Abstract
Because CD4+ T cells play a key role in aiding cellular immune responses, we wanted to assess whether increasing numbers of gene-engineered antigen-restricted CD4+ T cells could enhance an antitumor response mediated by similarly gene-engineered CD8+ T cells. In this study, we have used retroviral transduction to generate erbB2-reactive mouse T-cell populations composed of various proportions of CD4+ and CD8+ cells and then determined the antitumor reactivity of these mixtures. Gene-modified CD4+ and CD8+ T cells were shown to specifically secrete Tc1 (T cytotoxic-1) or Tc2 cytokines, proliferate, and lyse erbB2+ tumor targets following antigen ligation in vitro. In adoptive transfer experiments using severe combined immunodeficient (scid) mice, we demonstrated that injection of equivalent numbers of antigenspecific engineered CD8+ and CD4+ T cells led to significant improvement in survival of mice bearing established lung metastases compared with transfer of unfractionated (largely CD8+) engineered T cells. Transferred CD4+ T cells had to be antigen-specific (not just activated) and secrete interferon γ (IFN-γ) to potentiate the antitumor effect. Importantly, antitumor responses in these mice correlated with localization and persistence of geneengineered T cells at the tumor site. Strikingly, mice that survived primary tumor challenge could reject a subsequent rechallenge. Overall, this study has highlighted the therapeutic potential of using combined transfer of antigen-specific gene-modified CD8+ and CD4+ T cells to significantly enhance T-cell adoptive transfer strategies for cancer therapy.
Introduction
Much attention has been directed at the genetic modification of T cells and their therapeutic potential in the adoptive immunotherapy of cancer. T cells engineered to express chimeric surface receptors that incorporate an extracellular single-chain antibody domain (scFv) and a transmembrane and cytoplasmic signaling domain can specifically direct antitumor immune responses in a major histocompatibility complex (MHC)–independent manner at targets normally capable of evading immune recognition. These targets also often lack important costimulatory ligands for maximal T-cell activation. The therapeutic potential of engineered T cells extends from studies demonstrating specific antigen binding and target-cell lysis in vitro1-3 and in a range of different mouse tumor models4-8 to their successful transfer into patients with minimal side effects in phase 1 clinical trials.9
A number of studies have demonstrated the importance of CD4+ T-cell help in elimination of infectious disease and for antitumor immunity.10,11 CD4+ T cells have been demonstrated to be critical for maintenance of CD8+ T-cell numbers, their recruitment to the tumor site,12-14 and induction of a memory response.15,16 In patients infected with HIV, the coinfusion of genetically modified CD4+ and CD8+ T cells has been demonstrated to overcome the lack of T-cell persistence observed with transfusion of engineered CD8+ T cells alone.9,17 However, despite these promising studies, the issue of whether the addition of antigenspecific engineered CD4+ T-cell help may enhance antitumor immunity, long-term persistence, and secondary tumor rejection has not been properly tested in vivo for adoptively transferred T cells.
In the interest of generating an optimal antitumor response, we wanted to determine whether increasing the proportion of engineered CD4+ T cells could more effectively eradicate disease. In this study we have used retroviral transduction for expression of the scFv-CD28-ζ receptor, reactive against the erbB2 human breast cancer–associated antigen, on the surface of both CD8+ and CD4+ splenic mouse T cells. Strikingly, we show that coinfusing equivalent numbers of engineered CD8+ and CD4+ T cells led to complete eradication of established tumor in mice, whereas transfer of mainly CD8+ T cells was less effective. The enhanced efficacy was entirely dependent on antigenic specificity of the CD4+ T cells and correlated with tumor localization and long-term persistence of both CD8+ and CD4+ T cells. Interestingly, mice “cured” of primary tumor could subsequently reject a secondary challenge with erbB2+ tumor. Thus, these studies show that CD4+ T cells are an important component in successful adoptive immunotherapy with retrovirus-transduced CD8+ cytotoxic T lymphocytes (CTLs), thus raising important implications for optimizing adoptive T-cell therapy for cancer treatment in patients.
Materials and methods
Cell culture
MDA-MB-435 human mammary carcinoma cells, the erbB2-expressing MDA-MB-435 cells, 4T1.2 mouse mammary carcinoma cells, and the erbB2-expressing 4T1.2-erbB2 cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% (vol/vol) fetal calf serum (FCS), 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Life Technologies, Grand Island, NY). Retrovirus-producing GP+E86 cells were cultured in DMEM containing 0.5 mg/mL G418 (Life Technologies).
Mice
BALB/c and BALB/c scid/scid (scid) mice were purchased from the Walter and Eliza Hall Institute of Medical Research (Melbourne, Australia). BALB/c interferon γ (IFN-γ)–deficient (BALB/c IFN-γ–/–) mice were bred at the Peter MacCallum Cancer Centre. Mice 6 to 12 weeks of age were used in experiments that were performed in accordance with the Peter MacCallum Cancer Centre's animal experimental ethics committee guidelines.
Generation of scFv receptor-transduced CD8+ and CD4+ mouse T cells
A chimeric gene construct composed of the scFv–anti-erbB2 monoclonal antibody (mAb), a membrane-proximal hinge region of human CD8, and the transmembrane and cytoplasmic regions of the mouse CD28 signaling chain fused to the cytoplasmic region of human T-cell receptor ζ (TCR-ζ; scFv–anti-erbB2 CD28-ζ) was cloned into the retroviral vector pLXSN as previously described.5 A stable GP+E86 ecotrophic packaging cell line expressing the scFv–anti-erbB2 CD28-ζ receptor was isolated as previously described.5 Transduction of mouse splenic T lymphocytes was performed as described previously.7,18 To generate transduced CD8+ and CD4+ T cells, each T-cell subset was initially isolated by labeling with anti-CD4 or anti-CD8 magnetic beads (Miltenyi Biotec, Auburn, CA) and passed through a magnetic-activated cell sorting (MACS) depletion column. The efficiency of isolating separate T-cell subsets was verified by flow cytometry. Enriched CD8+, CD4+, or unfractionated T-cell cultures (107 cells) were immediately cocultured with retrovirus-producing packaging cells (5 × 105) for 72 hours in DMEM supplemented with 4 μg/mL polybrene, 5 μg/mL phytohemagglutinin (PHA; Sigma, Saint Louis, MO), and 100 U/mL human recombinant interleukin-2 (rIL-2; Chiron, Emeryville, CA).
Flow cytometry
Expression of the α-erbB2-CD28-ζ chimeric receptor on the surface of CD8+, CD4+, or unfractionated transduced mouse T cells was determined by indirect immunofluorescence with a c-myc tag antibody (Ab), followed by staining with a phycoerythrin (PE)–labeled anti–mouse immunoglobulin mAb (BD Biosciences, San Jose, CA). Background fluorescence was assessed using the PE-labeled anti–mouse immunoglobulin mAb alone. Cell-surface phenotyping of transduced cells was determined by direct staining with fluorescein isothiocyanate (FITC)–labeled anti-CD4 (RM4-5; PharMingen, San Diego, CA) and PE-labeled anti-CD8 (53-6.7; PharMingen) mAbs, as previously described.4,5,7,18
Antigen-specific cytotoxicity, cytokine secretion, and proliferation by transduced T cells
The ability of transduced T cells to specifically mediate target-cell lysis was assessed in a 6-hour chromium-release assay, as described previously.19 The capacity of transduced T cells to produce cytokines (IFN-γ, IL-2, granulocyte-macrophage colony-stimulating factor [GM-CSF], IL-4) after erbB2 antigen ligation was determined by enzyme-linked immunosorbent assay (ELISA) (PharMingen) and the proliferative capacity of transduced T cells was assessed in a [3H]-thymidine incorporation assay, as described previously.3,5
Immunohistochemistry
Hematoxylin and eosin staining and immunohistochemistry were performed on frozen sections. Antibodies used were FITC-antimouse CD4, biotin-antimouse CD8b.2, allophycocyanin (APC)–antimouse CD11b, FITC-antimouse CD11b (all from Becton Dickinson, San Jose, CA), biotin-antimyc tag (Abcam, Cambridge, United Kingdom), isotype controls, and a streptavidin Alexa 594 secondary (Molecular Probes, Eugene, OR). The microscopes used were a Leica DMBRE (Leica, Wetzlar, Germany) with a Plan (PL) FLUOTAR, numerical aperture (NA) 0.7 objective lens and Bio-Rad MRC 1024 confocal (Bio-Rad, Hercules, CA) for confocal images and a Zeiss Axioskop 2 (Zeiss, Hertfordshire, United Kingdom) with a Plan-NEC FLUAR, NA 0.7/40 × objective lens and RT SE Diagnostic Instruments SPOT camera (Diagnostic Images, Sterling Heights, MI) for H&E images. Original magnification was × 400. Tissue sections were mounted with DakoCytomation Fluorescent Mounting Medium (DakoCytomation, Glostrup, Denmark) or stained for H&E. Image acquisition and processing software was LaserSharp 2000 or SPOT Basic Version 4.1 and Adobe Photoshop CS2, respectively.
PCR
The detection of gene-engineered T cells following adoptive transfer in mice was assessed by polymerase chain reaction (PCR) amplification of the neomycin phosphotransferase gene. Mice bearing 5-day MDA-MB-435 tumor were given injections of scFv-CD28-ζ–transduced CD8+ and CD4+ T cells and were eye bled or had spleens removed at various time points. Red blood cells were lysed using ACK lysis buffer (room-temperature ammonium chloride potassium for 5 minutes), washed, and cells resuspended at 5 × 106 cells/200 μL. gDNA from peripheral blood or splenocytes was subsequently purified for PCR using QIAamp DNA Blood MiniKit per the manufacturer's instructions (Qiagen, Clifton Hill, Australia). Neomycin sense primer was 5′-TGGCTGCTATTGGGCGAAGT-3′; antisense was 5′-TATCACGGGTAGCCAACGCT-3′, mouse β-actin sense primer was 5′-AGGCGGTGCTGTCCTTGTAT-3′; and antisense was 5′-GGAAGGAAGGCTGGAAGAGT-3′. The forward and reverse primers for both neomycin and β-actin genes were designed to amplify fragments of approximately 400 base pairs (bp) using Platinum Pfx DNA polymerase (Invitrogen Life Sciences, Carlsbad, CA).
Adoptive transfer model
The antitumor activity of transduced T cells was assessed against the MDA-MB-435-erbB2 tumor cell line as previously described.5 Briefly, scid mice were given intravenous injections of 5 × 106 human MDA-MB-435-erbB2 breast carcinoma cells to establish pulmonary metastases. Unfractionated scFv-CD28-ζ–transduced T cells (107), transduced CD8+ or CD4+ T cells alone (107), or a 1:1 combination of transduced CD8+ and CD4+ T cells (5 × 106 of each) from BALB/c donor mice were injected intravenously into groups of 5 to 10 mice at day 5 after tumor inoculation. For experiments evaluating the minimum number of engineered CD4 cells required to achieve total tumor regression, mice received different ratios of transduced CD8+ and CD4+ T cells. The adoptive transfer of T cells transduced with an irrelevant scFv receptor (scFv-α-CEA-γ) or mock transduced with an empty vector pLXSN served as controls. In addition, adoptive transfer of scFv-transduced CD8+ and CD4+ T lymphocytes (5 × 106 of each, day 5) from either BALB/c-wild-type or BALB/c IFN-γ–/– donor mice was used to evaluate the role of IFN-γ released by either T-cell subset in the antitumor effect. All mice were monitored daily for tumor growth. Tumor growth was assessed as follows: (1) in survival experiments, mice that were morbid were killed and the day of death and lung weights recorded or (2) mice were killed at days 6, 12, or 16 and harvested lungs were fixed in 10% formalin, embedded, sectioned, and stained with hematoxylin and eosin for histologic examination or frozen sections of lungs were made for immunohistochemical analysis.
Tumor rechallenge experiments
Mice surviving the primary MDA-MB-435 tumor long-term (> 100 days) were rechallenged with an intravenous injection of 5 × 106 human MDA-MB-435-erbB2 tumor cells and survival monitored over 100 days. In another set of experiments, mice were rechallenged with a subcutaneous injection of the mouse mammary carcinoma 4T1.2-erbB2 cells or 4T1.2 parental cells at 5 × 104 (high dose) or 5 × 103 (low dose). Survival of mice was monitored daily and defined as the period with no overt signs of distress, as assessed by 2 independent observers. Subcutaneous tumor growth was measured by a caliper square along the perpendicular axes of the tumors. Data were recorded as either percentage survival or the mean tumor size (mm2, product of the 2 perpendicular diameters) ± SEM.
Results
Expression of the scFv-CD28-ζ receptor in transduced CD8+ and CD4+ primary mouse T cells
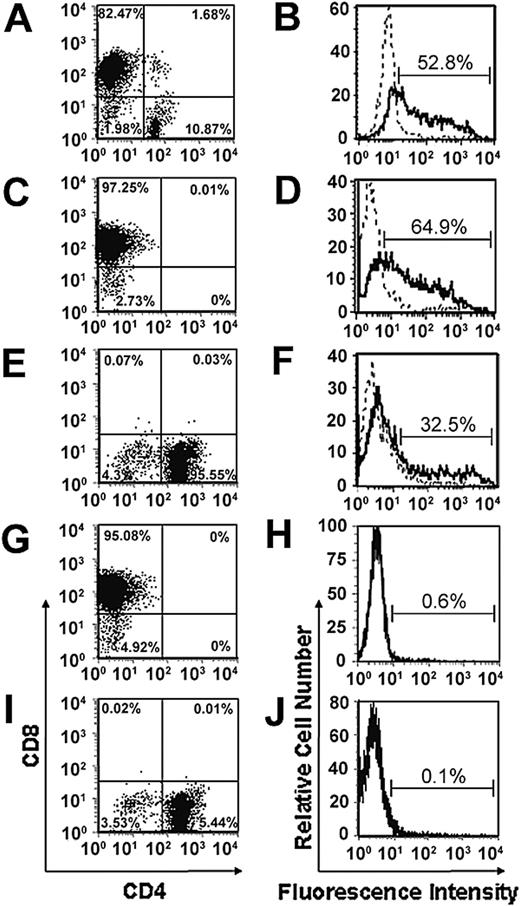
Consistent with our previous studies, we have demonstrated that retroviral transduction of unfractionated PHA/IL-2–stimulated splenic T cells consistently resulted in a high proportion of CD8+ T cells (80%-85%) but low numbers of CD4+ T cells (10%-15%; Figure 1A-B).4,5,7,18 To determine whether we could achieve expression of the chimeric α-erbB2-CD28-ζ receptor in a greater percentage of CD4+ T cells, CD8+ and CD4+ T cells were isolated by magnetic bead depletion prior to transduction. Using this approach, we were able to achieve good levels of expression of the chimeric α-erbB2-CD28-ζ receptor in CD8+ and CD4+ T cells after staining with a c-myc anti-tag antibody (Figure 1D-F) compared to mock-transduced CD8+ or CD4+ T cells (Figure 1H,J), although expression was consistently found to be higher in CD8+ T cells than CD4+ T cells. This may reflect less efficient cell cycling by CD4+ T cells following PHA/IL-2 stimulation during the transduction process. Importantly, the T-cell populations consisted of more than 95% CD8+ or CD4+ T cells following transduction (Figure 1C,E,G,I). There was negligible expression of the chimeric receptor in either the CD8– or CD4– populations (data not shown).
Expression of the scFv-CD28-ζ receptor in transduced CD8+ and CD4+ primary mouse T cells. Splenic T cells enriched from BALB/c mice were depleted into CD4+ and CD8+ T-cell subsets or left as unfractionated T cells prior to retroviral transduction with the scFv-CD28-ζ receptor. Transduced unfractionated T cells consisted of 80% to 85% CD8+ T cells and about 10% CD4+ T cells (A), whereas isolated populations consisted of more than 90% CD8+ (C,G) or CD4+ T cells (E,I). Chimeric scFv receptor expression was detected in unfractionated transduced T cells (B), CD8+ (D), and CD4+ (F) T cells by flow cytometry following staining with an anti-tag mAb and PE-labeled sheep anti–mouse immunoglobulin (solid line) or with the PE-labeled secondary alone (dashed line). Negligible receptor expression was detected in isolated populations of CD8+ (H) and CD4+ (J) T cells transduced with the empty LXSN vector. Similar results were obtained in 10 experiments.
Expression of the scFv-CD28-ζ receptor in transduced CD8+ and CD4+ primary mouse T cells. Splenic T cells enriched from BALB/c mice were depleted into CD4+ and CD8+ T-cell subsets or left as unfractionated T cells prior to retroviral transduction with the scFv-CD28-ζ receptor. Transduced unfractionated T cells consisted of 80% to 85% CD8+ T cells and about 10% CD4+ T cells (A), whereas isolated populations consisted of more than 90% CD8+ (C,G) or CD4+ T cells (E,I). Chimeric scFv receptor expression was detected in unfractionated transduced T cells (B), CD8+ (D), and CD4+ (F) T cells by flow cytometry following staining with an anti-tag mAb and PE-labeled sheep anti–mouse immunoglobulin (solid line) or with the PE-labeled secondary alone (dashed line). Negligible receptor expression was detected in isolated populations of CD8+ (H) and CD4+ (J) T cells transduced with the empty LXSN vector. Similar results were obtained in 10 experiments.
Transduced CD8+ and CD4+ mouse T cells mediate antigen-specific cytokine secretion, proliferation, and tumor-cell lysis
The functional capacity of either unfractionated or CD4+- or CD8+-transduced T cells was compared in a number of different in vitro assays. We have previously shown the ability of scFv-CD28-ζ unfractionated engineered T cells to secrete Tc1 (T cytotoxic-1) cytokines (IFN-γ and GM-CSF) on antigen stimulation with erbB2+ target cells.5,6 Thus, it was of interest to determine the cytokine secretion pattern of isolated CD8+ and CD4+ T-cell populations transduced with the scFv-CD28-ζ receptor. Following coculture with MDA-MB-435-erbB2 tumor cells, transduced CD8+ T cells produced high levels of Tc1 cytokines IFN-γ and GM-CSF (Figure 2A-B). However, transduced CD4+ T cells produced high levels of both Tc1 (IFN-γ, GM-CSF, IL-2; Figure 2A-B,D) and Tc2 (IL-4; Figure 2C) cytokines. Consistent with previous studies,20 unfractionated and CD8+-transduced T cells produced high levels of IFN-γ and GM-CSF but no IL-4 or IL-2 cytokines following stimulation with antigen-positive tumor targets (Figure 2A-D). In contrast, Finney et al demonstrated significant IL-2 release for transduced CD8+ T cells following stimulation using plate-bound antigen.21 This difference may be due to plate-bound antigen or immobilized antibody (or both) providing a stronger signal to transduced T cells compared with antigen-positive tumor targets. The secretion of cytokines by scFv-CD28-ζ–transduced CD8+ and CD4+ cells was antigen-specific because transduced T cells cocultured with erbB2–MDA-MB-435 parental tumor cells demonstrated no significant cytokine secretion. In addition, mocktransduced CD8+ and CD4+ cells cocultured with either cell line secreted no measurable cytokine. We next examined the proliferative capacity of the various T-cell populations transduced with the scFv-CD28-ζ receptor by measuring [3H]-thymidine incorporation following stimulation with plate-bound anti-tag antibody. Interestingly, there was no significant difference in the level of proliferation observed by transduced CD4+, CD8+, or unfractionated T cells following receptor ligation and proliferation by all transduced T-cell populations was comparable to stimulation through the endogenous T-cell and CD28 receptors (Figure 2E). No proliferation was observed by mock-transduced CD4+, CD8+, or unfractionated T cells following stimulation with anti-tag mAb (Figure 2E). In another experiment, the addition of irradiated engineered CD4+ T cells (5 × 104) to transduced CD8+ T cells (5 × 104) did not increase the level of CD8+ T-cell proliferation compared with transduced CD8+ T cells alone following stimulation with anti-tag mAb (ie, irradiated transduced CD4+ and CD8+ T cells, 30 021 ± 3266 cpm versus transduced CD8+ T cells alone, 33 620 ± 5347 cpm). Lastly, we compared the ability of unfractionated, CD4+, and CD8+ T cells engineered with the scFv-CD28-ζ receptor to mediate specific lysis of MDA-MB-435-erbB2 target cells in a 6-hour 51Cr-release assay. Interestingly, transduced unfractionated and CD8+ T cells mediated greater cytolytic activity of MDA-MB-435-erbB2 target cells compared with transduced CD4+ T cells (Figure 2F). No lysis of erbB2–MDA-MB-435 parental tumor cells was observed by any of the scFv-CD28-ζ engineered T-cell populations and mock-transduced CD8+ and CD4+ T cells could not lyse either cell line (Figure 2G). Taken together, these data demonstrated that both CD8+ and CD4+ isolated T cells could be effectively transduced with the scFv-CD28-ζ receptor and mediate antigen-specific cytokine release, proliferation, and cytolytic activity.
Antigen-specific cytokine secretion, proliferation, and cytotoxicity by transduced mouse CD8+ and CD4+ T cells. To evaluate cytokine secretion, unfractionated T cells (□), CD8+ T cells (dotted bars) and CD4+ T cells (diagonally striped bars) transduced with the scFv-CD28-ζ receptor or mock-transduced unfractionated T cells (▪), CD8+ T cells (cross-hatched bars), and CD4+ T cells (▤) were cocultured with media alone or irradiated MDA-MB-435-erbB2 cells or MDA-MB-435 parental cells or stimulated with plate-bound anti-CD3 and anti-CD28 mAbs in 12-well plates for 24 hours. Supernatants were harvested and assessed for IFN-γ (A), GM-CSF (B), IL-4 (C), and IL-2 (D) production by ELISA. Results are expressed as picogram per milliliter of cytokine secreted ± SE for duplicate samples of 3 representative experiments. The proliferative capacity of each transduced T-cell population was evaluated in a 72-hour [3H]-thymidine incorporation assay (E). Transduced unfractionated T cells (□), CD8+ T cells (dotted bars), and CD4+ T cells (diagonally striped bars) or mock-transduced unfractionated T cells (▪), CD8+ T cells (cross-hatched bars), and CD4+ T cells (▤) were incubated with media alone or stimulated with plate-bound anti-CD3 and anti-CD28 mAbs or anti-tag mAb for 3 days. Results are expressed as means ± SE of triplicate samples and are representative of 3 experiments. Specific cytolytic function of scFv-CD28-ζ–transduced mouse T cells was evaluated in a 6-hour 51Cr-release assay. Transduced unfractionated (▵) and CD8+ (○) T cells demonstrated greater lysis of MDA-MB-435-erbB2 target cells than CD4+-transduced (▪) T cells (F). No lysis of MDA-MB-435 parental target cells was observed by chimeric receptor-transduced T cells (G) and mock-transduced unfractionated T cells (▪), CD8+ T cells (▴), or CD4+ T cells (□) did not lyse either cell line. The spontaneous lysis was less than 10% in all assays. Results are expressed as percent specific 51Cr release ± SE (%) of triplicate samples representative of at least 2 experiments.
Antigen-specific cytokine secretion, proliferation, and cytotoxicity by transduced mouse CD8+ and CD4+ T cells. To evaluate cytokine secretion, unfractionated T cells (□), CD8+ T cells (dotted bars) and CD4+ T cells (diagonally striped bars) transduced with the scFv-CD28-ζ receptor or mock-transduced unfractionated T cells (▪), CD8+ T cells (cross-hatched bars), and CD4+ T cells (▤) were cocultured with media alone or irradiated MDA-MB-435-erbB2 cells or MDA-MB-435 parental cells or stimulated with plate-bound anti-CD3 and anti-CD28 mAbs in 12-well plates for 24 hours. Supernatants were harvested and assessed for IFN-γ (A), GM-CSF (B), IL-4 (C), and IL-2 (D) production by ELISA. Results are expressed as picogram per milliliter of cytokine secreted ± SE for duplicate samples of 3 representative experiments. The proliferative capacity of each transduced T-cell population was evaluated in a 72-hour [3H]-thymidine incorporation assay (E). Transduced unfractionated T cells (□), CD8+ T cells (dotted bars), and CD4+ T cells (diagonally striped bars) or mock-transduced unfractionated T cells (▪), CD8+ T cells (cross-hatched bars), and CD4+ T cells (▤) were incubated with media alone or stimulated with plate-bound anti-CD3 and anti-CD28 mAbs or anti-tag mAb for 3 days. Results are expressed as means ± SE of triplicate samples and are representative of 3 experiments. Specific cytolytic function of scFv-CD28-ζ–transduced mouse T cells was evaluated in a 6-hour 51Cr-release assay. Transduced unfractionated (▵) and CD8+ (○) T cells demonstrated greater lysis of MDA-MB-435-erbB2 target cells than CD4+-transduced (▪) T cells (F). No lysis of MDA-MB-435 parental target cells was observed by chimeric receptor-transduced T cells (G) and mock-transduced unfractionated T cells (▪), CD8+ T cells (▴), or CD4+ T cells (□) did not lyse either cell line. The spontaneous lysis was less than 10% in all assays. Results are expressed as percent specific 51Cr release ± SE (%) of triplicate samples representative of at least 2 experiments.
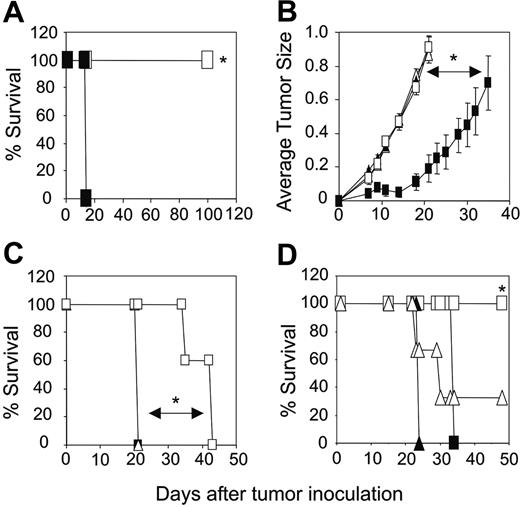
Increased transfer of engineered CD4+ T cells enhances tumor-free survival of mice
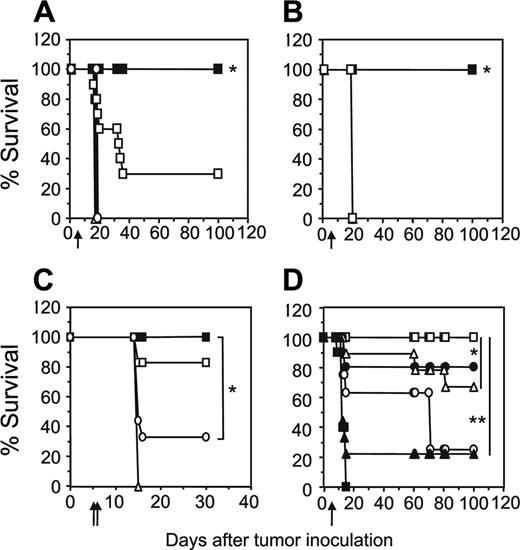
The adoptive transfer of unfractionated scFv-α-erbB2-CD28-ζ–engineered T cells containing about 10% transduced CD4+ T cells into scid mice bearing 5-day MDA-MB-435-erbB2 lung metastases has previously demonstrated between 20% and 50% long-term survival of mice.5 To test whether increasing the proportion of gene-modified CD4+ T cells could enhance the antitumor response in this model, we compared survival of mice treated with unfractionated scFv-CD28-ζ–transduced T cells (107) or the same total number of CD8+ and CD4+ T cells (5 × 106 cells of each). Strikingly, we demonstrated 100% survival of mice bearing 5-day MDA-MB-435-erbB2 lung metastases that received a 1:1 ratio of scFv-CD28-ζ–engineered CD8+ and CD4+ T cells compared to 30% survival of mice treated with unfractionated transduced T cells (P < .005; Figure 3A). The enhanced antitumor response was entirely dependent on the transfer of appropriately targeted CD4+ T cells, because mice treated with a 1:1 combination of transduced CD8+ T cells and naïve CD4+ T cells or transduced CD8+ T cells and CD4+ T cells engineered with an irrelevant scFv-α-CEA-γ receptor did not survive beyond 20 days (Figure 3B). In addition, mice receiving a 1:1 ratio of transduced CD8+ and CD4+ T cells of irrelevant specificity or no T cells did not survive (Figure 3A). Interestingly, no antitumor effect was observed in mice that received either 107 transduced CD4+ or CD8+ T cells alone indicating a requirement for cooperation of the 2 types of T cell against such established tumors (Figure 3A). In another experiment, increasing the dose by administering 2 injections of 107 gene-engineered CD8+ T cells alone at days 5 and 6 resulted in improved survival of a small number of mice (Figure 3C). However, mice receiving both engineered CD8+ and CD4+ T cells at a 1:1 ratio at just a single dose again resulted in 100% survival. Thus, clearly in our model, optimal antitumor effects were observed by inclusion of engineered CD4+ T cells in the treatment regimen. In these experiments, it may have been likely that 5-day established tumor was too advanced for transduced CD8+ T cells to exert an effective antitumor effect, which is consistent with other adoptive transfer studies involving antigen-specific CD8+ T cells.14,22
To determine the optimal ratio of scFv-CD28-ζ–transduced CD4+ and CD8+ T cells required to achieve 100% survival, mice bearing 5-day MDA-MB-435-erbB2 tumor were treated with different ratios of scFv-CD28-ζ–transduced CD8+ and CD4+ T cells. Interestingly, only a 1:1 ratio of transduced CD8+ and CD4+ T cells (5 × 106 of each T-cell subset) resulted in 100% survival consistent with previous experiments (Figure 3D). In contrast, the percentage of survivors decreased significantly when mice received a greater proportion of either CD8+- or CD4+-transduced T cells (Figure 3D). Collectively, these results demonstrated for the first time in vivo that optimal transfer of engineered antigen-specific CD4+ T cells could significantly improve redirected T-cell therapy for treatment of cancer.
IFN-γ released by CD8+-but not CD4+-engineered T cells iscritical for antitumor rejection
A critical role for IFN-γ has previously been shown in the antitumor response by adoptively transferred unfractionated engineered T cells.5 To further dissect the importance of antigenspecific release of IFN-γ secreted by either the CD8+ or CD4+ subsets, we transduced and adoptively transferred different combinations of CD8+ and CD4+ donor T cells from BALB/c wild-type and IFN-γ knockout mice. It is important to note that the expression of the scFv-CD28-ζ receptor in CD8+ and CD4+ T cells from IFN-γ–/– knockout mice was equivalent to that detected in wild-type CD8+ and CD4+ T cells (data not shown). Consistent with previous experiments all mice bearing 5-day MDA-MB-435erbB2 tumor survived following treatment with 1:1 transfer of IFN-γ wild-type CD8+- and CD4+-transduced T cells (Figure 4). This was also found to be the case with mice treated with 1:1 transfer of CD8+IFN-γ+/+- and CD4+IFN-γ–/–-transduced T cells, suggesting that IFN-γ produced by engineered CD4+ T cells was not critical for this antitumor effect. In contrast, only 50% of mice treated with CD8+IFN-γ–/–- and CD4+IFN-γ+/+-transduced T cells survived, indicating that IFN-γ secreted by engineered CD8+ T cells was absolutely essential to achieve 100% survival of mice (Figure 4). Nevertheless, IFN-γ produced by engineered antigenspecific CD4+ T cells was able to partially compensate given the fact that transfer of scFv-CD28-ζ–transduced CD8+IFN-γ–/– and CD4+IFN-γ–/– T cells had no effect.
Increased transfer of engineered CD4+ T cells enhances tumor-free survival of mice. (A) Groups of 10 scid mice were given intravenous injections of 5 × 106 MDA-MB-435-erbB2 cells at day 0 prior to intravenous injection of scFv-CD28-ζ–transduced T cells at day 5. Mice treated with a 1:1 ratio of CD4+-transduced (5 × 106) and CD8+-transduced (5 × 106) T cells (▪) demonstrated 100% survival compared to 30% survival of mice receiving unfractionated transduced T cells (□; *P < .005, Mann-Whitney test). No survival of mice was observed for those receiving CD4+-transduced T cells alone (▪), CD8+-transduced T cells alone (○), control transduced T cells (▵), or no T cells (▴). (B) Enhanced survival of mice was due to increased transfer of antigen-specific CD4+ T cells. All scid mice bearing 5-day MDA-MB-435-erbB2 tumor that received a 1:1 ratio of CD8+-transduced (5 × 106) and CD4+-transduced (5 × 106) T cells (▪) survived compared with mice receiving either a 1:1 combination of transduced CD8+ T cells (5 × 106) and naïve CD4+ T cells (5 × 106; □), 1:1 combination of transduced CD8+ (5 × 106) T cells and CD4+ T cells (5 × 106) transduced with an irrelevant scFv-anti-CEA-γ receptor (▵) or no T-cell treatment (▴; *P < .001, Mann-Whitney test). (C) Transfer of a greater number of transduced CD8+ T cells alone does not rescue all mice from disease. Mice were given intravenous injections of 5 × 106 MDA-MB-435-erbB2 cells at day 0 prior to intravenous injection of scFv-CD28-ζ–transduced T cells. Mice treated at day 5 with a single dose of CD4+-transduced (5 × 106) and CD8+-transduced (5 × 106) T cells (▪) in a 1:1 ratio demonstrated 100% survival compared to about 30% survival for mice treated at days 5 and 6 with transduced CD8+ T cells alone (107/injection; ○)or about 80% survival for mice treated at days 5 and 6 with transduced unfractionated T cells (107/injection; □). Mice receiving control T cells (▵) did not survive. The difference between groups of mice receiving a 1:1 ratio of transduced CD8+ and CD4+ T cells and transduced CD8+ T cells alone was significant (*P < .01, Mann-Whitney test). (D) To determine the optimal ratio of transduced CD4+ and CD8+ T cells required to achieve 100% survival of mice bearing 5-day established MDA-MB-435-erbB2 lung metastases, mice were treated with transduced CD8+ and CD4+ T cells at the following ratios: 1:1 (5 × 106 CD8+,5 × 106 CD4+ T cells; □), 9:1 (9 × 106 CD8+, 1 × 106 CD4+ T cells; ▴), 3:1 (7.5 × 106 CD8+, 2.5 × 106 CD4+ T cells; ▵), 1:3 (2.5 × 106 CD8+, 7.5 × 106 CD4+ T cells; ▪), or 1:9 (1 × 106 CD8+, 9 × 106 CD4+ T cells; ○). Control mice were treated with mock-transduced CD4+ and CD8+ T cells at a 1:1 ratio (▪; *P < .05, **P < .001, Mann-Whitney test). All results are calculated as the percentage of each group surviving and are representative of 2 experiments performed. Arrows depict days of T-cell transfer.
Increased transfer of engineered CD4+ T cells enhances tumor-free survival of mice. (A) Groups of 10 scid mice were given intravenous injections of 5 × 106 MDA-MB-435-erbB2 cells at day 0 prior to intravenous injection of scFv-CD28-ζ–transduced T cells at day 5. Mice treated with a 1:1 ratio of CD4+-transduced (5 × 106) and CD8+-transduced (5 × 106) T cells (▪) demonstrated 100% survival compared to 30% survival of mice receiving unfractionated transduced T cells (□; *P < .005, Mann-Whitney test). No survival of mice was observed for those receiving CD4+-transduced T cells alone (▪), CD8+-transduced T cells alone (○), control transduced T cells (▵), or no T cells (▴). (B) Enhanced survival of mice was due to increased transfer of antigen-specific CD4+ T cells. All scid mice bearing 5-day MDA-MB-435-erbB2 tumor that received a 1:1 ratio of CD8+-transduced (5 × 106) and CD4+-transduced (5 × 106) T cells (▪) survived compared with mice receiving either a 1:1 combination of transduced CD8+ T cells (5 × 106) and naïve CD4+ T cells (5 × 106; □), 1:1 combination of transduced CD8+ (5 × 106) T cells and CD4+ T cells (5 × 106) transduced with an irrelevant scFv-anti-CEA-γ receptor (▵) or no T-cell treatment (▴; *P < .001, Mann-Whitney test). (C) Transfer of a greater number of transduced CD8+ T cells alone does not rescue all mice from disease. Mice were given intravenous injections of 5 × 106 MDA-MB-435-erbB2 cells at day 0 prior to intravenous injection of scFv-CD28-ζ–transduced T cells. Mice treated at day 5 with a single dose of CD4+-transduced (5 × 106) and CD8+-transduced (5 × 106) T cells (▪) in a 1:1 ratio demonstrated 100% survival compared to about 30% survival for mice treated at days 5 and 6 with transduced CD8+ T cells alone (107/injection; ○)or about 80% survival for mice treated at days 5 and 6 with transduced unfractionated T cells (107/injection; □). Mice receiving control T cells (▵) did not survive. The difference between groups of mice receiving a 1:1 ratio of transduced CD8+ and CD4+ T cells and transduced CD8+ T cells alone was significant (*P < .01, Mann-Whitney test). (D) To determine the optimal ratio of transduced CD4+ and CD8+ T cells required to achieve 100% survival of mice bearing 5-day established MDA-MB-435-erbB2 lung metastases, mice were treated with transduced CD8+ and CD4+ T cells at the following ratios: 1:1 (5 × 106 CD8+,5 × 106 CD4+ T cells; □), 9:1 (9 × 106 CD8+, 1 × 106 CD4+ T cells; ▴), 3:1 (7.5 × 106 CD8+, 2.5 × 106 CD4+ T cells; ▵), 1:3 (2.5 × 106 CD8+, 7.5 × 106 CD4+ T cells; ▪), or 1:9 (1 × 106 CD8+, 9 × 106 CD4+ T cells; ○). Control mice were treated with mock-transduced CD4+ and CD8+ T cells at a 1:1 ratio (▪; *P < .05, **P < .001, Mann-Whitney test). All results are calculated as the percentage of each group surviving and are representative of 2 experiments performed. Arrows depict days of T-cell transfer.
Detection of transduced CD8+ and CD4+ T cells in vivo
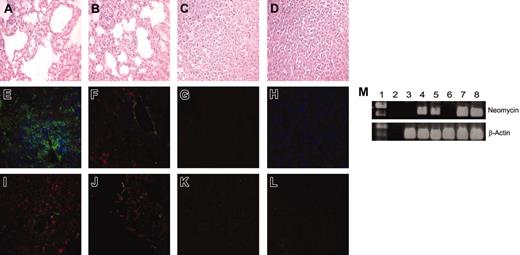
Given the striking response of adoptively transferred engineered CD8+ and CD4+ T cells against established lung metastases, we next tested whether transduced T cells could be detected at the tumor site. Hematoxylin and eosin (H&E) staining of mouse lung sections 16 days after tumor inoculation demonstrated significant reduction in lung tumor burden in mice treated with 1:1 transfer of scFv-α-erbB2-CD28-ζ–engineered CD8+ and CD4+ T cells compared to control T cells (Figure 5A,D). In contrast, the number of mice responding after transfer of transduced unfractionated T cells was significantly reduced as demonstrated by 2 representative H&E lung sections at day 16 (Figure 5B-C). Confocal microscopy following immunohistochemical staining demonstrated the presence of both CD8+ and CD4+ T cells in day 16 lung sections of mice treated with 1:1 transfer of transduced CD8+ and CD4+ T cells (Figure 5E). The detection of α-tag staining in lung sections of treated mice suggested that these T cells were expressing the chimeric scFv-CD28-ζ receptor (Figure 5I). Only CD8+ T cells and α-tag+ staining were detected in lungs of mice that responded to treatment with unfractionated T cells (Figure 5F,J). No staining was observed in mice that did not respond to treatment with unfractionated T-cell transfer (Figure 5G,K). In addition, no detection of control CD8+ and CD4+ T cells was observed in lung sections from mice (Figure 5H,L). Interestingly, neither transduced CD8+ nor CD4+ T cells injected alone were detected in day 16 lung sections, consistent with no antitumor effect observed in these mice (data not shown). As controls, both CD8+ and CD4+ T cells were detected in spleens of BALB/c mice using the same immunohistochemical staining procedure, but not in lungs from normal scid mice (data not shown).
To further evaluate persistence of transduced T cells in longterm surviving mice, we performed PCR amplification of the neomycin gene. The presence of an about 400-bp fragment corresponding to the neomycin gene in both peripheral blood and spleens of mice surviving tumor for more than 100 days after treatment with transduced CD8+ and CD4+ T cells indicated that these T cells could persist long-term even after tumor eradication (Figure 5M). In addition, quantitation of transduced T cells in the spleen and blood of long-term surviving mice by PCR using neomycin-specific primers indicated that the proportion of neomycin-positive cells was more than 0.01% (data not shown). The neomycin gene was not detected in peripheral blood and spleens of normal BALB/c or scid mice (Figure 5M). The persistence of these T cells was further supported by flow cytometry that demonstrated the presence of both CD8+ and CD4+ T cells in peripheral blood of long-term surviving mice (data not shown). Overall, these data suggested that the antitumor effect in mice was dependent on the localization at the site of tumor and long-term persistence of adoptively transferred engineered CD8+ and CD4+ T cells.
Antigen-specific release of IFN-γ by transduced CD8+ T cells is critical for enhanced survival of mice. The scid mice were given intravenous injections of 5 × 106 MDA-MB-435-erbB2 tumor cells followed by combined transfer of transduced CD8+ (5 × 106) and CD4+ (5 × 106) donor T cells at day 5 from BALB/c wild-type or BALB/c IFN-γ–/–mice. Mice received either 1:1 transfer of scFv-CD28-ζ–transduced CD8+IFN-γ+/+/CD4+IFN-γ+/+ T cells (▪), CD8+IFN-γ–/–/CD4+IFN-γ–/–T cells (▴), CD8+IFN-γ–/–/CD4+IFN-γ+/+ T cells (○), or CD8+IFN-γ+/+/CD4+IFN-γ–/–(▵) T cells. Control mice received a 1:1 transfer of wild-type CD8+ and CD4+ T cells transduced with the scFv-anti-CEA-γ receptor (□). Results are calculated as the percentage of each group surviving and are representative of 2 experiments. Arrows depict the day of T-cell transfer (*P < .05, Mann-Whitney test).
Antigen-specific release of IFN-γ by transduced CD8+ T cells is critical for enhanced survival of mice. The scid mice were given intravenous injections of 5 × 106 MDA-MB-435-erbB2 tumor cells followed by combined transfer of transduced CD8+ (5 × 106) and CD4+ (5 × 106) donor T cells at day 5 from BALB/c wild-type or BALB/c IFN-γ–/–mice. Mice received either 1:1 transfer of scFv-CD28-ζ–transduced CD8+IFN-γ+/+/CD4+IFN-γ+/+ T cells (▪), CD8+IFN-γ–/–/CD4+IFN-γ–/–T cells (▴), CD8+IFN-γ–/–/CD4+IFN-γ+/+ T cells (○), or CD8+IFN-γ+/+/CD4+IFN-γ–/–(▵) T cells. Control mice received a 1:1 transfer of wild-type CD8+ and CD4+ T cells transduced with the scFv-anti-CEA-γ receptor (□). Results are calculated as the percentage of each group surviving and are representative of 2 experiments. Arrows depict the day of T-cell transfer (*P < .05, Mann-Whitney test).
Detection of transduced CD8+ and CD4+ T cells in vivo. The scid mice were given intravenous injections of MDA-MB-435-erbB2 tumor cells (5 × 106 cells) at day 0 followed by intravenous injection of transduced T cells at day 5. Lungs were harvested and prepared for immunohistologic analysis 16 days after tumor inoculation. Lung sections from mice that received 1:1 CD4+ (5 × 106) and CD8+ (5 × 106) scFv-CD28-ζ transduced T cells (A,E,I), unfractionated transduced T cells (107), mouse no. 1 (B-C,F), mouse no. 2 (G,J-K), or 1:1 CD4+ (5 × 106) and CD8+ (5 × 106) scFv-α-CEA-γ–transduced T cells (D,H,L) were stained with H&E, with anti-CD4 (green), anti-CD8 (red), and anti-CD11b (blue) mAbs (E-H), or with anti-tag (red) and anti-CD11b (green) mAbs (I-L). Representative fields of 5 sections analyzed are shown. Original magnification × 400. (M) Peripheral blood and spleens were harvested from mice at day 100 and used for detection of neomycin phosphotransferase mRNA (∼400 bp). Both peripheral blood (lanes 4 and 5) and spleens (lanes 7 and 8) of 2 representative mice treated with scFv-CD28-ζ–transduced CD4+ and CD8+ T cells demonstrated the presence of the neomycin phosphotransferase gene. There was no neomycin phosphotransferase detected in either the peripheral blood (lane 3) or spleens (lane 6) of normal scid control mice. As controls, β-actin was detected in all peripheral blood (lane 3) and spleen samples (lane 8) tested. No neomycin phosphotransferase or β-actin was detected in empty DNA controls (lane 2). Lane 1 = 1 kilobase (kb) Plus markers.
Detection of transduced CD8+ and CD4+ T cells in vivo. The scid mice were given intravenous injections of MDA-MB-435-erbB2 tumor cells (5 × 106 cells) at day 0 followed by intravenous injection of transduced T cells at day 5. Lungs were harvested and prepared for immunohistologic analysis 16 days after tumor inoculation. Lung sections from mice that received 1:1 CD4+ (5 × 106) and CD8+ (5 × 106) scFv-CD28-ζ transduced T cells (A,E,I), unfractionated transduced T cells (107), mouse no. 1 (B-C,F), mouse no. 2 (G,J-K), or 1:1 CD4+ (5 × 106) and CD8+ (5 × 106) scFv-α-CEA-γ–transduced T cells (D,H,L) were stained with H&E, with anti-CD4 (green), anti-CD8 (red), and anti-CD11b (blue) mAbs (E-H), or with anti-tag (red) and anti-CD11b (green) mAbs (I-L). Representative fields of 5 sections analyzed are shown. Original magnification × 400. (M) Peripheral blood and spleens were harvested from mice at day 100 and used for detection of neomycin phosphotransferase mRNA (∼400 bp). Both peripheral blood (lanes 4 and 5) and spleens (lanes 7 and 8) of 2 representative mice treated with scFv-CD28-ζ–transduced CD4+ and CD8+ T cells demonstrated the presence of the neomycin phosphotransferase gene. There was no neomycin phosphotransferase detected in either the peripheral blood (lane 3) or spleens (lane 6) of normal scid control mice. As controls, β-actin was detected in all peripheral blood (lane 3) and spleen samples (lane 8) tested. No neomycin phosphotransferase or β-actin was detected in empty DNA controls (lane 2). Lane 1 = 1 kilobase (kb) Plus markers.
Antigen-specific response to tumor rechallenge
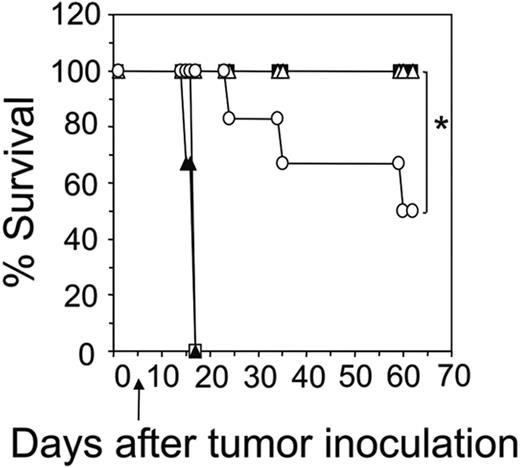
An important issue with regard to any cancer therapy is whether the specific treatment can induce a secondary response to tumor relapse. To test whether our scFv approach could effectively mediate a response to secondary tumor rechallenge, long-term surviving mice (> 100 days) initially treated with transduced CD8+ and CD4+ T cells were given intravenous injections of 5 × 106 MDA-MB-435-erbB2 tumor cells. Remarkably, all mice were able to totally eradicate a second dose of MDA-MB-435-erbB2 tumor (Figure 6A). Normal scid mice given injections of MDA-MB-435-erbB2 cells did not survive (Figure 6A). As a further test of specificity, long-term surviving mice were rechallenged with a subcutaneous dose (5 × 104) of mouse mammary carcinoma tumor cells expressing the human erbB2 antigen (4T1.2-erbB2) or 4T1.2 parental cells. As demonstrated for Figure 6A, all mice could significantly inhibit the subcutaneous growth of 4T1.2-erbB2 tumor compared to 4T1.2 parental tumor cells and their survival was significantly prolonged (Figure 6B-C). The subcutaneous growth of 4T1.2-erbB2 or 4T1.2 parental tumor cells in normal scid mice was not comparatively inhibited (Figure 6B-C). To determine whether long-term surviving mice could totally eradicate a lower dose of 4T1.2-erbB2 tumor, mice were rechallenged with 5 × 103 4T1.2-erbB2 tumor cells. Strikingly, the subcutaneous growth of 4T1.2-erbB2 at this lower dose was totally inhibited compared to subcutaneous injection of 5 × 104 4T1.2-erbB2 tumor cells (Figure 6D). The subcutaneous growth of 4T1.2-erbB2 or 4T1.2 parental tumor cells at both high and low doses was not affected in control scid mice (Figure 6D). These results have clearly demonstrated for the first time that mice treated with gene-engineered T cells could mount a sustained and potent antigen-specific secondary response to tumor rechallenge.
Discussion
Adoptive immunotherapy strategies involving the genetic modification of autologous T cells with scFv chimeric receptors or TCR genes is gaining wider acceptance as a promising treatment for cancer. Indeed, recent advances in the design of scFv receptors comprising both primary TCR-ζ and CD28 costimulatory signaling domains have demonstrated striking results against early subcutaneous tumor growth and tumor metastases in mice after adoptive transfer.4-6 Interestingly, these experiments involved adoptive transfer of unfractionated transduced T cells consisting largely of CD8+ T cells. CD8+ T cells have been demonstrated to be important for antitumor effects in a number of mouse models and can alone be sufficient for tumor rejection.22-24 However, from our work and that of some other investigators, adoptive transfer of CD8+ T cells alone is largely ineffective against more established disease.5,14,22 Given that CD4+ T cells have been well documented to play a critical role in antitumor immunity,25 we wanted to test whether increasing the proportion of engineered CD4+ T cells could further improve the antitumor response against later stage disease. In this study, we have shown a dramatic improvement in survival of mice bearing established pulmonary metastases following combined adoptive transfer of antigen-specific scFv-CD28-ζ engineered CD8+ and CD4+ T cells. Optimal antitumor efficacy was achieved with the addition of equivalent numbers of engineered CD4+ and CD8+ T cells, and the improved antitumor response observed in mice was entirely dependent on the transfer of erbB2 antigenspecific CD4+ T cells. Importantly, tumor eradication in mice correlated with localization at the tumor site and long-term persistence of engineered CD8+ and CD4+ T cells. In addition, we have demonstrated for the first time that gene-engineered T cells could completely reject a subsequent rechallenge with erbB2+ tumor.
Long-term surviving mice can induce an antigen-specific secondary response to tumor rechallenge. (A) Long-term surviving mice (100 days after primary MDA-MB-435-erbB2 tumor inoculation) treated with combined transfer of CD4+-transduced and CD8+-transduced T cells were able to reject a secondary intravenous injection of MDA-MB-435-erbB2 tumor cells (5 × 106 cells; □). Normal mice did not reject intravenous injection of MDA-MB-435-erbB2 tumor cells (5 × 106; ▪; *P < .001, Mann-Whitney test). (B) As a further test of specificity, long-term surviving mice received subcutaneous injections of 5 × 104 mouse mammary carcinoma 4T1.2-erbB2 (▪) or 4T1.2 parental cells (□) and their growth was statistically compared (*P < .005, Mann-Whitney test). As controls, normal scid mice received either 5 × 104 4T1.2-erbB2 (▴) or 4T1.2-parental tumor cells (▵). (C) Survival of long-term surviving mice given subcutaneous injections of 5 × 104 4T1.2-erbB2 (□) or 4T1 parental cells (▵) was compared (*P < .005, Mann-Whitney test). As controls, normal scid mice were challenged with 5 × 104 4T1.2-erbB2 (▪)or 4T1 parental tumor cells (▴). (D) To test the ability of mice to eradicate a lower dose of 4T1.2-erbB2 tumor, long-term surviving mice were challenged subcutaneously with either 5 × 104 cells (▵) or 5 × 103 4T1.2-erbB2 tumor cells (□) and survival was statistically compared (*P < .001, Mann-Whitney test). As controls the growth of 4T1.2-erbB2 tumor cells in normal scid mice at 5 × 104 cells (▴)or5 × 103 cells (▪) was assessed. All results are calculated as the percentage of each group surviving or represented as the mean size (mm2) ± SEM and are representative of 2 experiments.
Long-term surviving mice can induce an antigen-specific secondary response to tumor rechallenge. (A) Long-term surviving mice (100 days after primary MDA-MB-435-erbB2 tumor inoculation) treated with combined transfer of CD4+-transduced and CD8+-transduced T cells were able to reject a secondary intravenous injection of MDA-MB-435-erbB2 tumor cells (5 × 106 cells; □). Normal mice did not reject intravenous injection of MDA-MB-435-erbB2 tumor cells (5 × 106; ▪; *P < .001, Mann-Whitney test). (B) As a further test of specificity, long-term surviving mice received subcutaneous injections of 5 × 104 mouse mammary carcinoma 4T1.2-erbB2 (▪) or 4T1.2 parental cells (□) and their growth was statistically compared (*P < .005, Mann-Whitney test). As controls, normal scid mice received either 5 × 104 4T1.2-erbB2 (▴) or 4T1.2-parental tumor cells (▵). (C) Survival of long-term surviving mice given subcutaneous injections of 5 × 104 4T1.2-erbB2 (□) or 4T1 parental cells (▵) was compared (*P < .005, Mann-Whitney test). As controls, normal scid mice were challenged with 5 × 104 4T1.2-erbB2 (▪)or 4T1 parental tumor cells (▴). (D) To test the ability of mice to eradicate a lower dose of 4T1.2-erbB2 tumor, long-term surviving mice were challenged subcutaneously with either 5 × 104 cells (▵) or 5 × 103 4T1.2-erbB2 tumor cells (□) and survival was statistically compared (*P < .001, Mann-Whitney test). As controls the growth of 4T1.2-erbB2 tumor cells in normal scid mice at 5 × 104 cells (▴)or5 × 103 cells (▪) was assessed. All results are calculated as the percentage of each group surviving or represented as the mean size (mm2) ± SEM and are representative of 2 experiments.
Current immunotherapeutic strategies involving adoptive transfer of T-cell clones have demonstrated promising results in the clinic, particularly in patients with melanoma.26-28 Nevertheless, the broad application of this approach and other vaccination strategies have been far less successful against more refractory cancers such as breast, colon, and ovarian carcinomas. Several factors may account for these poor antitumor responses, which include (1) tumor down-regulation of MHC class I or class II/peptide from the cell surface,29 (2) lack of appropriate T-cell costimulation or CD4+ T-cell help for full T-cell activation,30 and (3) immunosuppressive effects of T-immunoregulatory cells.31,32 Our approach uses scFv-engineered T cells incorporating built-in “costimulatory” activity, which are able to recognize tumor targets in a non-MHC–restricted manner, clearly addressing the problems associated with MHC class I down-regulation and lack of expression of critical costimulatory ligands by tumor cells. Despite this, our studies revealed that adoptive transfer of either scFv-CD28-ζ–transduced CD8+ or CD4+ T cells alone had little antitumor effect, which was consistent with a lack of persistence of these geneengineered cells at the tumor site.
A number of reports have demonstrated in different models that CD4+ T cells are important for maintenance and full activation of cytotoxic T cells25 and induction of an effective memory response,15,16,33,34 and have demonstrated the importance of antigenspecific help (both Th1 and Th2 cells) for tumor eradication in vivo.14,35 However, the importance of CD4+ T-cell help for adoptively transferred gene-modified T cells has not been properly assessed in vivo. In this study, we have clearly demonstrated that adoptive transfer of both engineered antigen-specific CD8+ and CD4+ T cells was critical to achieve complete eradication of established tumor. Interestingly, scFv-transduced CD8+ T cells could specifically mediate high levels of cytotoxicity and IFN-γ release in vitro but no IL-2 secretion following antigen ligation. In contrast, scFv-transduced CD4+ T cells demonstrated reduced cytotoxicity but high levels of both IFN-γ and IL-2 secretion. We were able to demonstrate in vivo using donor T cells from IFN-γ–/– mice that IFN-γ released by engineered CD4+ T cells could contribute to the antitumor effect in the absence of IFN-γ from CD8+ T cells. Although the mechanism of IFN-γ release by transduced CD8+ and CD4+ T cells in our model remains unclear, this may involve recruitment of innate effector cells,36 regulation of leukocyte-endothelium interactions,37 or an antiangiogenic effect.38 Given that IL-2 has been previously shown to be important for maintaining CD8+ T-cell function in vivo,39,40 it also remains possible that the provision of IL-2 `help' from transduced CD4+ T cells may play a role for the improved antitumor efficacy observed following combined transfer of both engineered CD8+ and CD4+ T cells. Interestingly, we demonstrated that exogenous administration of low- or high-dose human rIL-2, together with transduced CD8+ T cells, could not mimic the effect of transferring both transduced CD8+ and CD4+ T cells (data not shown). This may indicate that local production of IL-2 by transduced CD4+ T cells is more critical for sustaining an effective antitumor response.
A recent study by Gyobu et al,20 has claimed improved antitumor responses in vivo following combined transfer of transduced human CD8+ and CD4+ T cells compared to either T-cell subset alone. Nevertheless, there are several problems with testing the efficacy of human T cells in mice that undermine this study. We have previously reported poor persistence of genemodified human T cells after systemic transfer in mice41 and another study demonstrated antitumor effects only following intratumoral injection of gene-modified human T cells and IL-2.42 Consistent with these findings, antitumor effects in mice were only observed by Gyobu et al20 following preincubation of effector and target cells prior to injection. Thus, there was no requirement for trafficking of T cells to the tumor site and the extent of target-cell death prior to transfer in vivo was unknown. In contrast, we have demonstrated in this study that complete eradication of established tumor at a distant site required systemic injection of a 1:1 transfer of antigen-specific transduced mouse CD8+ and CD4+ T cells. In addition, we were able to importantly demonstrate long-term persistence and function of these gene-engineered T cells after tumor rechallenge. Another issue for adoptive transfer of human T cells in mice is the inability of human IFN-γ released by transduced human T cells to interact with the endogenous host IFN-γ receptors. Indeed, we have demonstrated in this study using donor T cells from IFN-γ–/– mice a critical role for IFN-γ secreted by gene-engineered mouse CD8+ T cells for antitumor rejection. However, this effector arm and possibly important effects from other cytokines were absent in the study by Gyobu and colleagues.20
An important issue for application of gene therapy in the clinic involving retroviral gene transfer is the potential risk of insertional oncogenesis. This risk was recently highlighted when 2 of 10 SCID-X1 patients given transfusions of gene-modified autologous bone marrow-derived progenitor and stem cells developed T-cell leukemia.43 However, in the case of T-cell adoptive immunotherapy approaches there has been no report of T-cell transformation in animal models or in patients.4,5,9,26,44 Consistent with this we observed no signs of transformation in our long-term surviving mice even after tumor rechallenge despite the fact we could clearly detect persistence of our gene-modified cells in both the spleen and peripheral blood. In any case, if a problem were to arise with the transfer of genetically modified T cells in patients, a suicide gene strategy involving hsv-tk45 or the cytoplasmic domain of Fas46 could be used. Alternatively, the design of new integration vectors that target sites of the genome that are considered “safe” could be tested in future trials.
One of the striking features of this study was the ability of our gene-engineered CD8+ and CD4+ T cells to persist long-term and specifically reject a subsequent tumor rechallenge. This has never been reported previously for adoptively transferred gene-engineered T cells and potentially represents a significant step for application of this type of therapy in the clinic for treatment of not only patients with primary tumors but in cases of tumor relapse where cancer deposits have metastasized to multiple sites. Central to this process was the addition of engineered antigen-specific CD4+ T cells because adoptive transfer of scFv-CD28-ζ–engineered CD8+ T cells alone did not persist or mediate a strong antitumor effect. Our study supports a large body of work demonstrating the importance of CD4+ T-cell help for maintenance of CD8+ T-cell function and generation of a recall response.13,15,16,25,33,34
In this study immunodeficient scid mice were used for T-cell transfer experiments due to the immunogenic nature of the human erbB2 target antigen. Although an appropriate model does not exist, it would be interesting in future experiments to determine whether CD8+ and CD4+ gene-engineered T cells can persist and induce potent recall responses in an immunocompetent host. Nevertheless, the experiments described here using the scid mouse are relevant and can be mimicked in patients by a nonmyeloablative regimen involving cyclophosphamide administration. Such regimens are currently being used in patients receiving melanoma-specific T cells, which may enhance the therapeutic effect by dampening the immunosuppressive effect of T-regulatory cells.28
In conclusion, the experiments described in this study demonstrate for the first time in vivo that the provision of engineered antigen-specific CD4+ T-cell help can significantly improve antitumor responses, maintain persistence of transferred T cells, and induce a potent recall response after tumor rechallenge. Other studies have demonstrated that help may be provided to CD8+ T cells in other ways, including the use of antigen-positive CD80+ artificial antigen-presenting cells (AAPCs) and IL-15 before adoptive transfer24 or the addition of exogenous cytokines such as IL-2.47 The toxicity associated with exogenous IL-2 administration makes this latter approach less appealing.48 Our approach alleviates IL-2 toxicity and now with current technology for efficiently transducing both CD8+ and CD4+ human T cells,2,21,41,49 this strategy is clinically feasible. Our preclinical studies demonstrating the importance of engineered CD4+ T-cell help represents a significant step for enhancing redirected CTL therapy for treatment of cancer.
Prepublished online as Blood First Edition Paper, July 19, 2005; DOI 10.1182/blood-2004-12-4906.
Supported by the Susan G. Komen Breast Cancer Foundation and the Cancer Council of Victoria research grants. M.H.K. and P.K.D. were supported by National Health and Medical Research Council of Australia (NHMRC) R. D.
Wright Research Fellowships. M.J.S. and J.A.T. were supported by NHMRC Principal Research and Senior Principal Research Fellowships, respectively.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge the Peter MacCallum Cancer Centre Experimental Animal Facility technicians for animal care.

![Figure 2. Antigen-specific cytokine secretion, proliferation, and cytotoxicity by transduced mouse CD8+ and CD4+ T cells. To evaluate cytokine secretion, unfractionated T cells (□), CD8+ T cells (dotted bars) and CD4+ T cells (diagonally striped bars) transduced with the scFv-CD28-ζ receptor or mock-transduced unfractionated T cells (▪), CD8+ T cells (cross-hatched bars), and CD4+ T cells (▤) were cocultured with media alone or irradiated MDA-MB-435-erbB2 cells or MDA-MB-435 parental cells or stimulated with plate-bound anti-CD3 and anti-CD28 mAbs in 12-well plates for 24 hours. Supernatants were harvested and assessed for IFN-γ (A), GM-CSF (B), IL-4 (C), and IL-2 (D) production by ELISA. Results are expressed as picogram per milliliter of cytokine secreted ± SE for duplicate samples of 3 representative experiments. The proliferative capacity of each transduced T-cell population was evaluated in a 72-hour [3H]-thymidine incorporation assay (E). Transduced unfractionated T cells (□), CD8+ T cells (dotted bars), and CD4+ T cells (diagonally striped bars) or mock-transduced unfractionated T cells (▪), CD8+ T cells (cross-hatched bars), and CD4+ T cells (▤) were incubated with media alone or stimulated with plate-bound anti-CD3 and anti-CD28 mAbs or anti-tag mAb for 3 days. Results are expressed as means ± SE of triplicate samples and are representative of 3 experiments. Specific cytolytic function of scFv-CD28-ζ–transduced mouse T cells was evaluated in a 6-hour 51Cr-release assay. Transduced unfractionated (▵) and CD8+ (○) T cells demonstrated greater lysis of MDA-MB-435-erbB2 target cells than CD4+-transduced (▪) T cells (F). No lysis of MDA-MB-435 parental target cells was observed by chimeric receptor-transduced T cells (G) and mock-transduced unfractionated T cells (▪), CD8+ T cells (▴), or CD4+ T cells (□) did not lyse either cell line. The spontaneous lysis was less than 10% in all assays. Results are expressed as percent specific 51Cr release ± SE (%) of triplicate samples representative of at least 2 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/9/10.1182_blood-2004-12-4906/6/m_zh80210586070002.jpeg?Expires=1769115096&Signature=aOlSxcPaj3m~0d2~rzrUBLH92LmxbmEWRWx-RED3iZQzZWIPiOxUUGB98ShfzmxroKyzOgeiMYYUhLQwjRt6Dy5UNV5ue3hNJd4gJwlXr8KA1vENplrpJkUGxZxDYES3n0jcbKwu6IjvTP4XGTn1PKKrqVL7W4-bS2ospsEuskfE~dFqkUbqRhhzUuWKwiS5OndgeGFud4U~MvPfoEJC6Zn53R5jxdJqurhr8fhqnHI3FP306s6iqCD5wlYw7GgBxpU29EoZkxIe-p1aHsUmI44kuaH2uPC8DiAz1bb4jTfM2aRz-I8I8QwCuTtUWtCoF7HSxOj31dTJl1jgxkACNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal