Abstract
Because the causes of most lymphoid neoplasms remain unknown, comparison of incidence patterns by disease subtype may provide critical clues for future etiologic investigations. We therefore conducted a comprehensive assessment of 114 548 lymphoid neoplasms diagnosed during 1992-2001 in 12 Surveillance, Epidemiology, and End Results (SEER) registries according to the internationally recognized World Health Organization (WHO) lymphoma classification introduced in 2001. Cases coded in International Classification of Diseases for Oncology, Second Edition (ICD-O-2), were converted to ICD-O-3 for WHO subtype assignment. Age-specific and age-adjusted rates were compared by sex and race (white, black, Asian). Age-adjusted trends in incidence were estimated by sex and race using weighted least squares log-linear regression. Diverse incidence patterns and trends were observed by lymphoid neoplasm subtype and population. In the elderly (75 years or older), rates of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma increased 1.4% and 1.8% per year, respectively, whereas rates of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) declined 2.1% per year. Although whites bear the highest incidence burden for most lymphoid neoplasm subtypes, most notably for hairy cell leukemia and follicular lymphoma, black predominance was observed for plasma cell and T-cell neoplasms. Asians have considerably lower rates than whites and blacks for CLL/SLL and Hodgkin lymphoma. We conclude that the striking differences in incidence patterns by histologic subtype strongly suggest that there is etiologic heterogeneity among lymphoid neoplasms and support the pursuit of epidemiologic analysis by subtype.
Introduction
Neoplasms originating in lymphoid tissue comprise a diverse yet closely related group of neoplasms, including non-Hodgkin lymphoma (NHL), Hodgkin lymphoma, multiple myeloma, and acute and chronic lymphocytic leukemia. Together, lymphoid neoplasms will yield approximately 93 420 incident cases and 38 000 deaths in the United States during 2005.1 The etiology of lymphoid neoplasms remains largely unknown, although risk factors include certain infections as well as treatments and diseases that cause severe immunosuppression.2-9
Beginning prior to 1950, an epidemic of NHL but not other hematopoietic neoplasms has been well documented in many populations,10,11 with an estimated 50% increase in United States age-adjusted incidence from 1970 to 1990 11-14 (Figure 1). Changes in diagnostic practice over time and the emergence of the human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) pandemic in the early 1980s contributed to the rise in NHL but are not sufficient to explain entirely these dramatic increases.12,15-19 Recent reports suggest that the steep rise in incidence may have slowed in the late 1990s,15,20-22 as shown in Figure 1. Interpretation of NHL rates as a single disease entity requires caution, however, because the rates reflect a combination of diagnostic and etiologic factors that may differ by disease subtype.
Categorizing the various lymphoid neoplasms has proven to be enormously challenging and has resulted in the evolution of numerous classification schemes over the past 50 years.23-27 In 2001, the World Health Organization (WHO) introduced a new system for classifying hematopoietic neoplasms. Whereas previous classifications relied predominantly on morphology, the new WHO classification incorporates morphology, immunophenotype, cytogenetic and molecular features, clinical behavior, and some known aspects of etiology and pathogenesis into the definition of each disease subtype.28 Additionally, the WHO classification highlights the interrelatedness of the various lymphoid neoplasms based on the cell of origin.
In light of the changing classifications, few comprehensive descriptive analyses of the patterns and time trends of lymphoid neoplasm subtypes have been conducted. Some of us previously evaluated the incidence patterns of lymphoid neoplasms diagnosed during 1978-1995 in 9 United States population-based cancer registries29 using the earlier Working Formulation classification.26 Even with this less satisfactory classification scheme, the feasibility and importance of distinguishing among lymphoid neoplasms has been demonstrated. Other reports on incidence patterns of individual subtypes during the last 15 years also suggest that patterns vary among the lymphoid neoplasms.11-13,15,17,30-39
To date, no systematic descriptive analysis has considered incidence patterns for the entirety of lymphoid neoplasm subtypes as defined by the WHO classification.28 Because the causes of most lymphoid neoplasms remain largely unknown, comparison of incidence rates and patterns for specific subtypes may provide critical clues for future etiologic investigations. Recent adoption of the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), by United States cancer registries and the ability to convert cases coded in ICD-O-2 to ICD-O-3 40 make it feasible for the first time to assess incidence patterns and trends for lymphoid neoplasms according to the internationally recognized World Health Organization (WHO) classification introduced in 2001. We therefore conducted an analysis of the patterns and time trends of 114 548 incident lymphoid neoplasms diagnosed during 1992-2001 in 12 United States population-based cancer registries using the new WHO classification.28
Trends in incidence of hematopoietic neoplasms by broad subtype category, 9 SEER registries, 1978-1979 to 2000-2001.*All incidence rates are age adjusted to the 2000 United States population and presented for 12 fixed 2-year time periods (1978-1979 to 2000-2001). †Lymphoid neoplasms excepting Hodgkin lymphoma and plasma-cell neoplasms. ‡Predominantly myeloid leukemia. §Predominantly multiple myeloma.
Trends in incidence of hematopoietic neoplasms by broad subtype category, 9 SEER registries, 1978-1979 to 2000-2001.*All incidence rates are age adjusted to the 2000 United States population and presented for 12 fixed 2-year time periods (1978-1979 to 2000-2001). †Lymphoid neoplasms excepting Hodgkin lymphoma and plasma-cell neoplasms. ‡Predominantly myeloid leukemia. §Predominantly multiple myeloma.
Patients, materials, and methods
Incidence data were obtained from the United States National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program.41 SEER data collection began in the early 1970s with population-based registries in 5 states (Connecticut, Hawaii, Iowa, New Mexico, and Utah) and 4 metropolitan areas (Detroit, MI; San Francisco/Oakland, CA; Seattle/Puget Sound, WA; and Atlanta, GA).14 SEER expanded in 1992 to include two additional metropolitan areas (Los Angeles County and San Jose/Monterey, CA) and the Alaska Native Tumor Registry.42 Together these 12 regions account for approximately 14% of the United States population.43 Our analysis considers the incidence of all lymphoid neoplasms diagnosed during the 10-year study period beginning January 1, 1992, when data first were combined from all 12 registries, and ending December 31, 2001, the most recent time for which complete data are available; this analysis utilizes data from the November 2003 SEER data submission, released in April 2004.42
For each newly identified lymphoid neoplasm case, SEER registries report patient demographic data, including age, race, ethnicity, and sex, and information on the tumor histologic type, primary site, behavior (benign or malignant), and immunophenotype (B cell, T/natural killer [NK] cell, or unknown). Data regarding B-cell versus T/NK-cell lineage have been recorded in SEER registries since the 1970s, although prior to 1990 the proportion of cases with immunophenotype data are small.29 Cases with known race were grouped into whites, blacks, Asians or Pacific Islanders (hereafter referred to as “Asians”), and American Indians or Alaska Natives; a total of 650 (1.0%) men and 519 (1.0%) women were excluded from race-specific analyses because they were recorded as unknown race. Analysis of the incidence of lymphoid neoplasms by Hispanic ethnicity is beyond the scope of this paper because ethnicity is not recorded reliably in all 12 of the registries used in this analysis.41 Data on histologic type and primary site were coded according to ICD-O-2,44 for cases diagnosed during 1992-2000, or according to ICD-O-345 for cases diagnosed in 2001. SEER uses a conversion algorithm to translate cases coded using ICD-O-2 to ICD-O-3.40
Lymphoid neoplasm classification
The history of classifying lymphoid neoplasms since the 1940s has been reviewed previously.23,25,27 Briefly, beginning in 1982, the United States National Cancer Institute proposed the Working Formulation, which emphasized groupings according to morphology and clinical prognosis, to translate among the various lymphoma classifications in use at the time (eg, Rappaport, Lukes-Collins, Kiel).26 Prior to 1994, the Working Formulation was adopted in the United States, whereas an updated Kiel classification was used in Europe.46 Based on advances in knowledge, the Working Formulation and Kiel classifications were replaced in 1994 with the Revised European-American Lymphoma (REAL) classification, which incorporated morphologic, immunophenotypic, genotypic, and clinical features into disease subtype definitions.24 With the advent of the REAL classification, several changes were made to the classification of lymphomas used by SEER registries, including the addition of ICD-O-2 codes for marginal zone lymphoma (ICD-O-2 codes 9710, 9711, 9715) and the addition of the term “mantle cell” (in contrast to “mantle zone”) lymphoma.40 Since the 1970s the leukemias have been classified predominantly using the French-American-British (FAB) classification.47,48 Updated terminology from the FAB classification also has been incorporated into ICD-O periodically.40,47-49
The WHO classification introduced in 2001 builds on the REAL and FAB classifications and is the current gold standard for classifying all hematopoietic neoplasms.28 The WHO system distinguishes hematologic malignancies according to cell lineage: lymphoid, myeloid, histiocytic/dendritic, and mast cells. Within the lymphoid neoplasms, morphologic and immunologic characteristics distinguish Hodgkin lymphomas from the NHLs. Stage of differentiation and additional morphologic, phenotypic, genotypic, and clinical features distinguish among the NHL subtypes. Lymphomas and corresponding lymphoid leukemias are presently considered different phases (solid versus circulating, respectively) of the same disease entity. By incorporating WHO terminology into the classification of hematologic malignancies used by SEER registries, ICD-O-3 provides a valuable link between WHO subtypes and historical data in the United States.
The ICD-O-3 codes and immunophenotype data reported by 12 SEER registries were used to categorize cases according to the WHO classification.28 The ICD-O-3 codes included in each WHO disease entity were reviewed by an expert hematopathologist (D.D.W.). The classification of WHO disease entities and ICD-O-3 codes considered in the current analysis are presented in Table 1. All data refer to the incidence of neoplasms with malignant behavior.
Incidence of hematopoietic neoplasms by subtype and ICD-O-3 codes, 12 SEER registries, 1992-2001
Hematopoietic neoplasm subtype . | ICD-O-3 codes*† . | No. . | Rate‡ . |
|---|---|---|---|
| Lymphoid neoplasms, total | – | 114 548 | 33.65 |
| B-cell lymphoid neoplasms, total | 9590-9591(B), 9596(B), 9670-9671, 9673, 9675(B), 9678-9680, 9684, 9687, 9689-9691, 9695, 9698-9699, 9727(B), 9728, 9731-9734, 9761, 9764, 9820(B), 9823, 9826, 9832(B), 9833, 9835(B), 9836, 9940, 9970(B) | 87 666 | 26.13 |
| DLBCL | 9675(B), 9678-9680, 9684 | 24 246 | 7.14 |
| Marginal zone lymphoma | 9689, 9699 | 3247 | 0.97 |
| Follicular lymphoma | 9690-9691, 9695, 9698 | 10 705 | 3.18 |
| CLL/SLL | 9670, 9823 | 16 984 | 5.17 |
| Mantle cell lymphoma | 9673 | 1691 | 0.51 |
| Burkitt lymphoma/leukemia | 9687, 9826 | 1102 | 0.30 |
| Plasma cell neoplasms | 9731-9734 | 18 644 | 5.66 |
| Multiple myeloma | 9732 | 17 407 | 5.29 |
| Plasma cell leukemia | 9733 | 107 | 0.03 |
| Plasmacytoma | 9731, 9734 | 1130 | 0.34 |
| Hairy cell leukemia | 9940 | 1116 | 0.33 |
| Lymphoplasmacytic lymphoma | 9671 | 911 | 0.27 |
| Waldenstrom macroglobulinemia | 9761 | 1144 | 0.35 |
| B-cell lymphoid neoplasms, NOS | 9590-9591(B), 9596(B), 9764, 9820(B), 9970(B) | 4872 | 1.45 |
| T/NK-cell lymphoid neoplasms, total | 9590-9591(T/NK), 9596(T/NK), 9675(T/NK), 9700-9702, 9705, 9708-9709, 9714, 9716-9719, 9727(T/NK), 9729, 9820(T/NK), 9827, 9831, 9832(T/NK), 9834, 9835(T/NK), 9837, 9948, 9970(T/NK) | 6228 | 1.79 |
| Mycosis fungoides/Sézary syndrome | 9700-9701 | 1773 | 0.52 |
| Peripheral T-cell lymphoma | 9675(T/NK), 9702, 9705, 9708, 9714, 9716, 9827 | 2071 | 0.60 |
| Angioimmunoblastic lymphoma | 9705 | 176 | 0.05 |
| Anaplastic large-cell lymphoma | 9714 | 864 | 0.25 |
| Peripheral T-cell lymphoma, NOS | 9675(T/NK), 9702, 9708, 9716, 9827 | 1031 | 0.30 |
| T/NK-cell lymphoid neoplasms, NOS | 9590-9591(T/NK), 9596(T/NK), 9709, 9717-9719, 9820(T/NK), 9831, 9948, 9970(T/NK) | 1501 | 0.44 |
| Lymphoblastic leukemia/lymphoma§ | 9727-9729, 9835-9837 | 6127 | 1.61 |
| B-cell lymphoblastic leukemia/lymphoma | 9727(B), 9728, 9835(B), 9836 | 2907 | 0.76 |
| T-cell lymphoblastic leukemia/lymphoma | 9727(T/NK), 9729, 9835(T/NK), 9837 | 852 | 0.22 |
| Unknown type lymphoblastic leukemia/lymphoma | 9727, 9835(unknown) | 2368 | 0.63 |
| Prolymphocytic leukemia | 9832-9834 | 237 | 0.07 |
| Hodgkin lymphoma | 9650-9655, 9659, 9661-9665, 9667 | 10 042 | 2.67 |
| Classical Hodgkin lymphoma | 9650-9655, 9661-9665, 9667 | 9734 | 2.59 |
| Nodular sclerosis | 9663-9665, 9667 | 6270 | 1.63 |
| Mixed cellularity/lymphocyte depleted | 9652-9655 | 1906 | 0.53 |
| Classical Hodgkin lymphoma, NOS | 9650-9651, 9661-9662 | 1558 | 0.43 |
| Nodular lymphocyte predominant Hodgkin lymphoma | 9659 | 308 | 0.08 |
| Unknown type lymphoid neoplasms | 9590-9591(unknown), 9596(unknown), 9675(unknown), 9820(unknown), 9970(unknown) | 8135 | 2.40 |
| Nonlymphoid hematopoietic neoplasms, total | – | 22 437 | 6.63 |
| Chronic myeloproliferative diseases∥ | 9950, 9960-9964, 9975 | ||
| Myelodysplastic syndromes∥ | 9980, 9982-9987, 9989 | ||
| Myeloid leukemias | 9840, 9860-9861, 9863, 9866-9867, 9870-9876, 9891, 9895-9897, 9910, 9920, 9930-9931, 9945-9946 | 19 640 | 5.78 |
| Acute myeloid leukemias | 9840, 9861, 9866-9867, 9870-9874, 9891, 9895-9897, 9910, 9920, 9930-9931 | 13 358 | 3.93 |
| Chronic myeloid leukemias | 9863, 9875-9876, 9945-9946 | 5863 | 1.72 |
| Myeloid leukemias, NOS | 9860 | 419 | 0.13 |
| Leukemias, NOS¶ | 9800-9801, 9805 | 2597 | 0.79 |
| Mast cell neoplasms | 9740-9742 | 65 | 0.02 |
| Histiocytic and dendritic cell neoplasms | 9750-9758 | 122 | 0.03 |
| Other hematopoietic diseases¶ | 9760, 9762, 9765-9769 | 13 | 0.00 |
| Hematopoietic neoplasms total | – | 136 985 | 40.28 |
Hematopoietic neoplasm subtype . | ICD-O-3 codes*† . | No. . | Rate‡ . |
|---|---|---|---|
| Lymphoid neoplasms, total | – | 114 548 | 33.65 |
| B-cell lymphoid neoplasms, total | 9590-9591(B), 9596(B), 9670-9671, 9673, 9675(B), 9678-9680, 9684, 9687, 9689-9691, 9695, 9698-9699, 9727(B), 9728, 9731-9734, 9761, 9764, 9820(B), 9823, 9826, 9832(B), 9833, 9835(B), 9836, 9940, 9970(B) | 87 666 | 26.13 |
| DLBCL | 9675(B), 9678-9680, 9684 | 24 246 | 7.14 |
| Marginal zone lymphoma | 9689, 9699 | 3247 | 0.97 |
| Follicular lymphoma | 9690-9691, 9695, 9698 | 10 705 | 3.18 |
| CLL/SLL | 9670, 9823 | 16 984 | 5.17 |
| Mantle cell lymphoma | 9673 | 1691 | 0.51 |
| Burkitt lymphoma/leukemia | 9687, 9826 | 1102 | 0.30 |
| Plasma cell neoplasms | 9731-9734 | 18 644 | 5.66 |
| Multiple myeloma | 9732 | 17 407 | 5.29 |
| Plasma cell leukemia | 9733 | 107 | 0.03 |
| Plasmacytoma | 9731, 9734 | 1130 | 0.34 |
| Hairy cell leukemia | 9940 | 1116 | 0.33 |
| Lymphoplasmacytic lymphoma | 9671 | 911 | 0.27 |
| Waldenstrom macroglobulinemia | 9761 | 1144 | 0.35 |
| B-cell lymphoid neoplasms, NOS | 9590-9591(B), 9596(B), 9764, 9820(B), 9970(B) | 4872 | 1.45 |
| T/NK-cell lymphoid neoplasms, total | 9590-9591(T/NK), 9596(T/NK), 9675(T/NK), 9700-9702, 9705, 9708-9709, 9714, 9716-9719, 9727(T/NK), 9729, 9820(T/NK), 9827, 9831, 9832(T/NK), 9834, 9835(T/NK), 9837, 9948, 9970(T/NK) | 6228 | 1.79 |
| Mycosis fungoides/Sézary syndrome | 9700-9701 | 1773 | 0.52 |
| Peripheral T-cell lymphoma | 9675(T/NK), 9702, 9705, 9708, 9714, 9716, 9827 | 2071 | 0.60 |
| Angioimmunoblastic lymphoma | 9705 | 176 | 0.05 |
| Anaplastic large-cell lymphoma | 9714 | 864 | 0.25 |
| Peripheral T-cell lymphoma, NOS | 9675(T/NK), 9702, 9708, 9716, 9827 | 1031 | 0.30 |
| T/NK-cell lymphoid neoplasms, NOS | 9590-9591(T/NK), 9596(T/NK), 9709, 9717-9719, 9820(T/NK), 9831, 9948, 9970(T/NK) | 1501 | 0.44 |
| Lymphoblastic leukemia/lymphoma§ | 9727-9729, 9835-9837 | 6127 | 1.61 |
| B-cell lymphoblastic leukemia/lymphoma | 9727(B), 9728, 9835(B), 9836 | 2907 | 0.76 |
| T-cell lymphoblastic leukemia/lymphoma | 9727(T/NK), 9729, 9835(T/NK), 9837 | 852 | 0.22 |
| Unknown type lymphoblastic leukemia/lymphoma | 9727, 9835(unknown) | 2368 | 0.63 |
| Prolymphocytic leukemia | 9832-9834 | 237 | 0.07 |
| Hodgkin lymphoma | 9650-9655, 9659, 9661-9665, 9667 | 10 042 | 2.67 |
| Classical Hodgkin lymphoma | 9650-9655, 9661-9665, 9667 | 9734 | 2.59 |
| Nodular sclerosis | 9663-9665, 9667 | 6270 | 1.63 |
| Mixed cellularity/lymphocyte depleted | 9652-9655 | 1906 | 0.53 |
| Classical Hodgkin lymphoma, NOS | 9650-9651, 9661-9662 | 1558 | 0.43 |
| Nodular lymphocyte predominant Hodgkin lymphoma | 9659 | 308 | 0.08 |
| Unknown type lymphoid neoplasms | 9590-9591(unknown), 9596(unknown), 9675(unknown), 9820(unknown), 9970(unknown) | 8135 | 2.40 |
| Nonlymphoid hematopoietic neoplasms, total | – | 22 437 | 6.63 |
| Chronic myeloproliferative diseases∥ | 9950, 9960-9964, 9975 | ||
| Myelodysplastic syndromes∥ | 9980, 9982-9987, 9989 | ||
| Myeloid leukemias | 9840, 9860-9861, 9863, 9866-9867, 9870-9876, 9891, 9895-9897, 9910, 9920, 9930-9931, 9945-9946 | 19 640 | 5.78 |
| Acute myeloid leukemias | 9840, 9861, 9866-9867, 9870-9874, 9891, 9895-9897, 9910, 9920, 9930-9931 | 13 358 | 3.93 |
| Chronic myeloid leukemias | 9863, 9875-9876, 9945-9946 | 5863 | 1.72 |
| Myeloid leukemias, NOS | 9860 | 419 | 0.13 |
| Leukemias, NOS¶ | 9800-9801, 9805 | 2597 | 0.79 |
| Mast cell neoplasms | 9740-9742 | 65 | 0.02 |
| Histiocytic and dendritic cell neoplasms | 9750-9758 | 122 | 0.03 |
| Other hematopoietic diseases¶ | 9760, 9762, 9765-9769 | 13 | 0.00 |
| Hematopoietic neoplasms total | – | 136 985 | 40.28 |
ICD-O-3 indicates International Classification of Diseases for Oncology, Third Edition; SEER, Surveillance, Epidemiology, and End Results; DLBCL, diffuse large B-cell lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; NOS, not otherwise specified; NK, natural killer (cell).
Data on histologic type and primary site were coded to ICD-O-2 (44) for cases diagnosed between 1992 and 2000 or ICD-O-3 (45) for cases diagnosed in 2001. SEER used a conversion algorithm to translate cases coded using ICD-O-2 to ICD-O-3 (40)
Codes followed by parentheses indicate that immunophenotyping data (B-cell, T/NK-cell, or unknown) were used to assign cases to that lymphoid neoplasm subtype
All incidence rates are age adjusted to the 2000 United States population and expressed per 100 000 person-years
Also known as acute lymphoblastic leukemia (ALL)
Chronic myeloproliferative diseases and myelodysplastic syndromes, now classified as malignant in ICD-O-3, were classified as uncertain behavior in ICD-O-2 and thus were not reportable to SEER until 2001. In 2001, 634 cases (1.77 cases per 100 000 persons) of chronic myeloproliferative diseases and 1024 cases (2.91 cases per 100 000 persons) of myelodysplastic syndromes were diagnosed. These disease entities were excluded from our analysis
Lymphoid versus myeloid origin not determined
Statistical analysis
Incidence rates, computed by aggregating county resident population estimates from the United States Census Bureau, were directly age adjusted to the 2000 United States standard population and expressed per 100 000 person-years.41 Age-specific rates were computed for 8 groups (less than 15, 15 to 24, 25 to 34, 35 to 44, 45 to 54, 55 to 64, 65 to 74, and 75 or more years); the age-specific rates for these 8 groups were age adjusted by 5-year groups. Certain analyses were conducted separately for the elderly (persons aged 75 years or older) and for persons less than 25 years of age, as has been done in some other investigations assessing combined groupings of leukemias and lymphomas in children and young persons (eg, Roman et al).50 Rates, standard errors, and 95% confidence intervals (95% CIs) were computed using SEER*Stat software, version 5.2.2.51 Incidence rates were compared by sex for each of 3 racial groups (whites, blacks, Asians) and by race for each sex using incidence rate ratios (IRRs) and an approximate 95% CI for the IRR.52 The small number of cases precluded analysis of the incidence of lymphoid neoplasms for American Indians/Alaska Natives.
Temporal trends were quantified by estimates of the annual percent change (APC) computed using weighted least squares log-linear regression.51 Briefly, this method fits a single, straight-line model that regresses the calendar year against the natural logarithm of the annual rate, weighted by the reciprocal of the variance of the natural logarithm of the incidence rate. The slope of this line and the standard error are used to compute a point estimate and 95% CI for the APC. The incidence rate was determined to have changed over time if the APC differed significantly from zero based on a 2-sided P value less than .05.
Figures are presented with the incidence rate per 100 000 person-years on the y-axis using a log scale and age or year of diagnosis on the x-axis. Temporal trends are portrayed visually using fixed time periods (Figure 1: 1978-1979 to 2000-2001; Figure 4: 1992-1995, 1996-1998, 1999-2001). Age-specific incidence is portrayed visually using 8 10-year age groups, from less than 15 to 75 or more years of age. A uniform scaling ratio (1 log cycle on the y-axis has the same length as 40 years on the x-axis, with a 10-degree slope representing an annual change of 1%) was used to facilitate comparison among figures for different disease subtypes.53
Results
A total of 136 985 cases of hematopoietic neoplasms were diagnosed during 1992-2001 among residents of 12 SEER registries, of which 114 548 (84%) were lymphoid neoplasms (Table 1). Of the remaining 22 437 hematopoietic neoplasms, 88% were myeloid leukemias. In the present analysis, we focused on the incidence of lymphoid neoplasms. Mature B-cell neoplasms accounted for approximately three quarters of all lymphoid neoplasms, Hodgkin lymphoma accounted for 8%, mature T/NK-cell neoplasms and lymphoblastic leukemia/lymphoma each accounted for 5%, and lymphoid neoplasms of unknown type accounted for 7%. Mature B-cell neoplasms comprised diffuse large B-cell lymphoma (DLBCL) (28%), plasma cell neoplasms (predominantly multiple myeloma) (22%), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) (20%), follicular lymphoma (13%), and various other known or unknown types (16%).
Incidence rate ratios by sex
Males had higher rates than females for most lymphoid neoplasm subtypes. The male-to-female (M/F) IRR was 1.5 to 1.6 for all lymphoid neoplasms combined and significantly greater than unity for virtually all subtypes (Tables 2, 3). Among B-cell neoplasms, male predominance was most pronounced for Burkitt lymphoma/leukemia and hairy cell leukemia, with the M/F IRR among whites exceeding 3. The M/F IRRs ranged between 2 and 3 for mantle cell lymphoma, plasmacytoma, and Waldenström macroglobulinemia. Rates differed little by sex for marginal zone and follicular lymphomas. Among T-cell neoplasms, the M/F IRR among whites was 1.8 to 2.0 for the major subtypes. Although no sex difference was observed for nodular sclerosis Hodgkin lymphoma, a 2-fold male predominance in whites was seen for mixed cellularity/lymphocyte depleted Hodgkin lymphoma and a 3-fold male predominance in whites was seen for nodular lymphocyte predominant Hodgkin lymphoma. The M/F IRRs among blacks and Asians generally were similar to those among whites for most subtypes, with only a few notable exceptions. The M/F IRRs among blacks were lower for mycosis fungoides/Sézary syndrome and nodular lymphocyte predominant Hodgkin lymphoma and higher for follicular lymphoma, angioimmunoblastic lymphoma, and mixed cellularity/lymphocyte depleted Hodgkin lymphoma compared with whites. The M/F IRRs among Asians were lower for Burkitt lymphoma/leukemia, anaplastic large-cell lymphoma, and nodular lymphocyte predominant Hodgkin lymphoma and higher for lymphoplasmacytic lymphoma.
Incidence of lymphoid neoplasms by subtype, race, and sex, 12 SEER registries, 1992-2001
. | Men . | . | . | . | . | . | . | . | Women . | . | . | . | . | . | . | . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | White . | . | Black . | . | Asian . | . | AI/AN . | . | White . | . | Black . | . | Asian . | . | AI/AN . | . | ||||||||||||||
| Lymphoid neoplasm subtype . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | ||||||||||||||
| Lymphoid neoplasms, total | 53 875 | 44.01 | 5390 | 41.09 | 3688 | 25.38 | 244 | 16.25 | 42 561 | 27.97 | 4468 | 26.53 | 2932 | 16.76 | 221 | 12.45 | ||||||||||||||
| B-cell lymphoid neoplasms, total | 41 127 | 34.31 | 3893 | 31.94 | 2813 | 19.77 | 168 | 11.99 | 33 058 | 21.66 | 3345 | 20.75 | 2318 | 13.46 | 174 | 10.43 | ||||||||||||||
| DLBCL | 11 529 | 9.35 | 940 | 6.45 | 1010 | 7.09 | 43 | 3.10 | 9099 | 5.93 | 625 | 3.60 | 840 | 4.92 | 46 | 2.71 | ||||||||||||||
| Marginal zone lymphoma | 1153 | 0.97 | 96 | 0.82 | 167 | 1.19 | 5 | 0.30 | 1483 | 0.98 | 120 | 0.72 | 178 | 1.01 | 6 | 0.41 | ||||||||||||||
| Follicular lymphoma | 4738 | 3.85 | 244 | 1.87 | 233 | 1.59 | 15 | 1.04 | 4902 | 3.31 | 202 | 1.20 | 245 | 1.37 | 27 | 1.78 | ||||||||||||||
| CLL/SLL | 8796 | 7.72 | 646 | 5.86 | 251 | 1.77 | 16 | 1.19 | 6361 | 4.09 | 461 | 3.00 | 157 | 0.95 | 16 | 1.13 | ||||||||||||||
| Mantle cell lymphoma | 989 | 0.84 | 52 | 0.45 | 48 | 0.32 | 4 | 0.36 | 518 | 0.34 | 37 | 0.23 | 31 | 0.18 | 4 | 0.26 | ||||||||||||||
| Burkitt lymphoma/leukemia | 677 | 0.49 | 76 | 0.42 | 66 | 0.38 | 5 | 0.20 | 221 | 0.15 | 21 | 0.11 | 28 | 0.17 | 0 | 0.00 | ||||||||||||||
| Plasma cell neoplasms | 7739 | 6.72 | 1445 | 13.40 | 560 | 4.21 | 49 | 4.13 | 6601 | 4.28 | 1605 | 10.35 | 478 | 2.84 | 47 | 3.10 | ||||||||||||||
| Multiple myeloma | 7098 | 6.20 | 1367 | 12.74 | 520 | 3.93 | 43 | 3.76 | 6231 | 4.04 | 1529 | 9.89 | 458 | 2.73 | 46 | 3.01 | ||||||||||||||
| Plasma cell leukemia | 46 | 0.04 | 8 | 0.08 | 0 | 0.00 | 0 | 0.00 | 37 | 0.02 | 13 | 0.08 | 3 | 0.02 | 0 | 0.00 | ||||||||||||||
| Plasmacytoma | 595 | 0.49 | 70 | 0.58 | 40 | 0.29 | 6 | 0.37 | 333 | 0.22 | 63 | 0.38 | 17 | 0.10 | 1 | 0.10 | ||||||||||||||
| Hairy cell leukemia | 782 | 0.62 | 25 | 0.21 | 27 | 0.20 | 1 | 0.06 | 243 | 0.16 | 10 | 0.06 | 7 | 0.04 | 1 | 0.04 | ||||||||||||||
| Lymphoplasmacytic lymphoma | 407 | 0.35 | 37 | 0.29 | 39 | 0.30 | 0 | 0.00 | 350 | 0.23 | 45 | 0.27 | 16 | 0.10 | 1 | 0.03 | ||||||||||||||
| Waldenström macroglobulinemia | 629 | 0.56 | 31 | 0.25 | 42 | 0.32 | 2 | 0.18 | 388 | 0.25 | 15 | 0.10 | 22 | 0.14 | 0 | 0.00 | ||||||||||||||
| B-cell lymphoid neoplasms, NOS | 2236 | 1.84 | 213 | 1.51 | 224 | 1.65 | 12 | 0.99 | 1826 | 1.20 | 131 | 0.78 | 182 | 1.07 | 8 | 0.42 | ||||||||||||||
| T/NK-cell lymphoid neoplasms, total | 2950 | 2.33 | 405 | 2.71 | 327 | 2.14 | 31 | 2.06 | 1820 | 1.23 | 324 | 1.81 | 208 | 1.14 | 13 | 0.57 | ||||||||||||||
| Mycosis fungoides/Sézary syndrome | 781 | 0.64 | 123 | 0.88 | 66 | 0.46 | 6 | 0.35 | 525 | 0.36 | 129 | 0.73 | 45 | 0.25 | 4 | 0.15 | ||||||||||||||
| Peripheral T-cell lymphoma | 942 | 0.75 | 139 | 0.96 | 119 | 0.82 | 13 | 1.11 | 638 | 0.42 | 102 | 0.57 | 90 | 0.51 | 6 | 0.33 | ||||||||||||||
| Angioimmunoblastic lymphoma | 66 | 0.05 | 8 | 0.07 | 15 | 0.11 | 0 | 0.00 | 68 | 0.04 | 7 | 0.04 | 11 | 0.06 | 0 | 0.00 | ||||||||||||||
| Anaplastic large-cell lymphoma | 435 | 0.34 | 58 | 0.38 | 28 | 0.17 | 6 | 0.53 | 267 | 0.18 | 37 | 0.20 | 26 | 0.13 | 2 | 0.13 | ||||||||||||||
| Peripheral T-cell lymphoma, NOS | 441 | 0.36 | 73 | 0.51 | 76 | 0.54 | 7 | 0.59 | 303 | 0.20 | 58 | 0.34 | 53 | 0.31 | 4 | 0.20 | ||||||||||||||
| T/NK-cell lymphoid neoplasms, NOS | 750 | 0.61 | 74 | 0.55 | 83 | 0.56 | 7 | 0.44 | 456 | 0.31 | 56 | 0.33 | 44 | 0.23 | 1 | 0.05 | ||||||||||||||
| Lymphoblastic leukemia/lymphoma | 2929 | 2.04 | 235 | 1.09 | 312 | 1.61 | 34 | 0.91 | 2144 | 1.47 | 182 | 0.81 | 231 | 1.18 | 31 | 1.01 | ||||||||||||||
| B-cell lymphoblastic leukemia/lymphoma | 1390 | 0.95 | 87 | 0.41 | 144 | 0.73 | 16 | 0.45 | 1038 | 0.72 | 71 | 0.32 | 133 | 0.68 | 18 | 0.56 | ||||||||||||||
| T-cell lymphoblastic leukemia/lymphoma | 463 | 0.32 | 66 | 0.30 | 58 | 0.28 | 4 | 0.11 | 192 | 0.13 | 35 | 0.16 | 28 | 0.14 | 2 | 0.05 | ||||||||||||||
| Unknown type lymphoblastic leukemia/lymphoma | 1076 | 0.76 | 82 | 0.39 | 110 | 0.60 | 14 | 0.36 | 914 | 0.62 | 76 | 0.34 | 70 | 0.36 | 11 | 0.40 | ||||||||||||||
| Prolymphocytic leukemia | 137 | 0.12 | 8 | 0.07 | 5 | 0.04 | 1 | 0.06 | 75 | 0.05 | 8 | 0.05 | 2 | 0.01 | 0 | 0.00 | ||||||||||||||
| Hodgkin lymphoma | 4668 | 3.29 | 525 | 2.83 | 207 | 1.16 | 7 | 0.39 | 3887 | 2.63 | 457 | 2.07 | 183 | 0.90 | 14 | 0.51 | ||||||||||||||
| Classical Hodgkin lymphoma | 4503 | 3.17 | 488 | 2.64 | 202 | 1.14 | 7 | 0.39 | 3830 | 2.60 | 423 | 1.91 | 178 | 0.87 | 13 | 0.45 | ||||||||||||||
| Nodular sclerosis | 2650 | 1.80 | 245 | 1.25 | 114 | 0.61 | 4 | 0.14 | 2776 | 1.89 | 298 | 1.31 | 123 | 0.56 | 12 | 0.40 | ||||||||||||||
| Mixed cellularity/lymphocyte depleted | 1050 | 0.78 | 132 | 0.78 | 53 | 0.33 | 1 | 0.04 | 567 | 0.38 | 56 | 0.27 | 32 | 0.18 | 0 | 0.00 | ||||||||||||||
| Classical Hodgkin lymphoma, NOS | 803 | 0.59 | 111 | 0.61 | 35 | 0.20 | 2 | 0.21 | 487 | 0.33 | 69 | 0.32 | 23 | 0.13 | 1 | 0.05 | ||||||||||||||
| Nodular lymphocyte predominant Hodgkin lymphoma | 165 | 0.12 | 37 | 0.19 | 5 | 0.02 | 0 | 0.00 | 57 | 0.04 | 34 | 0.16 | 5 | 0.03 | 1 | 0.06 | ||||||||||||||
| Unknown type lymphoid neoplasms | 3993 | 3.27 | 481 | 3.18 | 229 | 1.70 | 24 | 1.44 | 2844 | 1.80 | 262 | 1.54 | 153 | 0.91 | 9 | 0.54 | ||||||||||||||
. | Men . | . | . | . | . | . | . | . | Women . | . | . | . | . | . | . | . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | White . | . | Black . | . | Asian . | . | AI/AN . | . | White . | . | Black . | . | Asian . | . | AI/AN . | . | ||||||||||||||
| Lymphoid neoplasm subtype . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | No. . | Rate* . | ||||||||||||||
| Lymphoid neoplasms, total | 53 875 | 44.01 | 5390 | 41.09 | 3688 | 25.38 | 244 | 16.25 | 42 561 | 27.97 | 4468 | 26.53 | 2932 | 16.76 | 221 | 12.45 | ||||||||||||||
| B-cell lymphoid neoplasms, total | 41 127 | 34.31 | 3893 | 31.94 | 2813 | 19.77 | 168 | 11.99 | 33 058 | 21.66 | 3345 | 20.75 | 2318 | 13.46 | 174 | 10.43 | ||||||||||||||
| DLBCL | 11 529 | 9.35 | 940 | 6.45 | 1010 | 7.09 | 43 | 3.10 | 9099 | 5.93 | 625 | 3.60 | 840 | 4.92 | 46 | 2.71 | ||||||||||||||
| Marginal zone lymphoma | 1153 | 0.97 | 96 | 0.82 | 167 | 1.19 | 5 | 0.30 | 1483 | 0.98 | 120 | 0.72 | 178 | 1.01 | 6 | 0.41 | ||||||||||||||
| Follicular lymphoma | 4738 | 3.85 | 244 | 1.87 | 233 | 1.59 | 15 | 1.04 | 4902 | 3.31 | 202 | 1.20 | 245 | 1.37 | 27 | 1.78 | ||||||||||||||
| CLL/SLL | 8796 | 7.72 | 646 | 5.86 | 251 | 1.77 | 16 | 1.19 | 6361 | 4.09 | 461 | 3.00 | 157 | 0.95 | 16 | 1.13 | ||||||||||||||
| Mantle cell lymphoma | 989 | 0.84 | 52 | 0.45 | 48 | 0.32 | 4 | 0.36 | 518 | 0.34 | 37 | 0.23 | 31 | 0.18 | 4 | 0.26 | ||||||||||||||
| Burkitt lymphoma/leukemia | 677 | 0.49 | 76 | 0.42 | 66 | 0.38 | 5 | 0.20 | 221 | 0.15 | 21 | 0.11 | 28 | 0.17 | 0 | 0.00 | ||||||||||||||
| Plasma cell neoplasms | 7739 | 6.72 | 1445 | 13.40 | 560 | 4.21 | 49 | 4.13 | 6601 | 4.28 | 1605 | 10.35 | 478 | 2.84 | 47 | 3.10 | ||||||||||||||
| Multiple myeloma | 7098 | 6.20 | 1367 | 12.74 | 520 | 3.93 | 43 | 3.76 | 6231 | 4.04 | 1529 | 9.89 | 458 | 2.73 | 46 | 3.01 | ||||||||||||||
| Plasma cell leukemia | 46 | 0.04 | 8 | 0.08 | 0 | 0.00 | 0 | 0.00 | 37 | 0.02 | 13 | 0.08 | 3 | 0.02 | 0 | 0.00 | ||||||||||||||
| Plasmacytoma | 595 | 0.49 | 70 | 0.58 | 40 | 0.29 | 6 | 0.37 | 333 | 0.22 | 63 | 0.38 | 17 | 0.10 | 1 | 0.10 | ||||||||||||||
| Hairy cell leukemia | 782 | 0.62 | 25 | 0.21 | 27 | 0.20 | 1 | 0.06 | 243 | 0.16 | 10 | 0.06 | 7 | 0.04 | 1 | 0.04 | ||||||||||||||
| Lymphoplasmacytic lymphoma | 407 | 0.35 | 37 | 0.29 | 39 | 0.30 | 0 | 0.00 | 350 | 0.23 | 45 | 0.27 | 16 | 0.10 | 1 | 0.03 | ||||||||||||||
| Waldenström macroglobulinemia | 629 | 0.56 | 31 | 0.25 | 42 | 0.32 | 2 | 0.18 | 388 | 0.25 | 15 | 0.10 | 22 | 0.14 | 0 | 0.00 | ||||||||||||||
| B-cell lymphoid neoplasms, NOS | 2236 | 1.84 | 213 | 1.51 | 224 | 1.65 | 12 | 0.99 | 1826 | 1.20 | 131 | 0.78 | 182 | 1.07 | 8 | 0.42 | ||||||||||||||
| T/NK-cell lymphoid neoplasms, total | 2950 | 2.33 | 405 | 2.71 | 327 | 2.14 | 31 | 2.06 | 1820 | 1.23 | 324 | 1.81 | 208 | 1.14 | 13 | 0.57 | ||||||||||||||
| Mycosis fungoides/Sézary syndrome | 781 | 0.64 | 123 | 0.88 | 66 | 0.46 | 6 | 0.35 | 525 | 0.36 | 129 | 0.73 | 45 | 0.25 | 4 | 0.15 | ||||||||||||||
| Peripheral T-cell lymphoma | 942 | 0.75 | 139 | 0.96 | 119 | 0.82 | 13 | 1.11 | 638 | 0.42 | 102 | 0.57 | 90 | 0.51 | 6 | 0.33 | ||||||||||||||
| Angioimmunoblastic lymphoma | 66 | 0.05 | 8 | 0.07 | 15 | 0.11 | 0 | 0.00 | 68 | 0.04 | 7 | 0.04 | 11 | 0.06 | 0 | 0.00 | ||||||||||||||
| Anaplastic large-cell lymphoma | 435 | 0.34 | 58 | 0.38 | 28 | 0.17 | 6 | 0.53 | 267 | 0.18 | 37 | 0.20 | 26 | 0.13 | 2 | 0.13 | ||||||||||||||
| Peripheral T-cell lymphoma, NOS | 441 | 0.36 | 73 | 0.51 | 76 | 0.54 | 7 | 0.59 | 303 | 0.20 | 58 | 0.34 | 53 | 0.31 | 4 | 0.20 | ||||||||||||||
| T/NK-cell lymphoid neoplasms, NOS | 750 | 0.61 | 74 | 0.55 | 83 | 0.56 | 7 | 0.44 | 456 | 0.31 | 56 | 0.33 | 44 | 0.23 | 1 | 0.05 | ||||||||||||||
| Lymphoblastic leukemia/lymphoma | 2929 | 2.04 | 235 | 1.09 | 312 | 1.61 | 34 | 0.91 | 2144 | 1.47 | 182 | 0.81 | 231 | 1.18 | 31 | 1.01 | ||||||||||||||
| B-cell lymphoblastic leukemia/lymphoma | 1390 | 0.95 | 87 | 0.41 | 144 | 0.73 | 16 | 0.45 | 1038 | 0.72 | 71 | 0.32 | 133 | 0.68 | 18 | 0.56 | ||||||||||||||
| T-cell lymphoblastic leukemia/lymphoma | 463 | 0.32 | 66 | 0.30 | 58 | 0.28 | 4 | 0.11 | 192 | 0.13 | 35 | 0.16 | 28 | 0.14 | 2 | 0.05 | ||||||||||||||
| Unknown type lymphoblastic leukemia/lymphoma | 1076 | 0.76 | 82 | 0.39 | 110 | 0.60 | 14 | 0.36 | 914 | 0.62 | 76 | 0.34 | 70 | 0.36 | 11 | 0.40 | ||||||||||||||
| Prolymphocytic leukemia | 137 | 0.12 | 8 | 0.07 | 5 | 0.04 | 1 | 0.06 | 75 | 0.05 | 8 | 0.05 | 2 | 0.01 | 0 | 0.00 | ||||||||||||||
| Hodgkin lymphoma | 4668 | 3.29 | 525 | 2.83 | 207 | 1.16 | 7 | 0.39 | 3887 | 2.63 | 457 | 2.07 | 183 | 0.90 | 14 | 0.51 | ||||||||||||||
| Classical Hodgkin lymphoma | 4503 | 3.17 | 488 | 2.64 | 202 | 1.14 | 7 | 0.39 | 3830 | 2.60 | 423 | 1.91 | 178 | 0.87 | 13 | 0.45 | ||||||||||||||
| Nodular sclerosis | 2650 | 1.80 | 245 | 1.25 | 114 | 0.61 | 4 | 0.14 | 2776 | 1.89 | 298 | 1.31 | 123 | 0.56 | 12 | 0.40 | ||||||||||||||
| Mixed cellularity/lymphocyte depleted | 1050 | 0.78 | 132 | 0.78 | 53 | 0.33 | 1 | 0.04 | 567 | 0.38 | 56 | 0.27 | 32 | 0.18 | 0 | 0.00 | ||||||||||||||
| Classical Hodgkin lymphoma, NOS | 803 | 0.59 | 111 | 0.61 | 35 | 0.20 | 2 | 0.21 | 487 | 0.33 | 69 | 0.32 | 23 | 0.13 | 1 | 0.05 | ||||||||||||||
| Nodular lymphocyte predominant Hodgkin lymphoma | 165 | 0.12 | 37 | 0.19 | 5 | 0.02 | 0 | 0.00 | 57 | 0.04 | 34 | 0.16 | 5 | 0.03 | 1 | 0.06 | ||||||||||||||
| Unknown type lymphoid neoplasms | 3993 | 3.27 | 481 | 3.18 | 229 | 1.70 | 24 | 1.44 | 2844 | 1.80 | 262 | 1.54 | 153 | 0.91 | 9 | 0.54 | ||||||||||||||
AI/AN indicates American Indian or Alaska Native; other abbreviations are explained in Table 1.
All incidence rates are age adjusted to the 2000 United States population and expressed per 100 000 person-years
Incidence rate*ratios for lymphoid neoplasms by subtype, race, and sex, 12 SEER registries, 1992-2001
. | Male-female IRR . | . | . | White-black IRR . | . | White-Asian IRR . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphoid neoplasm subtype . | White . | Black . | Asian . | Males . | Females . | Males . | Females . | ||||
| Lymphoid neoplasms, total | 1.6 | 1.5 | 1.5 | 1.1 | 1.1 | 1.7 | 1.7 | ||||
| B-cell lymphoid neoplasms, total | 1.6 | 1.5 | 1.5 | 1.1 | 1.0 | 1.7 | 1.6 | ||||
| DLBCL | 1.6 | 1.8 | 1.4 | 1.5 | 1.6 | 1.3 | 1.2 | ||||
| Marginal zone lymphoma | 1.0 | 1.1 | 1.2 | 1.2 | 1.4 | 0.8 | 1.0 | ||||
| Follicular lymphoma | 1.2 | 1.6 | 1.2 | 2.1 | 2.8 | 2.4 | 2.4 | ||||
| CLL/SLL | 1.9 | 2.0 | 1.9 | 1.3 | 1.4 | 4.4 | 4.3 | ||||
| Mantle cell lymphoma | 2.5 | 1.9 | 1.8 | 1.9 | 1.5 | 2.6 | 1.9 | ||||
| Burkitt lymphoma/leukemia | 3.3 | 3.8 | 2.2 | 1.2 | 1.4 | 1.3 | 0.9 | ||||
| Plasma cell neoplasms | 1.6 | 1.3 | 1.5 | 0.5 | 0.4 | 1.6 | 1.5 | ||||
| Multiple myeloma | 1.5 | 1.3 | 1.4 | 0.5 | 0.4 | 1.6 | 1.5 | ||||
| Plasma cell leukemia | 1.6 | 1.0 | –† | 0.5 | 0.3 | –† | 1.2 | ||||
| Plasmacytoma | 2.2 | 1.5 | 3.0 | 0.8 | 0.6 | 1.7 | 2.3 | ||||
| Hairy cell leukemia | 3.8 | 3.7 | 5.5 | 3.0 | 2.9 | 3.1 | 4.5 | ||||
| Lymphoplasmacytic lymphoma | 1.5 | 1.1 | 3.1 | 1.2 | 0.8 | 1.2 | 2.3 | ||||
| Waldenström macroglobulinemia | 2.2 | 2.6 | 2.3 | 2.2 | 2.6 | 1.8 | 1.8 | ||||
| B-cell lymphoid neoplasms, NOS | 1.5 | 1.9 | 1.5 | 1.2 | 1.5 | 1.1 | 1.1 | ||||
| T/NK-cell lymphoid neoplasms, total | 1.9 | 1.5 | 1.9 | 0.9 | 0.7 | 1.1 | 1.1 | ||||
| Mycosis fungoides/Sézary syndrome | 1.8 | 1.2 | 1.8 | 0.7 | 0.5 | 1.4 | 1.4 | ||||
| Peripheral T-cell lymphoma | 1.8 | 1.7 | 1.6 | 0.8 | 0.7 | 0.9 | 0.8 | ||||
| Angioimmunoblastic lymphoma | 1.3 | 2.0 | 1.7 | 0.8 | 1.2 | 0.5 | 0.7 | ||||
| Anaplastic large-cell lymphoma | 1.9 | 2.0 | 1.3 | 0.9 | 0.9 | 1.9 | 1.3 | ||||
| Peripheral T-cell lymphoma, NOS | 1.8 | 1.5 | 1.7 | 0.7 | 0.6 | 0.7 | 0.6 | ||||
| T/NK-cell lymphoid neoplasms, NOS | 2.0 | 1.7 | 2.4 | 1.1 | 0.9 | 1.1 | 1.3 | ||||
| Lymphoblastic leukemia/lymphoma | 1.4 | 1.3 | 1.4 | 1.9 | 1.8 | 1.3 | 1.3 | ||||
| B-cell lymphoblastic leukemia/lymphoma | 1.3 | 1.3 | 1.1 | 2.3 | 2.3 | 1.3 | 1.1 | ||||
| T-cell lymphoblastic leukemia/lymphoma | 2.4 | 1.8 | 2.0 | 1.1 | 0.8 | 1.1 | 1.0 | ||||
| Unknown type lymphoblastic leukemia/lymphoma | 1.2 | 1.2 | 1.6 | 2.0 | 1.8 | 1.3 | 1.7 | ||||
| Prolymphocytic leukemia | 2.6 | 1.4 | 3.2 | 1.7 | 0.9 | 3.3 | 4.1 | ||||
| Hodgkin lymphoma | 1.2 | 1.4 | 1.3 | 1.2 | 1.3 | 2.8 | 2.9 | ||||
| Classical Hodgkin lymphoma | 1.2 | 1.4 | 1.3 | 1.2 | 1.4 | 2.8 | 3.0 | ||||
| Nodular sclerosis | 1.0 | 1.0 | 1.1 | 1.4 | 1.4 | 3.0 | 3.4 | ||||
| Mixed cellularity/lymphocyte depleted | 2.0 | 2.9 | 1.8 | 1.0 | 1.4 | 2.4 | 2.1 | ||||
| Classical Hodgkin lymphoma, NOS | 1.8 | 1.9 | 1.6 | 1.0 | 1.0 | 2.9 | 2.5 | ||||
| Nodular lymphocyte predominant Hodgkin lymphoma | 3.0 | 1.2 | 0.9 | 0.6 | 0.2 | 5.0 | 1.5 | ||||
| Unknown type lymphoid neoplasms | 1.8 | 2.1 | 1.9 | 1.0 | 1.2 | 1.9 | 2.0 | ||||
. | Male-female IRR . | . | . | White-black IRR . | . | White-Asian IRR . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphoid neoplasm subtype . | White . | Black . | Asian . | Males . | Females . | Males . | Females . | ||||
| Lymphoid neoplasms, total | 1.6 | 1.5 | 1.5 | 1.1 | 1.1 | 1.7 | 1.7 | ||||
| B-cell lymphoid neoplasms, total | 1.6 | 1.5 | 1.5 | 1.1 | 1.0 | 1.7 | 1.6 | ||||
| DLBCL | 1.6 | 1.8 | 1.4 | 1.5 | 1.6 | 1.3 | 1.2 | ||||
| Marginal zone lymphoma | 1.0 | 1.1 | 1.2 | 1.2 | 1.4 | 0.8 | 1.0 | ||||
| Follicular lymphoma | 1.2 | 1.6 | 1.2 | 2.1 | 2.8 | 2.4 | 2.4 | ||||
| CLL/SLL | 1.9 | 2.0 | 1.9 | 1.3 | 1.4 | 4.4 | 4.3 | ||||
| Mantle cell lymphoma | 2.5 | 1.9 | 1.8 | 1.9 | 1.5 | 2.6 | 1.9 | ||||
| Burkitt lymphoma/leukemia | 3.3 | 3.8 | 2.2 | 1.2 | 1.4 | 1.3 | 0.9 | ||||
| Plasma cell neoplasms | 1.6 | 1.3 | 1.5 | 0.5 | 0.4 | 1.6 | 1.5 | ||||
| Multiple myeloma | 1.5 | 1.3 | 1.4 | 0.5 | 0.4 | 1.6 | 1.5 | ||||
| Plasma cell leukemia | 1.6 | 1.0 | –† | 0.5 | 0.3 | –† | 1.2 | ||||
| Plasmacytoma | 2.2 | 1.5 | 3.0 | 0.8 | 0.6 | 1.7 | 2.3 | ||||
| Hairy cell leukemia | 3.8 | 3.7 | 5.5 | 3.0 | 2.9 | 3.1 | 4.5 | ||||
| Lymphoplasmacytic lymphoma | 1.5 | 1.1 | 3.1 | 1.2 | 0.8 | 1.2 | 2.3 | ||||
| Waldenström macroglobulinemia | 2.2 | 2.6 | 2.3 | 2.2 | 2.6 | 1.8 | 1.8 | ||||
| B-cell lymphoid neoplasms, NOS | 1.5 | 1.9 | 1.5 | 1.2 | 1.5 | 1.1 | 1.1 | ||||
| T/NK-cell lymphoid neoplasms, total | 1.9 | 1.5 | 1.9 | 0.9 | 0.7 | 1.1 | 1.1 | ||||
| Mycosis fungoides/Sézary syndrome | 1.8 | 1.2 | 1.8 | 0.7 | 0.5 | 1.4 | 1.4 | ||||
| Peripheral T-cell lymphoma | 1.8 | 1.7 | 1.6 | 0.8 | 0.7 | 0.9 | 0.8 | ||||
| Angioimmunoblastic lymphoma | 1.3 | 2.0 | 1.7 | 0.8 | 1.2 | 0.5 | 0.7 | ||||
| Anaplastic large-cell lymphoma | 1.9 | 2.0 | 1.3 | 0.9 | 0.9 | 1.9 | 1.3 | ||||
| Peripheral T-cell lymphoma, NOS | 1.8 | 1.5 | 1.7 | 0.7 | 0.6 | 0.7 | 0.6 | ||||
| T/NK-cell lymphoid neoplasms, NOS | 2.0 | 1.7 | 2.4 | 1.1 | 0.9 | 1.1 | 1.3 | ||||
| Lymphoblastic leukemia/lymphoma | 1.4 | 1.3 | 1.4 | 1.9 | 1.8 | 1.3 | 1.3 | ||||
| B-cell lymphoblastic leukemia/lymphoma | 1.3 | 1.3 | 1.1 | 2.3 | 2.3 | 1.3 | 1.1 | ||||
| T-cell lymphoblastic leukemia/lymphoma | 2.4 | 1.8 | 2.0 | 1.1 | 0.8 | 1.1 | 1.0 | ||||
| Unknown type lymphoblastic leukemia/lymphoma | 1.2 | 1.2 | 1.6 | 2.0 | 1.8 | 1.3 | 1.7 | ||||
| Prolymphocytic leukemia | 2.6 | 1.4 | 3.2 | 1.7 | 0.9 | 3.3 | 4.1 | ||||
| Hodgkin lymphoma | 1.2 | 1.4 | 1.3 | 1.2 | 1.3 | 2.8 | 2.9 | ||||
| Classical Hodgkin lymphoma | 1.2 | 1.4 | 1.3 | 1.2 | 1.4 | 2.8 | 3.0 | ||||
| Nodular sclerosis | 1.0 | 1.0 | 1.1 | 1.4 | 1.4 | 3.0 | 3.4 | ||||
| Mixed cellularity/lymphocyte depleted | 2.0 | 2.9 | 1.8 | 1.0 | 1.4 | 2.4 | 2.1 | ||||
| Classical Hodgkin lymphoma, NOS | 1.8 | 1.9 | 1.6 | 1.0 | 1.0 | 2.9 | 2.5 | ||||
| Nodular lymphocyte predominant Hodgkin lymphoma | 3.0 | 1.2 | 0.9 | 0.6 | 0.2 | 5.0 | 1.5 | ||||
| Unknown type lymphoid neoplasms | 1.8 | 2.1 | 1.9 | 1.0 | 1.2 | 1.9 | 2.0 | ||||
Italicized IRRs were not statistically significant at P < .05 (95% CI included 1.0); 95% CIs available from the author.
IRR indicates incidence rate ratio; other abbreviations are explained in Table 1.
All incidence rates are age adjusted to the 2000 United States population
Calculation of the IRR was precluded by zero cases diagnosed among Asian males
Incidence rate ratios by race
Total lymphoid neoplasm rates were 10% higher for whites than for blacks (Tables 2, 3). Among B-cell neoplasms, white predominance was most pronounced for hairy cell leukemia, follicular lymphoma, mantle cell lymphoma, and Waldenström macroglobulinemia (white-to-black [W/B] IRR among males, 2 to 3). In contrast, black predominance was observed for plasma cell neoplasms (W/B IRR among males, 0.5) and for mycosis fungoides/Sézary syndrome and peripheral T-cell lymphoma (W/B IRR among males, 0.7 to 0.8). Among B- and T-cell neoplasms, the W/B IRRs among females generally were similar to those among males, except for a higher W/B IRR for follicular lymphoma and a lower W/B IRR for mycosis fungoides/Sézary syndrome among females compared with males. Among the Hodgkin lymphomas, regardless of sex, the W/B IRRs ranged from 1.0 to 1.4 for the classical Hodgkin lymphomas, whereas a black predominance was observed for nodular lymphocyte predominant Hodgkin lymphoma (W/B IRR, 0.6 and 0.2 for males and females, respectively).
Total lymphoid neoplasm rates were 70% higher for whites than for Asians (Tables 2, 3). Among B-cell neoplasms, the white-to-Asian (W/A) IRR among males was more than 3 for CLL/SLL and hairy cell leukemia and ranged from 2 to 3 for follicular and mantle cell lymphomas. Notably, rates differed little between whites and Asians for DLBCL, marginal zone lymphoma, Burkitt lymphoma/leukemia, and lymphoplasmacytic lymphoma. Among B-cell neoplasms, the W/A IRRs among females were similar to those among males, except for a higher W/A IRR for lymphoplasmacytic lymphoma for females compared with males. Among T-cell neoplasms, regardless of sex, the W/A IRR for mycosis fungoides/Sézary syndrome was 1.4, whereas the W/A IRR approached unity for peripheral T-cell lymphoma. Among classic Hodgkin lymphomas, regardless of sex, the W/A IRRs ranged from 2.1 to 3.4. In contrast, the W/A IRRs for nodular lymphocyte predominant Hodgkin lymphoma were 5.0 and 1.5 among males and females, respectively.
Age-specific incidence
The incidence of total lymphoid neoplasms increased monotonically with age in all race and sex subgroups (Figure 2A). Steep increases in incidence with age were observed for most subtypes, with several notable exceptions. Burkitt lymphoma/leukemia and mixed cellularity/lymphocyte depleted Hodgkin lymphoma rates increased more gradually with age (Figure 2G,N). B-cell and T-cell lymphoblastic lymphoma/leukemia were diagnosed predominantly in children (Figure 2K,L). Nodular sclerosis and nodular lymphocyte predominant Hodgkin lymphomas were diagnosed predominantly in persons aged 15 to 34 years and 15 to 64 years, respectively (Figure 2M,O).
We observed similar patterns in the race and sex subgroups for most but not all lymphoid neoplasm subtypes. Substantially higher rates were observed among white and black men aged 25 to 54 years for DLBCL, Burkitt lymphoma/leukemia, mixed cellularity/lymphocyte depleted Hodgkin lymphoma, and unknown type lymphoid neoplasms (Figure 2B,G,N,P).
Relative distribution of lymphoid neoplasm subtypes
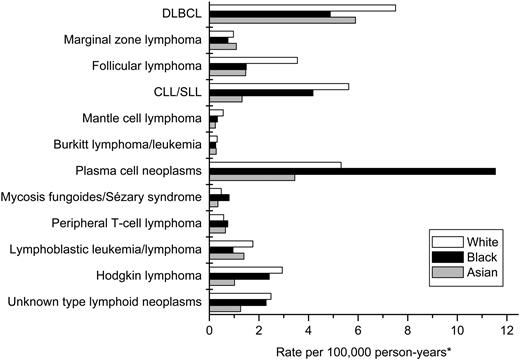
The burden of disease accounted for by selected subtypes varied by race and age. DLBCL accounted for 21% of all lymphoid neoplasms in whites, and CLL/SLL, plasma cell neoplasms, and follicular lymphoma accounted for 16%, 15%, and 10%, respectively (Figure 3). In contrast, plasma cell neoplasms accounted for 35% of lymphoid neoplasms in blacks, with DLBCL and CLL/SLL accounting for 15% and 13%, respectively; follicular lymphoma accounted for only 5%. The most common lymphoid neoplasm subtypes in Asians were DLBCL (29%) and plasma cell neoplasms (17%), with follicular lymphoma, CLL/SLL, and lymphoblastic leukemia/lymphoma each accounting for 6% to 7% of lymphoid neoplasms.
DLBCL, CLL/SLL, and plasma cell neoplasms each accounted for roughly 20% of lymphoid neoplasms in the elderly (data not shown). In contrast, lymphoblastic leukemia/lymphoma and Hodgkin lymphoma accounted for 50% and 31%, respectively, of lymphoid neoplasms in children (data not shown). The relative distribution of lymphoid neoplasm subtypes for males and females was similar within these race and age groups.
Temporal trends
Incidence trends varied substantially among lymphoid neoplasm subtypes. Overall, total lymphoid neoplasm rates decreased at the rate of 1% per year among males during 1992-2001, from 43.2 to 39.7 cases per 100 000 person-years, but remained unchanged among females (Table 4; Figure 4A).
Annual percent changes in lymphoid neoplasm incidence rates*by subtype, race, and sex, 12 SEER registries, 1992-2001
. | . | Men . | . | . | Women . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphoid neoplasm subtype . | Total† . | White . | Black . | Asian . | White . | Black . | Asian . | ||||
| Lymphoid neoplasms, total | –0.50‡ | –0.84§ | –1.84 | –0.33 | 0.08 | 0.14 | 1.39 | ||||
| B-cell lymphoid neoplasms, total | 0.51‡ | 0.23 | –1.25 | 0.86 | 1.01§ | 0.43 | 2.11‡ | ||||
| DLBCL | –0.46§ | –0.73§ | –2.28 | –1.99 | 0.19 | 1.27 | 1.75 | ||||
| Marginal zone lymphoma | 21.31§ | 21.36§ | –∥ | 18.14‡ | 22.17§ | –∥ | 17.49‡ | ||||
| Follicular lymphoma | 0.51 | 0.10 | –1.31 | 4.48 | 1.24‡ | 0.19 | –0.05 | ||||
| CLL/SLL | –2.67§ | –2.20§ | –3.68‡ | –0.76 | –3.11§ | –1.78 | –8.99§ | ||||
| Mantle cell lymphoma | 8.08§ | 12.21§ | 5.74 | 13.67‡ | 2.02 | –∥ | –∥ | ||||
| Burkitt lymphoma/leukemia | 8.36§ | 5.47‡ | 7.95 | 17.85§ | 15.61§ | –∥ | 11.48 | ||||
| Plasma cell neoplasms | –0.73 | –0.78 | –1.86 | –2.06 | –0.67 | –1.59 | 2.79 | ||||
| Hairy cell leukemia | –1.84 | –2.77‡ | –∥ | –1.13 | 2.35 | –∥ | –∥ | ||||
| Lymphoplasmacytic lymphoma | 4.42‡ | 5.30‡ | 5.41 | 5.25 | 2.86 | –5.31 | –∥ | ||||
| Waldenström macroglobulinemia | –0.21 | 1.01 | –3.25 | –6.51 | –1.11 | –∥ | –∥ | ||||
| B-cell lymphoid neoplasms, NOS | 1.64 | 1.36 | 3.48 | –1.34 | 2.13 | 7.53 | 0.92 | ||||
| T/NK-cell lymphoid neoplasms, total | 3.80§ | 3.45§ | 1.09 | 3.73‡ | 4.32 | 6.74‡ | 1.05 | ||||
| Mycosis fungoides/Sézary syndrome | 1.11 | 1.16 | –1.73 | –5.20 | 1.32 | 6.08 | –2.09 | ||||
| Peripheral T-cell lymphoma | 6.55§ | 6.21§ | 0.77 | 5.09 | 6.15‡ | 13.53‡ | 2.24 | ||||
| T/NK-cell lymphoid neoplasms, NOS | 3.26‡ | 3.01 | 4.46 | 7.32 | 4.52 | –0.69 | 3.05 | ||||
| Lymphoblastic leukemia/lymphoma | 0.08 | –0.82 | –4.03 | 0.94 | 2.24 | 2.17 | –1.90 | ||||
| Prolymphocytic leukemia | 3.93 | 0.73 | –∥ | –∥ | 3.26 | –∥ | –∥ | ||||
| Hodgkin lymphoma | –0.49 | –0.27 | 1.18 | –0.63 | –0.52 | –3.12 | 6.61‡ | ||||
| Classical Hodgkin lymphoma | –0.60‡ | –0.41 | 0.94 | –0.41 | –0.53 | –3.89‡ | 7.00‡ | ||||
| Nodular sclerosis | 0.17 | 0.66 | 3.67 | 0.67 | –0.32 | –2.65 | 5.64 | ||||
| Mixed cellularity/lymphocyte depleted | –6.02§ | –6.09§ | –6.54‡ | –8.15 | –4.09‡ | –11.27 | 2.76 | ||||
| Classical Hodgkin lymphoma, NOS | 3.52‡ | 3.96‡ | 6.24 | –∥ | 2.89 | –1.05 | –∥ | ||||
| Nodular lymphocyte predominant Hodgkin lymphoma | 3.20 | 4.00 | 5.06 | –∥ | 0.80 | 4.61 | –∥ | ||||
| Unknown type lymphoid neoplasms | –12.41§ | –13.86§ | –11.00§ | –14.28§ | –10.78§ | –6.82‡ | –10.17§ | ||||
. | . | Men . | . | . | Women . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphoid neoplasm subtype . | Total† . | White . | Black . | Asian . | White . | Black . | Asian . | ||||
| Lymphoid neoplasms, total | –0.50‡ | –0.84§ | –1.84 | –0.33 | 0.08 | 0.14 | 1.39 | ||||
| B-cell lymphoid neoplasms, total | 0.51‡ | 0.23 | –1.25 | 0.86 | 1.01§ | 0.43 | 2.11‡ | ||||
| DLBCL | –0.46§ | –0.73§ | –2.28 | –1.99 | 0.19 | 1.27 | 1.75 | ||||
| Marginal zone lymphoma | 21.31§ | 21.36§ | –∥ | 18.14‡ | 22.17§ | –∥ | 17.49‡ | ||||
| Follicular lymphoma | 0.51 | 0.10 | –1.31 | 4.48 | 1.24‡ | 0.19 | –0.05 | ||||
| CLL/SLL | –2.67§ | –2.20§ | –3.68‡ | –0.76 | –3.11§ | –1.78 | –8.99§ | ||||
| Mantle cell lymphoma | 8.08§ | 12.21§ | 5.74 | 13.67‡ | 2.02 | –∥ | –∥ | ||||
| Burkitt lymphoma/leukemia | 8.36§ | 5.47‡ | 7.95 | 17.85§ | 15.61§ | –∥ | 11.48 | ||||
| Plasma cell neoplasms | –0.73 | –0.78 | –1.86 | –2.06 | –0.67 | –1.59 | 2.79 | ||||
| Hairy cell leukemia | –1.84 | –2.77‡ | –∥ | –1.13 | 2.35 | –∥ | –∥ | ||||
| Lymphoplasmacytic lymphoma | 4.42‡ | 5.30‡ | 5.41 | 5.25 | 2.86 | –5.31 | –∥ | ||||
| Waldenström macroglobulinemia | –0.21 | 1.01 | –3.25 | –6.51 | –1.11 | –∥ | –∥ | ||||
| B-cell lymphoid neoplasms, NOS | 1.64 | 1.36 | 3.48 | –1.34 | 2.13 | 7.53 | 0.92 | ||||
| T/NK-cell lymphoid neoplasms, total | 3.80§ | 3.45§ | 1.09 | 3.73‡ | 4.32 | 6.74‡ | 1.05 | ||||
| Mycosis fungoides/Sézary syndrome | 1.11 | 1.16 | –1.73 | –5.20 | 1.32 | 6.08 | –2.09 | ||||
| Peripheral T-cell lymphoma | 6.55§ | 6.21§ | 0.77 | 5.09 | 6.15‡ | 13.53‡ | 2.24 | ||||
| T/NK-cell lymphoid neoplasms, NOS | 3.26‡ | 3.01 | 4.46 | 7.32 | 4.52 | –0.69 | 3.05 | ||||
| Lymphoblastic leukemia/lymphoma | 0.08 | –0.82 | –4.03 | 0.94 | 2.24 | 2.17 | –1.90 | ||||
| Prolymphocytic leukemia | 3.93 | 0.73 | –∥ | –∥ | 3.26 | –∥ | –∥ | ||||
| Hodgkin lymphoma | –0.49 | –0.27 | 1.18 | –0.63 | –0.52 | –3.12 | 6.61‡ | ||||
| Classical Hodgkin lymphoma | –0.60‡ | –0.41 | 0.94 | –0.41 | –0.53 | –3.89‡ | 7.00‡ | ||||
| Nodular sclerosis | 0.17 | 0.66 | 3.67 | 0.67 | –0.32 | –2.65 | 5.64 | ||||
| Mixed cellularity/lymphocyte depleted | –6.02§ | –6.09§ | –6.54‡ | –8.15 | –4.09‡ | –11.27 | 2.76 | ||||
| Classical Hodgkin lymphoma, NOS | 3.52‡ | 3.96‡ | 6.24 | –∥ | 2.89 | –1.05 | –∥ | ||||
| Nodular lymphocyte predominant Hodgkin lymphoma | 3.20 | 4.00 | 5.06 | –∥ | 0.80 | 4.61 | –∥ | ||||
| Unknown type lymphoid neoplasms | –12.41§ | –13.86§ | –11.00§ | –14.28§ | –10.78§ | –6.82‡ | –10.17§ | ||||
APC indicates annual percent change; other abbreviations are explained in Table 1.
All incidence rates are age adjusted to the 2000 United States population
The total APC is based on the incidence of lymphoid neoplasms among individuals of all races (whites, blacks, Asians, and other or unknown race)
P < .05
P < .01
Calculation of the APC was precluded by at least one annual rate of zero
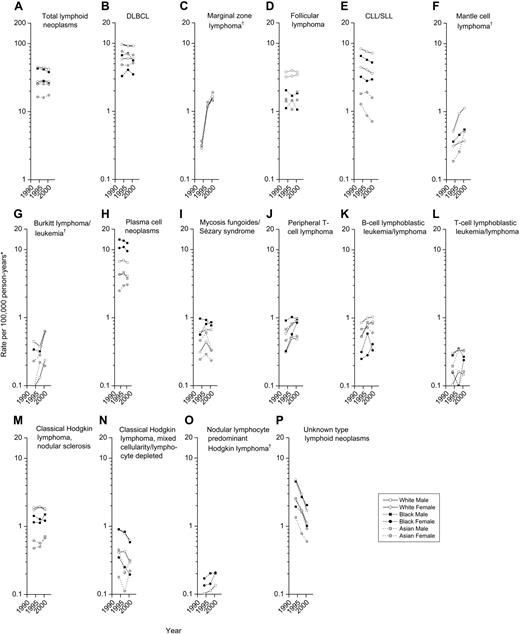
Among B-cell lymphomas, incidence rates during the 10-year period rose significantly for marginal zone lymphoma, mantle cell lymphoma, and Burkitt lymphoma/leukemia (Figure 4C,F,G) and declined significantly for DLBCL and CLL/SLL (Figure 4B,E). Marginal zone lymphoma increased in all population subgroups from 1992-1996, as this disease subtype became recognized as a distinct entity, and continued to increase 4% to 10% per year during 1996-2001 (data not shown). Incidence of both mantle cell lymphoma and Burkitt lymphoma/leukemia increased approximately 8% per year in the total population, particularly in the elderly. In the total population, DLBCL declined 0.5% per year during 1992-2001. This decline was observed predominantly in men aged 25 to 54, whereas DLBCL increased 1.4% per year in the elderly (data not shown). Rates for immunoblastic lymphoma (ICD-O-3 code 9684), a morphologic variant of DLBCL, declined 17.2% per year (data not shown). During 1992-2001, CLL/SLL decreased 2% to 3% per year for whites and for black men and 9% per year for Asian women, amplifying the excess incidence among whites compared with Asians. Incidence of follicular lymphoma and plasma cell neoplasms did not change significantly overall (Figure 4D,H), although follicular lymphoma increased 1.8% per year during 1992-2001 among the elderly (data not shown).
Incidence of T/NK-cell neoplasms rose 4% per year, from 2.0 to 2.6 cases per 100 000 person-years in males and from 1.0 to 1.4 cases per 100 000 person-years in females, due especially to increases in peripheral T-cell lymphomas; mycosis fungoides/Sézary syndrome rates remained unchanged (Figure 4J,I).
Overall, Hodgkin lymphoma rates remained stable during 1992-2001. A slight decline in classical Hodgkin lymphoma reflected a 6% per year decline in mixed cellularity/lymphocyte-depleted Hodgkin lymphoma rates (Figure 4N), no change in nodular sclerosis Hodgkin lymphoma rates (Figure 4M), and a 3% per year increase in classical Hodgkin lymphoma-not otherwise specified (NOS) rates. This pattern was substantially different for Asian women, where classical Hodgkin lymphoma increased 7% per year. Rates of nodular lymphocyte predominant Hodgkin lymphoma did not change significantly overall (Figure 4O) but increased 11% per year among children (data not shown).
During 1992-2001, unknown type lymphoid neoplasms decreased 13% per year in males, from 4.7 to 1.2 cases per 100 000 person-years, and 10% per year in females, from 2.5 to 0.9 cases per 100 000 person-years (Figure 4P). Although similar rates of decline were observed at all ages, the proportion of unknown type lymphoid neoplasms in 2001 remained substantially higher for the elderly than for children (4.4% versus 0.3%, respectively).
Incidence of lymphoid neoplasms by subtype, race, sex, and age, 12 SEER registries, 1992-2001.*All incidence rates are age adjusted to the 2000 United States population within age groups. Abbreviations are defined in Table 1.
Incidence of lymphoid neoplasms by subtype, race, sex, and age, 12 SEER registries, 1992-2001.*All incidence rates are age adjusted to the 2000 United States population within age groups. Abbreviations are defined in Table 1.
Immunophenotype data
During 1992-2001, 39.2% of all lymphoid neoplasm diagnoses were supported by immunophenotype data (data not shown). Although completion of immunophenotype data varied by disease subtype, ranging from less than 1% for plasma cell neoplasms to 76% for marginal zone lymphomas, the concordance between the SEER immunophenotype data and WHO-defined cell lineage was 97% for B-cell and 90% for T/NK-cell lymphoid neoplasms.
Discussion
We observed diverse incidence patterns among more than 114 000 lymphoid neoplasms diagnosed in the United States during 1992-2001. The substantial differences in incidence patterns among the lymphoid neoplasms support clinical, laboratory, and epidemiologic research that increasingly suggests that there is etiologic heterogeneity by disease subtype. This population-based study provides the first comprehensive descriptive analysis of incidence patterns for the entirety of lymphoid neoplasms as defined by the recently introduced WHO classification. Additionally, we present the first broad description of lymphoid neoplasm incidence patterns and trends for Asian Americans, with detailed evaluation of incidence according to disease subtype and in comparison with incidence among whites and blacks.
The most common lymphoid neoplasm subtypes in Asians were DLBCL and plasma cell neoplasms. We confirm that the substantially lower rates of CLL/SLL in Asia compared with other continents persist among Asian Americans, particularly for the elderly.54 It is commonly thought that Asians have higher rates of T/NK-cell neoplasms compared with other populations.9 We demonstrate that Asians in the United States have a higher relative incidence of T/NK-cell neoplasms compared with whites (8% versus 5% of lymphoid neoplasms) but a similar absolute incidence of T/NK-cell neoplasms (1.6 versus 1.7 cases per 100 000 person-years). The higher relative incidence of T/NK-cell neoplasms among Asian Americans results from substantially lower incidence compared with whites for B-cell neoplasms (16.3 versus 27.2 cases per 100 000 person-years) and Hodgkin lymphoma (1.0 versus 2.9 cases per 100 000 person-years). Incidence trends among Asian Americans during 1992-2001 were consistent with trends in other populations, with the exception of a 7% per year increase in classical Hodgkin lymphoma for Asian women. Incidence patterns may vary among Asian Americans according to population origin,54-58 but small numbers and the rarity of some disease subtypes precluded analysis of this potential variation. Epidemiologic investigations have demonstrated little difference in lymphoid neoplasm incidence among foreign-born and United States-born Asians,55-58 supporting the role of host susceptibility in lymphoid neoplasm etiology.
Incidence of lymphoid neoplasms by subtype and race, 12 SEER registries, 1992-2001.*All incidence rates are age adjusted to the 2000 United States population. Abbreviations are explained in Table 1.
Incidence of lymphoid neoplasms by subtype and race, 12 SEER registries, 1992-2001.*All incidence rates are age adjusted to the 2000 United States population. Abbreviations are explained in Table 1.
Additional research focused on environmental factors and host susceptibility is needed and may benefit from consideration of the lymphoid neoplasms for which rates differ greatly by race or sex. Marked white predominance is observed for hairy cell leukemia and follicular lymphoma, whereas marked black predominance is observed for plasma cell neoplasms. Black predominance also is observed for T/NK-cell neoplasms, most notably for mycosis fungoides/Sézary syndrome. Asians have considerably lower rates than whites and blacks for CLL/SLL, particularly in the elderly, and Hodgkin lymphoma, particularly in children. Striking male predominance is observed for Burkitt lymphoma/leukemia and hairy cell leukemia, regardless of race. White males also have substantially higher rates than any other sex or race group for mantle cell lymphoma and nodular lymphocyte predominant Hodgkin lymphoma. In contrast, rates differed little between males and females for follicular lymphoma, marginal zone lymphoma, and nodular sclerosis Hodgkin lymphoma.
Lymphoid neoplasm incidence in the elderly represents a notable public health burden due to the high rates (170.6 cases per 100 000 person-years) and aging United States population. Further investigations regarding the biology of aging and age-related effects such as immune senescence are particularly important for the subtypes where rates increase most steeply with age, including DLBCL, follicular lymphoma, CLL/SLL, and plasma cell neoplasms. Because the chronic inflammation, DNA damage, and diminished immune surveillance that occur with older age59 are also linked with cancer,60 we believe they are important avenues of research for these lymphoma subtypes. During 1992-2001, rates of DLBCL and follicular lymphoma in the elderly increased 1.4% and 1.8% per year, respectively. Improved diagnostic specificity during the 1990s28,61 did not appear to affect the increasing rates of DLBCL in the elderly. In contrast, rates of CLL/SLL declined 2.1% per year, and rates of plasma cell neoplasms did not change significantly for the elderly. The decline in CLL/SLL is surprising in light of increased recognition of this underdiagnosed disease.13,62 This decline suggests that exposures to the still unknown cause(s) of CLL/SLL may have similarly declined.
Trends in incidence of lymphoid neoplasms by subtype, race, and sex, 12 SEER registries, 1992-1995 to 1999-2001.*All incidence rates are age adjusted to the 2000 United States population and presented for 3 fixed time periods (1992-1995, 1996-1998, 1999-2001). †Presentation of trends for certain populations was precluded by at least one annual rate of zero. Abbreviations are defined in Table 1.
Trends in incidence of lymphoid neoplasms by subtype, race, and sex, 12 SEER registries, 1992-1995 to 1999-2001.*All incidence rates are age adjusted to the 2000 United States population and presented for 3 fixed time periods (1992-1995, 1996-1998, 1999-2001). †Presentation of trends for certain populations was precluded by at least one annual rate of zero. Abbreviations are defined in Table 1.
Some of the greatest variation in incidence among lymphoid neoplasms is seen in children. Incidence of lymphoblastic leukemia/lymphoma is higher in children than in any other age group; in contrast, incidence for several subtypes, including follicular lymphoma, CLL/SLL, and plasma cell neoplasms, is virtually nonexistent in children. During 1992-2001, lymphoblastic leukemia/lymphoma accounted for 50% and Hodgkin lymphoma accounted for 31% of lymphoid neoplasms in children (2.9 cases and 1.8 cases per 100 000 person-years, respectively). Substantial international variation for both absolute rates and relative frequencies of childhood lymphoid neoplasms has been demonstrated, providing important etiologic clues.63 Incidence of lymphoblastic leukemia/lymphoma is higher in areas with higher socioeconomic development, with rates among United States whites 4 times higher than rates in sub-Saharan Africa.63 In contrast, Burkitt lymphoma/leukemia is the most common childhood cancer in tropical Africa, where incidence is related to EBV and malaria.64 We confirm the continued stability of lymphoblastic leukemia/lymphoma and Burkitt lymphoma/leukemia rates in United States children65 and a continuation of the decades-long decline in classical Hodgkin lymphoma, particularly in the mixed cellularity/lymphocyte-depleted subtype, though the causes of this decline remain unknown.33,34,65 During 1992-2001, lymphoid neoplasms accounted for approximately one third of all cancers diagnosed in children in the United States42 ; thus, understanding the effects of perinatal and early life exposures in the etiology of these neoplasms may be important.
Although the HIV status is not known for cancer cases reported to SEER registries, the observed excess incidence among white and black men aged 25 to 54 compared with other race, sex, and age groups for DLBCL (including immunoblastic lymphoma), Burkitt lymphoma, and mixed cellularity/lymphocyte depleted Hodgkin lymphoma is consistent with previous reports identifying these subtypes as AIDS-related lymphomas.15,29,30,37,66-71 The observed excess incidence in this population for unknown type lymphoid neoplasms also suggests that AIDS cases are disproportionately less likely to be assigned a specific WHO subtype. Declining incidence of DLBCL and mixed cellularity/lymphocyte depleted Hodgkin lymphoma in this population subgroup, particularly during 1995-2001, may reflect the impact of highly active antiretroviral therapy introduced in the mid 1990s, which increases immune function in patients with AIDS and reduces the risk of AIDS-related lymphomas.67-69,71 Additional analyses that can explicitly identify HIV-positive individuals are better suited to examining the incidence patterns for AIDS-related lymphomas.
The main limitation of this analysis is a lack of central pathology review for all cases. Nevertheless, although diagnostic practices have changed considerably during the past 15 years, agreement among hematopathologists is much improved in the REAL and WHO classifications compared with earlier classifications.29,61 Diagnostic concordance among expert hematologists61 and between registry coding and expert pathologic review72 has been estimated to be more than 85% for most REAL and WHO lymphoid neoplasm subtypes. Only for Burkitt lymphoma/leukemia was substantially lower agreement (about 50%) reported.61,72 The use of cases classified according to ICD-O-2, prior to the introduction of the WHO classification, is another limitation of this analysis. For cases originally classified in ICD-O-2, a comparison of the ICD-O-3 code assigned by the conversion algorithm40 and the ICD-O-3 code that would have been assigned by a SEER reviewer suggests that agreement is generally high for the major subtypes (follicular lymphoma [88%], DLBCL [87%], CLL/SLL [80%], peripheral T-cell lymphoma [74%], and mycosis fungoides/Sézary syndrome [73%]).73 Additional limitations of this analysis include the variation between hospitals in the quality and level of expert hematopathologic review, potential errors in the coding of lymphoid neoplasms, missing or incomplete data in the registry, and the potential underestimate of incidence rates for more recent years due to reporting delays.41 To minimize these potential errors, SEER registries are rigorously evaluated for data quality and completeness, with case ascertainment of 98% or greater in all registries, and SEER reviewers undergo extensive training for general cancer registration and for specific cancer sites.
Changes in diagnostic practice likely are responsible for the increasing rates of marginal zone and mantle cell lymphomas and decreasing rates of unknown type lymphoid neoplasms. Increased incidence of marginal zone lymphoma during 1992-1995 reflects the implementation of ICD-O-2 codes specific for marginal zone lymphoma by SEER registries in 1995.40 It is plausible that the 4% to 10% increases per year for marginal zone lymphoma during 1996-2001 reflect improving recognition by pathologists, although true changes in incidence resulting from changes in risk factor prevalence cannot be ruled out. Risk of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT), the most common type of marginal zone lymphoma, is related to inflammation resulting from autoimmune diseases or gastritis associated with infections such as Helicobacter pylori.28,74 The incidence of autoimmune conditions may be increasing slightly in the United States,75 whereas the incidence of H pylori infections has decreased in developed countries in more recent birth cohorts.76 Thus, the impact of changes in risk factor prevalence on trends in marginal zone lymphoma is unclear. Nevertheless, the similar seroprevalence of H pylori between men and women in the United States77 and the predominance of autoimmune diseases among women75 may explain why the male-to-female IRR for marginal zone lymphoma is lower than that of any other B-cell lymphoid neoplasm. Increased incidence of mantle cell lymphoma during 1992-2001 likely reflects improved diagnosis of this subtype, particularly with the introduction of immunohistochemical staining for cyclin D1, which is overexpressed in virtually all mantle cell lymphomas.78-80 During 1992-2001, rates for unknown type lymphoid neoplasms in the total population decreased 12% per year; a similar rate of decline was observed in all race, sex, and age groups. This decrease is likely due to increased diagnostic specificity resulting from the incorporation of entities described by the REAL and WHO classifications and utilization of cytogenetic and immunophenotypic data. The decline in unknown type lymphoid neoplasms could therefore contribute artifactually to the increase we observed for some subtypes and attenuate the estimated decline we observed for other subtypes, particularly for AIDS-related subtypes. Increased efforts to classify cases are necessary to resolve this uncertainty; nevertheless, unknown type lymphoid neoplasms still made up more than 3% of lymphoid neoplasms in 2001.
Despite our use of the recently introduced WHO classification for our analysis, several important categories, such as DLBCL, remain heterogeneous with respect to molecular characteristics and gene-expression profiles.81-88 Future research is needed to understand the etiologic relevance of this heterogeneity and its consequences for lymphoid neoplasm classification.
We conclude that the striking differences in incidence patterns by histologic subtype strongly suggest that there is etiologic heterogeneity among lymphoid neoplasms and support the pursuit of epidemiologic analysis by subtype. The established specificity of certain infections74,89,90 and increasing evidence suggesting that certain risk factors91 and host susceptibility92 may be related to specific subtypes further support this conclusion, although it remains plausible that some risk factors are related to multiple subtypes or all lymphomas.70 Research within large consortium efforts such as InterLymph, the International Lymphoma Epidemiology Consortium,93,94 will likely contribute to identifying the elusive etiologic causes of lymphoid neoplasms.
Prepublished online as Blood First Edition Paper, September 8, 2005; DOI 10.1182/blood-2005-06-2508.
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal