Abstract
Small G proteins serve as critical control points in signal transduction, integrating a wide range of stimuli to dictate discrete cellular outcomes. The outcomes of small G-protein signaling can both potentiate and antagonize one another. Studies in hematopoietic cells have uncovered multiple functions for the small G protein, Rap1 (Ras-proximate-1). Because Rap1 can regulate cell proliferation, differentiation, and adhesion through distinct mechanisms, it serves as a paradigm for the need for tight cellular control of small G-protein function. Rap1 has received recent attention for its role in enhancing integrin-dependent signals. This action of Rap1 augments a variety of processes that characterize hematopoietic-cell function, including aggregation, migration, extravasation, and homing to target tissues. Rap1 may also regulate cellular differentiation and proliferation via pathways that are distinct from those mediating adhesion, and involve regulation of the mitogen-activated protein (MAP) kinase or ERK (extracellular signal-regulated kinase) cascade. These actions of Rap1 occur in selected cell types to enhance or diminish ERK signaling, depending on the expression pattern of the MAP kinase kinase kinases of the Raf family: Raf-1 and B-Raf. This review will examine the functions of Rap1 in hematopoietic cells, and focus on 3 cellular scenarios where the multiple actions of Rap1 function have been proposed. Recent studies implicating Rap1 in the maturation of megakaryocytes, the pathogenesis of chronic myelogenous leukemia (CML), and activation of peripheral T cells will receive particular attention.
Introduction
Rap1 (Ras-proximate-1) is a small G protein in the Ras superfamily. Rap1 is expressed in cells as one of 2 isoforms, Rap1a and Rap1b. It is most closely related to the 2 isoforms of Rap2 (Rap2a and Rap2b), small G proteins with overlapping but distinct functions.3 Like all G proteins, Rap1 exists in an inactive guanine nucleotide diphosphate (GDP)–bound state and is activated when GDP is exchanged for guanine nucleotide triphosphate (GTP). Rap1, like other small G proteins, is targeted to lipid membranes by the covalent attachment of lipid moieties to the carboxyl terminus via geranyl-geranyl lipids to the terminal cysteine during Rap1 synthesis and transport through the Golgi.4 This posttranslational modification anchors Rap1 to both endosomal and plasma membranes.4 Movement of Rap1 from endosomal membranes to the plasma membrane upon Rap1 activation has been reported in a number of cell types, including Jurkat T cells and megakaryocytes.4-6 Upon activation by GTP binding, Rap1 undergoes a conformational change that exposes an effector-binding loop, allowing the recruitment of a variety of potential effectors. This recruitment triggers the signal pathways linking Rap1 to integrin signaling, ERK activation, and other effector pathways,7 some of which are shown in Figure 1. In the following sections, we will outline some of the mechanisms that regulate Rap1 activity in hematopoietic cells.
Regulation of Rap1
Rap1 GEFs
A diverse family of guanine nucleotide exchange factors (GEFs) stimulates the replacement of GDP for GTP to activate small G proteins. Several Rap1 GEFs have been identified (Table 1), enabling Rap1 to respond to a diverse set of stimuli.3 Three representative Rap1 GEFs are depicted in Figure 1.
Rap1 guanine nucleotide exchange factors
Rap GEFs . | Synonyms . | Substrates . | Activators . | Reference no. . |
|---|---|---|---|---|
| RapGEF1 | C3G, GRF2 | Rap1, Rap2 | PKA, RTKs | 8 |
| RapGEF2 | PDZ-GEF1, RA-GEF1 | Rap1, Rap2 | ? | 9,10 |
| RapGEF3 | EPAC1, cAMP-GEF1 | Rap1, Rap2 | cAMP | 11,12 |
| RapGEF4 | EPAC2, cAMP-GEF2 | Rap1, Rap2 | cAMP | 12 |
| RapGEF5 | Mr-GEF | Rap1 | Ras | 9 |
| RapGEF6 | PDZ-GEF2, RA-GEF2, nRapGEP | Rap1, Rap2 | ? | 13 |
| RasGRP2 | CalDAG-GEF I* | Rap1, Rap2 | Calcium, DAG | 14,15 |
| RasGRP3 | CalDAG-GEFIII | Ras, Rap1 | Calcium, DAG | 16 |
| Dock-4 | None | Rap1 | ? | 17 |
| AND-34 | None | Rap1 | Cas | 18 |
| PLC | None | Rap1 | Ras, Rap1 | 19 |
Rap GEFs . | Synonyms . | Substrates . | Activators . | Reference no. . |
|---|---|---|---|---|
| RapGEF1 | C3G, GRF2 | Rap1, Rap2 | PKA, RTKs | 8 |
| RapGEF2 | PDZ-GEF1, RA-GEF1 | Rap1, Rap2 | ? | 9,10 |
| RapGEF3 | EPAC1, cAMP-GEF1 | Rap1, Rap2 | cAMP | 11,12 |
| RapGEF4 | EPAC2, cAMP-GEF2 | Rap1, Rap2 | cAMP | 12 |
| RapGEF5 | Mr-GEF | Rap1 | Ras | 9 |
| RapGEF6 | PDZ-GEF2, RA-GEF2, nRapGEP | Rap1, Rap2 | ? | 13 |
| RasGRP2 | CalDAG-GEF I* | Rap1, Rap2 | Calcium, DAG | 14,15 |
| RasGRP3 | CalDAG-GEFIII | Ras, Rap1 | Calcium, DAG | 16 |
| Dock-4 | None | Rap1 | ? | 17 |
| AND-34 | None | Rap1 | Cas | 18 |
| PLC | None | Rap1 | Ras, Rap1 | 19 |
PKA indicates protein kinase A; RTKs, receptor tyrosine kinases; DAG, diacyl glycerol; and ?, activator unknown.
CalDAG-GEFI is a shorter alternatively spliced form of RasGRP2.
C3G (RapGEF1), the first Rap1 GEF identified,8 is activated through its association via the Src homology 2 (SH2) domains of the Crk family of adaptor proteins, which include CrkI (CT10 regulator of kinase), CrkII, and CrkL.20,21 In addition to Rap1, C3G may activate other small G proteins, including Rap2, R-Ras, TC21, and the Cdc42-related protein, TC10.3 The inducible association of Crk/C3G to sites of tyrosine phosphorylation at the plasma and intracellular membranes22 occurs following ligand activation of a variety of receptors, including the receptor tyrosine kinases (RTKs) for epidermal growth factor (EGF), nerve growth factor (NGF), and insulin7 ; cytokine receptors for erythropoietin (EPO), interleukin 3 (IL-3),20 interferon-γ,23 as well as the T-cell receptor (TCR)24 and the B-cell receptor.25 For cytokine receptors, tyrosine phosphorylation is initiated by the Janus kinase (JAK) family kinases.
For both RTKs and cytokine receptors, the phospho-tyrosine residues that provide the docking sites for Crk/C3G can occur on specialized scaffolding molecules, including Cbl (Casitas B-lineage lymphoma proto-oncogene),26 Cas,27 and Gab1.28 Phosphorylation of Crk binding sites on these molecules can be triggered by a variety of tyrosine kinases, including RTKs themselves as well as cytoplasmic tyrosine kinases, including c-src29,30 and BCR/ABL.31
Calcium and diacylglycerol (CalDAG)–GEFs comprise another family of GEFs that respond to calcium and diacylglycerol, making them potential targets of phospholipase C (PLC) action (Figure 1). Four CalDAG-GEF gene products have been identified. CalDAG-GEFI, an alternatively spliced form of RasGRP2,15 is strongly expressed in megakaryocytes and platelets32 and modestly expressed in neutrophils.33 It shows preferential activation of Rap1, with some activity toward N-Ras, H-Ras, R-Ras, TC21, and Rap2.12 CalDAG-GEFII (RasGRP1) acts on Ras and R-Ras, but not Rap1, and is a critical mediator of TCR activation of Ras and ERKs in lymphocytes.34 CalDAG-GEFIII (RasGRP3) can activate Rap1, Ras, and R-Ras3 and has been implicated in B-cell development.35 RasGRP4 has been identified as a mast-cell–specific GEF that activates Ras.36
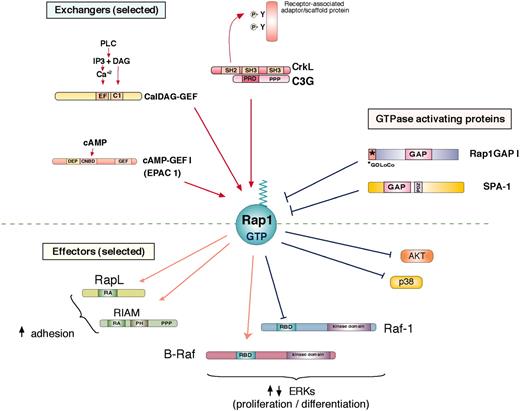
Rap1 regulation and activation of effector proteins. Rap1 is activated (Rap1-GTP) by specific guanine nucleotide exchange factors (GEFs) and inhibited by specific GTPase activating proteins to regulated GTP-dependent binding to potential effector proteins. A diverse family of GEFs activates Rap1 by exchanging GDP for GTP (selected GEFs are shown). C3G, the first Rap1 GEF identified, is activated through its association with CrkL, a member of the Crk family of adaptor proteins, via a proline-rich (PPP) region on C3G and an SH3 region on CrkL. The inducible association of Crk/C3G to sites of tyrosine phosphorylation on receptor-associated adaptor/scaffold proteins occurs following ligand activation of a variety of receptors. CalDAG-GEFs respond to calcium (Ca2+) and diacylglycerol (DAG), making them targets of phospholipase C (PLC) action. cAMP-GEFs or EPACs (exchange proteins directly activated by cAMP; EPAC 1 shown here) are activated by directly binding cAMP to cyclic nucleotide binding domains (CNDB). Two Rap1GAPs are shown here: Rap1GAP1 and SPA-1. Rap1GAP1 is expressed widely and contains a GOLoCo motif that can associate with heterotrimeric G-protein alpha subunits.124 SPA-1 is highly expressed in hematopoietic cells and contains a PDZ (domain found in PSD-95, Discs large, and ZO-1) domain that can mediate interactions with membrane-associated proteins. Following GTP binding, Rap1-GTP associates with a number of potential effectors, some of which are shown here. One of the best-studied effector pathways of Rap1 is the stimulation of cell adhesion. RapL and RIAM (Rap1-GTP–interacting adaptor molecule) have been shown to mediate integrin-mediated adhesion in hematopoietic cells. Both contain a Ras association (RA) domain, and RIAM also contains a Pleckstrin-homology (PH) domain. B-Raf and Raf-1 are related kinases that contribute to the MAP kinase pathway upstream of ERK. Both are recruited to Rap1-GTP via their Ras binding domain (RBD). In cells expressing B-Raf, Rap1 activates B-Raf–dependent signals to ERKs. In B-Raf–negative cells, such as T cells, Rap1 cannot couple to ERK, but can antagonize Ras-dependent signals to ERK, in part by binding to Raf-1. Rap1 can also sequester other effectors of Ras, such as the upstream activators of the p38 MAP kinase, to antagonize the activation of p38 by IL-1. In addition, Rap1 has been proposed to inhibit the phosphatidylinositol 3-kinase–dependent phosphorylation of the survival factor Akt, in B cells.25 DEP indicates domain found in Disheveled, Eg-10, and pleckstrin; IP3, inositol 1,4,5 triphosphate.
Rap1 regulation and activation of effector proteins. Rap1 is activated (Rap1-GTP) by specific guanine nucleotide exchange factors (GEFs) and inhibited by specific GTPase activating proteins to regulated GTP-dependent binding to potential effector proteins. A diverse family of GEFs activates Rap1 by exchanging GDP for GTP (selected GEFs are shown). C3G, the first Rap1 GEF identified, is activated through its association with CrkL, a member of the Crk family of adaptor proteins, via a proline-rich (PPP) region on C3G and an SH3 region on CrkL. The inducible association of Crk/C3G to sites of tyrosine phosphorylation on receptor-associated adaptor/scaffold proteins occurs following ligand activation of a variety of receptors. CalDAG-GEFs respond to calcium (Ca2+) and diacylglycerol (DAG), making them targets of phospholipase C (PLC) action. cAMP-GEFs or EPACs (exchange proteins directly activated by cAMP; EPAC 1 shown here) are activated by directly binding cAMP to cyclic nucleotide binding domains (CNDB). Two Rap1GAPs are shown here: Rap1GAP1 and SPA-1. Rap1GAP1 is expressed widely and contains a GOLoCo motif that can associate with heterotrimeric G-protein alpha subunits.124 SPA-1 is highly expressed in hematopoietic cells and contains a PDZ (domain found in PSD-95, Discs large, and ZO-1) domain that can mediate interactions with membrane-associated proteins. Following GTP binding, Rap1-GTP associates with a number of potential effectors, some of which are shown here. One of the best-studied effector pathways of Rap1 is the stimulation of cell adhesion. RapL and RIAM (Rap1-GTP–interacting adaptor molecule) have been shown to mediate integrin-mediated adhesion in hematopoietic cells. Both contain a Ras association (RA) domain, and RIAM also contains a Pleckstrin-homology (PH) domain. B-Raf and Raf-1 are related kinases that contribute to the MAP kinase pathway upstream of ERK. Both are recruited to Rap1-GTP via their Ras binding domain (RBD). In cells expressing B-Raf, Rap1 activates B-Raf–dependent signals to ERKs. In B-Raf–negative cells, such as T cells, Rap1 cannot couple to ERK, but can antagonize Ras-dependent signals to ERK, in part by binding to Raf-1. Rap1 can also sequester other effectors of Ras, such as the upstream activators of the p38 MAP kinase, to antagonize the activation of p38 by IL-1. In addition, Rap1 has been proposed to inhibit the phosphatidylinositol 3-kinase–dependent phosphorylation of the survival factor Akt, in B cells.25 DEP indicates domain found in Disheveled, Eg-10, and pleckstrin; IP3, inositol 1,4,5 triphosphate.
Another class of Rap1GEFs includes cAMP (cyclic adenosine monophosphate)–GEFs or EPACs (exchange proteins directly activated by cAMP) that are activated by direct binding of cAMP. cAMP-GEFI (EPAC1) is widely expressed,11 while cAMP-GEFII (EPAC2) is enriched in brain and adrenal12 and both are selective for Rap1.14 A role of EPACs has been proposed for Rap1-dependent cell adhesion to laminin in both epithelial cells37 and red blood cells,38 and in the regulation of vascular endothelial barrier function.39,40
Rap GAPs
Rap1 signaling is terminated by the hydrolysis of bound GTP to GDP. This is achieved through specific GTPase-activating proteins (GAPs). The specificity of Rap GAPs for Rap1 is dictated by unique sequences present in both the Rap GAPs and Rap1 that provides specificity for Rap1.41
The principal Rap1GAPs that have been identified in hematopoietic cells are Rap1GAP1 and SPA-1 (signal-induced proliferation-associated gene-1). Rap1GAP1 was originally identified on plasma membranes isolated from differentiated promyelocytic HL-60 cells.42 The expression of SPA-1 is restricted to hematopoietic tissues.43,44 Its expression is highest in lymphoid organs and regulated during differentiation. Immature bone marrow cells predominantly express SPA-1, whereas mature (lineage-positive) bone marrow cells predominantly express Rap1GAP.45 This shift in expression of SPA-1 to Rap1GAP is also seen upon inducing granulocyte differentiation with granulocyte–colony-stimulating factor (G-CSF) of the IL-3–dependent promyeloblastic cell line 32D45 and in HL-60 cells.43,44 Recently, a novel Rap1GAP, RapGAP2, was identified in platelets and lymphocytes.46
Rap1 effector functions
Figure 1 highlights some of the proposed effectors that bind selectively to Rap1GTP. For the purposes of this review, we have limited our discussion to the actions of Rap1 that regulate cell adhesion and the MAP kinase cascade. A more complete listing of Rap1 functions can be found in recent reviews.1,3,7,47
Rap1 regulation of integrin-mediated adhesion
One of the best-studied effector pathways of Rap1 is the stimulation of cell adhesion. Activation of cell-surface receptors generate intracellular signals that increase integrin-mediated adhesion, termed “inside-out” signaling. A number of studies confirm a role for Rap1 in this process. It has been proposed that Rap1 activation initiates inside-out signaling to integrins, resulting in conformational changes that convert integrin to high-affinity states. In addition, integrins can undergo changes in lateral mobility that increase their avidity for ligand binding.48,49 Rap2 has also been shown to play a role in the adhesion and migration of B cells.50,51
Inside-out signaling enhances the function of a wide range of integrins including αLβ2 (LFA-1), VLA-4, and VLA-5 on T cells,49,52-54 αIIbβ3 on megakaryocytes and platelets,55,56 CD11b on monocytes and macrophages,57 and others.1 Integrins bind to a variety of ligands found in the extracellular matrix or on the surface of adhering cells.1 In contrast, “outside-in” signaling refers to intracellular signals that are generated following the binding of integrins with their extracellular ligands.48 Rap1 itself is a target of outside-in signaling,58,59 suggesting the possibility of a positive feedback loop further enhancing integrin function.
A mediator of Rap1-dependent adhesion in T cells has recently been identified as RapL (also called Nore 1b).60 RapL is an alternatively spliced product of RassF5 and is highly expressed in immune cells and binds Rap1 in a GTP-dependent manner. In T cells, this binding is stimulated by TCR and chemokine receptor activation. Following binding, it has been proposed to translocate to the plasma membrane, where it binds the T-cell integrin LFA-1 and enhances its avidity/affinity for its ligand intercellular adhesion molecule (ICAM).61 Expression of interfering mutants of RapL in T cells block Rap1-, TCR-, and chemokine-dependent adhesion to ICAM-1,60 and RapL-null lymphocytes show reduced adhesion in both T and B cells as well as defects in homing of lymphocytes and dendritic cells.2
RapL binds to the cytoplasmic tail of the α chain of LFA-1 to regulate LFA-1 affinity.61 Upon stimulation, both RapL and LFA-1 relocalize to the leading edge of T cells.60 Mutants of LFA-1 that lack RapL binding do not relocalize, but, importantly, they do not prevent RapL from relocalizing, suggesting that the targeting of RapL to the plasma membrane may direct LFA-1 as well. These mutants abolish LFA-1 activation by Rap1 and TCR.61 Recent studies have also identified a role for RapL in the Rap1-dependent migration of vascular endothelial cells.62
Another effector of Rap1 implicated in integrin signaling is Rap1-GTP–interacting adaptor molecule (RIAM). RIAM induces β1 and β2 integrin–mediated adhesion in Jurkat T cells and is required for Rap1-dependent adhesion in these cells.63 RIAM also binds both profillin and Ena/VASP (enabled/vasodilator-stimulated phosphoprotein), modulators of the actin cytoskeleton, and this action may participate in the recruitment of Rap1 and possibly LFA-1 to the lymphocyte plasma membrane upon activation.5 Rap1 enhancement of cell-cell interaction is not limited to its actions on integrins, and a wide range of adhesion molecules have been implicated in Rap1 action.47
Rap1 regulation of the ERK cascade
Rap1 was first identified as an antagonist of Ras-induced transformation in fibroblasts.64 The recruitment of Raf-1 to GTP-bound Ras allows Raf-1 to activate the MAP/ERK kinase MEK, which subsequently activates the MAP kinase ERK. Active Rap1 can bind, but not activate Raf-1, and thereby may sequester Raf-1 from the Ras/ERK pathway.65,66 The antagonism of Ras-dependent activation of Raf-1 has been proposed to mediate some of the antiproliferative actions of Rap1 in T lymphocytes67 and other cell types,7 and may underlie the role of Rap1 in T-cell anergy,24 although other mechanisms have been proposed.68 The expression of Raf-1 is ubiquitous, yet the ability of Rap1 to inhibit the ERK cascade is not universal, even in cells where Raf-1 is the major Raf isoform expressed.69
The ability of Rap1 to sequester effectors of Ras may also be seen with upstream activators of the p38 MAP kinase. For example, in thymocytes, IL-1 activates p38 in a Ras-dependent manner that is antagonized by Rap1. However, the direct target of Rap1 signaling is not known.70 Another pathway in which Rap1 can antagonize Ras function in T cells is the suppression of Ras-dependent reactive oxygen species, an action that allows for T-cell proliferation.71
The effect of Rap1 on ERKs may be indirect. Adhesion of integrins can trigger signals back to the integrin-expressing cell through a variety of signaling cascades,72 collectively referred to as outside-in signaling. Multiple mechanisms for this type of outside-in signaling from integrins to ERKs have been proposed.73,74 Integrins can activate ERKs via both focal adhesion kinase (FAK)–dependent75 and FAK-independent mechanisms.74 Cross-talk with growth factor receptors also couples integrins to ERKs.76 These pathways are often mediated via Ras, although Rap1-dependent activation of ERKs through integrins has been reported.77,78
A major target of the antagonism of Ras and Rap1 signaling is integrin adhesion itself. Ras and Rap1 have opposing actions on integrin-dependent adhesion79,80 and cadherin-dependent adhesion.81 The antagonism may partially explain the initial observation that Rap1 antagonized Ras-dependent transformation.64
Rap1 can also bind a second member of the Raf family, B-Raf.82 Unlike Raf-1, the recruitment of B-Raf to Rap1 is capable of activating MEK and the ERK cascade. Although B-Raf was first identified in brain, it is expressed in a wide variety of hematopoietic cells and cell lines.83-85
The distinct and opposing actions of Rap1 on Raf-1 and B-Raf dictate that the outcome of Rap1 signaling to ERKs depends in part on the relative expression of these 2 Raf isoforms. The expression of B-Raf is critical for Rap1 to activate ERKs in megakaryocytes and myeloid precursors. In peripheral T cells that lack B-Raf, Rap1 antagonizes Ras-dependent activation of ERKs.
Given the diverse processes regulated by Rap1, it is likely that the choice of Rap1 effectors is highly regulated. In the following section, we will examine 3 hematopoietic cells that use multiple Rap1 effector pathways: megakaryocytes/platelets, leukocytes, and T lymphocytes. We will characterize Rap1 actions in these cells and speculate how the cell integrates these diverse actions to achieve the physiologic requirements of those cells.
Megakaryocytes/platelets
Megakaryocyte/platelet adhesion
The Rap1 isoform, Rap1b, is highly expressed in both mature megakaryocytes and platelets, and it is expressed at lower levels in immature megakaryocytes.6 Many studies support a role of Rap1 in megakaryocyte/platelet adhesion, which regulates critical aspects of platelet function, including aggregation, adhesion, and spreading. This occurs through direct interactions between platelet integrins and fibrin, fibrinogen, Von Willebrand factor, and collagen, as well as other ligands and receptors.86
In murine megakaryocytes and human MEG-01 cells, the constitutively active mutant RapV12 dramatically increases the binding of the αIIbβ3 integrin to fibrinogen in activated56 and resting cells.55 αIIβ3 activation is negatively regulated by nitric oxide and cyclic guanosine monophosphate (cGMP) via the inhibition of Rap1,87 possibly by protein kinase G (PKG)–dependent activation of Rap1GAP2.46
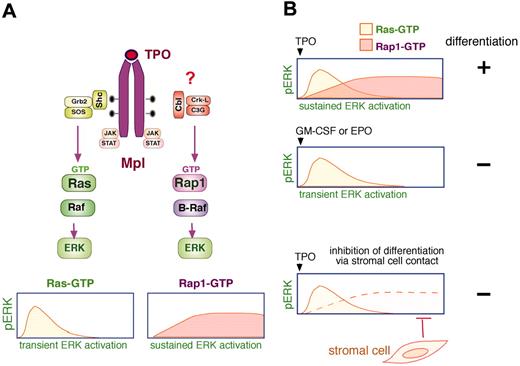
Differentiation of megakaryocyte precursors requires sustained ERK activation. (A) Thrombopoietin (TPO) activation of ERKs has 2 components, a Ras-dependent transient ERK activation and a Rap1-dependent sustained ERK activation. TPO binds its receptor (Mpl) to activate the JAK family kinases that promote tyrosine phosphorylation of the cytoplasmic tail. These phosphorylations serve as binding sites for signaling molecules, including Shc, which recruits Grb2 (growth factor receptor-bound protein-2)/SOS to activate Ras (Ras-GTP) resulting in a transient activation of ERKs (pERK). Sustained pERK requires the activation Rap1 (Rap1-GTP) and B-Raf. The recruitment of a Cbl/CrkL/C3G complex to Mpl has been proposed to trigger the activation of Rap1. (B) Stimulation of megakaryocytes with TPO induces sustained activation/phosphorylation of ERKs (pERK), which is required for megakaryocyte precursor differentiation (+). The sustained ERK activation by TPO has 2 components mediated by Ras and Rap1, as described in panel A. In contrast, stimulation of megakaryocytes with GM-CSF or EPO induces a Ras-dependent transient pERK, which does not lead to cellular differentiation (–). Direct contact between stromal cells and megakaryocytes inhibits the sustained pERK to prevent differentiation (–) by specifically disrupting Rap1/B-Raf signals. Shc indicates SH2-containing protein; STAT, signal transducer and activator of transcription.
Differentiation of megakaryocyte precursors requires sustained ERK activation. (A) Thrombopoietin (TPO) activation of ERKs has 2 components, a Ras-dependent transient ERK activation and a Rap1-dependent sustained ERK activation. TPO binds its receptor (Mpl) to activate the JAK family kinases that promote tyrosine phosphorylation of the cytoplasmic tail. These phosphorylations serve as binding sites for signaling molecules, including Shc, which recruits Grb2 (growth factor receptor-bound protein-2)/SOS to activate Ras (Ras-GTP) resulting in a transient activation of ERKs (pERK). Sustained pERK requires the activation Rap1 (Rap1-GTP) and B-Raf. The recruitment of a Cbl/CrkL/C3G complex to Mpl has been proposed to trigger the activation of Rap1. (B) Stimulation of megakaryocytes with TPO induces sustained activation/phosphorylation of ERKs (pERK), which is required for megakaryocyte precursor differentiation (+). The sustained ERK activation by TPO has 2 components mediated by Ras and Rap1, as described in panel A. In contrast, stimulation of megakaryocytes with GM-CSF or EPO induces a Ras-dependent transient pERK, which does not lead to cellular differentiation (–). Direct contact between stromal cells and megakaryocytes inhibits the sustained pERK to prevent differentiation (–) by specifically disrupting Rap1/B-Raf signals. Shc indicates SH2-containing protein; STAT, signal transducer and activator of transcription.
Many stimuli trigger Rap1 activation in platelets,48 including turbulence,55 epinephrine, adenosine diphosphate (ADP), thrombin, thromboxane A2, and platelet activating factor.88,89 ADP, thrombin, and thromboxane A2 are linked to PLC, protein kinase C (PKC) and rises in intracellular calcium via the G protein Gq. Indeed, platelets deficient in Gq show defects in platelet function.90 However, evidence for calcium-independent activation of Rap1 in platelets also exists, particularly for epinephrine and ADP.91
One Rap1GEF, CalDAG-GEFI, has recently been implicated in mediating the activation of Rap1 and agonist-induced αIIbβ3 binding to fibrinogen.32 Since both calcium and DAG activate CalDAG-GEFI, it is a candidate to link calcium signaling to Rap1. Initially, CalDAG-GEFI was implicated by gene profiling for candidate integrin regulators.92 The role for CalDAG-GEF in platelet aggregation and thrombus formation has emerged from studies in mice deficient for CalDAG-GEFI.33 Platelets from these mice were deficient in aggregation in response to calcium, collagen, ADP, thromboxane A2, and low but not high concentrations of thrombin or DAG analogs, suggesting that CalDAG-GEFI activation of Rap1 was a major mechanism for activation of αIIbβ3 integrins but that other mechanisms also exist. RapL is also expressed in platelets and may mediate these effects.33
Megakaryocyte maturation requires Rap1 activation of ERKs
The maturation of megakaryocytes in the bone marrow is tightly controlled by both extracellular ligands and interaction with stromal cells and a role for Rap1 regulation has been proposed for both processes (Figure 2). Differentiation of magakaryocytes by thrombopoietin (TPO), acting through the Mpl (myeloproliferative virus ligand) receptor, requires sustained ERK activation,93,94 whereas erythropoietin (EPO) and granulocyte macrophage (GM)–CSF, which do not induce differentiation, induce a transient activation of ERKs.95 A model has been proposed that sustained activation of ERKs during the lineage commitment phase of megakaryocytic differentiation results in the synthesis and secretion of autocrine factors that initiate terminal differentiation.94
The requirement for Rap1 in megakaryocytic differentiation has been examined in the human leukemic cell line UT7. Using these cells, it was demonstrated that Ras/Raf-1 activation was responsible for transient, and Rap1/B-Raf for sustained ERK activation.96 The participation of Rap1/B-Raf in sustained ERK signaling was shown using interfering mutants for both Rap1 and B-Raf, as wellas with a mutant Mpl receptor (Mpl-Δ3). TPO activation of Mpl-Δ3 was selectively deficient in sustained ERK activation,96 and lacked activation of Rap1 and B-Raf but retained full activation of Ras and Raf-1. The ability of TPO to activate Ras to mediate transient, and Rap1 to mediate sustained ERK signals is similar to that seen in the neuronal differentiation of PC12 cells stimulated by NGF, which also requires the activation of Rap1 and B-Raf.97 The specific Rap1GEFs downstream of TPO have not been identified; however, an association of a CrkL/C3G complex with Mpl has been described.31,98
Further support for the role of Rap1 in TPO-induced differentiation comes from coculture of megakaryocyte-precursor cell lines with bone marrow stromal cells, which blocks their differentiation. This block requires cell-cell contact and occurs via the inhibition of sustained ERK activation,99 and can be overcome by expression of constitutive mutants of the MAP kinase cascade.100 Stromal cells block sustained ERK activation by selectively interfering with the activation of Rap1, without affecting Ras.100 Although the mechanism by which Rap1 is selectively targeted by stromal inhibition is not known, disruption of protein complexes involving the adaptor protein Cbl following stromal-cell interaction has been shown.100 These studies strongly suggest that Rap1-dependent ERK activation participates in megakaryocytic maturation and is a target of stromal inhibition (Figure 2). It will be necessary to confirm these results using primary megakaryocyte precursors in culture.
Megakaryocytes: integration of signals
For megakaryocytes, the consequence of Rap1 activation may be temporally regulated. In megakaryocyte precursors, Rap1 signaling through B-Raf enhances ERK activation. Signals from TPO to Rap1 promote the sustained activation of ERKs that triggers megakaryocyte differentiation. Since cell-cell contact with stromal cells inhibits Rap1 activation of ERKs in cocultured megakaryocyte precursors, it is possible that the ability of Rap1 activation to enhance adhesion to stromal cells during this critical period is limited by this potential negative feedback loop. It will be important to determine whether stromal-cell contact with megakaryocyte precursors can be regulated by Rap1, and whether TPO activation of Rap1 enhances adhesion of these precursor cells.
In platelets, the major function of Rap1 appears to be the activation of integrin-mediated adhesion; Rap1-dependent regulation of ERKs has not been reported. Are these 2 unique actions of Rap1 regulated at the different stages of development in these cells? The ability to channel Rap1 to effectors upstream of ERKs at the precursor stage, and to integrin activation at later stages, may be achieved by the selective activation of distinct pools of Rap1 that are preferentially coupled to each effector pathway. It is likely that distinct Rap1GEFs may be responsible for activation of B-Raf/ERK signaling and αIIβ3 integrin enhancement. It is also possible that TPO activates selected pools of Rap1 in megakaryocyte precursors that can engage B-Raf, whereas in platelets, agonists may activate pools of Rap1 that are linked to RapL. Rap1 activation of ERK signaling has been shown to occur via C3G.31 Although C3G has been implicated in Rap1-dependent adhesion in many hematopoietic-cell types58,101,102 in platelets, increases in adhesion by platelet agonists are thought to involve CalDAG exchangers.32,33 Alternatively, effectors like B-Raf and RapL may also be differentially expressed during cell development. The megakaryocyte model provides the opportunity to address these general questions of Rap1 signaling.
Leukocyte cells
Rap1 and cell growth (leukemia)
Rap1 has been implicated indirectly in leukemic malignancies through the examination of genetic events resulting in leukemias. CalDAG-GEFI is recurrently activated by proviral insertion in the leukemia-prone BXH-2 mice.103 The SPA-1 gene is located on chromosome 11g13.3, a hot spot in human hematologic malignancies.104 However, the target of Rap1 activation relevant for the development of these leukemias has not been examined.
Human CML is characterized by the acquired translocations of the long arms of chromosomes 9 and 22 that generate the BCR/ABL fusion gene. The importance of dysregulated ABL kinase arising from this translocation is demonstrated by the efficacy of ABL kinase inhibitors in the treatment of this disease.105 CrkL mediates mitogenic signals implicated in BCR/ABL-dependent transformation106 and is highly phosphorylated on tyrosine in leukocytes from patients with CML, suggesting that it may be a substrate of BCR/ABL.31 Hematopoietic cells that have been transformed by expression of BCR/ABL show both constitutive phosphorylation of c-Cbl and its association with CrkL. C3G can also associate with BCR/ABL via CrkL.106-108 However, some studies see a dissolution of the CrkL/C3G complex in BCR/ABL-expressing cells.102
The ability of BCR/ABL to activate Rap1 has recently been demonstrated in the murine hematopoietic cell line TonB210. This activation required ABL kinase activity and resulted in the activation of B-Raf and ERKs.83 Inhibition of Rap1 activation by expression of SPA-1 or the interfering mutant Rap1N17 blocked ERK-dependent signaling,83 demonstrating that in addition to activating Ras/Raf-1,109 BCR/ABL can activate Rap1/B-Raf to activate ERKs. The mechanism of Rap1 activation by BCR/ABL was not examined, although the direct binding of CrkL/C3G to BCR/ABL has been reported108 (Figure 3).
The most provocative study linking Rap1 activation to hematologic malignancies comes from the examination of Spa-1–/– mice.45 In Spa-1–/– myeloid progenitor cells, basal levels of Rap1 activity were elevated. Nearly all mice developed overt leukemias, including those similar to CML in the chronic phase and chronic lymphocytic leukemia (CLL). The mechanism for leukemogenesis in these mice is unknown. However, preleukemic cells showed elevated basal Rap1 activation and constitutive ERK activation without changes in Ras activity. This suggests that in those cells the elevated Rap1 activity was contributing directly to the activation of ERKs, suggesting a possible role for Rap1/B-Raf signaling in the expansion of hematopoietic progenitor cells. However, examination of leukemic blast cell lines derived from Spa-1–/– mice revealed a different pattern of high ERK activity and high Ras activity,45 consistent with an indirect action of Rap1 on ERKs in these cells.
Leukocyte adhesion
Hematopoietic cytokines including IL-3, EPO, TPO, GM-CSF, and stem-cell factors all activate adhesion of hematopoietic cells via β1 integrins.20 β1 integrins are essential for fetal and adult hematopoiesis, in part by establishing proper homing and by increasing cell survival, and the function of leukocytes in trafficking, transendothelial migration, and targeting to sites of inflammation.
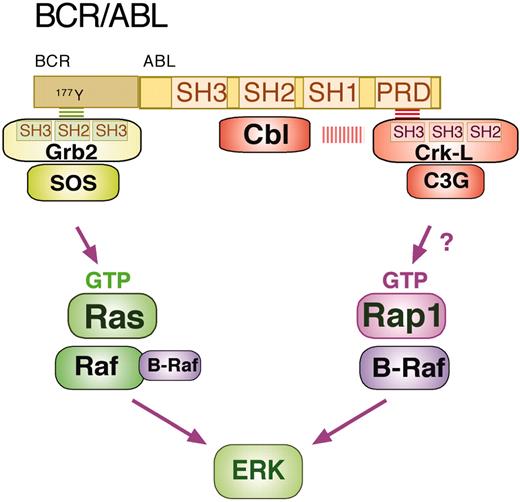
BCR/ABL can activate ERKs via Ras and Rap1. BCR/ABL is a fusion protein between the BCR and the kinase ABL. The ABL kinase contains Src homology domains 1, 2, and 3 (SH1, SH2, and SH3) and a proline-rich domain (PRD). BCR/ABL binds multiple proteins to trigger intracellular signaling cascades. The binding of Grb2, Cbl, and CrkL are shown here. Grb2 associates with BCR/ABL as a complex with the RasGEF SOS (son of sevenless). The SH2 domain of Grb2 binds to phosphotyrosine 177 in the BCR region of BCR-ABL to allow SOS to activate Ras (Ras-GTP), which in turn recruits Raf-1 (and possibly B-Raf) to activate ERK. CrkL exists as a complex with the Rap1 GEF C3G. Recruitment of CrkL/C3G to the PRD of BCR/ABL (via a CrkL SH3 domain) allows C3G to activate Rap1 (Rap1-GTP), which recruits B-Raf to activate ERK. The scaffold molecule Cbl binds the SH2 domain of BCR/ABL and may also participate in the complex with CrkL/C3G.
BCR/ABL can activate ERKs via Ras and Rap1. BCR/ABL is a fusion protein between the BCR and the kinase ABL. The ABL kinase contains Src homology domains 1, 2, and 3 (SH1, SH2, and SH3) and a proline-rich domain (PRD). BCR/ABL binds multiple proteins to trigger intracellular signaling cascades. The binding of Grb2, Cbl, and CrkL are shown here. Grb2 associates with BCR/ABL as a complex with the RasGEF SOS (son of sevenless). The SH2 domain of Grb2 binds to phosphotyrosine 177 in the BCR region of BCR-ABL to allow SOS to activate Ras (Ras-GTP), which in turn recruits Raf-1 (and possibly B-Raf) to activate ERK. CrkL exists as a complex with the Rap1 GEF C3G. Recruitment of CrkL/C3G to the PRD of BCR/ABL (via a CrkL SH3 domain) allows C3G to activate Rap1 (Rap1-GTP), which recruits B-Raf to activate ERK. The scaffold molecule Cbl binds the SH2 domain of BCR/ABL and may also participate in the complex with CrkL/C3G.
Rap1-dependent enhancement of β1 integrin function in leukocyte cells has been examined in the promyelocyte 32D cell line. 32D cells are nonadherent but become adherent following treatment with G-CSF. Blockade of Rap1 activation by retroviral expression of SPA-1 inhibits adhesion by G-CSF.110 Rap1 is also required for β1 integrin–mediated adhesion of 32D cells expressing the EPO receptor (EpoR)20 following stimulation with IL-3, and with EPO. EPO- and IL-3–dependent cell lines respond to cytokine stimulation by inducing c-Cbl association with CrkL/C3G.26 Integrin-mediated adhesion could be blocked by interfering mutants of Rap1, C3G, and CrkL and restored by constitutively active mutants of Rap1.20 Additionally, conditional overexpression of either CrkL or C3G enhanced cytokine activation of Rap1 and adhesion.101 A modest role of PLC in both cytokine activation of Rap1 and adhesion has also been shown,20 suggesting additional mechanisms for Rap1 activation in these cells. Direct evidence that CrkL is required for Rap1 activation by cytokines comes from studies in CrkL–/– mouse embryo fibroblast that show loss of Rap1 activation in response to interferon-α.111
As discussed in the section entitled “Rap1 and cell growth (leukemia),” the Spa-1–/– mouse model implicated Rap1 activation of ERKs in the expansion of Spa-1–/– progenitors.45 Additionally, ERK-independent actions of Rap1 in blast cells have also been implicated by those studies.45 Experiments performed in cell lines established from Spa-1–/– leukemic cells in blast crisis, showed that Rap1 was not enhancing ERKs but that additional changes had occurred that constitutively activated Ras/ERK signals. Restoring SPA-1 expression in blast cells decreased leukemogenesis, suggesting that at this stage, constitutive Rap1 activity was leukemogenic without regulating ERKs.45 It is possible that Rap1 activation of ERKs participates in the expansion of preleukemic cells but that other actions of Rap1 may enhance leukemogenesis at later stages. For example, Rap1 enhancement of adhesion may potentiate ERK activation via outside-in signals from integrins to Ras. Rap1 activation of adhesion may also contribute to the proliferative advantage of Spa-1–/– cells by enabling blast cells to home to niches conducive to leukemogenesis, possibly through direct interaction with stromal cells. Indeed, proper interactions with stromal cells are essential for the immortalization of SPA-1–/– blast cells.45
Leukocytes: integration of signals
Rap1 has recently been implicated in signaling downstream of BCR/ABL, and Rap1 activation of B-Raf and ERKs has been observed following expression of BCR/ABL in myelocyte cell lines.83 However, Rap1 activation of ERKs may not be limited to BCR/ABL-expressing cells. The myeloproliferative disorders and elevated ERK activation seen within myelocytes in Spa-1–/– mice suggest that the Rap1/B-Raf/ERK cascade can be activated in cells lacking BCR/ABL. These properties of Spa-1–/– myelocytes suggest that in normal myelocytes, endogenous SPA-1 limits the activation of this pathway. Moreover, it is also possible that endogenous SPA-1 may regulate discrete pools of Rap1 by its recruitment to specific subcellular locales.
Rap1 activation of ERKs and enhancement of adhesion within the bone marrow may also synergize to augment proliferation of myelocyte progenitors. It will be important to evaluate myelocyte development in cells lacking either RapL (to limit Rap-1–dependent adhesion), or B-Raf (to limit Rap-1–dependent ERK activation) to distinguish the contributions of each arm of Rap1 signaling during leukocyte development.
T lymphocytes
Rap1 inhibition of ERKs
Primary peripheral T cells are not thought to express B-Raf.112 In these cells Rap1 appears to antagonize Ras-dependent ERK activation,113 perhaps by sequestering Raf-1. A model of antagonism of T-cell function was first proposed for Rap1 when it was shown that Rap1 was constitutively active in nonfunctional or anergic T cells.24 T cells activated through the TCR in the absence of CD28 costimulation become anergic,68 which is characterized biochemically by constitutive Rap1 activity and decreased Ras, ERK, JNK, and AP-1 (activator protein-1) activation.68 The anergic phenotype can be mimicked by overexpression of constitutively active mutants of Rap1.24,54 Selected forms of anergy can be suppressed by transgenic expression of B-Raf, allowing Rap1 to couple to ERKs.113
Additional evidence for Rap1 antagonism of ERKs in T cells comes from studies using the Spa-1–/– mice. These mice, which have provided a valuable model of CML, as described in the section entitled “Leukocyte cells,” provide insight into Rap1 signaling in T cells as well. The phenotype of Spa-1–/– T cells is growth inhibition, in contrast to the hyperproliferative defect seen in Spa-1–/– myelocytes. This difference in the actions of Rap1 correlates with the differences in ERK activation and B-Raf expression of the two cell types, but may also be due to other cell-type–specific actions of Rap1. Compared with wild-type T cells, Spa-1–/– T cells were refractive to TCR stimulation, and were associated with constitutive Rap1 activation and diminished ERK activation despite normal or enhanced Ras activity.114 This suggests that the high levels of Rap1 activation achieved by eliminating SPA-1 can antagonize Ras signaling to ERKs in peripheral T cells. However, in that study these effects were age dependent. The age-dependent increase in anergic T cells at 9 to 12 months was attributed to the constitutive activation of Rap1.
Stimulation through the TCR activates Rap1.113,115 Recent studies using Rap1GAP1-expressing T cells showed loss of Rap1 activation by TCR with Rap1GAP1 expression and enhanced TCR-mediated ERK activation.116 These and related studies67 have demonstrated a role for Rap1 to uncouple Ras-mediated ERK activation in peripheral T cells.113 TCR function is enhanced by stimulation of the coreceptor CD28, which is characterized by enhanced ERK activation and IL-2 production67,117 and the inhibition of Rap152,54 without effecting Ras.113,116 It has been proposed that CD28 does this by inducing endogenous Rap1GAP activity.67
In peripheral T cells Rap1 has 2 opposing functions: one is to enhance integrin-mediated adhesion and the other is the regulation of Ras-dependent ERK activation. (A) T-cell activation requires multiple interactions with antigen-presenting cells (APCs). Antigen-dependent interactions are mediated by TCR recognition of antigen bound by the major histocompatibility complex (MHC) on the surface of APCs. Antigen-dependent signals downstream of TCR activate both Rap1 and Ras. Rap1 can enhance T-cell adhesion by stimulating the association of RapL with the α-chain of the integrin LFA-1, enhancing its affinity/avidity for ICAM. TCR activation of Ras and ERKs leads to the activation of multiple transcription factors, including the induction of the transcription factor Fos, which, in conjunction with other transcription factors (NFAT [nuclear factor of activated T cells] and nuclear factor κB [NFκB]), stimulates the transcription and production of IL-2, the major proliferative cytokine of activated T cells. TCR-dependent Rap1 activation antagonizes Ras signaling to ERKs, limiting IL-2 production. APCs express a transmembrane ligand B7 that binds 2 coreceptors, CD28 and CTLA-4, on T cells. Both CD28 and CTLA-4 regulate Rap1. CD28 augments signals from the TCR to IL-2 via multiple pathways, including ERKs shown here. CD28 inhibits TCR-dependent Rap1 activation, providing a mechanism for enhancing ERK-dependent IL-2 production. CTLA-4 antagonizes multiple stimulatory signals from the TCR to IL-2, including ERKs. In contrast to CD28, CTLA-4 activates Rap1, leading to down-regulation of ERK activity, providing a mechanism for the CTLA-4–dependent inhibition of IL-2 production. (B) The balance between the inhibition of ERK-dependent T-cell activation and the enhancement of LFA-dependent T-cell activation optimizes the T-cell response to antigen.
In peripheral T cells Rap1 has 2 opposing functions: one is to enhance integrin-mediated adhesion and the other is the regulation of Ras-dependent ERK activation. (A) T-cell activation requires multiple interactions with antigen-presenting cells (APCs). Antigen-dependent interactions are mediated by TCR recognition of antigen bound by the major histocompatibility complex (MHC) on the surface of APCs. Antigen-dependent signals downstream of TCR activate both Rap1 and Ras. Rap1 can enhance T-cell adhesion by stimulating the association of RapL with the α-chain of the integrin LFA-1, enhancing its affinity/avidity for ICAM. TCR activation of Ras and ERKs leads to the activation of multiple transcription factors, including the induction of the transcription factor Fos, which, in conjunction with other transcription factors (NFAT [nuclear factor of activated T cells] and nuclear factor κB [NFκB]), stimulates the transcription and production of IL-2, the major proliferative cytokine of activated T cells. TCR-dependent Rap1 activation antagonizes Ras signaling to ERKs, limiting IL-2 production. APCs express a transmembrane ligand B7 that binds 2 coreceptors, CD28 and CTLA-4, on T cells. Both CD28 and CTLA-4 regulate Rap1. CD28 augments signals from the TCR to IL-2 via multiple pathways, including ERKs shown here. CD28 inhibits TCR-dependent Rap1 activation, providing a mechanism for enhancing ERK-dependent IL-2 production. CTLA-4 antagonizes multiple stimulatory signals from the TCR to IL-2, including ERKs. In contrast to CD28, CTLA-4 activates Rap1, leading to down-regulation of ERK activity, providing a mechanism for the CTLA-4–dependent inhibition of IL-2 production. (B) The balance between the inhibition of ERK-dependent T-cell activation and the enhancement of LFA-dependent T-cell activation optimizes the T-cell response to antigen.
In contrast to CD28, the coreceptor CTLA-4 (cytotoxic T lymphocyte associated protein-4) inhibits T-cell activation by limiting multiple signals downstream of TCR, including ERKs.118,119 Recent studies have demonstrated that Rap1 mediates the inhibition of ERKs by CTLA-4.116 Rap1GAP1 expression in T cells blocks ERK inhibition by CTLA-4, and partially rescues IL-2 production in these cells. These studies suggest that TCR activation of Rap1 is inhibited by CD28 and activated by CTLA-4, to increase and decrease ERK activation, respectively (Figure 4).
T-cell adhesion
In T cells, Rap1 has been implicated in the enhancement of the function of a variety of integrins, including αLβ2 (LFA-1), α4β1 (VLA-4), and α5β1 (VLA-5).49,52-54,120 Rap1-dependent adhesion via different integrin subsets facilitates T-cell migration and augments antigen-dependent T-cell activation.49,54,121 In the human leukocyte adhesion deficiency, LAD III, a defect in Rap1 activation is responsible for the profound defect in integrin adhesiveness.122
Antigen-dependent activation of Rap1 causes T cells to form stable conjugates with antigen-presenting cells (APCs), which are inhibited by interfering mutants of Rap1, as well as SPA-1 and Rap1GAP1.54,110,116 Transgenic expression of constitutively active mutants of Rap1 augments integrin-mediated T-cell adhesion by increasing the avidity of LFA-1.49,54 Recent studies have shown that the expression of RapV12, a constitutively active mutant of Rap1, can potentiate T-cell responses to antigen, including enhanced thymocyte positive selection, increased proliferation, and IL-2 production.49 In that study,49 the expression of RapV12 had no inhibitory effect on ERK activation, in contrast to studies showing a negative regulatory role of Rap1 on ERK activation.54,114,116 These differences may be due to differences in the level of Rap1-GTP achieved.114 It was proposed that the strong positive effect of Rap1 occurred via T-cell activation by enhancing adhesion to thymic epithelial cells and APCs. These actions on adhesion are thought to be mediated by the Rap1 effector RapL (see the section entitled “Rap1 effector functions”).2,60 This study demonstrates that constitutive activation of Rap1 can induce Ras-independent effects on T-cell activation.
Integration of Rap1 signaling in T cells
Rap1 antagonism of Ras-dependent ERK signaling provides a negative signal for T-cell activation, whereas enhancement of T-cell adhesion provides a positive signal for T-cell activation. These 2 actions of Rap1 are seemingly at odds with each other. T-cell adhesion to APCs is essential for T-cell activation, and constitutive activation of Rap increases T-cell adhesion and T-cell activation.49,54 How can this action of Rap1 be coordinated with its ability to limit T-cell activation through its inhibition of ERKs?
One possibility is that the balance between these opposing functions may be influenced by the characteristics of the antigenic peptide that triggers the T-cell response. While LFA-1/ICAM interactions are essential for T-cell responses by low affinity peptides,49,121 these interactions may not be necessary when high-affinity peptide is used.121 When interactions between APCs and T cells are of high affinity, T cells may become overstimulated by enhanced Rap1-mediated adhesion, resulting in cell death or anergy.24,54 Therefore, in T lymphocytes the 2 functions of Rap1 may work together to enhance T-cell activation in response to weak stimuli, and depress T-cell activation in response to hyperstimulation. In this way, Rap1 activation may optimize T-cell responses to antigenic stimuli (Figure 4).
Early studies showing Rap1 antagonism of Ras/ERK signaling have relied on the use of constitutively active Rap1 mutants. However, subsequent studies in T cells and other cell types have used selective inhibition of Rap1 to establish this function of Rap1.30,123-125 Additionally, Raf-1/ERK may not be the only Ras pathway antagonized by Rap1 in T cells. Rap1 inhibition of Ras-dependent signaling to p38 and reactive oxygen species has been described.70,71 However, it is clear that the physiologic role of Rap1 is not limited to the antagonism of Ras signaling, but can activate T cells to proliferate via pathways that are independent of Ras.
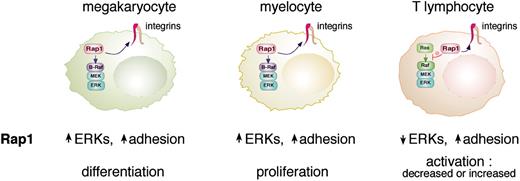
Rap1 has multiple functions in hematopoietic cells. Rap1 can enhance integrin-mediated adhesion in all hematopoietic cells examined to date. Rap1 can also regulate ERK activation in the 3 types of cells shown here (megakaryocytes, myelocytes, and T lymphocytes). Rap1 activates ERK via B-Raf in megakaryocytes and myelocytes that express B-Raf. In T cells that do not express B-Raf, Rap1 antagonizes Ras-dependent ERK activation. The proposed actions of Rap1 on ERK and adhesion and the overall cellular responses to Rap1 are listed below each cell type.
Rap1 has multiple functions in hematopoietic cells. Rap1 can enhance integrin-mediated adhesion in all hematopoietic cells examined to date. Rap1 can also regulate ERK activation in the 3 types of cells shown here (megakaryocytes, myelocytes, and T lymphocytes). Rap1 activates ERK via B-Raf in megakaryocytes and myelocytes that express B-Raf. In T cells that do not express B-Raf, Rap1 antagonizes Ras-dependent ERK activation. The proposed actions of Rap1 on ERK and adhesion and the overall cellular responses to Rap1 are listed below each cell type.
The enhancement of T-cell activation via enhanced integrin/APC interactions forms the basis of Rap1's enhancement of T-cell function. It is generally thought that enhancing integrin adhesion can activate T-cell function by strengthening signals downstream of the TCR. However, the enhanced T-cell function seen in RapV12-overexpressing transgenic T cells is not associated with increased ERK signaling.49 Therefore, it appears that Ras-independent signaling cascades are triggered by outside-in signaling to enhance IL-2 production in that model.
Both functions of Rap1 (enhancement of integrin function and antagonism of Ras-mediated signals) may also act in a temporal manner to optimize T-cell responses. The antagonism of Ras-mediated signals by Rap1 may set a threshold for activation of primed T cells that is regulated by coreceptors such as CD28 and CTLA-4. The enhancement of integrin function by Rap1 may also play a role in the initial TCR engagement and formation of the immunologic synapse by enhancing adhesion during early T-cell activation.
Conclusion
In the 3 examples included in this review, Rap1 displays dual actions: the regulation of ERK signaling and the augmentation of cell adhesion. The ability of Rap1 to activate or inhibit ERK signaling in each of these cells is dictated, in part, by the expression of B-Raf in megakaryocytes and myelocytes, and the absence of B-Raf in peripheral T cells (Figure 5). The activation of ERKs by Rap1/B-Raf participates in differentiation of megakaryocytes and, possibly, in the proliferation of myelocytes, whereas the inhibition of ERKs can limit the proliferation of a subset of peripheral T cells. Rap1 enhancement of cell adhesion also occurs within all of the 3 cell types via a distinct effector pathway possibly involving RapL. What is the consequence of this enhanced adhesion and how does the cell coordinate this action with that of ERK regulation to produce an integrated response to Rap1 activation? It is likely that each cell uses distinct strategies to ensure that Rap1 activation results in a coherent cellular response. Hematopoietic cells offer a unique opportunity to examine these potential distinct strategies by which the outcome of signaling pathways to Rap1 are restricted temporally and spatially to achieve cell-specific outcomes.
Note added in proof. Two recent reports support the ability of Rap1 to antagonize T cell function. According to one report, the transgenic expression of RapE63 in T lymphocytes blocked ERK activation, IL-2 production, and proliferation in peripheral T cells.126 The other report confirms that CTLA-4 activates Rap1 and proposes that Rap1 activation contributes to decreased IL-2 production upon CTLA-4 engagement.127 It is important to note that both studies demonstrate that the Rap1-dependent decrease in T-cell function was accompanied by increased LFA-1-mediated adhesion.
Prepublished online as Blood First Edition Paper, August 2, 2005; DOI 10.1182/blood-2005-03-1062.
Supported in part by National Institutes of Health, National Institute of Allergy and Infectious Disease, grant number AI047 337 (P.J.S.S.).

![Figure 4. In peripheral T cells Rap1 has 2 opposing functions: one is to enhance integrin-mediated adhesion and the other is the regulation of Ras-dependent ERK activation. (A) T-cell activation requires multiple interactions with antigen-presenting cells (APCs). Antigen-dependent interactions are mediated by TCR recognition of antigen bound by the major histocompatibility complex (MHC) on the surface of APCs. Antigen-dependent signals downstream of TCR activate both Rap1 and Ras. Rap1 can enhance T-cell adhesion by stimulating the association of RapL with the α-chain of the integrin LFA-1, enhancing its affinity/avidity for ICAM. TCR activation of Ras and ERKs leads to the activation of multiple transcription factors, including the induction of the transcription factor Fos, which, in conjunction with other transcription factors (NFAT [nuclear factor of activated T cells] and nuclear factor κB [NFκB]), stimulates the transcription and production of IL-2, the major proliferative cytokine of activated T cells. TCR-dependent Rap1 activation antagonizes Ras signaling to ERKs, limiting IL-2 production. APCs express a transmembrane ligand B7 that binds 2 coreceptors, CD28 and CTLA-4, on T cells. Both CD28 and CTLA-4 regulate Rap1. CD28 augments signals from the TCR to IL-2 via multiple pathways, including ERKs shown here. CD28 inhibits TCR-dependent Rap1 activation, providing a mechanism for enhancing ERK-dependent IL-2 production. CTLA-4 antagonizes multiple stimulatory signals from the TCR to IL-2, including ERKs. In contrast to CD28, CTLA-4 activates Rap1, leading to down-regulation of ERK activity, providing a mechanism for the CTLA-4–dependent inhibition of IL-2 production. (B) The balance between the inhibition of ERK-dependent T-cell activation and the enhancement of LFA-dependent T-cell activation optimizes the T-cell response to antigen.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/9/10.1182_blood-2005-03-1062/6/m_zh80210586160004.jpeg?Expires=1769118780&Signature=MVSZxOTFsBx3rUQbWTqnQAFM~Hl8bnMZPgv4XlHS14Ea0EQJ4IzD4qCFHHl1ETSba4DvPdhsQy8s6Qob6rWzeKJvzLvNb4Q5deO~rJ~ahYuJHU2xjjLyJnhPfHm5jKUU5eFboObs8lpp4wgKO6W4i5v3OObYls04h17cEVjLfQEdaT6wlhogJ3wVSIRao~xSkJ1SnJ~YmLVJPKv0j5bNdgFKiY8mvyaruN3WwKfvHyL832ceEsjW8Ub493HkaTHcgzcKxFo~1SszlTGRsqBrY14XVzHjbIwuZUZwNq6XwiVIENRR53kFswMuFASYagVi4dRm~i0gNYgbsi-e3EzEsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal