Abstract
Mantle-cell lymphoma (MCL) is a mature B-cell lymphoma with an aggressive course and generally poor prognosis. Conventional chemotherapy has little efficacy. Bortezomib is a novel, reversible, and highly specific proteasome inhibitor that appears as a new hope for MCL treatment. We have analyzed the in vitro sensitivity to bortezomib in 4 MCL cell lines and in primary tumor cells from 10 MCL patients. Bortezomib induced phosphatidylserine exposure, mitochondrial depolarization, ROS generation, Bax and Bak conformational changes, and caspase activation. In addition, ROS scavengers, but not pancaspase inhibitors, blocked all apoptosis hallmarks. Protein and mRNA-expression analysis, revealed marked up-regulation of the BH3-only protein Noxa, between 4 to 6 hours after bortezomib addition, independent of p53 status. However, this up-regulation was faster and higher in cells with functional p53. Noxa RNA interference markedly decreased sensitivity to bortezomib, pointing to this protein as a key mediator between proteasome inhibition and mitochondrial depolarization in MCL cells. Noxa interacts with the antiapoptotic protein Mcl-1 and promotes Bak release from Mcl-1, suggesting that up-regulation of Noxa might counteract Mcl-1 accumulation after bortezomib treatment. These findings should be useful to extend the therapeutic strategies in MCL patients and to improve their prognosis.
Introduction
Mantle-cell lymphoma (MCL) is a lymphoid malignancy derived from a subset of mature B cells with coexpression of CD5.1,2 MCL is characterized by the chromosomal translocation t(11;14)(q13; q32), which results in cyclin D1 overexpression,3,4 with the consequent deregulation of cell-cycle control at G1-S checkpoint. In addition to classic MCL, a blastoid variant of this disease has been described that is characterized by high mitotic rate and is associated with INK4a/ARF deletions, p53 mutations, and complex karyotypes.5-7 MCL is characterized by an aggressive clinical course and poor response to conventional chemotherapy due to either rapid relapse after an initial response or primary resistance to drugs.8,9 Thus, there is currently a strong effort to develop compounds that target novel biologic pathways.
Protein degradation is crucial for controlling the availability of regulatory proteins in the cell. The ubiquitin-proteasomal system represents the major nonlysosomal pathway through which intracellular proteins are degraded in eukaryotic cells.10 The proteasome is a large, multicatalytic complex, present in both the cytoplasm and nucleus of all eukaryotic cells. It is composed of 2 functional components: a 20S catalytic core and 2 19S regulatory subunits. The protease activity resides in a channel at the center of 20S subunit, and exhibits 3 enzymatic activities: chymotrypsin-like, trypsinlike, and post-glutamyl peptide hydrolase-like. Many proteins degraded by proteasome are implicated in crucial processes: cell-cycle-regulatory proteins (cyclins A, B, D, and E; p21 and p27), the tumor suppressor p53, NF-κB activation, and cell-adhesion molecules (ICAM-1, VCAM-1).11,12 Furthermore, transformed cells are much more sensitive to proteasome inhibition than normal cells. This is in part due to the high replication rate of malignant cells, which implies rapid protein synthesis and turnover, but also because of the genetic changes that accompany transformation that disable diverse protective checkpoint mechanisms. Accordingly, the proteasome has emerged as an attractive target for cancer therapy.
Bortezomib (PS-341, Velcade) is a proteasome inhibitor that belongs to the family of peptide boronic acid analogs. Bortezomib inhibits proteasome by binding reversibly to the chymotrypsin-like site in the 20S core.13 Bortezomib was the first proteasome inhibitor used in clinical trials for multiple myeloma and solid tumors,14-17 and it has been approved for the treatment of patients with refractory multiple myeloma.18,19 Recently, the results of 2 separate phase 2 clinical trials conducted in relapsed or refractory MCL treated with bortezomib have been published, both showing promising results of bortezomib used as a single agent, with an overall response rate higher than 40% and a relatively long duration of the responses for patients intensively treated.20,21 Considering this, it is important to elucidate the molecular mechanism by which bortezomib induces cytotoxicity in MCL. The aim of this study was to characterize the main pathway by which proteasome inhibition leads to apoptosis commitment in MCL cells. A better understanding of these mechanisms may establish the basis for a rational use of bortezomib in new combination therapies, also improving the prognosis of MCL.
Patients, materials, and methods
Cell lines
Granta-519, Jeko, REC-1, and JVM-2 cell lines, all with the t(11;14)(q13; q32) translocation, were used. Genetic alterations of these cell lines are showed in Table 1.
Characteristics of MCL cell lines
. | . | Checkpoint alterations . | . | . | ||
|---|---|---|---|---|---|---|
| MCL cell line . | LD 50, nM . | p53 . | ATM . | p16 . | ||
| JVM-2 | 18.2 ± 1.3 | wt | wt | wt | ||
| GRANTA-519 | 19.4 ± 1.8 | wt | mut | del | ||
| JEKO | 26.6 ± 2.2 | mut | wt | wt | ||
| REC-1 | 60.1 ± 1.6 | wt | wt | del | ||
. | . | Checkpoint alterations . | . | . | ||
|---|---|---|---|---|---|---|
| MCL cell line . | LD 50, nM . | p53 . | ATM . | p16 . | ||
| JVM-2 | 18.2 ± 1.3 | wt | wt | wt | ||
| GRANTA-519 | 19.4 ± 1.8 | wt | mut | del | ||
| JEKO | 26.6 ± 2.2 | mut | wt | wt | ||
| REC-1 | 60.1 ± 1.6 | wt | wt | del | ||
wt indicates wild type; mut, mutated; and del, deletion.
All cell lines (0.3-0.5 × 106 cells/mL) were cultured in RPMI 1640 culture medium (Gibco, Paisley, Scotland, United Kingdom), supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco), 2 mM glutamine, 50 μg/mL penicillin-streptomycin, and 100 μg/mL normocin (Amaxa, Köln, Germany). In Granta-519 cells, DMEM culture medium (Gibco) was used instead of RPMI 1640. All cultures were routinely tested for mycoplasma contamination.
Patients
Ten patients diagnosed with MCL according to the World Health Organization classification22 pp 168-170 who had not received treatment for the previous 3 months were studied. Tumor cells were obtained from peripheral blood or spleen. The percentage of malignant cells (CD19+, CD5+, CD23- showing light chain restriction) was quantified by flow cytometry. Cyclin D1 overexpression was demonstrated in all cases by immunohistochemistry. The clinical characteristics of these patients are listed in Table 2. An informed consent following the Declaration of Helsinki was obtained from each patient in accordance with the Ethical Committee of the Hospital Clinic (Barcelona, Spain).
Characteristics of MCL patients
Patient no. . | Disease status . | Cell source* . | Morphologic variant . | % of tumoral cells . | p53 status† . |
|---|---|---|---|---|---|
| 1 | Diagnosis | PB | C | 95 | wt |
| 2 | Relapse | PB | C | 95 | CGT→CAT (codon 273) |
| 3 | Diagnosis | PB | C | 84 | wt |
| 4 | Diagnosis | PB | C | 85 | wt |
| 5 | Diagnosis | Spleen | C | 95 | wt |
| 6 | Diagnosis | Spleen | C | 80 | wt |
| 7 | Diagnosis | PB | C | 86 | wt |
| 8 | Relapse | PB | B | 70 | wt |
| 9 | Diagnosis | Spleen | C | 95 | wt |
| 10 | Diagnosis | PB | C | 75 | wt |
Patient no. . | Disease status . | Cell source* . | Morphologic variant . | % of tumoral cells . | p53 status† . |
|---|---|---|---|---|---|
| 1 | Diagnosis | PB | C | 95 | wt |
| 2 | Relapse | PB | C | 95 | CGT→CAT (codon 273) |
| 3 | Diagnosis | PB | C | 84 | wt |
| 4 | Diagnosis | PB | C | 85 | wt |
| 5 | Diagnosis | Spleen | C | 95 | wt |
| 6 | Diagnosis | Spleen | C | 80 | wt |
| 7 | Diagnosis | PB | C | 86 | wt |
| 8 | Relapse | PB | B | 70 | wt |
| 9 | Diagnosis | Spleen | C | 95 | wt |
| 10 | Diagnosis | PB | C | 75 | wt |
PB indicates peripheral blood; C, classical; and B, blastoid.
Source of the cells used for the in vitro analysis
p53 status assessed by fluorescence in situ hybridization (FISH) and mutational status analyzed bysingle-strand conformation polymorphism (SSCP) and sequencing
Isolation and culture of MCL primary cells
Mononuclear cells from peripheral-blood samples were isolated by Ficoll/Hypaque sedimentation (Seromed, Berlin, Germany). Tumor cells were obtained after squirting spleen biopsies with RPMI 1640 culture medium using a fine needle. Cells were either used directly or cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide and 20% heat-inactivated FCS. Manipulation due to freezing/thawing did not influence cell response.
Mononuclear cells from patients with MCL (1-2 × 106 cells/mL) were cultured in X-vivo 10 medium (Biowhittaker, Cambrex, Bioscience, Verviers, Belgium). All MCL cell lines and primary cells were cultured at 37°C in a humidified atmosphere containing 5% carbon dioxide. Cells were incubated for 1 to 20 hours with bortezomib (Millennium, Cambridge, MA). When benzyloxy-carbonyl-Val-Ala-Asp-fluoro-methylketone (z-VAD.fmk; Bachem, Bubendorf, Switzerland), N-acetylcysteine (NAC), or glutathione-reduced ethyl ester (GSH; Sigma, St Louis, MO) was used, cells were preincubated for 1 hour prior to the addition of bortezomib.
Analysis of apoptosis features by flow cytometry
Phosphatidylserine (PS) exposure was quantified by surface annexin V/propidium iodide (PI) staining as previously described.23 Lethal dose 50 (LD50) was defined as the concentration of drug required to reduce at 50% the cell viability.
Changes in mitochondrial transmembrane potential (ΔΨm) were evaluated by staining cells with 20 nM 3,3′-diexyloxacarbocyanine iodide (DiOC6[3]; Molecular Probes, Eugene, OR). ROS production was determined by staining cells with 2 μM dihydroethidine (DHE; Molecular Probes) as previously described.24 Ten thousand cells per sample were acquired in a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Experiments were performed in triplicate.
Detection of intracellular proteins by flow cytometry
Cells were fixed and permeabilized as previously described.25 Cells (0.5 × 106) were stained with 1 μg/mL of antibodies against the active form of caspase-3 (BD-Pharmingen, San Diego, CA), Bax (BD-Pharmingen), and Bak (Oncogene Research, Boston, MA) for 30 minutes at room temperature, followed by goat anti-rabbit FITC (SuperTechs, Bethesda, MD) or goat anti-mouse FITC (DAKO, Glostrup, Denmark), and analyzed in a FACScan flow cytometer.
Immunoblotting and immunoprecipitation
Cells were lysed in 50 mM Tris (tris-hydroxymethyl-aminomethane)-HCl (pH 7.6) buffer containing 0.15 M NaCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 10 μg/mL leupeptin and benzamidine, 1 mM PMSF, and Triton X-100 1%. Solubilized proteins were analyzed in polyacrylamide gels (12%-15%). Western Blot analysis was performed as previously described.23 Chemiluminescence was detected using LAS3000 Fujifilm (Tokyo, Japan) device. Equal protein loading was confirmed with α-tubulin. Relative protein quantification was done with Image Gauge software (Fujifilm).
The following monoclonal and polyclonal antibodies were used: active caspase-3, Bax, and Bid (BD-Pharmingen, San Diego, CA); Bcl-XL (Santa Cruz Biotechnology, CA); Bak (Ab-1), p53 (Ab-2), and α-tubulin (Oncogene Research); Bcl-2 (DAKO); Bax (clone YTH-6A7; Trevigen, Gaithersburg, MD); Puma (Proscience, Poway, CA), p21 (clone EA10), and Bim (Calbiochem, La Jolla, CA); and Phospho-IκB-α (clone 5A5; Cell Signaling, Beverly, MA).
For immunoprecipitation, CHAPS buffer was used (1% CHAPS [wt/vol], 10 mM Hepes [pH 7.4], 150 mM NaCl, and protease inhibitors). Protein extracts were firstly precleared by incubation with G protein (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) for 1 hour and then were incubated overnight with anti-Mcl-1 monoclonal antibody (clone 22; BD-Pharmingen) or anti-Bak polyclonal rabbit antibody (Calbiochem). Afterward, G-protein beads were added for 2 hours. Supernatant (nonimmunoprecipitated fraction) was recovered by centrifugation, and G-protein beads (immunoprecipitated fraction) were washed thrice with CHAPS buffer. Nonreducing 5X sample buffer (400 mM Tris HCl [pH 6.8], 10% SDS, 50% glycerol, 0.1% Bromophenol blue) was added to both fractions and analyzed in 15% polyacrylamide gels followed by Western blotting. Membranes of Mcl-1 immunoprecipitation were probed with polyclonal anti-Mcl-1 antibody (S-19; Santa Cruz Biotechnology) to verify immunoprecipitation quality and with monoclonal anti-Noxa antibody (clone 114C307; Alexis Biochemicals, Lausen, Switzerland). Membranes of Bak immunoprecipitation were probed with anti-Bak (clone G317-2; BD-Pharmingen) and anti-Mcl-1 (clone 22; BD-Pharmingen) monoclonal antibodies.
mRNA quantification by real-time reverse-transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using the guanidinium thiocyanate method (Ultraspec; Bioteck Laboratories, Houston, TX). RNA (1 μg) was retrotranscribed to cDNA using the Taqman reverse-transcription reagents, and mRNA expression was analyzed using predesigned Assay-on-demand (Applied Biosystems, Foster City, CA). The comparative Ct method (ΔΔCt) for relative quantification of gene expression was used. β-glucoronidase (GUS) was used as an internal control, and mRNA-expression levels were given as arbitrary units as previously described.26
20S proteasome activity assay
Cytosolic extracts (without protease inhibitors) were used to measure proteasome activity using 20S proteasome assay kit (Chemicon International, Temecula, CA) following the manufacturer's instructions. The assay is based on detection of the fluorophore 7-amino-4-methylcoumarin (AMC) after cleavage from the labeled substrate LLVY-AMC. The free AMC fluorescence was quantified using a 380/460-nm filter set in a fluorometer.
RNA interference assays
The sense strand of the siRNA used to silence NOXA gene (Noxa siRNA) was 5′-CUU CCG GCAGAAACU UCU G-3′, corresponding to the positions 250 to 270 relative to the start codon of the Noxa mRNA. As a negative control, nonsilencing siRNA (ns siRNA) was used (5′-UUC UCC GAA CGU GUC ACG U-3′). Both siRNAs with 2 3′-dTdT overhangs were synthesized by Qiagen (Hilden, Germany). Jeko and Jurkat cells (clon ED-1, TIB-152) were electroporated using a Nucleofector system (Amaxa) according to the manufacturer's instructions. Briefly, 5 × 106 Jeko or Jurkat cells were resuspended in 100 μL R/T cell nucleofector solution containing 3 μg (2 μM) double-stranded siRNAs and electroporated with A23 or C16 Nucleofector programs, respectively. Prewarmed cultured medium (500 μL) was added to each cuvette and cells were transferred to culture plates and cultured at 3 × 106 cells/mL for 3 hours. Dead cells were removed by low-speed centrifugation and viable cells were diluted at 1 × 106 cells/mL and incubated for 3 hours before experiments with bortezomib incubation.
Results
Bortezomib induces apoptosis in mantle-cell lymphoma
To assess the efficacy of the proteasome inhibitor bortezomib in MCL cells, the toxicity of this drug was analyzed in several MCL cell lines and in primary cells from 10 MCL patients. JVM-2, Granta-519, Jeko, and REC-1 cell lines were exposed to bortezomib for 20 hours, at doses ranging from 5 nM to 100 nM. The lethal doses 50 (LD50) for these MCL cell lines are shown in Table 1. The median LD50 was 31 nM (range, 18.2-60.1 nM). No correlation was observed between sensitivity to bortezomib and genetic alterations in cell-cycle checkpoints and DNA damage sensors genes (Table 1).
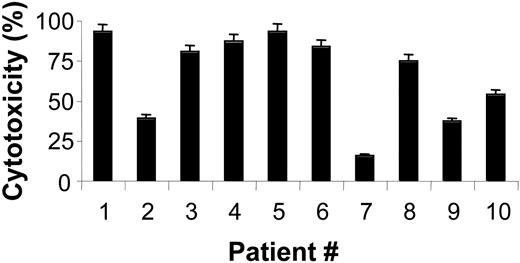
Primary cells from 10 MCL patients were incubated with different doses of bortezomib. The characteristics of these patients are summarized in Table 2. Incubation of primary MCL cells with 20 nM bortezomib for 20 hours induced different cytotoxic effects, with a median ± SD of 54.5% ± 2.37% of control (Figure 1). In cells from 7 MCL patients, a high sensitivity to bortezomib was observed at low doses (20 nM). In cells from patients no. 2, no. 7, and no. 9, higher doses (100 nM) of bortezomib were needed (data not shown) in order to obtain a similar cytotoxic effect.
Cytotoxic effect of bortezomib in cells from MCL patients. Cytotoxic effect of 20 nM bortezomib after 20-hour incubation of primary MCL cells. Cytotoxicity was assessed by annexin V/PI staining as described in “Patients, materials, and methods.” The results shown are the mean value ± SD of duplicate experiments.
Cytotoxic effect of bortezomib in cells from MCL patients. Cytotoxic effect of 20 nM bortezomib after 20-hour incubation of primary MCL cells. Cytotoxicity was assessed by annexin V/PI staining as described in “Patients, materials, and methods.” The results shown are the mean value ± SD of duplicate experiments.
Bortezomib blocks proteasome activity at low doses and short time points in MCL cells
It has been described that bortezomib, upon entering the cell, binds reversibly to the threonine hydroxyl group in the chymotrypsin-like active site of proteasome blocking its activity. MCL cells were treated with several doses of bortezomib, and proteasome activity was assessed after incubation of cells from 30 minutes to 3 hours. As shown in Figure 2A, incubation of Granta-519 cells with 10 nM bortezomib for 30 minutes caused a 40% reduction in proteasome activity. This inhibition was time and dose dependent, reaching almost 80% proteasome inhibition (maximum inhibition in therapeutic trials) after 30-minute exposure to 50 nM bortezomib. Proteasome inhibition was also analyzed in primary MCL cells (patient no. 4). An inhibition of more than 50% was also detected after incubation of these primary MCL cells with several doses of bortezomib (Figure 2B). To further validate the assay, the levels of several proteins known to be regulated by proteasome were analyzed after bortezomib incubation. Figure 2C-D show the accumulation of the tumor suppressor p53, p21 (a cyclin-dependent kinase inhibitor), and the phosphorylated form of IκB (p-IκB, the inhibitor of NFκB) after a 6-hour incubation with 20 nM bortezomib both in primary MCL cells and MCL cell lines. However, at this time point, no inhibition of NF-κB activity was detected by a chemiluminescence NFκB activity assay (Transfactor NFκB Family Kit; BD-Pharmingen) (data not shown). Moreover, despite p21 accumulation, only a moderate G2/M arrest was detected in these experimental conditions (data not shown).
Exposure of MCL cells to bortezomib activates the mitochondrial apoptotic pathway
To elucidate the molecular mechanism by which bortezomib induces apoptosis in MCL cells, several hallmarks of the mitochondrial apoptotic pathway were analyzed. Incubation of Jeko and Granta-519 cell lines with 20 nM bortezomib induced phosphatidylserine exposure (PS), loss of ΔΨm, and generation of ROS (Figure 3). The role of Bax and Bak proteins was analyzed by labeling cells with antibodies directed against the NH2-terminal of these proteins.23
As shown in Figure 3, the conformational change of Bax and Bak proteins was detected after incubation of Jeko and Granta-519 cells with bortezomib.
Finally, the involvement of caspases in the apoptotic process was confirmed by detection of active caspase-3 by flow cytometry (Figure 3). The same results were detected in all primary MCL cells and MCL cell lines analyzed.
Inhibition of proteasome activity by bortezomib and accumulation of proteasome-degraded proteins. (A) Granta-519 cells were incubated with bortezomib (10-50 nM) from 30 minutes to 3 hours, and proteasome activity was analyzed as described in “Patients, materials, and methods.” (B) Total protein extracts (50 μg) from Granta-519 cells were incubated in the absence (-) or presence (+) of 20 nM bortezomib for 6 hours and analyzed by Western blotting. Membranes were probed for p53, p21, and pIκB to confirm proteasome activity inhibition. α-tubulin was also probed as an equal loading control. (C) Primary MCL cells from patient no. 4 were incubated with bortezomib (20-100 nM) for 3 and 6 hours, and proteasome activity was analyzed as described in “Patients, materials, and methods.” (D) Cells from patient 4 were incubated with the indicated doses of bortezomib for 6 hours. Whole protein extracts were obtained, and p53, p21, and pIκB protein levels were analyzed by Western blotting. Data are given as the mean value ± SD of 2 independent experiments.
Inhibition of proteasome activity by bortezomib and accumulation of proteasome-degraded proteins. (A) Granta-519 cells were incubated with bortezomib (10-50 nM) from 30 minutes to 3 hours, and proteasome activity was analyzed as described in “Patients, materials, and methods.” (B) Total protein extracts (50 μg) from Granta-519 cells were incubated in the absence (-) or presence (+) of 20 nM bortezomib for 6 hours and analyzed by Western blotting. Membranes were probed for p53, p21, and pIκB to confirm proteasome activity inhibition. α-tubulin was also probed as an equal loading control. (C) Primary MCL cells from patient no. 4 were incubated with bortezomib (20-100 nM) for 3 and 6 hours, and proteasome activity was analyzed as described in “Patients, materials, and methods.” (D) Cells from patient 4 were incubated with the indicated doses of bortezomib for 6 hours. Whole protein extracts were obtained, and p53, p21, and pIκB protein levels were analyzed by Western blotting. Data are given as the mean value ± SD of 2 independent experiments.
ROS scavengers but not caspase inhibitors block all bortezomib-induced apoptosis hallmarks
In order to establish the sequence of events that leads the cell to the “point of no return,” the effect of caspase inhibition was analyzed. Jeko cells were preincubated for 1 hour with 50 μM z-VAD.fmk before bortezomib incubation. Inhibition of caspases completely prevented caspase-3 activation but only partially blocked PS exposure, ΔΨm loss, and ROS generation. Moreover, no effect was observed on Bax and Bak conformational changes (Figure 3).
Since z-VAD.fmk did not abolish events associated with the apoptotic mitochondrial pathway, the effect of ROS scavengers on bortezomib-induced apoptosis was analyzed. Preincubation with glutathione-reduced ethyl ester (GSH), which acts simultaneously as a ROS scavenger and as a regulator of the intracellular redox state,27 prevented loss of ΔΨm, exposure of PS residues, the conformational changes of Bax and Bak, and caspase activation (Figure 3) in all MCL cell lines tested, as well as in primary MCL cells. Similar results were obtained using N-acetylcysteine, a nonspecific ROS scavenger (data not shown). Conversely, diphenileneiodonium (DPI), an inhibitor of NADH oxidase, did not show any protective effect (data not shown). These results suggest that ROS generation is one of the first events that follows proteasome inhibition after incubation of MCL cells with bortezomib.
Bortezomib activates the intrinsic pathway in MCL cells. Effect of caspase inhibition and ROS elimination on bortezomib-induced apoptosis in MCL. Jeko and Granta-519 cells were treated with 20 nM bortezomib for 18 hours in the absence or presence of z-VAD-fmk (50 μM) or glutathione-reduced ethyl ester (GSH, 2 mM). These inhibitors were preincubated for 1 hour before bortezomib addition. Cell viability was determined by annexin V/PI staining. ΔΨm was assessed by DiOC6(3) staining, and ROS generation was quantified using DHE. Bax/Bak conformational changes and caspase-3 activation were determined by staining permeabilized cells with anti-Bax, anti-Bak, and anti-active caspase-3 antibodies. In treated cells, the histogram (black) is superimposed with the control (gray). The percentage of positive cells for each labeling is indicated inside the chart.
Bortezomib activates the intrinsic pathway in MCL cells. Effect of caspase inhibition and ROS elimination on bortezomib-induced apoptosis in MCL. Jeko and Granta-519 cells were treated with 20 nM bortezomib for 18 hours in the absence or presence of z-VAD-fmk (50 μM) or glutathione-reduced ethyl ester (GSH, 2 mM). These inhibitors were preincubated for 1 hour before bortezomib addition. Cell viability was determined by annexin V/PI staining. ΔΨm was assessed by DiOC6(3) staining, and ROS generation was quantified using DHE. Bax/Bak conformational changes and caspase-3 activation were determined by staining permeabilized cells with anti-Bax, anti-Bak, and anti-active caspase-3 antibodies. In treated cells, the histogram (black) is superimposed with the control (gray). The percentage of positive cells for each labeling is indicated inside the chart.
Modulation of levels of Bcl-2 family proteins by bortezomib
The balance between proapoptotic and antiapoptotic Bcl-2 proteins is crucial for determining the fate of a cell. We have analyzed the effect of bortezomib on the levels of the antiapoptotic proteins of the Bcl-2 family and on the so-called BH3-only proteins.28
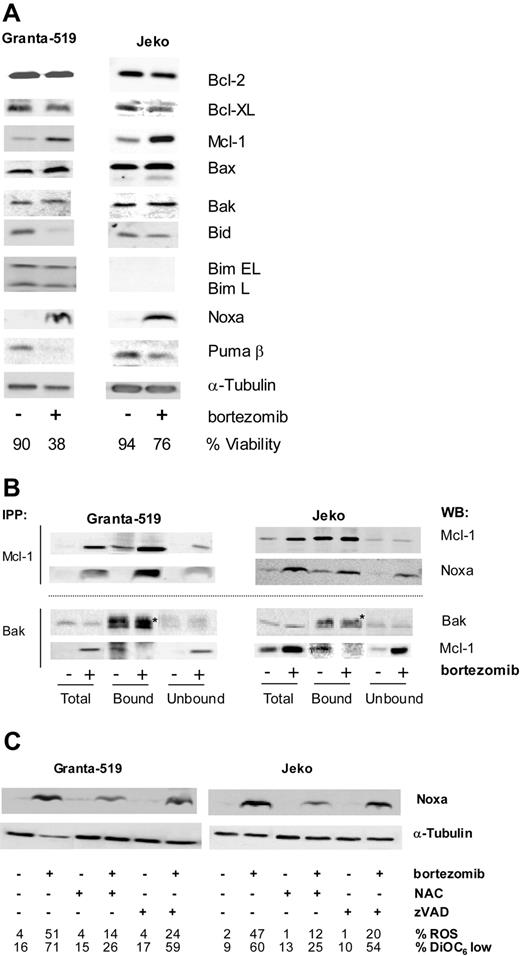
Granta-519 and Jeko cells were treated with 20 nM bortezomib for 18 hours and levels of Bcl-2, Bcl-XL, Mcl-1, Bax, Bak, Bim, Bid, Noxa, and Puma were analyzed by Western blotting (Figure 4A). After bortezomib incubation, Bcl-2 levels were not affected, but Bcl-XL levels decreased slightly. Bortezomib induced the accumulation of the antiapoptotic protein Mcl-1. This accumulation is due to the fact that Mcl-1 protein contains PEST sequences (sequences rich in proline, glutamate [E], serine [S], and threonine [T]) that target its degradation by proteasome.29 Although conformational changes of Bax and Bak proteins were observed after bortezomib treatment, no changes in the overall levels of these proteins were detected. Bortezomib also induced a decrease of Bid protein level, as a consequence of its cleavage mediated by caspase-3 activation, since this effect was prevented by Z-VAD incubation (data not shown). No Bim expression was detected in Jeko cells according to the existence of a homozygous deletion at 2q13.30 In Granta-519 cells, a slight diminution of Bim was detected after bortezomib incubation. Analysis of the BH3-only protein Puma demonstrated that the β isoform is the most prominent in MCL cell lines and that the expression of this isoform also decreased after bortezomib treatment. Finally, and most outstanding, we have observed an accumulation of the BH3-only protein Noxa, a protein whose levels were almost undetectable in untreated MCL cells. Moreover, bortezomib-induced Noxa up-regulation was detected in both MCL cell lines with wild-type p53 (wtp53) (Granta-519) or mutated p53 (mutp53) (Jeko), excluding a p53-only-dependent process. This protein does not contain PEST sequences, and consequently its accumulation could not be directly explained by proteasome inhibition.
Bortezomib modulates Bcl-2 family protein levels and interactions. Effect of the ROS scavenger NAC and z-VAD-fmk on Noxa modulation. (A) Granta-519 and Jeko cells were treated with 20 nM bortezomib for 18 hours and total protein extracts were analyzed by Western blotting using suitable antibodies. Viability was quantified by annexin V/PI staining. (B) Granta-519 and Jeko cells were treated with 20 nM bortezomib for 16 hours, and Mcl-1 and Bak immunoprecipitations were performed as described in “Patients, materials, and methods.” Total extracts, and bound and unbound fractions were analyzed by Western blotting for Mcl-1 and Noxa or Bak and Mcl-1, respectively. *Unspecific band of immunoglobulin light chain from Bak antibody. (C) Granta-519 and Jeko cells were treated with 20 nM bortezomib for 20 hours in the presence or absence of 10 mM NAC or 50 μM z-VAD-fmk (both added 1 hour before bortezomib), and total protein extracts were analyzed for Noxa expression by Western blotting. The percentage of ROS and the loss of ΔΨm were determined by DHE and DiOC6(3) staining, respectively, followed by fluorescence-activated cell sorter (FACs) analysis.
Bortezomib modulates Bcl-2 family protein levels and interactions. Effect of the ROS scavenger NAC and z-VAD-fmk on Noxa modulation. (A) Granta-519 and Jeko cells were treated with 20 nM bortezomib for 18 hours and total protein extracts were analyzed by Western blotting using suitable antibodies. Viability was quantified by annexin V/PI staining. (B) Granta-519 and Jeko cells were treated with 20 nM bortezomib for 16 hours, and Mcl-1 and Bak immunoprecipitations were performed as described in “Patients, materials, and methods.” Total extracts, and bound and unbound fractions were analyzed by Western blotting for Mcl-1 and Noxa or Bak and Mcl-1, respectively. *Unspecific band of immunoglobulin light chain from Bak antibody. (C) Granta-519 and Jeko cells were treated with 20 nM bortezomib for 20 hours in the presence or absence of 10 mM NAC or 50 μM z-VAD-fmk (both added 1 hour before bortezomib), and total protein extracts were analyzed for Noxa expression by Western blotting. The percentage of ROS and the loss of ΔΨm were determined by DHE and DiOC6(3) staining, respectively, followed by fluorescence-activated cell sorter (FACs) analysis.
Noxa up-regulation is critical in the onset of bortezomib-induced apoptosis by interacting with Mcl-1
It has been described that proapoptotic and antiapoptotic members of the Bcl-2 protein superfamily interact with each other following certain rules.31 To explore whether up-regulation of the proapoptotic Noxa protein could counteract the accumulation of the antiapoptotic Mcl-1 protein after bortezomib treatment, we analyzed the possible interactions between these proteins. As shown in Figure 4B, we confirmed that bortezomib induced Noxa up-regulation in MCL cells and that the major part of this protein coimmunoprecipitates with Mcl-1. Immunoprecipitation with Bak revealed that Mcl-1 coimmunoprecipitates with Bak in control cells, but after bortezomib treatment, Bak is release from Mcl-1, presumably due to Noxa up-regulation and coupling to Mcl-1. No interaction was detected between Bak and Noxa (data not shown).
ROS scavengers but not pancaspase inhibitors block Noxa up-regulation
To study a possible relationship between ROS generation and Noxa up-regulation, Noxa protein expression was analyzed in bortezomib-treated cells, in the presence or absence of ROS scavengers. NAC significantly reduced bortezomib-induced Noxa accumulation, both in wtp53 cells (Granta-519) and in mutp53 cells (Jeko) (Figure 4C). Similar results were obtained when GSH was used (data not shown). The effect of caspase inhibition was also evaluated by preincubating cells with z-VAD.fmk before bortezomib addition. Conversely, caspase inhibition did not affect Noxa up-regulation induced by bortezomib in these MCL cell lines (Figure 4C).
Noxa up-regulation induced by bortezomib is detected earlier in cells harboring wtp53
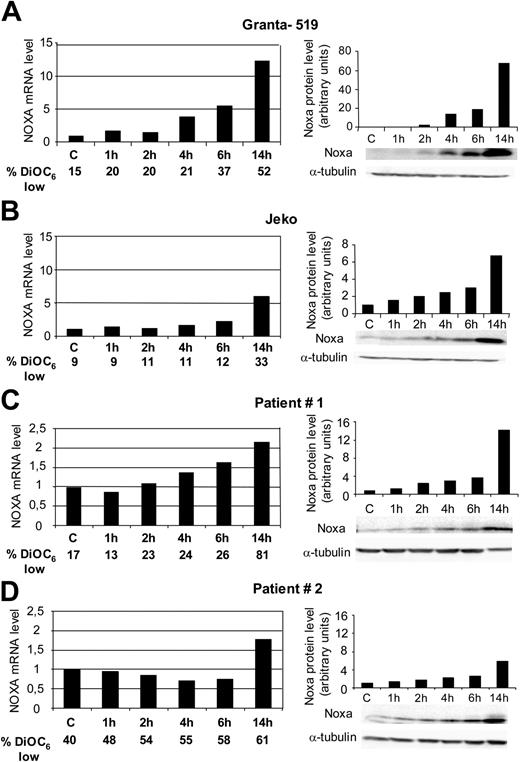
Considering the possible role of Noxa in apoptosis induction, a bortezomib time course of Noxa up-regulation was performed in wtp53 (Granta-519) and mutp53 (Jeko) cells. In both MCL cell lines, Noxa up-regulation was detected between 2 to 4 hours after bortezomib addition, both at protein and mRNA levels. This up-regulation was faster and higher in cells with functional p53 (Granta-519). Furthermore, Noxa up-regulation precedes loss of ΔΨm (Figure 5A-B).
Up-regulation of Noxa was also detected in primary cells from MCL patients. Figure 5C and 5D show the results observed in patient no. 1 (wtp53) and patient no. 2 (mutp53), respectively. At protein level, a time-dependent Noxa up-regulation was observed. At short time points (1-6 hours), the increase in Noxa protein was slightly higher in patient no. 1 (wtp53) than in patient no. 2 (mutp53), and after longer bortezomib exposure (14 hours), Noxa up-regulation in patient no. 1 (wtp53) was more than 2-fold compared with the up-regulation detected in patient no. 2 (mutp53). Similarly, Noxa up-regulation at mRNA level was higher in cells from patient no. 1 (wtp53) than in cells from patient no. 2 (mutp53).
This up-regulation was detected as early as 6 hours after bortezomib addition and continued with time, exceeding a 2-fold increase after 14 hours of bortezomib incubation. Conversely, in cells from patient no. 2 with a mutated p53, we could not detect a significant increase in Noxa levels before 14 hours of bortezomib incubation. All together, these results indicate that up-regulation of Noxa was faster and higher in primary and established MCL cells with functional p53.
Time course of Noxa activation at protein and mRNA levels. Cells from Granta-519 (A), Jeko (B), and primary cells from MCL patient 1 (C) were treated with 20 nM bortezomib, and primary cells from MCL patient 2 (D), with 50 nM bortezomib. RNA and protein were isolated after 1, 2, 4, 6, and 14 hours of incubation. Noxa mRNA levels were evaluated at these time points by quantitative RT-PCR (Taqman technology) using GUS as housekeeping gene. mRNA-expression levels are given in arbitrary units, using cDNA from untreated cells as reference control. Noxa protein levels were evaluated by Western blotting using α-tubulin as equal loading control. Relative protein quantification was analyzed with Image Gauge software from Fujifilm LAS 3000 chemiluminescence detector. The percentage of loss of ΔΨm was determined by DiOC6(3) staining, followed by FACs analysis.
Time course of Noxa activation at protein and mRNA levels. Cells from Granta-519 (A), Jeko (B), and primary cells from MCL patient 1 (C) were treated with 20 nM bortezomib, and primary cells from MCL patient 2 (D), with 50 nM bortezomib. RNA and protein were isolated after 1, 2, 4, 6, and 14 hours of incubation. Noxa mRNA levels were evaluated at these time points by quantitative RT-PCR (Taqman technology) using GUS as housekeeping gene. mRNA-expression levels are given in arbitrary units, using cDNA from untreated cells as reference control. Noxa protein levels were evaluated by Western blotting using α-tubulin as equal loading control. Relative protein quantification was analyzed with Image Gauge software from Fujifilm LAS 3000 chemiluminescence detector. The percentage of loss of ΔΨm was determined by DiOC6(3) staining, followed by FACs analysis.
Noxa RNAi demonstrates its pivotal role in bortezomib-induced apoptosis in MCL cells
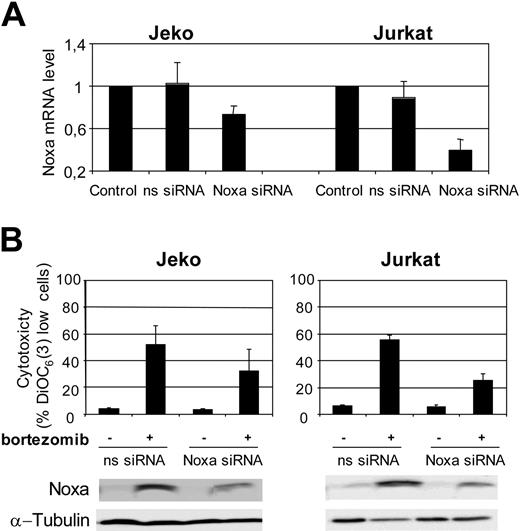
To further validate the role of Noxa in bortezomib-induced apoptosis in MCL cells, we performed RNA interference (RNAi) experiments to knock down the expression of this protein. Due to the fact that MCL cell lines are difficult to transfect, in parallel we have done Noxa siRNA assays in Jeko and in Jurkat cells, because transfection conditions for Jurkat cells are well established.32-34 Noxa and nonsilencing siRNA oligonucleotides were introduced in Jeko and Jurkat cells by electroporation with Nucleofector as described in “Patients, materials, and methods.” As shown in Figure 6A, a reduction of almost 30% and more than 50% in Noxa mRNA levels was observed in Jeko and Jurkat cells, respectively, after transfection with Noxa siRNA.
The effects of Noxa down-regulation were analyzed after treatment with 20 nM bortezomib for 18 hours in Noxa siRNA-transfected Jeko and Jurkat cells. As shown in Figure 6B, Noxa siRNA in Jeko cells reduced bortezomib toxicity by 35% compared with nonsilencing siRNA. A reduction in Noxa protein induction was also observed in cells transfected with Noxa siRNA.
This protective effect of Noxa siRNA against bortezomib-induced apoptosis was more prominent in Jurkat cells, in accordance with the higher efficiency of Noxa silencing in these cells (Figure 6A). In Jurkat cells, the protection observed exceeded 50% when compared with control-transfected cells. Similarly to Jeko cells, bortezomib-induced Noxa up-regulation was notably inhibited in Jurkat cells transfected with Noxa siRNA.
Discussion
MCL is an aggressive B-cell non-Hodgkin lymphoma with very poor prognosis and resistance to current chemotherapy regimens.1,9 For this reason, novel rationally based treatment approaches are needed to improve patient outcome. Proteasome inhibitors have emerged as a new group of chemotherapeutic agents for human cancer.35,36 Bortezomib is a small molecule proteasome inhibitor that has shown antitumor activity in a number of cell types and is currently undergoing clinical trials.14,16,20 Recent clinical data has shown promising results for the treatment of refractory MCL.20,21 The mechanisms by which bortezomib kills tumor cells are not totally defined. It has been proposed to be related to inactivation of NF-κB activity,37 stabilization of p53, and activation of stress-related pathways.38 In the present work, we show that low doses of bortezomib induce apoptosis in cells from MCL patients, as well as in MCL cell lines carrying the t(11;14)(q13;q32). It is remarkable that bortezomib showed antitumoral activity in MCL cell lines and MCL primary cells with defective DNA damage sensors genes (p53 [Jeko] and ATM [Granta-519]) and cell-cycle checkpoints (p16 deletions [REC-1 and Granta-519]). These “control points” are often impaired in MCL.5,7 We also demonstrate that this compound causes a very rapid proteasome inhibition in MCL cells and a marked accumulation of crucial cellular proteins linked to cell proliferation and apoptosis, such as the tumor suppressor p53, the cyclin-dependent kinase inhibitor p21, and pIκB, a NF-κB inhibitor. These results are in agreement with those published previously in MCL cells.39,40
Our experiments also demonstrate that bortezomib exerts its cytotoxic effect in MCL cells by activating the mitochondrial apoptotic pathway. Thus, bortezomib-induced apoptosis is associated with caspase activation and occurs via alteration of mitochondrial membrane potential and conformational changes of Bax and Bak proteins. In addition, ROS generation plays a critical role in bortezomib-induced apoptosis, since the disruption of mitochondrial function and the induction of apoptosis can be rescued almost totally by ROS scavengers. The mechanism underlying ROS generation after inhibition of proteasome is not known. It has been proposed that ROS generation induced by bortezomib may be due to induction of O2- and alteration of the oxidation-reduction metabolic pathways, bortezomib interference with mitochondrial electron transport system, or by endoplasmic reticulum stress due to protein accumulation in this organelle.38,41,42 Our results also demonstrate that caspases participate in a postmitochondrial phase, since z-VAD.fmk does not prevent bortezomib-induced ROS generation and loss of ΔΨm. These results indicate that ROS generation is upstream of caspase activation in bortezomib-induced apoptosis. This is consistent with the results published previously in other models.43
Noxa RNAi decreases response to bortezomib. (A) Noxa siRNA and nonsilencing-siRNA were transferred to Jeko and Jurkat cells by electroporation as described in “Patients, materials, and methods.” Total RNA was isolated 6 hours after transfection. Noxa mRNA levels were determined by quantitative RT-PCR (Taqman technology) using GUS as housekeeping gene. The results showed are the mean of 2 different experiments. (B) Jeko and Jurkat cells, transfected with nonsilencing siRNA (ns-siRNA) and with Noxa siRNA (Noxa-siRNA), were treated with 20 nM bortezomib for 18 hours. Viability was assessed by DiOC6(3) staining and FACs analysis. The results showed are the mean of 2 different experiments. In the same conditions, Noxa protein levels were also analyzed by Western blotting.
Noxa RNAi decreases response to bortezomib. (A) Noxa siRNA and nonsilencing-siRNA were transferred to Jeko and Jurkat cells by electroporation as described in “Patients, materials, and methods.” Total RNA was isolated 6 hours after transfection. Noxa mRNA levels were determined by quantitative RT-PCR (Taqman technology) using GUS as housekeeping gene. The results showed are the mean of 2 different experiments. (B) Jeko and Jurkat cells, transfected with nonsilencing siRNA (ns-siRNA) and with Noxa siRNA (Noxa-siRNA), were treated with 20 nM bortezomib for 18 hours. Viability was assessed by DiOC6(3) staining and FACs analysis. The results showed are the mean of 2 different experiments. In the same conditions, Noxa protein levels were also analyzed by Western blotting.
Bcl-2 proteins are implicated in the surveillance of mitochondrial integrity by means of a wise balance between proapoptotic and antiapoptotic members.44 However, this balance is often disrupted by a wide variety of cellular insults. Proteasome inhibition causes undoubtedly a great cellular stress, due to the accumulation of undergraded proteins, and this stress ends in apoptotic cell death. How this stress could induce mitochondrial dysfunction has not been addressed yet. Bortezomib induced a marked accumulation of Mcl-1 in MCL cells, which may be explained by the existence of PEST sequences in this protein. Thus, under proteasome inhibition Mcl-1 cannot be degraded and accumulates in the cell.45 Remarkably, this fact did not block apoptosis induced by bortezomib, but it could slow down apoptosis induction.
BH3-only proteins are known to be the sensors that mediate apoptotic signals and mitochondrial depolarization, although it is not entirely clear how BH3-only proteins activate the apoptotic machinery. It has been proposed that they may block the antiapoptotic effect of members of Bcl-2 superfamily, and/or induce Bax/Bak conformational change.46 Noxa is a BH3-only mitochondrial protein that contributes to apoptosis by disrupting mitochondrial outer membrane integrity. We demonstrate that bortezomib is able to trigger Noxa induction in MCL cells and this up-regulation is not due to proteasome inhibition, since Noxa does not contain PEST sequences. Noxa was firstly identified as a critical mediator of p53-dependent apoptosis.47,48 Our results demonstrated that up-regulation of Noxa is independent of p53 mutational status, albeit with a delayed onset in cells carrying mutated p53 (Jeko and patient no. 2). Recently, several studies have described up-regulation of Noxa as a process independent of p53 status.49 Our RNAi experiments demonstrate that Noxa siRNAs decrease bortezomib's cytotoxic effect in MCL cells, suggesting that up-regulation of Noxa is a key mediator between proteasome inhibition and apoptosis induction in MCL cells.
Interactions between proapoptotic and antiapoptotic members of Bcl-2 superfamily are essential for mitochondrial depolarization. It has been recently published that Noxa selectively binds to Mcl-1 and Bfl-1/A1 proteins31 and is also capable of displacing proapoptotic Bak from Mcl-1.50 Our results show that incubation of MCL cells with bortezomib triggers Noxa induction and the majority of this protein binds to Mcl-1. In addition, this coupling allows Bak to be released from Mcl-1. Once liberated, Bak may oligomerize and induce mitochondrial depolarization. These results support the role of Noxa as a key mediator between bortezomib apoptosis signal and mitochondrial depolarization.
In summary, our results demonstrate that bortezomib exerts a cytotoxic effect in primary MCL cells as well as in MCL cell lines through ROS generation and Noxa induction, which is supported by Noxa expression knock down. The interaction of Noxa with Mcl-1 suggests that after bortezomib treatment, Noxa may counteract the unwanted accumulation of Mcl-1 promoting Bak release. These findings confirm the potential use of bortezomib as a therapeutic agent in MCL and also establish the basis for the design of new, rationale-based bortezomib combination therapies.
Prepublished online as Blood First Edition Paper, September 15, 2005; DOI 10.1182/blood-2005-05-2091.
Supported by Fondo Investigaciones Sanitarias (FIS) 03/0398 (D.C.); European Comission contract SLMM-CT-2004-503351; the Lymphoma Research Foundation; and Redes temáticas de Centros: Genómica del cancer (03/10), Red Estudio de neoplasias linfoides (03/179), and Instituto de Salud Carlos III. P.P.-G. held a Beca de formación en investigación (BEFI) from FIS and now holds a Juan de la Cierva postdoctoral contract from the Ministerio de Educación y Ciencia (MEC). G.R. holds a postdoctoral contract from the CRED program (Contractació i reincorporació de doctors estrangers, Generalitat de Catalunya).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal