Abstract
Interactions between the endogenous estradiol metabolite 2-medroxyestradiol (2-ME) and histone deacetylase inhibitors (HDACIs) have been investigated in human leukemia cells. Coadministration of subtoxic or marginally toxic concentrations of 2-ME and SAHA or sodium butyrate in diverse human leukemia-cell types resulted in a marked increase in oxidative damage (eg, generation of reactive oxygen species [ROSs]), mitochondrial injury (eg, cytochrome c release and Bax translocation), caspase activation, and apoptosis. These interactions were also noted in primary human leukemia cells but not in normal bone marrow CD34+ cells. Synergistic interactions between these agents were associated with inactivation of Akt and activation of c-Jun N-terminal kinase (JNK). Essentially all of these events were reversed by free radical scavengers such as the manganese superoxide dismutase (MnSOD) mimetic TBAP and catalase. Notably, treatment with 2-ME/HDACIs resulted in down-regulation of thioredoxin, MnSOD, and glutathione peroxidase. Enforced activation of Akt blocked 2-ME/HDACI-mediated mitochondrial injury, caspase activation, and JNK up-regulation, but not generation of ROSs. Pharmacologic or genetic (siRNA) interruption of the JNK pathway also significantly attenuated the lethality of this regimen. Together, these findings support a model in which antileukemic synergism between 2-ME and HDACIs stems primarily from induction of oxidative damage, leading in turn to Akt inactivation and JNK activation, culminating in mitochondrial injury and apoptosis. They also raise the possibility that these events may preferentially occur in leukemic versus normal hematopoietic cells.
Introduction
Histone deacetylase inhibitors (HDACIs) represent a diverse class of agents that inhibit the activity of histone deacetylases (HDACs), enzymes that, in conjunction with histone acetylases (HATs), reciprocally regulate the acetylation of histones.1 HDACIs promote histone acetylation, allowing them to assume a more relaxed, open configuration, which in many, although not all, cases results in enhanced gene transcription.2 HDACIs may also interfere with the capacity of HDACs to participate in corepressor complexes that have been implicated in the differentiation block exhibited by certain forms of acute myeloid leukemia (AML; eg, those associated with AML-1/ETO).3 HDACIs such as short-chain fatty acid members of the butyrate family are potent inducers of leukemic-cell maturation in vitro.4 Second-generation HDACIs, such as suberoylanilide hydroxamic acid (SAHA), which are approximately 3 logs more potent than butyrate derivatives, revealed a biphasic effect in leukemia in that low HDACI concentrations resulted in maturation and higher concentrations led to apoptosis.5 HDACI lethality is regulated by multiple mechanisms including activation of stress-related or inactivation of cytoprotective pathways,6 up-regulation of death receptors,7 induction of p21CIP1,8 ceramide generation,9 and disruption of heat shock proteins (eg, Hsp90),10 among others. HDACIs also induce oxidative damage in neoplastic cells including the generation of reactive oxygen species (ROSs),11 possibly the result of perturbations in antioxidant genes, including thioredoxin (Trx).12 Recently, HDACIs including SAHA were shown to induce Trx selectively in normal but not in transformed cells, resulting in greater induction of ROSs in the latter.13 Thus, an increased susceptibility of neoplastic cells to HDACI-mediated oxidative injury might account for the therapeutic selectivity of these agents. Several HDACIs have now entered clinical trials in humans,1 and initial encouraging results in patients with AML14 and lymphoma have been reported.15
2-Methoxyestradiol (2-ME) is an estrogen derivative that does not bind the estrogen receptor16 and that exerts multiple activities in various cell systems, including induction of cell-cycle arrest,17 modulation of MAPKs including c-Jun N-terminal kinase (JNK),18 and binding to tubulin.19 A recent study demonstrated that 2-ME potently induced apoptosis in several human leukemia cell types through a mechanism involving generation of ROSs and induction of mitochondrial injury.20 In leukemia cells, these effects have been related to the inhibitory actions of 2-ME toward manganese superoxide dismutase (MnSOD),20 an antioxidant enzyme that plays an important role in cellular defenses against oxidative stress by reducing superoxide anions (O2-) to H2O2.21 Interestingly, 2-ME was found to be more toxic to leukemic cells than to their normal hematopoietic counterparts,20 which may reflect low MnSOD activity in transformed cells.22 Recently, down-regulation of the Akt signaling pathway has been implicated in 2-ME-mediated oxidative injury and apoptosis in human leukemia cells.23 Akt is a serine/threonine kinase that exerts multiple antiapoptotic actions including inactivation of Bad and caspase-9 among others.24 The selective toxicity of 2-ME toward leukemia cells20 suggests it may play a role in leukemia treatment.
Collectively, these findings indicate that both HDACIs13 and 2-ME20,23 kill neoplastic cells, at least in part, through generation of ROSs, effects that may be selective for transformed cells due to differential modulation of antioxidant enzymes.13,20 The suggestion that combining 2-ME with agents that induce free radicals might lead to synergistic antineoplastic effects20 prompted us to hypothesize that simultaneous exposure to HDACIs and 2-ME might enhance antileukemic activity and possibly selectivity. The goals of this study were to determine whether combined exposure of human leukemia cells to these agents would lead to synergistic antileukemic effects and to characterize the role of perturbations in signaling cascades implicated in oxidative injury responses, particularly the JNK and Akt pathways,25 in these actions. Our results indicate that combined treatment of human leukemia cells with 2-ME and the HDACIs, sodium butyrate (NaB) and SAHA, leads to a pronounced increase in oxidative injury and apoptosis, and that inactivation of the cytoprotective Akt pathway accompanied by activation of the JNK cascade play important functional roles in these events.
Materials and methods
Reagents
2-ME was purchased from Steraloids (Newport, RI). NaB was purchased from Biomol (Plymouth Meeting, PA), and SAHA was from Biovision (Mountain View, CA). SP600125, Z-VAD-FMK, and Mn-TBAP were from EMD Biosciences (La Jolla, CA). DCFH-DA was obtained from Molecular Probes (Eugene, OR). Sodium formate was from Sigma (St Louis, MO). Catalase was from Roche Molecular Biochemicals (Indianapolis, IN). Antibodies against cytochrome c, AIF, Bcl-xL, Akt, phospho-ERK, phospho-JNK, JNK, Trx, and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA); cleaved caspase-3, phospho-Akt, ERK, phospho-p38, and p38 were from Cell Signaling Technology (Beverly, MA); caspase-9, XIAP, Mcl-1, p21, p27, and Bax were from Pharmingen (San Diego, CA); PARP was from Biomol (Plymouth Meeting, PA); caspase-8 was from Alexis (Carlsbad, CA); Bcl-2 was from Dako (Carpinteria, CA); Cu/Zn SOD and MnSOD were from Upstate Biotechnology (Lake Placid, NY); and glutathione peroxidase 1 (GPx1) was from Abcam (Cambridge, MA).
Cells
U937, HL-60, and Jurkat human leukemia cells were purchased from American Type Culture Collection (ATCC; Manassas, VA) and cultured in RPMI 1640 medium supplemented with sodium pyruvate, minimum essential medium (MEM), essential vitamins, l-glutamine, penicillin, streptomycin, and 10% fetal bovine serum (FBS). U937 cells were stably transfected with a constitutively active form of Akt,9 and clones were selected with 400 μg/mL geneticin as we have previously reported.26
Peripheral-blood samples were obtained with informed consent from 5 patients with AML, 2 patients with chronic myeloid leukemia (CML), and 1 patient with acute lymphoma leukemia (ALL) undergoing routine diagnostic aspirations with approval from the Institutional Review Board of Virginia Commonwealth University. Informed consent was provided according to the Declaration of Helsinki. AML, CML, and ALL blasts were isolated by density gradient centrifugation over Histopaque-1077 (Sigma Diagnostics, St Louis, MO) at 400g for 38 minutes. Isolated mononuclear cells were washed and assayed for total number and viability using a Guava personal cytometer (Guava Technologies, Hayward, CA). Cells were suspended at 8 × 105/mL for treatment. Granulocyte colony-stimulating factor (G-CSF)-mobilized CD34+ cells from peripheral blood were purchased from Cambrex (Walkersville, MD). After washing and enumerating as described for mononuclear cells, cells were suspended at 8 × 105/mL prior to treatment.
Assessment of apoptosis
For annexin V/propidium iodide (PI) assays, cells were stained with annexin V-fluorescein isothiocyanate (FITC) and PI, and evaluated for apoptosis by flow cytometry according to the manufacturer's protocol (BD PharMingen, San Diego, CA). Briefly, 1 × 106 cells were washed twice with cold PBS and stained with 5 μL annexin V-FITC and 10 μLPI (5 μg/mL) in 1 × binding buffer (10 mM HEPES, pH 7.4, 140 mM NaOH, 2.5 mM CaCl2) for 15 minutes at room temperature in the dark. The apoptotic cells were determined using a Becton Dickinson FACScan cytoflurometer (Mansfield, MA). Both early apoptotic (annexin V+,PI-) and late (annexin V+ and PI+) apoptotic cells were included in cell death determinations. Annexin V/PE assay was performed to analyze the apoptosis in CD34+ using a Guava personal cytometer (Guava Technologies) according to the manufacturer's instructions.
Detection of intracellular ROSs
Intracellular production of ROSs was measured using DCFH-DA.23 To determine production of ROSs, control and drug-treated cells were incubated with DCFH-DA (5 μM) for 60 minutes, washed twice with cold PBS, and analyzed within 1 hour using a Becton Dickinson FACScan flow cytometer (Hialeah, FL). The effects of ROS scavengers (Mn-TBAP, catalase, formate) on generation of ROSs was determined as we have previously described in detail.27
Western blot analysis
Western blot analysis was performed using the NuPAGE Bis-Tris electrophoresis system (Invitrogen, Carlsbad, CA). The total cellular samples were washed twice with cold PBS and lysed in 1 × NuPAGE LDS sample buffer supplemented with 50 mM dithiothreitol (DTT; Fisher Biotech, Pittsburgh, PA). The protein concentration was determined using Coomassie Protein Assay Reagent (Pierce, Rockford, IL). The total cellular protein extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane in 20 mM Tris-HCl (pH 8.0) containing 150 mM glycine and 20% (vol/vol) methanol. Membranes were blocked with 5% nonfat dry milk in 1 × TBS containing 0.05% Tween 20 and incubated with antibodies described in “Reagents.” Protein bands were detected by incubating with horseradish peroxidase-conjugated antibodies (Kirkegaard and Perry Laboratories, Gaithersburg, MD), and visualized with enhanced chemiluminescence reagent (PerkinElmer Life Sciences, Boston, MA).
Analysis of cytosolic cytochrome c, AIF, Bax, and membrane Bax
After treatment, cells (2 × 106) were washed twice with cold PBS and lysed by incubating for 5 minutes in lysis buffer (75 mM NaCl, 8 mM Na2HPO4, 1 mM NaH2PO4, 1 mM EDTA, and 350 μg/mL digitonin). Cytosolic (supernatant) and membrane (pellet) fractions were separated by centrifugation at 12 000g for 1 minute. The protein concentration was determined using Coomassie Protein Assay Reagent (Pierce). Then, 30 μg cytosolic and membrane extracts were separated by SDS-PAGE, transferred to nitrocellulose membrane, and incubated with antibodies against cytochrome c (BD Pharmingen) and AIF (Santa Cruz Biotechnology) and Bax (BD Pharmingen).
siRNA studies
U937 cells (1.5 × 106) were transfected with 1 μg JNK1 annealed dsRNAi oligonucleotide 5′-CGUGGGAUUUAUGGUCUGUGTT-3′/3′-TTGCACCUAAAUACCAGACAC-5′ (Orbigen, San Diego, CA) using the Amaxa nucleofector (Köln, Germany) as recommended by the manufacturer. After incubation at 37°C for 24 hours, transfected cells were treated with 2-ME, NaB, and SAHA alone, and 2-ME plus NaB or SAHA, and subjected apoptosis and Western blot analysis.
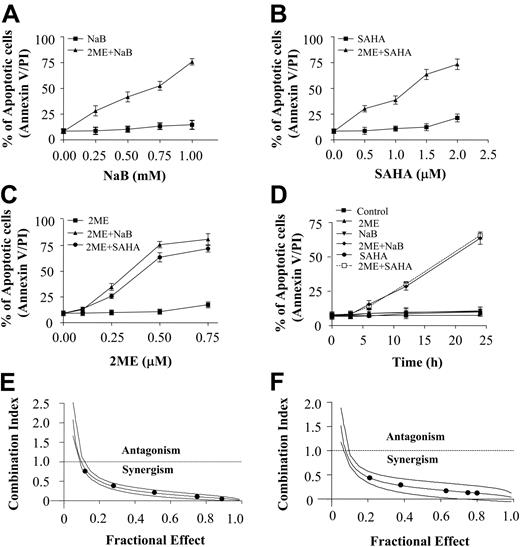
Coadministration of 2-ME and the HDACIs, NaB and SAHA, induces apoptosis in U937 cells in dose- and time-dependent manners. (A) U937 cells were treated with 0.5 μM 2-ME in the presence or absence of the designated concentration of NaB for 24 hours. (B) U937 cells were treated with 0.5 μM 2-ME in the presence or absence of the designated concentration of SAHA for 24 hours. (C) U937 cells were treated with either NaB (1 mM) or SAHA (1.5 μM) in the presence or absence of the designated concentration of 2-ME for 24 hours. (D) U937 cells were exposed to 0.5 μM 2-ME combined with either NaB (1 mM) or SAHA (1.5 μM) for the indicated time interval. After treatment as described, cells were stained with annexin V-FITC/PI, and apoptosis was determined using flow cytometry as described in “Materials and methods.” U937 cells were exposed to varying concentrations of 2-ME and NaB (E) or SAHA (F) at a fixed ratio (1:2 or 1:3, respectively) for 24 hours, after which the extent of apoptosis was determined by annexin V/PI staining and flow cytometry. CI values for each fraction affected were determined using commercially available software (Calcusyn, Biosoft). CI values less than 1.0 correspond to synergistic interactions.
Coadministration of 2-ME and the HDACIs, NaB and SAHA, induces apoptosis in U937 cells in dose- and time-dependent manners. (A) U937 cells were treated with 0.5 μM 2-ME in the presence or absence of the designated concentration of NaB for 24 hours. (B) U937 cells were treated with 0.5 μM 2-ME in the presence or absence of the designated concentration of SAHA for 24 hours. (C) U937 cells were treated with either NaB (1 mM) or SAHA (1.5 μM) in the presence or absence of the designated concentration of 2-ME for 24 hours. (D) U937 cells were exposed to 0.5 μM 2-ME combined with either NaB (1 mM) or SAHA (1.5 μM) for the indicated time interval. After treatment as described, cells were stained with annexin V-FITC/PI, and apoptosis was determined using flow cytometry as described in “Materials and methods.” U937 cells were exposed to varying concentrations of 2-ME and NaB (E) or SAHA (F) at a fixed ratio (1:2 or 1:3, respectively) for 24 hours, after which the extent of apoptosis was determined by annexin V/PI staining and flow cytometry. CI values for each fraction affected were determined using commercially available software (Calcusyn, Biosoft). CI values less than 1.0 correspond to synergistic interactions.
Statistical analysis
For analysis of apoptosis and ROS production, values were presented as means plus or minus SD for at least 3 separate experiments. Statistical differences between control and treated groups were determined by the Student t test. Differences were considered statistically significant for values of P less than .05 and P less than .01. Median dose effect analysis28 was used to analyze interactions between 2-ME and HDACIs administered simultaneously (24 hours) over a range of drug concentrations at a fixed concentration ratio with apoptosis as the end point using a commercially available software program (Calcysyn; Biosoft, Ferguson, MO). For each fraction affected (FA), a combination index (CI) was calculated. CI values less than 1.0 denote synergistic interactions.
Results
2-ME dramatically potentiates HDACI-mediated apoptosis in a concentration- and time-dependent manner
Interactions between 2-ME and the HDACIs, SAHA and NaB, were first investigated in U937 cells. Exposure (24 hours) to a subtoxic concentration of 2-ME (0.5 μM) significantly increased the lethality of essentially nontoxic concentrations of NaB and SAHA (0.25 mM and 0.5 μM, respectively), and the degree of potentiation increased as concentrations increased (Figure 1A-B). Parallel 2-ME dose-response studies revealed that concentrations as low as 0.25 μM 2-ME significantly increased the lethality of nontoxic concentrations of NaB and SAHA (1 mM and 1.5 μM, respectively), and apoptosis increased further with higher 2-ME concentrations (Figure 1C). Time-course studies revealed that potentiation of apoptosis was apparent after 12 hours of simultaneous exposure and was very extensive by 24 hours (Figure 1D). Finally, median dose effect analysis of apoptosis in cells exposed to 2-ME and NaB or SAHA administered for 24 hours at fixed ratios yielded CI values consistently less than 1.0, indicating synergistic interactions (Figure 1E-F). Together, these findings indicate that 2-ME interacts synergistically with the HDACIs, NaB and SAHA, to induce apoptosis in human myelomonocytic leukemia cells (U937).
2-ME interacts synergistically with HDACIs in myeloid and lymphoid cell lines as well as primary human leukemic cells
To determine whether these interactions were confined to U937 cells, parallel studies were performed with other human leukemia-cell types, including Jurkat T-lymphoblastic and HL-60 promyelocytic leukemia cells. In each case, coadministration of 2-ME (0.5 μM) with subtoxic concentrations of either NaB or SAHA (1 mM and 1.5 μM, respectively) for 24 hours resulted in a pronounced increase in apoptosis (Figure 2A). Parallel results were obtained when 5 AML (FAB classifications: M2, 4 patients, and M5, 1 patient; Figure 2B) and 2 CD34+ CML samples (chronic phase; Figure 2C) were evaluated following coadministration of 2-ME (1 μM) with subtoxic concentrations of either NaB or SAHA (1 mM and 1.5 μM, respectively) for 24 hours. Although effects were more modest, an increase in apoptosis with combined treatment was also observed in an ALL sample (FAB L2; Figure 2D). In contrast, 2-ME in combination with NaB or SAHA exerted little toxicity toward normal CD34+ bone marrow cells (Figure 2G) or normal peripheral-blood mononuclear cells (data not shown). Together, these findings indicate that interactions between 2-ME and HDACIs can occur in both myeloid and lymphoid human leukemia cell lines and in at least some primary leukemic cells. They also raise the possibility that as in the case of these agents administered individually13,20 this regimen may be relatively sparing toward normal hematopoietic cells.
Coadministration of 2-ME and the HDACIs, NaB and SAHA, induces apoptosis in U937, Jurkat, and HL-60 cells, and in AML, CML, and ALL blast samples. (A) U937, Jurkat, and HL-60 cells were treated with 0.5 μM 2-ME in the presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 24 hours, respectively, after which the percentage of apoptotic cells was determined by annexin V/PI staining and flow cytometry as described in “Materials and methods.” (B-D) Blasts from 5 patients with AML, 2 patients with CML, and 1 patient with ALL were isolated as described in “Materials and methods.” After washing and counting, isolated mononuclear cells were treated with 1 μM 2-ME in the presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 24 hours, respectively, after which the percentage of apoptotic cells was determined by annexin V/PI staining and flow cytometry as described in “Materials and methods.” (E) CD34+ cells were treated with 1 μM 2-ME in the presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 24 hours, after which the percentage of apoptotic cells was determined by annexin V/PE staining and flow cytometry as described in “Materials and methods.” Values represent the means ± SD for 3 replicate determinations.
Coadministration of 2-ME and the HDACIs, NaB and SAHA, induces apoptosis in U937, Jurkat, and HL-60 cells, and in AML, CML, and ALL blast samples. (A) U937, Jurkat, and HL-60 cells were treated with 0.5 μM 2-ME in the presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 24 hours, respectively, after which the percentage of apoptotic cells was determined by annexin V/PI staining and flow cytometry as described in “Materials and methods.” (B-D) Blasts from 5 patients with AML, 2 patients with CML, and 1 patient with ALL were isolated as described in “Materials and methods.” After washing and counting, isolated mononuclear cells were treated with 1 μM 2-ME in the presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 24 hours, respectively, after which the percentage of apoptotic cells was determined by annexin V/PI staining and flow cytometry as described in “Materials and methods.” (E) CD34+ cells were treated with 1 μM 2-ME in the presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 24 hours, after which the percentage of apoptotic cells was determined by annexin V/PE staining and flow cytometry as described in “Materials and methods.” Values represent the means ± SD for 3 replicate determinations.
Combined exposure of human leukemia cells to 2-ME and HDACIs results in a marked increase in mitochondrial injury and caspase activation
Western blot analysis was used to assess the effects of combined exposure to 2-ME and HDACIs on mitochondrial integrity and caspase activation. Whereas exposure of U937 cells to 2-ME (0.5 μM) or NaB/SAHA (1 mM or 1.5 μM) alone for 24 hours had little effect, combined treatment resulted in a pronounced increase in release of cytochrome c and AIF into the cytosolic S-100 fraction, accompanied by marked activation/cleavage of procaspases-9, -3, and -8, and degradation of PARP (Figure 3A).
Coadministration of 2-ME and NaB or SAHA results in cleavage of Bcl-2 and p27KIP1 and mitochondrial translocation of Bax
The effects of combining 2-ME with HDACIs was then examined in relation to effects on various survival and cell-cycle-regulatory proteins. Combined but not individual exposure to 2-ME (0.5 μM) and NaB or SAHA (1 mM and 1.5 μM, respectively) for 24 hours resulted in the cleavage of p27KIP1 and to a lesser extent Bcl-2 (Figure 3B). Induction of p21CIP1 was observed in cells treated with NaB or SAHA but was not substantially modified by coexposure to 2-ME. Expression of Bad, Bid, Bcl-xL, and total Bax also did not change appreciably with any treatment. On the other hand, cotreatment with 2-ME and either NaB or SAHA resulted in a very pronounced redistribution of Bax from the cytosolic to the mitochondrial compartment, a hallmark of apoptosis induction via the mitochondrial pathway.29
Combined exposure of leukemic cells to 2-ME and HDACIs results in a pronounced reduction in levels of phospho-Akt and a marked activation of JNK, but no effects on activation of ERK or p38 MAPK
Effects of combined treatment with 2-ME and HDACIs on expression of survival and stress-related signaling pathways was examined next. Exposure (24 hours) of U937 cells to 2-ME (0.5 μM) and NaB or SAHA (1 mM or 1.5 μM), but not the agents administered individually, resulted in a marked decrease in expression of phosphorylated Akt, but had no significant effect on total Akt, or ERK expression/activation (Figure 3C). In addition, combined (but not individual) treatment resulted in a dramatic increase in activation of JNK but had no effect on levels of phospho-p38 MAPK. Thus, combined treatment of leukemia cells with 2-ME and HDACIs resulted in a pronounced inactivation of the cytoprotective Akt pathway, and a reciprocal activation of the stress-related JNK pathway.
Time-course analysis of cells treated with HDACIs/2-ME revealed that, whereas PARP cleavage was first noted after 14 hours of treatment, Akt inactivation and JNK activation were observed at considerably earlier intervals (eg, 4-8 hours and 8 hours, respectively; Figure 3D) when the extent of apoptosis was minimal.
Signaling perturbations in 2-ME/HDACI-treated cells are largely caspase-independent
To evaluate the relationship between the preceding events and caspase activation, cells were exposed to 2-ME (1 μM) with or without HDACIs (1 mM NaB or 1.5 μM SAHA) for 14 hours in the presence or absence of the caspase inhibitor ZVAD. As shown in Figure 3E, PARP degradation and caspase-3 and caspase-8 cleavage were essentially abrogated by ZVAD. Similar effects were noted in the case of Bcl-2 and p27KIP1 cleavage (data not shown). In contrast, dephosphorylation/inactivation of Akt and JNK phosphorylation/activation were only minimally affected by ZVAD, suggesting that the latter events do not simply represent a consequence of the cell-death process.
Effects of 2-ME combined with the HDACIs, NaB and SAHA, on apoptosis-related gene expression and various signal-transduction pathways. (A) U937 cells were treated with 2-ME (0.5 μM) in presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 24 hours, after which total cellular extracts were prepared and subjected to Western blot assay using antibodies against PARP, caspase-8, caspase-9, and cleaved caspase-3. Cytosolic extracts were also prepared and subjected to Western blot assay using antibody against AIF and cytochrome c. (B) U937 cells were exposed to 2-ME (0.5 μM) combined with either NaB (1 mM) or SAHA (1.5 μM), after which total cellular extracts were prepared and subjected to Western blot assay using antibodies against p21, p27, Bcl-2, Bcl-xL, Bad, Bid, and bax. Cytosolic and membrane extracts were also prepared and subjected to Western blot assay using antibody against Bax. (C) After coadministration of 2-ME and NaB or SAHA for 24 hours, total cellular extracts were prepared and subjected to Western blot assay using antibodies against phospho-Akt, Akt, phospho-ERK, ERK, phospho-p38, p38, phospho-JNK, and JNK. (D) U937 cells were exposed to 2-ME (0.5 μM) combined with either NaB (1 mM) or SAHA (1.5 μM) for the indicated exposure interval, after which total cellular extracts were prepared and subjected to Western blot assay using antibodies against PARP, phosphor-Akt, Akt, phosphor-JNK, and JNK. (E) U937 cells were pretreated with the caspase inhibitor Z-VAD-FMK (10 μM) for 1 hour, followed by cotreatment with 0.5 μM 2-ME and 1 mM NaB or 1.5 μM SAHA for 24 hours, after which total cellular extracts were prepared and subjected to Western blot assay using antibodies against PARP, cleaved caspase-3, caspase-8, phosphor-Akt, Akt, phosphor-JNK, and JNK. For Western blot assay, each lane was loaded with 30 μg protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading and transfer. Two additional studies yielded equivalent results.
Effects of 2-ME combined with the HDACIs, NaB and SAHA, on apoptosis-related gene expression and various signal-transduction pathways. (A) U937 cells were treated with 2-ME (0.5 μM) in presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 24 hours, after which total cellular extracts were prepared and subjected to Western blot assay using antibodies against PARP, caspase-8, caspase-9, and cleaved caspase-3. Cytosolic extracts were also prepared and subjected to Western blot assay using antibody against AIF and cytochrome c. (B) U937 cells were exposed to 2-ME (0.5 μM) combined with either NaB (1 mM) or SAHA (1.5 μM), after which total cellular extracts were prepared and subjected to Western blot assay using antibodies against p21, p27, Bcl-2, Bcl-xL, Bad, Bid, and bax. Cytosolic and membrane extracts were also prepared and subjected to Western blot assay using antibody against Bax. (C) After coadministration of 2-ME and NaB or SAHA for 24 hours, total cellular extracts were prepared and subjected to Western blot assay using antibodies against phospho-Akt, Akt, phospho-ERK, ERK, phospho-p38, p38, phospho-JNK, and JNK. (D) U937 cells were exposed to 2-ME (0.5 μM) combined with either NaB (1 mM) or SAHA (1.5 μM) for the indicated exposure interval, after which total cellular extracts were prepared and subjected to Western blot assay using antibodies against PARP, phosphor-Akt, Akt, phosphor-JNK, and JNK. (E) U937 cells were pretreated with the caspase inhibitor Z-VAD-FMK (10 μM) for 1 hour, followed by cotreatment with 0.5 μM 2-ME and 1 mM NaB or 1.5 μM SAHA for 24 hours, after which total cellular extracts were prepared and subjected to Western blot assay using antibodies against PARP, cleaved caspase-3, caspase-8, phosphor-Akt, Akt, phosphor-JNK, and JNK. For Western blot assay, each lane was loaded with 30 μg protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading and transfer. Two additional studies yielded equivalent results.
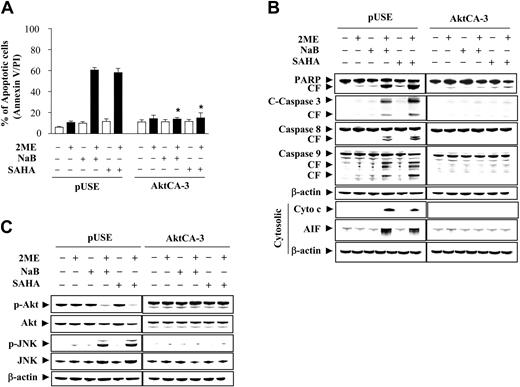
Enforced activation of Akt antagonizes the lethality of the 2-ME/HDACI regimen
To investigate further the functional significance of Akt inactivation in 2-ME/HDACI lethality, U937 cells stably expressing a constitutively active (myristolated) Akt construct were used (Akt-CA3). Notably, Akt-CA3 cells were largely immune to the lethal effects of both 2-ME/HDACI regimens, whereas empty vector controls (pUSE) were approximately as sensitive as parental cells (Figure 4A). Similar results were obtained with 2 other clones (eg, Atk-CA6 and CA11; data not shown). Consistent with these findings, enforced activation of Akt blocked 2-ME/HDACI-mediated PARP degradation, caspase cleavage/activation (Figure 4B), and cytochrome c and AIF release. Enforced Akt activation also prevented p27KIP1 and Bcl-2 degradation, but did not modify p21CIP1 expression (data not shown). Finally, Western blot analysis shown in Figure 4C demonstrated that enforced expression of constitutively active Akt blocked both Akt down-regulation and JNK activation in response to 2-ME/HDACI exposure. These findings argue that inactivation of the Akt pathway plays a critical role in synergistic antileukemic interactions between 2-ME and HDACIs and that this event lies upstream of JNK activation.
JNK activation plays an important functional role in antileukemic synergism between 2-ME and HDACIs
To determine what functional role, if any, JNK activation played in synergistic interactions between 2-ME and HDACIs, U937 cells were exposed to 2-ME (0.5 μM) plus either NaB (1 mM) or SAHA (1.5 μM) for 24 hours in the presence or absence of the JNK inhibitor SP600125. As shown in Figure 5A, Western blot analysis confirmed that cotreatment with SP600125 blocked 2-ME/HDACI-mediated activation of JNK, manifested by diminished phosphorylation of c-Jun, but had no effect on total c-Jun expression (Figure 5B). SP600125 also antagonized 2-ME/HDACI-mediated caspase activation and release of the proapoptotic mitochondrial proteins cytochrome c and AIF (Figure 5C). Because SP600125 is not an absolutely specific JNK inhibitor, parallel studies were performed using U937 cells transfected with JNK1 siRNA. Exposure to JNK1 siRNA significantly reduced levels of total JNK1 and phospho-JNK1 compared to control cells (Figure 5D). Levels of JNK2 were largely unaffected by treatment. Analysis of apoptosis by annexin V/PI revealed that transfection with JNK1 siRNA significantly diminished 2-ME/HDACI-mediated apoptosis compared to controls (Figure 5E; P < .01). Collectively, these findings suggest that enhanced JNK activation plays a significant functional role in 2-ME/HDACI-mediated mitochondrial injury, caspase activation, and apoptosis in human leukemia cells.
Induction of activated Akt markedly protect cells from apoptosis induced by coadministration of 2-ME and the HDACIs, NaB and SAHA. U937 cells were stably transfected with constitutively active forms of Akt (clone designated CA-3) or an empty vector (pUSE) as described in “Materials and methods.” Cells were then treated with 0.5 μM 2-ME in the presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 24 hours. (A) After treatment, apoptosis was analyzed using annexin V-FITC/PI assay as described in “Materials and methods.” *Values for Akt-CA3 cells treated with 2-ME in the presence of NaB or SAHA were significantly decreased compared with those for pUSE cells (Student t test; P < .01). (B) After treatment, total cellular or cytosolic extracts were prepared and subjected to Western blot analysis using antibodies against PARP, caspase-8, caspase-9, cleaved caspase-3, AIF, and cytochrome c, respectively. (C) After treatment, total cellular extracts were prepared and subjected to Western blot analysis using antibodies against phosphor-Akt, Akt, phospho-JNK, and JNK. For Western blot analysis, each lane was loaded with 30 μg protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading and transfer. Two additional studies yielded equivalent results.
Induction of activated Akt markedly protect cells from apoptosis induced by coadministration of 2-ME and the HDACIs, NaB and SAHA. U937 cells were stably transfected with constitutively active forms of Akt (clone designated CA-3) or an empty vector (pUSE) as described in “Materials and methods.” Cells were then treated with 0.5 μM 2-ME in the presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 24 hours. (A) After treatment, apoptosis was analyzed using annexin V-FITC/PI assay as described in “Materials and methods.” *Values for Akt-CA3 cells treated with 2-ME in the presence of NaB or SAHA were significantly decreased compared with those for pUSE cells (Student t test; P < .01). (B) After treatment, total cellular or cytosolic extracts were prepared and subjected to Western blot analysis using antibodies against PARP, caspase-8, caspase-9, cleaved caspase-3, AIF, and cytochrome c, respectively. (C) After treatment, total cellular extracts were prepared and subjected to Western blot analysis using antibodies against phosphor-Akt, Akt, phospho-JNK, and JNK. For Western blot analysis, each lane was loaded with 30 μg protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading and transfer. Two additional studies yielded equivalent results.
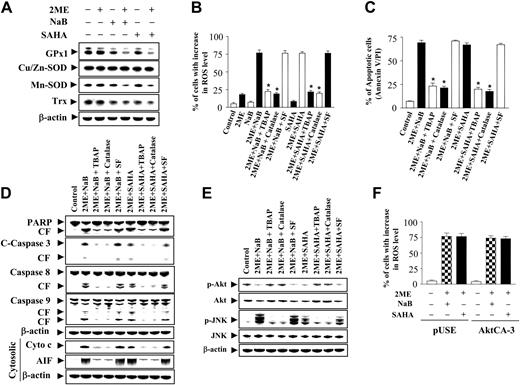
Coadministration of 2-ME and HDACI synergistically potentiates oxidative injury
Because both HDACIs11 and 2-ME20 have been shown to promote oxidative injury, the effects of combined exposure to these agents was examined in relation to expression of various antioxidant enzymes as well as generation of ROSs in leukemic cells. Individual treatment of cells with HDACIs or 2-ME for 6 hours, before the onset of apoptosis, resulted in either no or very modest effects on expression of GPx1, MnSOD, or Trx, whereas combined exposure resulted in reductions in all 3 (Figure 6A). No effects on Cu/Zn-SOD (SOD1) were observed for any drug treatment. Significantly, whereas treatment with 2-ME alone (6 hours) resulted in only a modest increase in ROS levels, and individual exposure to NaB or SAHA had virtually no effect, combined treatment resulted in a dramatic increase in the percentage of cells exhibiting oxidative injury (eg, ∼75%; Figure 6B). Furthermore, this effect was substantially attenuated by the cell-permeable MnSOD mimetic TBAP30 or by addition of the H202 scavenger catalase (Figure 6B; P < .01). Similar results were obtained with the more nonspecific free radical scavenger N-acetylcysteine (NAC; data not shown) or in cells ectopically expressing GPx1 (data not shown). In contrast, addition of sodium formate, a scavenger of hydroxyl radicals, had little effect. Attempts were then made to assess the functional significance of oxidative damage in HDACI/2-ME-induced cell death. Addition of TBAP or catalase (but not sodium formate) significantly reduced the lethality of both 2-ME/HDACI regimens (P < .01 in each case; Figure 6C). These findings indicate that oxidative injury plays a key role in the synergistic antileukemic effects of the 2-ME/HDACI regimen and implicate superoxide radicals and H2O2 in this process.
Pharmacologic inhibition of JNK and transfection of JNK1 siRNA significantly protect cells from apoptosis induced by coadministration of 2-ME and the HDACIs, NaB and SAHA. U937 cells were pretreated with 10 μM JNK inhibitor, SP600125 (SP), for 1 hour, followed by the addition of 0.5 μM 2-ME combined with either NaB (1 mM) or SAHA (1.5 μM) for 24 hours. (A) Cells were stained with annexin V/PI, and apoptosis was determined using flow cytometry as described in “Materials and methods.” The values obtained from annexin V/PI assays represent the mean ± SD for 3 separate experiments. *Values for cells cotreated with 2-ME, NaB or SAHA, and SP are significantly less than those obtained for cells treated with 2-ME and NaB or SAHA (Student t test; P < .01). (B) After treatment, total cellular extracts were prepared and subjected to Western blot analysis using antibodies against phospho-c-Jun, c-Jun. (C) After treatment, total cellular or cytosolic extracts were prepared and subjected to Western blot analysis using antibodies against PARP, full-length caspase-8 and caspase-9, cleaved caspase-3, AIF, and cytochrome c, respectively. U937 cells were transfected with JNK1 siRNA oligonucleotides or controls and incubated for 24 hours at 37°C, after which cells were treated with 0.5 μM 2-ME in the presence of 1 mM of NaB or 1.5 μM of SAHA for 24 hours. (D) After treatment, total cellular extracts were prepared and subjected to Western blot analysis using antibodies against phospho-JNK, JNK1, and JNK2. (E) Apoptosis was determined using the annexin V-FITC/PI assay as described in “Materials and methods.” *Values for cells treated with 2-ME in the presence of NaB or SAHA after transfection with JNK1 siRNA oligonucleotides are significantly decreased compared with those for control cells treated with 2-ME in the presence of NaB or SAHA (Student t test; P < .01). For Western blot assay, each lane was loaded with 30 μg protein; blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading. Two additional studies yielded equivalent results.
Pharmacologic inhibition of JNK and transfection of JNK1 siRNA significantly protect cells from apoptosis induced by coadministration of 2-ME and the HDACIs, NaB and SAHA. U937 cells were pretreated with 10 μM JNK inhibitor, SP600125 (SP), for 1 hour, followed by the addition of 0.5 μM 2-ME combined with either NaB (1 mM) or SAHA (1.5 μM) for 24 hours. (A) Cells were stained with annexin V/PI, and apoptosis was determined using flow cytometry as described in “Materials and methods.” The values obtained from annexin V/PI assays represent the mean ± SD for 3 separate experiments. *Values for cells cotreated with 2-ME, NaB or SAHA, and SP are significantly less than those obtained for cells treated with 2-ME and NaB or SAHA (Student t test; P < .01). (B) After treatment, total cellular extracts were prepared and subjected to Western blot analysis using antibodies against phospho-c-Jun, c-Jun. (C) After treatment, total cellular or cytosolic extracts were prepared and subjected to Western blot analysis using antibodies against PARP, full-length caspase-8 and caspase-9, cleaved caspase-3, AIF, and cytochrome c, respectively. U937 cells were transfected with JNK1 siRNA oligonucleotides or controls and incubated for 24 hours at 37°C, after which cells were treated with 0.5 μM 2-ME in the presence of 1 mM of NaB or 1.5 μM of SAHA for 24 hours. (D) After treatment, total cellular extracts were prepared and subjected to Western blot analysis using antibodies against phospho-JNK, JNK1, and JNK2. (E) Apoptosis was determined using the annexin V-FITC/PI assay as described in “Materials and methods.” *Values for cells treated with 2-ME in the presence of NaB or SAHA after transfection with JNK1 siRNA oligonucleotides are significantly decreased compared with those for control cells treated with 2-ME in the presence of NaB or SAHA (Student t test; P < .01). For Western blot assay, each lane was loaded with 30 μg protein; blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading. Two additional studies yielded equivalent results.
Consistent with these results, coadministration of TBAP or catalase (but not sodium formate) clearly reduced 2-ME/HDACI-mediated mitochondrial injury (ie, cytochrome c and AIF release), caspase-3, -9, and -8 activation, and PARP degradation (Figure 6D). Finally, analogous to the effects of NAC on cells treated with 2-ME alone, coadministration of TBAP or catalase but not formate substantially diminished 2-ME/HDACI-induced Akt inactivation and JNK activation (Figure 6E). Collectively, these findings indicate that enhanced oxidative damage induced by the 2-ME/HDACI regimen plays a primary functional role in multiple perturbations in signaling and cell-survival pathways that culminate in mitochondrial injury and induction of cell death.
HDACI/2-ME-mediated oxidative injury occurs upstream of Akt inactivation
To gain further insights into the hierarchy of events accompanying synergistic antileukemic interactions between HDACIs and 2-ME, generation of ROSs was determined in U937 cells expressing constitutively activeAkt (U937/AktCA-3). When exposed for 24 hours to 2-ME in combination with either NaB or SAHA, AktCA-3 cells displayed an increase in oxidative injury (ROS generation) equivalent to that of empty-vector controls (P > .05; Figure 6F). This finding, along with the observation that free radical scavengers largely block HDACI/2-ME-mediated Akt dephosphorylation, supports the view that oxidative injury represents a cause rather than a consequence of interruption of the Akt signaling pathway.
Discussion
The ability of HDACIs to induce differentiation or apoptosis in human leukemia cells5 has stimulated intense interest in their potential as antileukemic agents. Although it is often presumed that their mode of action, analogous to other epigenetic forms of therapy, involves derepression of silenced genes or disruption of corepressor complexes or both,31 it has also become apparent that HDACIs are potent inducers of apoptosis.2 Diverse mechanisms have been implicated in HDACI-mediated lethality, including up-regulation of death receptors and activation of the extrinsic apoptotic pathway,7 induction of oxidative injury,11 interference with chaperone protein function,32 and induction of perturbations in signaling and survival pathways,9,26 among others.33 These considerations could have important implications for the rational development of combination strategies involving HDACIs for leukemia and other hematopoietic malignancies. For example, in addition to interactions with more conventional cytotoxic agents,34 antileukemic synergism has been observed between HDACIs and multiple other novel agents, including imatinib mesylate,35 17-AAG,36 and CDK inhibitors such as flavopiridol37 to name a few.
Effects of antioxidants on ROS generation, apoptosis, and cell-signaling proteins induced by coadministration of 2-ME and the HDACIs, NaB or SAHA. (A) U937 cells were exposed to 2-ME (0.5 μM) combined with either NaB (1 mM) or SAHA (1.5 μM) for 6 hours, after which total cellular extracts were prepared and subjected to Western blot analysis using antibodies against GPx1, Cu/Zn-SOD, MnSOD, and Trx. (B) U937 cells were pretreated with antioxidants including TBAP (200 μM), catalase (5000 U/mL), or sodium formate (SF, 2 mM) for 2 hours, followed by the addition of 2-ME (0.5 μM) in the presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 6 hours. Cells were stained with DCFH-DA, after which ROS production was analyzed by flow cytometry as described in “Materials and methods.” (C) U937 cells were pretreated with antioxidants including TBAP (200 μM), catalase (5000 U/mL), or sodium formate (SF, 2 mM) for 2 hours, followed by the addition of 2-ME (0.5 μM) in the presence of NaB or SAHA for 24 hours, after which cells were stained with annexin V and PI and apoptosis was analyzed by flow cytometry as described in “Materials and methods.” *Values for cells treated with 2-ME and an antioxidant (TBAP or catalase) in the presence of NaB or SAHA are significantly less than those obtained for cells treated with 2-ME plus either NaB or SAHA in the absence of the antioxidant (Student t test; P < .01). U937 cells were pretreated with antioxidants including TBAP (200 μM), catalase (5000 U/mL), or sodium formate (SF, 2 mM) for 2 hours, followed by the addition of 2-ME (0.5 μM) in the presence of NaB or SAHA for 24 hours, after which total cellular or cytosolic extracts were prepared and subjected to Western blot analysis using antibodies against PARP, cleaved caspase-3, full-length caspase-8 and caspase-9, AIF, cytochrome c (D) or cell-signaling proteins including phospho-Akt, Akt, phospho-JNK, and JNK (E). (F) U937 cells were stably transfected with constitutively active forms of Akt (CA-3) or an empty vector (pUSE). Cells were treated with 0.5 μM 2-ME in the presence of NaB (1 mM) or SAHA (1.5 μM) for 6 hours, after which cells were stained with DCFH-DA, and ROS production was analyzed using flow cytometry as described in “Materials and methods.” For panels A, D, and E, each lane was loaded with 30 μg protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading and transfer. Two additional studies yielded equivalent results. For panels B, C, and F, results represent the means ± SD for 3 separate experiments performed in triplicate.
Effects of antioxidants on ROS generation, apoptosis, and cell-signaling proteins induced by coadministration of 2-ME and the HDACIs, NaB or SAHA. (A) U937 cells were exposed to 2-ME (0.5 μM) combined with either NaB (1 mM) or SAHA (1.5 μM) for 6 hours, after which total cellular extracts were prepared and subjected to Western blot analysis using antibodies against GPx1, Cu/Zn-SOD, MnSOD, and Trx. (B) U937 cells were pretreated with antioxidants including TBAP (200 μM), catalase (5000 U/mL), or sodium formate (SF, 2 mM) for 2 hours, followed by the addition of 2-ME (0.5 μM) in the presence or absence of NaB (1 mM) or SAHA (1.5 μM) for 6 hours. Cells were stained with DCFH-DA, after which ROS production was analyzed by flow cytometry as described in “Materials and methods.” (C) U937 cells were pretreated with antioxidants including TBAP (200 μM), catalase (5000 U/mL), or sodium formate (SF, 2 mM) for 2 hours, followed by the addition of 2-ME (0.5 μM) in the presence of NaB or SAHA for 24 hours, after which cells were stained with annexin V and PI and apoptosis was analyzed by flow cytometry as described in “Materials and methods.” *Values for cells treated with 2-ME and an antioxidant (TBAP or catalase) in the presence of NaB or SAHA are significantly less than those obtained for cells treated with 2-ME plus either NaB or SAHA in the absence of the antioxidant (Student t test; P < .01). U937 cells were pretreated with antioxidants including TBAP (200 μM), catalase (5000 U/mL), or sodium formate (SF, 2 mM) for 2 hours, followed by the addition of 2-ME (0.5 μM) in the presence of NaB or SAHA for 24 hours, after which total cellular or cytosolic extracts were prepared and subjected to Western blot analysis using antibodies against PARP, cleaved caspase-3, full-length caspase-8 and caspase-9, AIF, cytochrome c (D) or cell-signaling proteins including phospho-Akt, Akt, phospho-JNK, and JNK (E). (F) U937 cells were stably transfected with constitutively active forms of Akt (CA-3) or an empty vector (pUSE). Cells were treated with 0.5 μM 2-ME in the presence of NaB (1 mM) or SAHA (1.5 μM) for 6 hours, after which cells were stained with DCFH-DA, and ROS production was analyzed using flow cytometry as described in “Materials and methods.” For panels A, D, and E, each lane was loaded with 30 μg protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading and transfer. Two additional studies yielded equivalent results. For panels B, C, and F, results represent the means ± SD for 3 separate experiments performed in triplicate.
The present results indicate that 2-ME, an estrogen derivative that does not bind to the estrogen receptor, interacts in a highly synergistic manner with HDACIs to induce mitochondrial injury and apoptosis in human leukemia cells. Although 2-ME has been known to inhibit tumor-cell proliferation by triggering cell-cycle arrest17 and to disrupt microtubule function,38 recent studies indicate that in leukemia cells, 2-ME induces oxidative damage by blocking the activity of MnSOD, an important free radical scavenger.20 In this context, HDACIs are also known to kill cells through induction of ROSs.11 Consequently, it is tempting to speculate that synergistic antileukemic interactions between these agents stem from the combined effects of ROS generation accompanied by disabling of critical defense mechanisms responsible for protecting cells from oxidative injury.
The bulk of evidence suggests that 2-ME-related inactivation of Akt played a key role in potentiation of HDACI-induced lethality. In a recent study, the lethal effects of 2-ME toward human leukemia cells, as well as induction of oxidative damage, was shown to depend on Akt down-regulation.21 In this regard, the ability of Akt to protect cells from oxidative damage is well documented,39 although the mechanism by which this phenomenon occurs has not been fully elucidated. Furthermore, Akt activation has been shown to enhance the acetylation and transactivation potential of the p65 subunit of NF-κB,40 which in turn limits HDACI lethality.41 The finding that enforced activation of Akt dramatically reduced 2-ME/HDACI-induced apoptosis is consistent with these findings. Alternatively, the cytoprotective effects of Akt may involve diverse downstream Akt targets, including Bad,42 caspase-9,43 and members of the FOXO transcription factor family,44 all of which have been implicated in the response of cells to oxidative injury.24,42 It is noteworthy that the free radical scavengers TBAP and catalase substantially attenuated 2-ME/HDACI-associated Akt down-regulation; conversely, enforced expression of constitutively active Akt failed to attenuate 2-ME/HDACI-mediated ROS generation. Collectively, these findings indicate that oxidative damage plays a primary role in mediating the lethality of this regimen, whereas Akt inactivation appears to amplify the consequences of this event. Finally, interactions between HDACIs and 2-ME in human leukemia cells appeared qualitatively similar to those observed in the case of the alkyllysophospholipid perifosine, which down-regulates Akt activity by interfering with membrane recruitment.45 Notably, perifosine/HDACI-mediated lethality was also associated with oxidative injury.9 Taken together, such observations suggest that disabling the Akt pathway plays an important functional role in promoting the lethal consequences of HDACI-mediated oxidative damage.
The finding that combined treatment with HDACIs and 2-ME resulted in a marked increase in activation of the stress-related kinase JNK, and that genetic or pharmacologic interruption of this pathway significantly attenuated lethality, is consistent with evidence that JNK plays a critical role in oxidative damage responses.46 It is also in accord with evidence linking the cytotoxic effects of both HDACIs and 2-ME, administered individually, to JNK activation.21,47,48 The mechanism by which JNK activation promotes apoptosis is not known with certainty, but has been related to Bcl-2 phosphorylation and inactivation49 or to direct induction of mitochondrial injury (eg, cytochrome c release).50 The ability of enforced activation of Akt to block JNK activation in 2-ME/HDACI-treated cells suggests the presence of cross-talk between these signaling pathways, a phenomenon for which precedents exist. For example, ROS-induced activation of JNK has been shown to proceed via an Akt-dependent process in hepatoma cells.51 ROSs stimulated human hepatoma-cell proliferation via cross-talk between PI3-K/PKB and JNK signaling pathways.51 Together, these observations raise the possibility that down-regulation of Akt by the HDACI/2-ME regimen cooperates with JNK activation to lower the threshold for mitochondrial injury and apoptosis in human leukemia cells. Finally, the present results share certain similarities with recent reports linking inflammatory cytokine-mediated down-regulation of antioxidant enzymes (MnSOD and Trx) and ROS generation, to sustained JNK activation and cell death.52
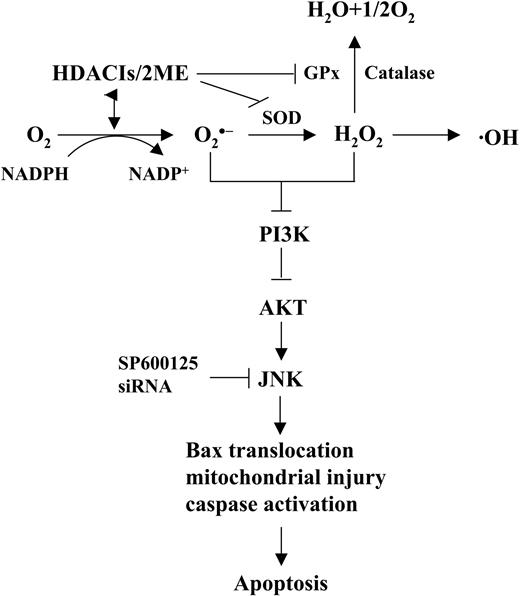
The finding that various free radical scavengers, including the MnSOD mimetic TBAP, as well as GPx and catalase, significantly reduced 2-ME/HDACI-induced ROS generation and lethality, argues for a functional role for oxidative stress in apoptosis induction. In this context, combined exposure to 2-ME and HDACI regimen resulted in down-regulation of MnSOD, Trx, and GPx in leukemic cells, which, in conjunction with inhibition of MnSOD by 2-ME20 in all likelihood contributed to the pronounced increase in ROS production. Interestingly, differential effects on Trx expression have recently been implicated in the selective lethality HDACIs exhibit toward neoplastic cells.13 It is therefore conceivable that 2-ME, which also selectively kills leukemia cells via oxidative injury secondary to MnSOD inhibition,20,23 might amplify the leukemia-specific actions of agents that induce oxidative damage such as HDACIs. Analogously, arsenic trioxide (As2O3), a compound that induces apoptosis in malignant hematopoietic cells by triggering oxidative injury,53 has also been shown to interact synergistically with 2-ME in human chronic lymphocytic leukemia (CLL) lymphocytes.54 Consistent with these concepts, the 2-ME/HDACI regimen was toxic to various primary human leukemia-cell specimens as well as continuously cultured cell lines, but relatively sparing to normal hematopoietic cells. It is tempting to speculate that preferential down-regulation or inhibition of various antioxidant enzymes in leukemic cells might contribute to the observed in vitro selectivity of this regimen. Collectively, these findings support a hypothetical model of 2-ME/HDACI lethality in leukemia cells (Figure 7). In this model, 2-ME and HDACIs cooperate to inhibit or down-regulate MnSOD, GPx, and Trx, leading to increased generation of various ROSs, including O2· -and H202, These in turn trigger, through as yet to be discovered mechanisms, down-regulation of the cytoprotective Akt pathway, followed by JNK activation, culminating in Bax translocation, mitochondrial injury, caspase activation, and apoptosis.
Hypothetical model of 2-ME and HDACI interactions in human leukemia cells. In this model, combined exposure to HDACIs and 2-ME results in down-regulation/inactivation of antioxidant enzymes such as MnSOD, Trx, and GPx1, leading in turn to generation of free radicals, particularly O2 · - and H202. These events trigger, through an as-yet—to-be-determined mechanism, down-regulation of Akt, which induces activation of the stress-related kinase JNK. Sustained activation of JNK culminates in Bax translocation, cytochrome c release, activation of the caspase cascade, and apoptosis.
Hypothetical model of 2-ME and HDACI interactions in human leukemia cells. In this model, combined exposure to HDACIs and 2-ME results in down-regulation/inactivation of antioxidant enzymes such as MnSOD, Trx, and GPx1, leading in turn to generation of free radicals, particularly O2 · - and H202. These events trigger, through an as-yet—to-be-determined mechanism, down-regulation of Akt, which induces activation of the stress-related kinase JNK. Sustained activation of JNK culminates in Bax translocation, cytochrome c release, activation of the caspase cascade, and apoptosis.
Although disruption of HDAC-associated repressor complexes provides a rationale for the use of HDACIs or other epigenetic forms of therapy in leukemia,2 it has become apparent that this class of agents kills leukemic cells through a variety of mechanisms.6-11 Moreover, evidence of preclinical antileukemic activity5 as well as antitumor selectivity13 provides a strong rationale for evaluating these agents in patients with refractory leukemia. Indeed, preliminary results suggest encouraging activity for HDACIs such as SAHA and depsipeptide in acute leukemia and cutaneous T-cell lymphoma, respectively.14,15 Furthermore, an improved understanding of the mechanisms of action of HDACIs has prompted the rational development of combination regimens incorporating these agents. Specifically, agents that disrupt key cytoprotective signaling pathways (eg, Akt, NF-κB) or promote oxidative damage, including tyrosine kinase inhibitors,35,55 alkyl-lysophospholipids,9 Hsp90 antagonists,10,36,56 and proteasome inhibitors,34 have all been shown to interact synergistically with HDACIs in various leukemia-cell model systems. In view of its shared capacity with HDACIs12 to induce selective oxidative injury in leukemia cells,20 as well as its ability to disable the cytoprotective Akt pathway,23 2-ME represents a logical candidate agent for such combination approaches. Accordingly, preclinical studies designed to assess the in vivo relevance of these in vitro findings are under development.
Prepublished online as Blood First Edition Paper, September 1, 2005; DOI 10.1182/blood-2005-06-2409.
Supported by Public Health Service grants CA-63753, CA-93738, CA-100866, and CA88906 from the National Cancer Institute; grant DK52825 from the National Institutes of Health; award 6045-03 from the Leukemia and Lymphoma Society of America; award DAMD-17-03-1-0209 from the Department of Defense; and a Translational Research award from the V-Foundation.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal