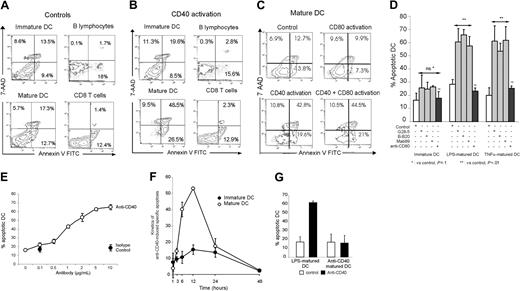
The effect of anti-CD40 on DC survival was examined by dual-staining flow cytometry with annexin V and the vital dye 7-AAD (Figure 2). Cell treatment for 6 hours with agonistic anti-CD40 mAbs induced significant apoptosis of LPS-matured DCs (Figure 2A-B,D). Similar results were obtained after DC maturation with TNF-α (Figure 2D). Apoptosis increased with the antibody concentration (Figure 2E). Induction of apoptosis increased with time, peaking 12 hours after CD40 ligation and then tailing off (Figure 2F). Apoptosis of immature DCs increased moderately after treatment with anti-CD40; however, this effect was far less marked than with mature cells (Figure 2A-B,D,F).
As shown in Figure 2A-B, activation with agonistic anti-CD40 had no proapoptotic effect on primary B cells or on human CD8 T cells activated with phytohemagglutinin (PHA) to induce CD40 expression (not shown). The use of cross-linked anti-CD40 with anti-IgG yielded the same results as in Figure 2 (not shown). In addition to isotype controls of irrelevant specificity, control experiments also included treatment of mature DCs with an agonistic anti-CD80 of the same isotype as anti-CD40. No significant apoptosis of mature DCs was found in response to anti-CD80 (Figure 2C). Combined treatment with anti-CD40 and a high concentration of anti-CD80 (10 μg/mL) had no influence on anti-CD40-mediated apoptosis (Figure 2C). Of interest, anti-CD40-matured DCs, in contrast to DCs matured with LPS or TNF-α, did not show a significant increase in apoptosis following subsequent CD40 activation (Figure 2G). Together, these results show that CD40 signaling triggered with an agonistic mAb leads to rapid and selective apoptosis of DCs matured with TLR agonists or proinflammatory cytokines.
Effect of CD40 activation on DC apoptosis. Cells were activated for 6 hours with the indicated mAbs or isotype controls, before annexin V FITC and 7-AAD staining and flow cytometry. This staining distinguishes between viable (7-AAD-/annexin V-), early apoptotic (annexin V+/7-AADdim), and late apoptotic (annexin V+/7-AAD+) DCs. (A-B) Apoptotic effect of the isotype control (A) or anti-CD40 clone B-B20 (B) on immature/LPS-matured DCs, B lymphocytes, and CD8+ T cells. Similar results were obtained in 10 independent experiments. (C) Effect of anti-CD40 (clone B-B20) and anti-CD80 antibodies on LPS-matured DC apoptosis. Similar results were obtained in 4 independent experiments. Numbers in the quadrants indicate the percentages of positive cells. (D) Effect of agonistic anti-CD40 (clones BB20, G28-5, and mAb89; gray bars), agonistic anti-CD80 (black bars), and isotype control (white bars) on apoptosis of immature, LPS-matured, or TNF-α-matured DCs. The percentage of apoptotic cells represents the sum of early and late apoptotic cells. Results are the means ± SEM of 4 independent experiments. (E) LPS-matured DCs were treated for 6 hours with increasing concentrations of anti-CD40 (B-B20) or the isotype control before staining with annexin V FITC and 7-AAD. Results are the means ± SEM of 3 independent experiments. (F) Immature and LPS-matured DCs were treated with 5 μg/mL anti-CD40 (B-B20) or the isotype control and were stained at various times with annexin V FITC and 7-AAD. Ten thousand events were acquired in the FS/SS gate. Results are expressed as anti-CD40-induced specific apoptosis (% apoptotic DCs following treatment with anti-CD40) - (% apoptotic DCs following treatment with the isotype control). DCs undergo apoptosis and form apoptotic bodies that are no longer detected in the FS/SS gate. This explains the fall in the percentage of apoptotic cells after 12 hours of activation. Results are means ± SEM of 5 independent experiments. (G) DCs were matured with LPS (1 μg/mL) or anti-CD40 B-B20 (5 μg/mL) for 24 hours, then activated for 6 hours with B-B20 or isotype control (5 μg/mL) and stained with annexin V FITC and 7-AAD. Results represent the mean ± SEM of 3 independent experiments.
Effect of CD40 activation on DC apoptosis. Cells were activated for 6 hours with the indicated mAbs or isotype controls, before annexin V FITC and 7-AAD staining and flow cytometry. This staining distinguishes between viable (7-AAD-/annexin V-), early apoptotic (annexin V+/7-AADdim), and late apoptotic (annexin V+/7-AAD+) DCs. (A-B) Apoptotic effect of the isotype control (A) or anti-CD40 clone B-B20 (B) on immature/LPS-matured DCs, B lymphocytes, and CD8+ T cells. Similar results were obtained in 10 independent experiments. (C) Effect of anti-CD40 (clone B-B20) and anti-CD80 antibodies on LPS-matured DC apoptosis. Similar results were obtained in 4 independent experiments. Numbers in the quadrants indicate the percentages of positive cells. (D) Effect of agonistic anti-CD40 (clones BB20, G28-5, and mAb89; gray bars), agonistic anti-CD80 (black bars), and isotype control (white bars) on apoptosis of immature, LPS-matured, or TNF-α-matured DCs. The percentage of apoptotic cells represents the sum of early and late apoptotic cells. Results are the means ± SEM of 4 independent experiments. (E) LPS-matured DCs were treated for 6 hours with increasing concentrations of anti-CD40 (B-B20) or the isotype control before staining with annexin V FITC and 7-AAD. Results are the means ± SEM of 3 independent experiments. (F) Immature and LPS-matured DCs were treated with 5 μg/mL anti-CD40 (B-B20) or the isotype control and were stained at various times with annexin V FITC and 7-AAD. Ten thousand events were acquired in the FS/SS gate. Results are expressed as anti-CD40-induced specific apoptosis (% apoptotic DCs following treatment with anti-CD40) - (% apoptotic DCs following treatment with the isotype control). DCs undergo apoptosis and form apoptotic bodies that are no longer detected in the FS/SS gate. This explains the fall in the percentage of apoptotic cells after 12 hours of activation. Results are means ± SEM of 5 independent experiments. (G) DCs were matured with LPS (1 μg/mL) or anti-CD40 B-B20 (5 μg/mL) for 24 hours, then activated for 6 hours with B-B20 or isotype control (5 μg/mL) and stained with annexin V FITC and 7-AAD. Results represent the mean ± SEM of 3 independent experiments.
Anti-CD40 triggers FADD-dependent caspase activation in mature DCs
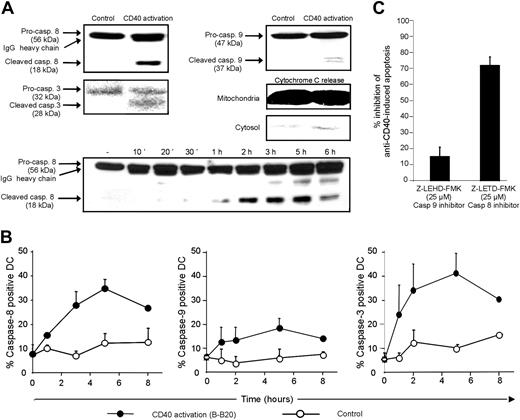
We then turned to the mechanisms of CD40-mediated apoptosis in mature DCs. We first examined the potential role of caspases. Western blotting showed that the upstream initiator caspase-8 and the downstream effector caspase-3 were cleaved in response to anti-CD40 (Figure 3A-B). Caspase-8 activation was detected as little as 1 hour after CD40 activation and started to decrease by 6 hours after activation (Figure 3B). Caspase-9 activation was not as marked as caspase-8 activation (Figure 3A). Caspase-9, a key component of the mitochondrial apoptosis pathway, is activated by autoprocessing within a cytosolic molecular complex involving apoptotic protease-activating factor 1 (APAF-1) and cytochrome c, following the release of the latter from mitochondria.25 As shown in Figure 3A, CD40 activation led to barely detectable cytochrome c release from mitochondria, suggesting that the mitochondrial pathway does not play a major role in CD40-mediated apoptosis of mature DCs. Flow cytometric analysis of cells stained at various times with fluorescent-labeled caspase inhibitor peptides, which bind with different affinities to the active enzymatic centers of caspases,26 gave similar results to those obtained by Western blotting (Figure 3B). Likewise, unlabeled Z-LETD-FMK peptide, which binds with high affinity to the caspase-8 active center, significantly reduced CD40-mediated apoptosis, in contrast to Z-LEHD-FMK peptide, which primarily binds the caspase-9 active center (Figure 3C).
The main apoptosis pathway known to trigger caspase-8 activation is that involving death domain-associated receptors, all of which belong to the TNF-R superfamily. Caspase-8 is activated by autocleavage following its recruitment by the adapter protein FADD (also called MORT-1) within the death-inducing signaling complex (DISC).27 By using confocal microscopy, we found that FADD was recruited to the cell surface in response to CD40 signaling, leading to apparent CD40-FADD colocalization (Figure 4A). Although FADD clearly moved to the cell surface, no physical association of FADD with CD40 could be demonstrated because the limited resolution of confocal microscopy did not allow us to discriminate between spatial proximity and physical association. We then performed a series of CD40 coimmunoprecipitation experiments with FADD, TRADD (another adaptor protein that binds FADD, enabling TNF-R1 to activate caspase-8), and caspase-8. Not surprisingly, the results were invariably negative (not shown), as CD40 has no known death domain in its intracellular portion that could permit FADD or TRADD binding. Together, these results raised the possibility of an indirect mechanism by which CD40 triggers FADD recruitment and caspase-8 activation.
Caspase activation following CD40 activation of mature DCs. (A) Mature DCs were activated for 4 hours with 5 μg/mL anti-CD40 (B-B20) or isotype control before cell lysis. Western blot was performed on protein extracts with anti-caspase-8, anti-caspase-9, and anti-caspase-3. Furthermore, caspase-8 activation was analyzed at various times. For cytochrome c release experiments, cytosolic and mitochondrial extracts were prepared (see “Materials and methods”) from mature DCs activated as described. Western blot was performed on protein extracts with anti-cytochrome c antibody. Similar results were obtained in 2 other independent experiments. (B) DCs were activated for the indicated times with agonistic anti-CD40 (B-B20) or isotype control. A fluorescent caspase inhibitor peptide was added 1 hour before flow cytometry. These results are the mean ± SEM of 3 independent experiments. (C) DCs were preincubated for 2 hours with Z-LEHD-FMK (caspase-9 inhibitor) or Z-IETD-FMK (caspase-8 inhibitor), then treated for 6 hours with anti-CD40 (B-B20) or isotype control before analyzing apoptosis as described. Results are the percentage inhibition of anti-CD40-induced apoptosis as compared to the isotype control. These results are the mean ± SEM of 3 independent experiments.
Caspase activation following CD40 activation of mature DCs. (A) Mature DCs were activated for 4 hours with 5 μg/mL anti-CD40 (B-B20) or isotype control before cell lysis. Western blot was performed on protein extracts with anti-caspase-8, anti-caspase-9, and anti-caspase-3. Furthermore, caspase-8 activation was analyzed at various times. For cytochrome c release experiments, cytosolic and mitochondrial extracts were prepared (see “Materials and methods”) from mature DCs activated as described. Western blot was performed on protein extracts with anti-cytochrome c antibody. Similar results were obtained in 2 other independent experiments. (B) DCs were activated for the indicated times with agonistic anti-CD40 (B-B20) or isotype control. A fluorescent caspase inhibitor peptide was added 1 hour before flow cytometry. These results are the mean ± SEM of 3 independent experiments. (C) DCs were preincubated for 2 hours with Z-LEHD-FMK (caspase-9 inhibitor) or Z-IETD-FMK (caspase-8 inhibitor), then treated for 6 hours with anti-CD40 (B-B20) or isotype control before analyzing apoptosis as described. Results are the percentage inhibition of anti-CD40-induced apoptosis as compared to the isotype control. These results are the mean ± SEM of 3 independent experiments.
CD40-induced apoptosis involves ligand-independent Fas activation
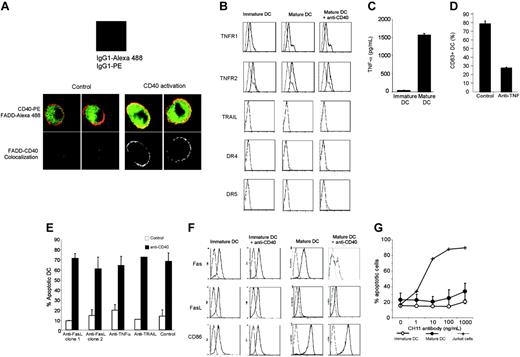
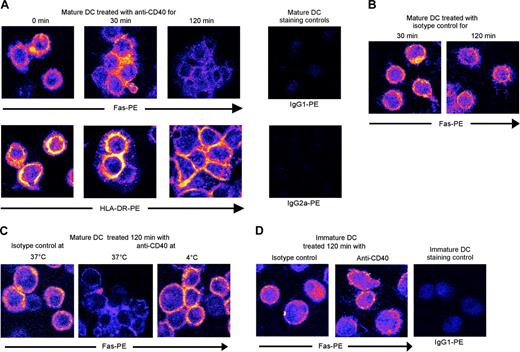
The best-characterized death domain-associated receptors are TNF-R1 (p55), Fas, TRAIL-R1, and TRAIL-R2 (DR4 and DR5). As shown in Figure 4B, moderate expression of TNF-R1 and TNF-R2 was detected by flow cytometry at the surface of both immature and mature DCs, and no clear change occurred 2 hours after CD40 activation. LPS-matured DCs produced TNF-α (Figure 4C). TNF receptor-associated factor 2 (TRAF2) is involved in the pathway of nuclear factor κB (NF-κB) induction by CD40 and TNFR,28,29 and also physically interacts with several antiapoptotic factors, including cellular inhibitor of apoptosis protein 1 (cIAP1) and cIAP2.30,31 Such antiapoptotic factors are recruited into the TRAF2-containing TNF-R1 signaling complex, thereby preventing efficient activation of caspase-8.31 Activated CD40 could therefore compete with activated TNF-R1 for the recruitment of cytosolic TRAF2-bound antiapoptotic factors, thereby promoting the formation of a caspase-8-activating TNF-R1 complex.31 We used a neutralizing anti-TNF-α to block soluble and membrane-anchored TNF-α. Figure 4D shows the functionality of this neutralizing antibody on the basis of its inhibitory effect on TNF-α-mediated DC maturation. However, preincubation of mature DCs with this antibody had no effect on CD40-mediated apoptosis (Figure 4E). In addition, we were unable to block CD40-mediated apoptosis by using a neutralizing anti-TRAIL (Figure 4E), which we tested despite the absence of flow cytometry-detectable DR4, DR5, or TRAIL at the cell surface (Figure 4B). Fas expression was detected at the surface of immature and mature DCs (Figure 4F). Fas ligand was also detected by flow cytometry at the surface of immature DCs and, to a lesser extent, mature DCs (Figure 4F). The Fas-Fas ligand expression ratio increased on maturation (Figure 4F). Flow cytometric analysis of Fas surface expression 2 hours after CD40 activation showed a heterogeneous profile, with a significant percentage of cells showing down-regulated Fas expression but no apparent change in the expression of Fas ligand or CD86 (used as cell surface control molecule; Figure 4F). In experiments using 2 distinct neutralizing antibodies, we treated mature DCs with anti-Fas ligand before CD40 activation to block the potential effect of membrane or soluble Fas ligand. This had no effect on apoptosis in response to anti-CD40 (Figure 4E). Also, treatment of immature DCs with both neutralizing anti-Fas ligand before LPS maturation and CD40 activation failed to reduce apoptosis (not shown). Of interest, both mature and immature DCs were resistant to apoptosis mediated by direct Fas triggering with an agonistic antibody (CH11 clone), in contrast to Jurkat cells (Figure 4G). However, the observed Fas down-regulation in mature DCs following CD40 activation was intriguing; indeed, following activation, Fas passes through several steps, including cell membrane polarization, before internalizing.32,33 We therefore examined by confocal microscopy Fas internalization in response to CD40. As shown in Figure 5, CD40 activation rapidly led DCs to aggregate. Strikingly, 2 hours after CD40 activation, Fas disappeared from the surface of DCs (Figure 5A-B), in contrast to HLA-DR (used as cell surface control molecule; Figure 5A). Fas internalization was blocked at 4°C (Figure 5C). Anti-CD40 treatment of immature DCs did not lead to significant Fas internalization (Figure 5D).
We also examined the cellular events involving Fas before the internalization step. Confocal microscopy colocalization studies showed that treatment of mature DCs for 1 hour with anti-CD40 triggered Fas clustering and translocation of the majority of Fas into lipid rafts (stained with Alexa 488-conjugated cholera toxin B; Figure 6A). In contrast, anti-CD40 had no clear effect on Fas distribution in immature DCs because large amounts of Fas remained excluded from lipid rafts on the cell surface (Figure 6A). Coimmunoprecipitation experiments showed that, on CD40 signaling, Fas physically interacted with FADD (Figure 6B), providing an explanation for the cell surface FADD recruitment that we had observed by confocal microscopy (Figure 4A). To inhibit Fas expression in mature DCs, we used a Fas-specific siRNA that led to a significant reduction in Fas expression at the cell surface, whereas it had no effect on CD86 expression (control; Figure 6C). This reduction in cell surface Fas expression was associated with significant inhibition of CD40-mediated apoptosis (Figure 6C). Altogether, these results show that CD40-mediated apoptosis involves a Fas-dependent mechanism involving FADD recruitment followed by caspase-8 activation, with no major mitochondrial participation.
Expression of death domain-associated receptors on DCs and ligand neutralization. (A) LPS-matured DCs were activated for 15 minutes with anti-CD40 (B-B20) or isotype control and stained for cell surface CD40 and for intracellular FADD (green), as described in “Materials and methods.” Cells were then analyzed by confocal microscopy. Colocalization is shown in the bottom panels. (B) Immature and LPS-matured DCs activated with anti-CD40 (B-B20) or isotype control for 2 hours were stained with anti-TNF-R1, anti-TNF-R2, anti-TRAIL, anti-DR4, and anti-DR5 (solid line) or with isotype control (dashed line). (C) TNF-α production by immature and LPS-matured DCs was measured by ELISA. Results are means ± SEM of 3 independent experiments. (D) To verify that anti-TNF-α was functional, immature DCs were treated for 30 minutes with 10 μg/mL neutralizing anti-TNF-α or control, then incubated for 24 hours with 50 ng/mL TNF-α. DCs were then stained for CD83 expression. Results are means ± SEM of 3 independent experiments. (E) Neutralization assays. DCs were preincubated for 2 hours with the indicated neutralizing antibodies or isotype control, then activated with anti-CD40 (BB-20) or an isotype control for 6 hours before analyzing apoptosis. Results are means ± SEM of 3 independent experiments. (F) Immature and LPS-matured DCs activated with anti-CD40 (B-B20) or isotype control for 2 hours were stained with anti-Fas, anti-Fas ligand, and anti-CD86 (control) (solid line) or with isotype control (dashed line). Results are representative of 3 independent experiments. (G) Immature or LPS-matured DCs were activated with increasing concentrations of anti-Fas antibody (CH11) and stained with annexin V-FITC and 7-AAD. Results are means ± SEM of 3 independent experiments. Jurkat cells were used as a positive control.
Expression of death domain-associated receptors on DCs and ligand neutralization. (A) LPS-matured DCs were activated for 15 minutes with anti-CD40 (B-B20) or isotype control and stained for cell surface CD40 and for intracellular FADD (green), as described in “Materials and methods.” Cells were then analyzed by confocal microscopy. Colocalization is shown in the bottom panels. (B) Immature and LPS-matured DCs activated with anti-CD40 (B-B20) or isotype control for 2 hours were stained with anti-TNF-R1, anti-TNF-R2, anti-TRAIL, anti-DR4, and anti-DR5 (solid line) or with isotype control (dashed line). (C) TNF-α production by immature and LPS-matured DCs was measured by ELISA. Results are means ± SEM of 3 independent experiments. (D) To verify that anti-TNF-α was functional, immature DCs were treated for 30 minutes with 10 μg/mL neutralizing anti-TNF-α or control, then incubated for 24 hours with 50 ng/mL TNF-α. DCs were then stained for CD83 expression. Results are means ± SEM of 3 independent experiments. (E) Neutralization assays. DCs were preincubated for 2 hours with the indicated neutralizing antibodies or isotype control, then activated with anti-CD40 (BB-20) or an isotype control for 6 hours before analyzing apoptosis. Results are means ± SEM of 3 independent experiments. (F) Immature and LPS-matured DCs activated with anti-CD40 (B-B20) or isotype control for 2 hours were stained with anti-Fas, anti-Fas ligand, and anti-CD86 (control) (solid line) or with isotype control (dashed line). Results are representative of 3 independent experiments. (G) Immature or LPS-matured DCs were activated with increasing concentrations of anti-Fas antibody (CH11) and stained with annexin V-FITC and 7-AAD. Results are means ± SEM of 3 independent experiments. Jurkat cells were used as a positive control.
Discussion
Here we observed a differential effect of agonistic anti-CD40 on mature and immature DCs. Whereas agonistic anti-CD40 led immature DCs to maturate and become immunogenic, the same treatment reduced the capacity of mature DCs to generate a T-cell response, by rapidly inducing apoptosis.
Anti-CD40 induced apoptosis of mature DC indirectly via Fas activation. This led to FADD recruitment, then rapid cleavage and activation of caspase-8 and caspase-3, but with no major involvement of the mitochondrial pathway of apoptosis. Mature DCs may therefore behave as type I cells.34
We found no evidence of Fas ligand involvement in the apoptosis triggered by anti-CD40. Such Fas ligand-independent Fas-dependent apoptosis has previously been observed in several models,35-38 in one case involving JNK (c-Jun NH2-terminal kinase).38 Here, although agonistic anti-CD40 treatment of mature DCs triggered activation of JNK and also p38 mitogen-activated protein (MAP) kinase and extracellular-regulated kinase (ERK; not shown), the use of potent selective inhibitor peptides of these MAP kinase family members had no significant inhibitory effect on CD40 mAb-triggered apoptosis (not shown).
DCs were resistant to apoptosis following direct Fas triggering. Similar results have been reported elsewhere.39,40 CD40 activation might therefore sensitize mature DCs to Fas-mediated apoptosis. Such an effect of CD40 has been reported in other cell types41-43 and may involve a shift in the balance between the antiapoptotic and proapoptotic proteins, FLICE inhibitory protein (FLIP) and FADD.43 This might also be related to ligand-independent redistribution of Fas into lipid rafts, as recently shown in T cells following T-cell receptor (TCR) activation.44 In support of this hypothesis, we observed almost complete translocation of Fas into lipid rafts of mature DCs following CD40 activation. This may explain the type I nature of the resulting apoptosis, as Fas has been shown to localize in lipid rafts in type I cells.45 In contrast, no marked Fas redistribution was observed at the surface of immature DCs in response to anti-CD40 (Figure 6). A differential effect on Fas distribution into lipid rafts, depending on DC maturation status, could therefore be one mechanism by which anti-CD40 signaling controls the fate of these cells.
Fas internalization in mature DCs treated with anti-CD40. (A) LPS-matured DCs activated for various times with anti-CD40 (B-B20) were stained for cell-surface Fas or HLA-DR expression before confocal microscopy. Images were pseudocolored after acquisition. Blue indicates negative staining and yellow strong staining. HLA-DR was used as a cell-surface control molecule. (B) LPS-matured DCs activated with anti-CD40 isotype control were stained for Fas, as described. (C) To control Fas internalization, LPS-matured DCs were activated for 2 hours with anti-CD40 at 4°C to block endocytosis. (D) Immature DCs were activated with anti-CD40 and processed as described.
Fas internalization in mature DCs treated with anti-CD40. (A) LPS-matured DCs activated for various times with anti-CD40 (B-B20) were stained for cell-surface Fas or HLA-DR expression before confocal microscopy. Images were pseudocolored after acquisition. Blue indicates negative staining and yellow strong staining. HLA-DR was used as a cell-surface control molecule. (B) LPS-matured DCs activated with anti-CD40 isotype control were stained for Fas, as described. (C) To control Fas internalization, LPS-matured DCs were activated for 2 hours with anti-CD40 at 4°C to block endocytosis. (D) Immature DCs were activated with anti-CD40 and processed as described.
Role of Fas in CD40-mediated apoptosis of mature DCs. (A) Fas redistribution into lipid rafts following CD40 activation of mature DCs. Immature or LPS-matured DCs were activated for 1 hour with 5 μg/mL anti-CD40 (BB20) or isotype control, then washed and stained with anti-Fas-PE and Alexa 488-conjugated cholera toxin B (CTxB) before confocal microscopy. CTxB staining, Fas staining, overlay of both, and colocalization analysis are shown for each image. The white bar in the bottom right corner corresponds to 10 μm. (B) FAS-FADD coimmunoprecipitation. LPS-matured DCs were activated for 1 hour with anti-CD40 before Fas immunoprecipitation. Complexes were resolved on SDS-PAGE and membranes were probed with anti-FADD antibody then reblotted with anti-Fas as reported in “Materials and methods.” For each experiment shown in this figure, similar results were obtained in 2 independent experiments. (C) Effect of Fas-specific siRNA on CD40-mediated apoptosis. TNF-α-matured DCs (see “Materials and methods”) were transfected with Fas-specific siRNA and stained 18 hours later for Fas and CD86 expression. Dashed line indicates isotype control IgG1-PE staining of siRNA-transfected DCs; thin line, isotype control IgG1-PE staining of mock-transfected DCs; thick line, Fas siRNA- or irrelevant siRNA-transfected cells; filled histogram, mock-transfected cells. Transfected cells were then incubated for 6 hours with 2 μg/mL anti-CD40 (BB20) or isotype control and stained with annexin V FITC and 7-AAD, as previously described. Results were expressed as the percent inhibition of anti-CD40-induced apoptosis in DCs transfected with Fas-specific siRNA or irrelevant siRNA, as compared to mock-transfected cells; that is, 100 - [[(% apoptotic siRNA-transfected DCs + anti-CD40) - (% apoptotic siRNA-transfected DCs + isotype control)] × 100]/[(% apoptotic mock-transfected DCs + anti-CD40) - (% apoptotic mock-transfected DCs + isotype control)]]. Results are the means ± SEM of 2 independent experiments.
Role of Fas in CD40-mediated apoptosis of mature DCs. (A) Fas redistribution into lipid rafts following CD40 activation of mature DCs. Immature or LPS-matured DCs were activated for 1 hour with 5 μg/mL anti-CD40 (BB20) or isotype control, then washed and stained with anti-Fas-PE and Alexa 488-conjugated cholera toxin B (CTxB) before confocal microscopy. CTxB staining, Fas staining, overlay of both, and colocalization analysis are shown for each image. The white bar in the bottom right corner corresponds to 10 μm. (B) FAS-FADD coimmunoprecipitation. LPS-matured DCs were activated for 1 hour with anti-CD40 before Fas immunoprecipitation. Complexes were resolved on SDS-PAGE and membranes were probed with anti-FADD antibody then reblotted with anti-Fas as reported in “Materials and methods.” For each experiment shown in this figure, similar results were obtained in 2 independent experiments. (C) Effect of Fas-specific siRNA on CD40-mediated apoptosis. TNF-α-matured DCs (see “Materials and methods”) were transfected with Fas-specific siRNA and stained 18 hours later for Fas and CD86 expression. Dashed line indicates isotype control IgG1-PE staining of siRNA-transfected DCs; thin line, isotype control IgG1-PE staining of mock-transfected DCs; thick line, Fas siRNA- or irrelevant siRNA-transfected cells; filled histogram, mock-transfected cells. Transfected cells were then incubated for 6 hours with 2 μg/mL anti-CD40 (BB20) or isotype control and stained with annexin V FITC and 7-AAD, as previously described. Results were expressed as the percent inhibition of anti-CD40-induced apoptosis in DCs transfected with Fas-specific siRNA or irrelevant siRNA, as compared to mock-transfected cells; that is, 100 - [[(% apoptotic siRNA-transfected DCs + anti-CD40) - (% apoptotic siRNA-transfected DCs + isotype control)] × 100]/[(% apoptotic mock-transfected DCs + anti-CD40) - (% apoptotic mock-transfected DCs + isotype control)]]. Results are the means ± SEM of 2 independent experiments.
Depletion of associated labile inhibitors such as Fas-associated phosphatase 1 (Fap-1), which has been postulated to play a role in distancing the death domains of trimerized Fas,46 might also be part of the cascade of events that leads to ligand-independent CD95 DISC formation and internalization of the corresponding complexes in mature DCs.46
Interestingly, in contrast to DCs matured with TLR agonists or proinflammatory cytokines, anti-CD40-matured DCs were resistant to CD40-mediated apoptosis, possibly owing to the reduction in CD40 cell surface expression following internalization of CD40-anti-CD40 complexes. Moreover, previous studies suggested that CD40 mAb signaling in immature DCs could provide an antiapoptotic environment that protects them from subsequent apoptotic signals.21,22
Immature DCs are present not only in peripheral tissues but also in lymph nodes, where they are thought to play a role in maintaining peripheral self-tolerance.1,19,20 Systemic administration of anti-CD40 may promote the maturation of DCs located both in peripheral tissues and in lymph nodes, enabling them to trigger efficient T-cell responses against antigens, including self-antigens, that are otherwise nonimmunogenic. By disrupting a physiologic mechanism of peripheral tolerance, agonistic anti-CD40 could therefore lead to better immune responses to pathogens or tumors that take advantage of this tolerance.47-49 This could explain the antitumoral effects of these antibodies.5-10 However, the other side of the coin is the high risk of autoimmunity.1,50,51
Anti-CD40 agonists, by shortening the lifespan of mature immunogenic DCs, could potentially reduce their capacity to generate effector and memory T cells directed against immunogenic antigens, including self antigens, that DCs have captured, for instance, in an inflammatory environment that favored their maturation. This might explain the immunosuppressive activity of agonistic anti-CD40 in chronic autoimmune inflammatory diseases such as rheumatoid arthritis,13 in which levels of TNF-α and other proinflammatory cytokines are elevated in peripheral blood and in the synovial compartment, and in which TNF-α blockade is beneficial. It has been suggested that TNF-α neutralization might partly exert its therapeutic effects by inhibiting DC maturation.52
An immunosuppressive effect of CD40/CD40 ligand interaction has also been observed in transgenic TNF/CD80 mice, a model of type 1 diabetes, expressing TNF-α and the costimulatory molecule CD80 in their pancreatic islets.15
Finally, our results suggest that CD40 mAb-based immunotherapy, through its differential effect on DCs, may have more complex effects than previously thought, calling for added caution. Indeed, anti-CD40 intervention might disrupt not only the deleterious peripheral tolerance of certain tumors or pathogens but also the beneficial natural tolerance of self antigens, creating a risk of autoimmunity. In addition, anti-CD40 therapy might down-regulate immune responses driven by mature DCs within inflammatory environments, with either beneficial or deleterious effects.
Prepublished online as Blood First Edition Paper, June 30, 2005; DOI 10.1182/blood-2004-12-4678.
Supported by grants from Agence Pour la Recherche Contre le SIDA (ANRS), INSERM, Fondation pour la Recherche Médicale, and Université du Québec A Montréal (UQAM; Montreal, QC, Canada).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 6. Role of Fas in CD40-mediated apoptosis of mature DCs. (A) Fas redistribution into lipid rafts following CD40 activation of mature DCs. Immature or LPS-matured DCs were activated for 1 hour with 5 μg/mL anti-CD40 (BB20) or isotype control, then washed and stained with anti-Fas-PE and Alexa 488-conjugated cholera toxin B (CTxB) before confocal microscopy. CTxB staining, Fas staining, overlay of both, and colocalization analysis are shown for each image. The white bar in the bottom right corner corresponds to 10 μm. (B) FAS-FADD coimmunoprecipitation. LPS-matured DCs were activated for 1 hour with anti-CD40 before Fas immunoprecipitation. Complexes were resolved on SDS-PAGE and membranes were probed with anti-FADD antibody then reblotted with anti-Fas as reported in “Materials and methods.” For each experiment shown in this figure, similar results were obtained in 2 independent experiments. (C) Effect of Fas-specific siRNA on CD40-mediated apoptosis. TNF-α-matured DCs (see “Materials and methods”) were transfected with Fas-specific siRNA and stained 18 hours later for Fas and CD86 expression. Dashed line indicates isotype control IgG1-PE staining of siRNA-transfected DCs; thin line, isotype control IgG1-PE staining of mock-transfected DCs; thick line, Fas siRNA- or irrelevant siRNA-transfected cells; filled histogram, mock-transfected cells. Transfected cells were then incubated for 6 hours with 2 μg/mL anti-CD40 (BB20) or isotype control and stained with annexin V FITC and 7-AAD, as previously described. Results were expressed as the percent inhibition of anti-CD40-induced apoptosis in DCs transfected with Fas-specific siRNA or irrelevant siRNA, as compared to mock-transfected cells; that is, 100 - [[(% apoptotic siRNA-transfected DCs + anti-CD40) - (% apoptotic siRNA-transfected DCs + isotype control)] × 100]/[(% apoptotic mock-transfected DCs + anti-CD40) - (% apoptotic mock-transfected DCs + isotype control)]]. Results are the means ± SEM of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/8/10.1182_blood-2004-12-4678/6/m_zh80200585550006.jpeg?Expires=1769103011&Signature=Ust4Dnz1ZT4igBWphoL1LxOEIbkfbrzp8ZaJOOs5I~hGupS6aq17iwPLgnWWELN-lRdTOuolR9TXSXdhjvckWl297c9YAeg9galK2fcbW2X57r3jod7ONBcMluo6h41BmzbHRanjWn~V9tK991sD6fXx226QQGJYDyimBJGESJfW75DR7hTFCmXBNpYRIXHwKzBS~gr6O6hZVeyg~GGUOD1kgXTCYttg-PvzJXh-xaXdQ7JunhILjcmyagcvZi2mWDP4LpoysvyPCeZM9QKGk-RrBCTI5ihVZr~I2QxlhzbAuYExPVMTMJSAz1jW42YoghnLZyzoLNTms2fmGEgMsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal