Abstract
Murine cytomegalovirus encodes a secreted, pro-inflammatory chemokine-like protein, MCK-2, that recruits leukocytes and facilitates viral dissemination. We have shown that MCK-2-enhanced recruitment of myelomonocytic leukocytes with an immature phenotype occurs early during infection and is associated with efficient viral dissemination. Expression of MCK-2 drives the mobilization of a population of leukocytes from bone marrow that express myeloid marker Mac-1 (CD11b), intermediate levels of Gr-1 (Ly6 G/C), platelet-endothelial-cell adhesion molecule-1 (PECAM-1, CD31), together with heterogeneous levels of stem-cell antigen-1 (Sca-1, Ly-6 A /E). Recombinant MCK-2 mediates recruitment of this population even in the absence of viral infection. Recruitment of this cell population and viral dissemination via the bloodstream to salivary glands proceeds normally in mice that lack CCR2 and MCP-1 (CCL2), suggesting that recruitment of macrophages is not a requisite component of pathogenesis. Thus, a systemic impact of MCK-2 enhances the normal host response and causes a marked increase in myelomonocytic recruitment with an immature phenotype to initial sites of infection. Mobilization influences levels of virus dissemination via the bloodstream to salivary glands and is dependent on a myelomonocytic cell type other than mature macrophages.
Introduction
Human cytomegalovirus (HCMV) is an opportunistic pathogen of widespread medical importance, causing congenital disease following a primary infection during pregnancy, as well as a wide range of organ-specific diseases following reactivation in immunocompromised patients.1 Murine CMV (MCMV) has proven to be an excellent model of HCMV infection and disease, providing information on the basic patterns of pathogenesis, latency, immunity, and dissemination.2-4 Dissemination of HCMV or MCMV requires a population of host mononuclear leukocytes that traverse the bloodstream.5-7 Based on comparisons of wild-type and mutant MCMV-infected mice, MCMV enhances dissemination levels by expressing a pro-inflammatory chemokine-like gene product called MCMV chemokine (MCK).2,7,8 MCK appears to increase the recruitment of leukocytes to initial sites of infection and to allow a greater number of virus-positive leukocytes to traverse the bloodstream to salivary glands (SGs),3,7 a site of prolonged replication and shedding. This process likely provides a source of virus for transmission to naive mice.2 The human pathogen HCMV also encodes chemokine homologs: one CC chemokine homolog (UL128) that may be analogous to MCK,9 as well as one very potent CXC chemokine (gpUL146/vCXCL1) that is as potent as human interleukin-8 (IL-8) and acts via CXCR2.10
The carboxyl terminal 81 aa m131 ORF encodes a predicted gene product, MCK-1,8 that elicits both calcium mobilization and adherence in resident or elicited peritoneal exudate cells (PECs).11 mRNA splicing adds 199 aa to this chemokine-like domain to make MCK-2 the natural, secreted gene product.12 Studies on virus-infected mice have suggested that MCK-2 influences levels of viremia as well as patterns of dissemination11,13 by increasing the inflammatory response at sites of infection.14 An MCK-2-dependent influx of leukocytes into initial sites of infection is followed by a mononuclear-cell (MNC)-associated viremia that peaks at day 5 after infection14 and leads to viral seeding of the SGs.2,15-17 Although MCK-2 is pro-inflammatory and a determinant of both viremia and dissemination11,14 and was also associated with both natural killer and adaptive immune defects,13 this chemokine-like function is not involved in immune evasion. On the one hand, MCK-2 control of viremia and dissemination is preserved in mice that lack the ability to mount an adaptive immune response.2,16 On the other hand, virus replication levels and clearance from inoculated foot pads (FPs) or sites of systemic infection, including draining lymph nodes (LNs), liver, lungs, spleen (SP), and bloodstream, are not influenced by expression of MCK-2.2,11,14 Overall levels of virulence, persistence, and latency of mck mutant viruses remain the same as wild-type virus.2
Most nonlymphoid MNCs in the PB are monocytes, a bone marrow (BM)-derived CD14+ cell that gives rise to tissue macrophages as well as to a subset of dendritic cells (DCs).18,19 PB monocytes and macrophages have been identified as host cells that support viral replication11,16,17,20-23 and latency22,24,25 ; however, these cells also have been implicated in host defense.20,23,26-28 The differentiation-dependent susceptibility of monocytes and macrophages to MCMV infection22,23 has led to a consensus view that nonpermissive monocytes may disseminate infection and differentiate into permissive mature cells upon entry into tissues. This pattern is similar to that proposed to explain viral reactivation from latency29 and has been reinforced by the apparent central role of myelomonocytic lineage cells in HCMV infection30-32 and latency.33-38
During infection or inflammatory insult, BM-derived leukocytes migrate out of the bloodstream into the affected tissues. Migration along blood vessels and diapedesis out of the bloodstream is controlled by sequential action of adhesion molecules and cytokines.39-42 This response involves a wide range of leukocyte types at many stages of differentiation, ranging from hematopoietic stem cells to monocytes.42-44 Accumulating evidence that the PB MNC is important in MCMV infection,16,17,20,21,23,45 combined with the impact of virus-encoded MCK-2 on recruitment, viremia, and dissemination,11,14 prompted a direct evaluation of the cell type(s) that becomes mobilized by infection and traverses the bloodstream to accumulate at initial sites of infection. We find that MCK-2 expression is associated with an intense response of immature BM-derived, myelomonocytic leukocytes that may influence levels of viremia and viral dissemination to the SGs.
Materials and methods
Mice, viruses, and experimental infection
Wild-type K181+16 and recombinant viruses RM427+, RM461, RQ461, RM4511, and RM4503 (Figure 1; Saederup et al,14 Stoddart et al,16 and van Den Pol et al46 ) were grown and titered on NIH3T3 cells from the American Type Culture Collection (CR1658) using tissue-culture conditions described previously.47 Medium from infected cultures was clarified 2500g, and virus was collected by sedimentation at 17 000g and stored at -80°C until use. Three- to 8-week-old BALB/c or C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were inoculated into a rear FP with 1 × 106 to 1 × 107 plaque-forming units (PFUs) as described previously.11 CCR2-/- mice, bred on a C57BL/6 background,48 and MCP-1-/- mice, bred on a BALB/c background49 (from Dr Izzi Charo, Gladstone Research Institutes (San Francisco, CA), and Dr Barrett Rollins, Harvard Medical School, Boston, MA, respectively), and MCP-1-/- CCR2-/- mice back-crossed with MCP-1-/- BALB/c mice were maintained under specific pathogen-free conditions with the approval of Stanford University Administrative Panel on Laboratory Animal Care. Control mice were sham inoculated with virus-free culture medium. After CO2 asphyxiation at indicated times, FP and PB cells were collected for either virus titer or infectious center assay (ICA) as described.16,47 SP, lung, and liver were collected as previously described.28,47 Peritoneal exudate cells (PECs) were harvested into RPMI1640 at 2 days after intraperitoneal injection of 3% thioglycollate (GIBCO, Grand Island, NY) solution (2 mL/mouse).28 All procedures were approved by the Stanford animal use committee.
Schematic representation of mutant virus genomes. The top line represents the K181+ strain MCMV genome16 with nucleotide numbers (GenBank accession number U68299). The MCMV ie1/ie2/ie3 transcriptional enhancer (hatched box) and transcripts (solid arrows) encoded by the wild-type viruses also are shown in the expanded region. Open boxes depict the positions of ORFs m131 and m129 that encode MCK-2.12 The mutations introduced into recombinant viruses RM461,16 RM427+,14 RM4503,46 and RM451111 are depicted below the transcripts. The lacZ insertion in RM461 or RM427+ (open box) and the EGFP-puro insertion in RM4503 or RM4511 (open box) are depicted below the map. Two nucleotide point mutations were introduced into RM4511 to alter the conserved CC chemokine motif in MCK-2 (C27R and C28G; 14).
Schematic representation of mutant virus genomes. The top line represents the K181+ strain MCMV genome16 with nucleotide numbers (GenBank accession number U68299). The MCMV ie1/ie2/ie3 transcriptional enhancer (hatched box) and transcripts (solid arrows) encoded by the wild-type viruses also are shown in the expanded region. Open boxes depict the positions of ORFs m131 and m129 that encode MCK-2.12 The mutations introduced into recombinant viruses RM461,16 RM427+,14 RM4503,46 and RM451111 are depicted below the transcripts. The lacZ insertion in RM461 or RM427+ (open box) and the EGFP-puro insertion in RM4503 or RM4511 (open box) are depicted below the map. Two nucleotide point mutations were introduced into RM4511 to alter the conserved CC chemokine motif in MCK-2 (C27R and C28G; 14).
Recombinant chemokines
N-Met-MCK-2 expressed in Escherichia coli was made by R&D Systems (Minneapolis, MN) and kindly provided by ChemoCentryx (Mountain View, CA). Prior to use, the biologic activity was assessed by a calcium mobilization assay using murine BM cells. BALB/c mice were injected into the FPs 4 times with 200 ng N-Met-MCK-2 (in 4 μL endotoxin-free phosphate-buffered saline [PBS]) at 0, 24, 48, and 72 hours in a time course. For isolation of cells, mice were killed by CO2 asphyxiation 3 hours after the last injection, and FP-resident leukocytes were isolated. Stocks of N-Met-MCK-2 contained less than 0.05 ng/dose of endotoxin, as measured using a commercial test kit (Sigma, St Louis, MO). C10 (487-C-050/CF, R&D Systems), a murine CC chemokine that induces macrophage-lineage cell infiltrates in vivo50 and recombinant HCMV vCXCL-1 (UL146; 620-CM-025, R&D Systems) and acts on human CXCR210 but is inactive on mouse cells,51 was a kind gift from ChemoCentryx.
Cell preparation and FCM analysis
Leukocytes were prepared from pooled, split FPs in Dulbecco modified minimal essential medium (BioWhittaker, Walkersville, MD) containing 1.5 mg/mL collagenase D (Roche, Indianapolis, IN) using 3 sequential digestions for 20 minutes at 37°C.52 After removal of undigested bones and hair, cells were collected by centrifugation, washed twice with HEPES-buffered RPMI1640 medium containing 2% fetal bovine serum (FBS), and finally suspended in 40% Percoll (Amersham Biosciences, Piscataway, NJ) made in 0.4 × Hanks balanced salt solution and 0.6 × HEPES-buffered RPMI1640 medium containing 2% FBS. The band of cells at the 40%/75% interface was collected after Percoll step gradient centrifugation at 1500 rpm (400g) for 30 minutes at 25°C and used for assays. PB leukocytes were prepared as described.47 Antibody staining and flow cytometric (FCM) analysis were by FACScan (Becton Dickinson, Mountain View, CA).53 20 000 (2-color) or 100 000 (3-color) events were collected for analysis using FlowJo (Treestar, San Carlos, CA) or CellQuest software (Becton Dickinson). Monoclonal antibodies (PharMingen, San Diego, CA), biotinylated or conjugated with either fluorescein isothiocyanate (FITC) or phycoerythrin (PE) against the following antigens were used: CD3-ϵ (2C11) CD4 (L3T4), CD8-α (Ly-2), Mac-1/CD11b (M1/70), CD11c (HL3), CD13 (R3-242), CD14 (rmC5-3), CD31/PECAM-1 (MEC13.3), CD40 (3/23), CD45/Ly-5 (leukocyte common antigen: LCA) (30-F11) B220/CD45R (RA3-6B2), c-kit/CD117 (2B8) Gr-1/Ly-6G/C (RB6-8C5), Mac-3 (M3/84), major histocompatibility class (MHC) class II/I-Ad/Ed (2G9), and Sca-1/Ly-6A/E (D7). PE-conjugated anti-CD34 (MEC14.7) and TriColor-conjugated anti-F4/80 were from Caltag (San Francisco, CA), and FITC-conjugated anti-CD205/DEC205 (NLDC-145) was from Serotec (Oxford, United Kingdom). The FcγRII/III-specific monoclonal antibody 2.4G2 (anti-CD16/CD23) was used as blocking reagent, and dead cells were excluded using a forward versus side scatter plot and propidium iodide (PI) staining.
Cell enrichment and separation
Mac-1+/Gr-1+ cells were enriched from FP or PB leukocytes incubated with biotin-conjugated anti-Mac-1 (M1/70) and anti-Gr-1 (RB6-8C5) at 4°C for 20 minutes, washed twice, and suspended in HBSS with 2% FBS (HBSS/FBS) to which streptavidin-conjugated immunomagnetic beads were added. After 20 minutes' incubation, beads were washed twice, suspended into 1 mL HBSS/FBS, and used for MACS separation (Miltenyi Biotec, Bergisch-Gladbach, Germany). To ensure purity, myeloid-cell populations were further purified by fluorescence-activated cell sorting (FACS Vantage, Becton Dickinson; FACS Shared User Group, Stanford University).54 Cells were then cytocentrifuged (1× 104 to 3× 104 cells/slide), and were fixed and stained with May-Grunwald-Giemsa (Sigma). Images were visualized using a Zeiss Axioskop 2 microscope equipped with a 60 × objective lens with a 10 × eyepiece (Zeiss, Thornwood, NY) and were captured with a SPOT Insight 3.2.0 camera (Diagnostic Instruments, Sterling Heights, MI) and tagged image-formatted files (TIFFs) were prepared for publication using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Results
Mac-1+/Gr-1int myeloid cells are recruited to sites of MCMV infection
We took advantage of the fact that mice infected with mck-expressing viruses exhibit 3 temporally distinct, MCK-2-enhanced properties compared with mck-mutant viruses: (1) increased inflammatory response at the site of infection within 2 days, (2) increased level of viremia within 5 days, and (3) increased level of virus dissemination to SGs within 7 to 14 days of inoculation.11,14 To identify cell populations that respond to MCK-2, leukocyte infiltrates into infected FPs were analyzed by FCM analysis, comparing mck-expressing and mck-mutant viruses (Figure 1). Consistent with previous investigations,14 twice as many leukocytes were recruited into FPs following inoculation of mck-expressing virus, and this was accompanied by greater swelling and increased cellularity (data not shown). When examined for individual myeloid-cell markers, 24% of infiltrating cells from mck-expressing virus-infected FPs were Mac-1+ (7 × 105 leukocytes/FP), twice the percentage and 4 times the number of such cells recruited by mck-mutant virus infection. A majority of Mac-1+ cells were found to co-express intermediate levels of Gr-1 (Figure 2A), and all expressed CD45 (leukocyte common antigen; data not shown). Cytologic examination (Figure 2B) confirmed that the 2 major populations of cells, Mac-1+/Gr-1int and Mac-1-/Gr-1-, were 10- to 17-μm MNCs with a high nucleus-to-cytoplasm ratio and large round- to kidney-shaped nuclei. These features, plus the lack of cytoplasmic granules, confirmed recruited cells to be predominantly monocytic rather than granulocytic (Figure 2B) and was consistent with an immature status. A Mac-1+/Gr-1int cell population consistently appeared in the FPs of virus-infected but not medium-treated mice and consistently accumulated to greater numbers when mck-expressing virus was compared to mck-mutant virus (Figure 2C). Neutrophils (Mac-1+/Gr-1high), which have long been recognized as a variable component in MCMV infection,55 were present in some experiments (Figure 2A) but not others (data not shown), depending on the batch of mice. Levels of Mac-1+/Gr-1int leukocytes were strongly influenced by mck expression, with twice the percentage and 4 times the number present in FPs infected with mck-expressing MCMV (parental K181+, RQ461, RM427+; see Figure 1A for recombinant virus genome organization) compared to mck-mutant viruses RM4511 or RM461 (data not shown). B220+ cells (B lymphocytes) were not detected over this time period. Approximately 3% to 4% of infiltrating cells in virus-infected mice were CD4+, 2% or less were CD8α+, and 7% to 10% were DX5+, suggesting overall similarity in recruitment of lymphocytes and natural killer (NK) cells, and all independent of MCK-2 (data not shown).
Markers and cytology of myeloid cells at sites of infection. (A) FCM analysis on medium (left), mck-mutant RM461-infected (middle), and mck-expressing RQ461 (right). Percentages of cells with each expression pattern are indicated in the Mac-1-/Gr-1+, Mac-1+/Gr-1+, and Mac-1+/Gr-1- quadrants. The Mac-1+/Gr-1int population is indicated by a dashed box. FP leukocytes were prepared from groups of 3 BALB/c mice at 48 hours after inoculation (inoculum of 1 × 106 PFUs). (B) May-Grunwald-Giemsa-stained FCM purified cells from pooled FPs (20 mice) at 48 hours after inoculation (inoculum of 1 × 106 PFUs), comparing 4 examples from the Mac-1+/Gr-1int population (left) and 4 examples from the Mac-1-/Gr-1- population (right) (× 1000). Mac-1+/Gr-1int cells were prepared by MACS separation, followed by FACSvantage sorting, and Mac-1-/Gr-1- cells were from the initial MACS separation step. (C) Summary data for the frequency of Mac-1+/Gr-1int cells of FP leukocytes. The mean (bars) with standard deviation (error bars) of 3 independent experiments is indicated.
Markers and cytology of myeloid cells at sites of infection. (A) FCM analysis on medium (left), mck-mutant RM461-infected (middle), and mck-expressing RQ461 (right). Percentages of cells with each expression pattern are indicated in the Mac-1-/Gr-1+, Mac-1+/Gr-1+, and Mac-1+/Gr-1- quadrants. The Mac-1+/Gr-1int population is indicated by a dashed box. FP leukocytes were prepared from groups of 3 BALB/c mice at 48 hours after inoculation (inoculum of 1 × 106 PFUs). (B) May-Grunwald-Giemsa-stained FCM purified cells from pooled FPs (20 mice) at 48 hours after inoculation (inoculum of 1 × 106 PFUs), comparing 4 examples from the Mac-1+/Gr-1int population (left) and 4 examples from the Mac-1-/Gr-1- population (right) (× 1000). Mac-1+/Gr-1int cells were prepared by MACS separation, followed by FACSvantage sorting, and Mac-1-/Gr-1- cells were from the initial MACS separation step. (C) Summary data for the frequency of Mac-1+/Gr-1int cells of FP leukocytes. The mean (bars) with standard deviation (error bars) of 3 independent experiments is indicated.
To determine whether MCK-2 was sufficient to induce recruitment of Mac-1+/Gr-1int cells, we tested a recombinant form of this protein, N-Met-MCK-2, purified from E coli. N-Met-MCK-2 was engineered to initiate at aa 22 in order to mimic the natural protein after signal sequence cleavage, with additional methionine to enable prokaryotic expression. Treatment with 100- or 200-ng doses of this recombinant protein every 24 hours over 4 days was sufficient to induce marked swelling within a short time that resolved within 24 hours following each injection (Figure 3A; data not shown). When FP infiltrates were examined 3 hours after the last injection (200-ng doses), approximately twice the number of cells were recruited by N-Met-MCK-2 (ranging from 1.0 × 106/FP to 2 × 106/FP) than by PBS. As shown in Figure 3B, administration of N-Met-MCK-2 induced the recruitment of leukocytes with Mac-1+/Gr-1int expression similar to the population of cells that was enhanced by virus infection. This distinct population was observed independent of virus infection in the presence of N-Met-MCK-2 or, to a lesser extent, C10, a CCR1-specific murine chemokine that recruits macrophage lineage cells.50 There was no recruitment in PBS controls (Figure 3). Other recombinant chemokines used as negative controls, such as vCXCL1 (HCMV UL146), did not induce a response following injection into FPs, as expected from known properties.51 Thus, a distinct Mac-1+/Gr-1int myeloid-cell population responded to MCK-2 as well as to C10.50
FCM analysis of N-Met-MCK-2 recruited Mac-1+/Gr-1int myeloid cells. (A) N-Met-MCK-2-induced swelling. BALB/c mice were injected into FPs 4 times with 200-ng doses of N-Met-MCK-2 (♦) or PBS carrier (⋄) using groups of mice as indicated by arrowheads below the graph. Standard deviation of mean swelling is indicated by error bars. (B) FCM analysis of cells recovered from FPs prepared from 2 independent experiments using groups of 4 (experiment 1) or 5 (experiment 2) BALB/c mice at 3 hours after the fourth N-Met-MCK-2 injection. FCM analysis and layout is described in the legend to Figure 2.
FCM analysis of N-Met-MCK-2 recruited Mac-1+/Gr-1int myeloid cells. (A) N-Met-MCK-2-induced swelling. BALB/c mice were injected into FPs 4 times with 200-ng doses of N-Met-MCK-2 (♦) or PBS carrier (⋄) using groups of mice as indicated by arrowheads below the graph. Standard deviation of mean swelling is indicated by error bars. (B) FCM analysis of cells recovered from FPs prepared from 2 independent experiments using groups of 4 (experiment 1) or 5 (experiment 2) BALB/c mice at 3 hours after the fourth N-Met-MCK-2 injection. FCM analysis and layout is described in the legend to Figure 2.
MCK-2-recruited Mac-1+/Gr-1int cells become preferentially infected
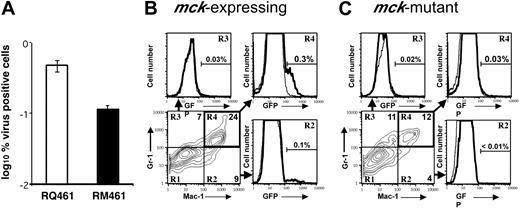
The mck-mutant and mck-expressing virus replication patterns in FPs, draining LNs, and SP are similar,14 suggesting that differences in recruitment are not due to an MCK-2-related defect in viral replication or gross alteration of leukocyte distribution. To determine whether the recruited population of MNCs became differentially infected, Mac-1+/Gr-1int infiltrates from FPs inoculated with mck-expressing viruses (RQ461, RM4503) or mck-mutant viruses (RM461, RM4511) were compared. Green fluorescent protein (GFP)-expressing RM4503 was found to recruit Mac-1+/Gr-1int cells to FPs in a pattern similar to RQ461, and RM4511 showed a mutant phenotype that was indistinguishable from RM461 (data not shown). Although replication levels in FPs of mice inoculated with mck-expressing and mutant virus were similar,14 we observed a 5-fold greater percentage of infectious leukocytes (Figure 4A) from FPs infected with RQ461 (0.5%) compared with mutant virus RM461 (0.1%). Consistent with this difference in infectivity, FCM (Figure 4B,C) revealed a 10-fold greater percentage of GFP-positive Mac-1+/Gr-1+ leukocytes in RM4503-infected FPs (0.3%) than were recovered from RM4511-infected FPs (0.03%). Although the overall percentages were lower, Mac-1+ leukocytes also exhibited a 10-fold difference (Figure 4B,C), but double-negative cells exhibited a lower 4-fold difference similar to the total cell population (data not shown). Despite the large numbers of MNCs recovered, a small proportion of these cells expressed GFP or scored as virus-positive by ICA, with the latter assay being more sensitive. Thus, MCK-2 may be responsible for enhanced recruitment as well as enhanced infection in Mac-1+/Gr-1int cells.
Mac-1+/Gr-1int cells mobilized into PB by MCK-2 are myeloid cells originating from BM. To determine the time course of Mac-1+/Gr-1int cell appearance following infection, FCM analysis was performed on FP and PB leukocytes harvested over the first 5 days of infection (Figure 5). Recruitment of Mac-1+/Gr-1int cells into FPs was enhanced by MCK-2 on days 2, 3, and 5 after inoculation. Twice as many (data not shown) and 3 times the percentage (Figure 5A) of Mac-1+/Gr-1int cells were recovered at day 2 or day 3 from mck-expressing virus-infected FPs. As shown in Figure 5B, the proportion of Mac-1+/Gr-1int cells that were detected in the PB increased from day 1 (RQ461, 7%; RM461, 8%) to day 2 (RQ461, 13%; RM461, 13%) in a pattern that was independent of MCK-2; however, between days 2 and 3, a strong MCK-2-dependent response by Mac-1+/Gr-1int cells was observed, with the percentage increasing to 21% in RQ461-infected mice but decreasing dramatically to 2% in RM461-infected mice. This reduction from day 2 to day 3 during infection in mck-mutant virus-infected FPs was observed repeatedly but was not understood. Thus, myeloid cells accumulating in FPs appeared to transit through PB in a pattern that was driven by MCK-2 to peak at day 3 after inoculation, as summarized in Figure 5C.
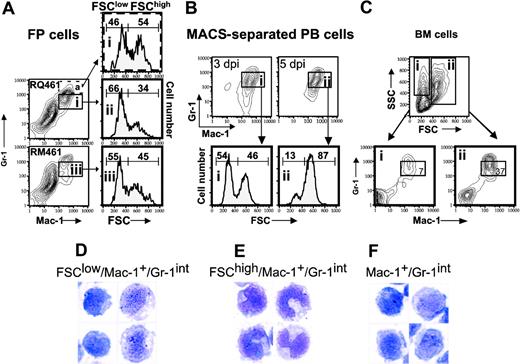
Next, we sought to identify the origin of the Mac-1+/Gr-1+ cells responding to MCMV infection by comparing the phenotype of cells isolated from a number of possible source tissues. As expected,56 cells with similar FSC characteristics were found in BM (Figure 6), but not in FPs, PB, LNs, or SP of uninfected mice (Figure 5; also Supplemental Figure S1, available on the Blood website by clicking the Supplemental Figures link at the top of the online article; and data not shown). The percentage of total Mac-1+/Gr-1+ cells recovered from BM at 5 days after inoculation (50%) was similar to levels in uninfected mice (data not shown). Although present in PB and FPs, Mac-1+/Gr-1+ cells were not present and did not accumulate in secondary lymphoid tissues during viral infection (Supplemental Figure S1). A majority of the MNCs recruited to PB or FPs and enhanced by MCK-2 at day 3 after inoculation had FSClow features (Figure 6A,B) in common with Mac-1+/Gr-1int BM-derived myeloid progenitors (Figure 6C). A preliminary analysis of CD45.1/Ly5.2+ bone marrow transplanted into CD45.2/Ly5.1+ recipients revealed that mck-expressing virus recruited a 3-fold higher percentage of CD45.1/Ly5.2+/Mac-1+/Gr-1+ cells to FPs (19%) as compared to the percentage recruited by mutant virus (7%). Of course, differential recruitment also was noticed in the CD45.2/Ly5.1+ population. This confirms the results shown for nonmanipulated BALB/c mice and further emphasizes the importance of BM-derived cells in MCK-2-enhanced recruitment. Further, FP cells sorted for Mac-1+/Gr-1+ expression at day 3 after inoculation had the FSClow phenotype of myeloid progenitors (data not shown). The pattern in PB shifted to a predominance of FSChigh pattern by 5 days after inoculation (Figure 6B), suggesting that cells had become more mature over the intervening 2 days. Taken together, these findings suggest that MNCs responding to viral infection are composed of myelomonocytic cells with characteristics of progenitors originating from the BM and are not found in other lymphoid organs.
Acquisition of virus by Mac-1+/Gr-1+ cells. (A) ICA on total FP leukocytes collected from 10 mice at 48 hours after inoculation with RQ461 or RM461 (inoculum of 1 × 106 PFUs) shown as geometric mean percentage virus-positive FP cells ± standard deviation of the geometric mean (error bars). (B) FCM analysis of mck-expressing RM4503-infected infiltrates prepared from independent groups of 5 BALB/c mice at 72 hours after inoculation (inoculum of 1 × 106 PFUs). Bottom left panel shows the Mac-1 and Gr-1 staining pattern for this population, and the 3 adjacent panels show FCM analysis of GFP in quadrants R2, R3, and R4, as indicated by arrows. A solid line indicates the histogram for RM4503, and a dashed line indicates the histogram for control RQ461-infected cells. (C) FCM analysis of mck-mutant RM4511-infected FP infiltrates prepared from 5 BALB/c mice at 72 hours after inoculation (inoculum of 1 × 106 PFUs). Bottom left panel shows the Mac-1 and Gr-1 staining pattern for this population, and the 3 adjacent panels show FCM analysis for GFP in quadrants R2, R3, and R4. A solid line indicates the histogram for RM4511, and a dashed line indicates the histogram for control RM461-infected cells. The percentage of GFP+ cells is indicated above the brackets in each of the panels.
Acquisition of virus by Mac-1+/Gr-1+ cells. (A) ICA on total FP leukocytes collected from 10 mice at 48 hours after inoculation with RQ461 or RM461 (inoculum of 1 × 106 PFUs) shown as geometric mean percentage virus-positive FP cells ± standard deviation of the geometric mean (error bars). (B) FCM analysis of mck-expressing RM4503-infected infiltrates prepared from independent groups of 5 BALB/c mice at 72 hours after inoculation (inoculum of 1 × 106 PFUs). Bottom left panel shows the Mac-1 and Gr-1 staining pattern for this population, and the 3 adjacent panels show FCM analysis of GFP in quadrants R2, R3, and R4, as indicated by arrows. A solid line indicates the histogram for RM4503, and a dashed line indicates the histogram for control RQ461-infected cells. (C) FCM analysis of mck-mutant RM4511-infected FP infiltrates prepared from 5 BALB/c mice at 72 hours after inoculation (inoculum of 1 × 106 PFUs). Bottom left panel shows the Mac-1 and Gr-1 staining pattern for this population, and the 3 adjacent panels show FCM analysis for GFP in quadrants R2, R3, and R4. A solid line indicates the histogram for RM4511, and a dashed line indicates the histogram for control RM461-infected cells. The percentage of GFP+ cells is indicated above the brackets in each of the panels.
Time course of Mac-1+/Gr-1int cell appearance following MCMV infection. Leukocytes were prepared from FPs (A) and PB (B) of groups of 5 infected BALB/c mice on days 0, 1, 2, 3, and 5 after inoculation with mck-expressing RQ461 or mck-mutant RM461 (inoculum of 1 × 106 PFUs). Mac-1+/Gr-1int cell populations are indicated by the boxed areas. (B) An arrow in the day 3 sample identified the peak in mobilization of the Mac-1+/Gr-1int cells into the bloodstream. (C) Summary of the kinetics of mobilization of Mac-1+/Gr-1int cells into PB and recruitment into FPs.
Time course of Mac-1+/Gr-1int cell appearance following MCMV infection. Leukocytes were prepared from FPs (A) and PB (B) of groups of 5 infected BALB/c mice on days 0, 1, 2, 3, and 5 after inoculation with mck-expressing RQ461 or mck-mutant RM461 (inoculum of 1 × 106 PFUs). Mac-1+/Gr-1int cell populations are indicated by the boxed areas. (B) An arrow in the day 3 sample identified the peak in mobilization of the Mac-1+/Gr-1int cells into the bloodstream. (C) Summary of the kinetics of mobilization of Mac-1+/Gr-1int cells into PB and recruitment into FPs.
Finally, the cytologic appearance of FSClow/Mac-1+/Gr-1int cells recruited into FPs (Figure 6D) was similar to total Mac-1+/Gr-1int cells (Figure 2B). These properties suggested a more immature differentiation state than FSChigh/Mac-1+/Gr-1int cells (Figure 6E). A comparable population of Mac-1+/Gr-1int cells was present in PB (Figure 6F; data not shown). Mac-1+/Gr-1high cells from either PB or FPs had segmented or donut-shaped nuclei typical of neutrophils and their immediate progenitors (data not shown). Collectively, these data further support a role for MCK-2 in enhancing the recruitment of myelomonocytic cells with an immature phenotype to initial sites of infection.
Mac-1+/Gr-1int cells recruited by MCK-2 coexpress CD31 (PECAM) and heterogeneous levels of Sca-1, but not other monocyte or macrophage markers
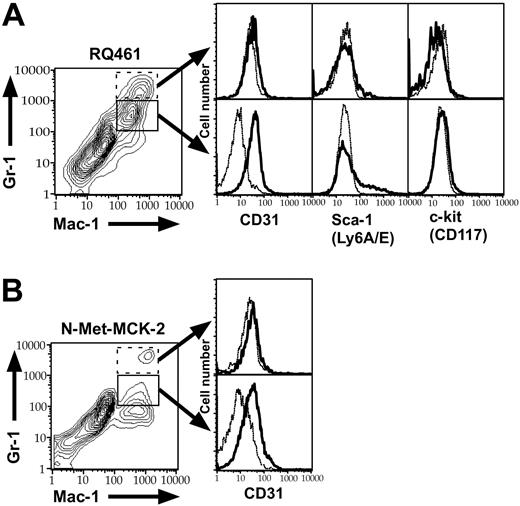
To further investigate the level of maturity of Mac-1+/Gr-1int cells recruited into PB and FPs, we performed FCM analysis for additional cell-lineage markers. In mck-expressing virus-infected mice, Mac-1+/Gr-1int cells coexpressed CD31 (platelet-endothelial-cell adhesion molecule-1; PECAM-1), as well as a heterogeneous pattern of the primitive hematopoietic-cell marker Sca-1 (Ly-6A/E) but not c-kit (CD117) or CD34 (Figure 7A; data not shown), consistent with these cells being myeloid progenitors.57 The heterogeneous presence of Sca-1 in the absence of more primitive markers suggested these were late in the myelomonocytic maturation pathway. mck-mutant virus recruited the same type of cell but at lower overall levels, consistent with the studies described in Figures 2 and 4, 5, 6 for Mac-1+/Gr-1+ cells. N-Met-MCK-2-recruited cells also coexpressed CD31 (Figure 7B) and variable levels of Sca-1 (data not shown). The Mac-1+/Gr-1int cell population that was detected in the bloodstream of mck-expressing virus-infected mice at day 3 after inoculation also was uniformly CD31+ and heterogeneous for Sca-1 (data not shown), further reinforcing the connection between MCK-2-mediated mobilization of late myelomonocytic progenitors (LMPs) into the bloodstream and appearance in tissues. Recruited CD31+/Mac-1+/Gr-1int cells lacked lineage markers of monocytes, macrophages, DCs, or neutrophils, including CD11c, CD205/DEC205, CD13, CD14, CD34, CD3-ϵ, CD4, CD8-α, CD45R/B220, F4/80, Mac-3, and MHC class II (I-A/E; data not shown). Mac-1+/Gr-1high neutrophils that were sometimes present in FPs lacked CD31 and Sca-1 (Figure 7A; data not shown), suggesting that these cells had migrated out of the bloodstream into tissues.58 Based on their light-scattering qualities, cytology, and lineage marker expression, CD31+/Mac-1+/Gr-1int MNCs were designated as LMPs. The uninfected cells mobilized at this early time point appear to be in the process of trafficking via the bloodstream to the FPs, where a small proportion acquire virus and appear as viremia that peaks at day 5 after inoculation.11,14
FCM light-scattering analysis and cytology of Mac-1+/Gr-1int cells. (A) FSC histograms of Mac-1+/Gr-1int (box) or Mac-1+/Gr-1high (broken box) cells collected from FPs of groups of 10 BALB/c mice on day 3 after inoculation with RQ461 or RM461 infection (inoculum of 1 × 106 PFUs). All histograms are from gated cell populations as indicated by boxed areas (i,ii,iii). (B) FSC histograms of MACS-enriched Mac-1+/Gr-1+ cells (boxed areas i and ii) from PB on day 3 or day 5 after inoculation with RQ461 (inoculum of 1 × 106 PFUs). (C) FCM analysis of BM cells from uninfected mice analyzed by FCM for Mac-1 and Gr-1 after gating on FSClow or FSChigh populations (boxed areas i and ii), indicating the percentage of total Mac-1+/Gr-1int cells (× 600) in each population. (D-F) May-Grunwald-Giemsa-stained cells from FPs or PB of groups of 20 mice at 48 hours after inoculation (inoculum of 1 × 106 PFUs), prepared as described for Figure 2. (D) FSClow/Mac-1+/Gr-1int cells from FPs. (E) FSChigh/Mac-1+/Gr-1int cells from FPs. (F) Mac-1+/Gr-1int cells from PB.
FCM light-scattering analysis and cytology of Mac-1+/Gr-1int cells. (A) FSC histograms of Mac-1+/Gr-1int (box) or Mac-1+/Gr-1high (broken box) cells collected from FPs of groups of 10 BALB/c mice on day 3 after inoculation with RQ461 or RM461 infection (inoculum of 1 × 106 PFUs). All histograms are from gated cell populations as indicated by boxed areas (i,ii,iii). (B) FSC histograms of MACS-enriched Mac-1+/Gr-1+ cells (boxed areas i and ii) from PB on day 3 or day 5 after inoculation with RQ461 (inoculum of 1 × 106 PFUs). (C) FCM analysis of BM cells from uninfected mice analyzed by FCM for Mac-1 and Gr-1 after gating on FSClow or FSChigh populations (boxed areas i and ii), indicating the percentage of total Mac-1+/Gr-1int cells (× 600) in each population. (D-F) May-Grunwald-Giemsa-stained cells from FPs or PB of groups of 20 mice at 48 hours after inoculation (inoculum of 1 × 106 PFUs), prepared as described for Figure 2. (D) FSClow/Mac-1+/Gr-1int cells from FPs. (E) FSChigh/Mac-1+/Gr-1int cells from FPs. (F) Mac-1+/Gr-1int cells from PB.
FCM analysis of Mac-1+/Gr-1int cells for CD31, Sca-1, and c-kit. FCM analysis of the FP infiltrates from groups of 10 infected BALB/c mice at 48 hours after inoculation with RQ461 (inoculum of 1 × 106 PFUs) or mice at 4 hours after the fourth N-Met-MCK-2 injection. (A) Histograms of Mac-1+/Gr-1int (solid box) or Mac-1+/Gr-1high population (dotted box) FP cells from RQ461-infected mice, showing expression levels of CD31 (PECAM-1), Sca-1 (Ly-6A/E), and c-kit (CD117). (B) Histograms of Mac-1+/Gr-1int (solid box) or Mac-1+/Gr-1high (dotted box) FP cells from N-Met-MCK2-treated mice showing expression levels of CD31 (PECAM-1). Background staining with an isotype antibody control is indicated as a dashed line in each histogram.
FCM analysis of Mac-1+/Gr-1int cells for CD31, Sca-1, and c-kit. FCM analysis of the FP infiltrates from groups of 10 infected BALB/c mice at 48 hours after inoculation with RQ461 (inoculum of 1 × 106 PFUs) or mice at 4 hours after the fourth N-Met-MCK-2 injection. (A) Histograms of Mac-1+/Gr-1int (solid box) or Mac-1+/Gr-1high population (dotted box) FP cells from RQ461-infected mice, showing expression levels of CD31 (PECAM-1), Sca-1 (Ly-6A/E), and c-kit (CD117). (B) Histograms of Mac-1+/Gr-1int (solid box) or Mac-1+/Gr-1high (dotted box) FP cells from N-Met-MCK2-treated mice showing expression levels of CD31 (PECAM-1). Background staining with an isotype antibody control is indicated as a dashed line in each histogram.
Recruitment of Mac-1+/Gr-1int cells and dissemination to SGs proceed normally in mice deficient in CCR2 and MCP-1. Because peak response of Mac-1+/Gr-1int cells was observed by day 3 after inoculation, we sought to assess the contribution of inflammatory monocyte/macrophage migration in MCK-2-enhanced recruitment of myelomonocytic cells and dissemination properties of MCMV. Mice deficient in MCP-1 (JE, CCL2)48 or CCR249 have fully developed myeloid-cell compartments but fail to mobilize monocytes or activated macrophages in response to thioglycolate injection.48,49 These mice are much more susceptible to bacterial infection and fail to sustain chronic inflammatory responses.59 By employing such mice, we sought to avoid nonspecific consequences of chemical depletion methods23,26,27 and focus on the relative contribution of immature and mature cells in the myelomonocytic lineage.
First, we confirmed a 5- to 10-fold defect in macrophage recruitment 72 hours after thioglycolate injection into MCP-1-/-, CCR2-/-, and cross-bred MCP-1-/- × CCR2-/- mice (data not shown). Following inoculation with MCMV, replication in PB and organs of these mice (Supplemental Figures S2A,B and S3A,B) was the same as control mice, suggesting that MCP-1/CCR2-dependent monocyte/macrophage migration did not contribute to infection. There was a 10- to 100-fold lower level of viremia at day 5 and a 100- to 1000-fold lower level of virus in the SGs at day 14 after inoculation in mck-mutant virus-infected CCR2-/- or MCP-1-/- mice, exactly the same as control mice (Supplemental Figures S2A and S3D; data not shown). MCMV-mediated recruitment of PEC macrophages at 96 hours after inoculation20,26 was severely compromised in both MCP-1-/- and CCR2-/- mice compared with control mice, even though overall numbers of infectious virus-positive cells remained similar (Supplemental Figure S3C; data not shown). Thus, all characteristics of viremia and dissemination were similar to patterns that had been previously characterized in BALB/c mice.14 Thus, chemokine-mediated monocyte/macrophage migration via MCP-1 and CCR2 are neutral host factors for pathogenesis of MCMV.
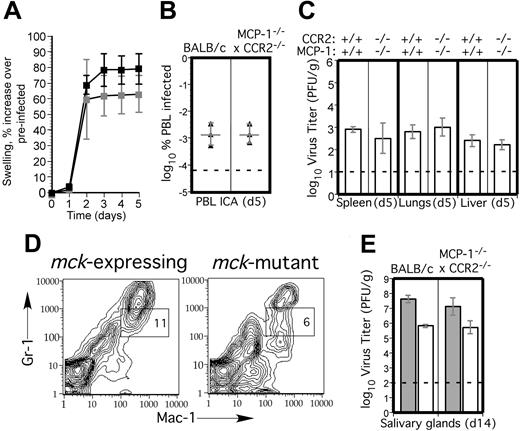
To control for the possibility that an alterative MCP-1 receptor or minor CCR2 ligand59 influenced these results, we crossed MCP-1-/- with CCR2-/- mice and tested the double-deficient mice for susceptibility to MCMV. Again, as for singly deficient mice, aspects of MCMV pathogenesis such as virus-induced FP swelling (Figure 8A), peak viremia (Figure 8B), and peak replication levels in SP, liver, and lungs (Figure 8C) were indistinguishable from BALB/c mice. Mac-1+/Gr-1int cell recruitment into FPs also was normal in MCP-1-/- CCR2-/- mice, and reduced cell recruitment by mck-mutant virus was preserved (Figure 8D). Taken together, these observations lend support to a role for Mac-1+/Gr-1int myeloid-cell recruitment in MCMV infection and show that recruitment of these cells is independent of monocyte/macrophage migration either in the presence or absence of MCK-2. Importantly, reduced dissemination of mck-mutant virus to salivary glands also was preserved in MCP-1-/- CCR2-/- mice (Figure 8E), showing that chemokine recruitment is not involved in myeloid-cell trafficking in MCMV dissemination or in host innate immune control. These data suggest that macrophages do not play a direct role in clearance and, most importantly, implicate a less mature cell or a different cell lineage than monocyte/macrophages as the vehicle for viral dissemination.
MCMV replication and dissemination in MCP-1-/- CCR2-/- mice. (A) Time-course analysis of FP swelling in 5 BALB/c (▪) and 5 MCP-1-/- CCR2-/- (▦) mice following inoculation with 1 × 106 PFUs of mck-expressing RM427+.14 The mean and standard deviation (error bars) are indicated. (B) Viremia levels determined by ICA on groups of 3 mice at day 5 after inoculation (intraperitoneal route) with 105 PFUs of wild-type RQ461 in BALB/c and MCP-1-/- CCR2-/- mice. Mean (vertical line) and standard deviation (error bars) are indicated, along with data points from individual mice. (C) Titers of virus in spleen, lungs, and liver of RQ461 in BALB/c and MCP-1-/- CCR2-/- mice (same infection as panel A). (D) FCM analysis of Mac-1+/Gr-1int myeloid cells recovered from FP of MCP-1-/- CCR2-/- mice. FP leukocytes were prepared from groups of 3 mice at day 5 after inoculation with mck-expressing RM427+ or mck-mutant RM461 lacZ-tagged viruses (inoculum of 1 × 106 PFUs). FCM analysis and layout is described in the legend to Figure 2. (E) Day 14 virus titers in SGs following inoculation (FP route) with 107 PFUs of RQ461 (▦) or RM461 (□).
MCMV replication and dissemination in MCP-1-/- CCR2-/- mice. (A) Time-course analysis of FP swelling in 5 BALB/c (▪) and 5 MCP-1-/- CCR2-/- (▦) mice following inoculation with 1 × 106 PFUs of mck-expressing RM427+.14 The mean and standard deviation (error bars) are indicated. (B) Viremia levels determined by ICA on groups of 3 mice at day 5 after inoculation (intraperitoneal route) with 105 PFUs of wild-type RQ461 in BALB/c and MCP-1-/- CCR2-/- mice. Mean (vertical line) and standard deviation (error bars) are indicated, along with data points from individual mice. (C) Titers of virus in spleen, lungs, and liver of RQ461 in BALB/c and MCP-1-/- CCR2-/- mice (same infection as panel A). (D) FCM analysis of Mac-1+/Gr-1int myeloid cells recovered from FP of MCP-1-/- CCR2-/- mice. FP leukocytes were prepared from groups of 3 mice at day 5 after inoculation with mck-expressing RM427+ or mck-mutant RM461 lacZ-tagged viruses (inoculum of 1 × 106 PFUs). FCM analysis and layout is described in the legend to Figure 2. (E) Day 14 virus titers in SGs following inoculation (FP route) with 107 PFUs of RQ461 (▦) or RM461 (□).
Discussion
We have shown that the virus-encoded chemokine MCK-2 exploits the host by inducing the mobilization and migration of LMPs, which are late-stage, immature myelomonocytic cells. Based on markers we employed, LMPs appear to be more mature MNCs than common myeloid progenitors or granulocyte-macrophage progenitors.60 Our previous studies of mck-expressing and -mutant viruses, together with our work on mice deficient in CCR2 and MCP-1, revealed no impact of MCK-2 on innate or adaptive immune clearance of MCMV,2,11,14 suggesting that LMPs are not responsible for immune control even though they constitute a component of the natural innate response.39,41 MCMV recruitment of LMPs starting between day 1 and day 2 after infection is enhanced by MCK-2 expression at sites of infection and increases the mobilization when BM-resident late myeloid progenitors become mobilized. These leukocytes then become infected in an MCK-2-enhanced fashion, either following transendothelial migration or by coming into contact with virus-infected endothelial cells that would line the vasculature in the initial site of infection. Endothelial cells play an important role during MCMV infection. In either scenario, we believe virus-positive LMPs or more mature macrophages or DCs derived from LMPs mediate efficient dissemination by traversing the bloodstream to SGs, where virus replication leads to shedding in saliva. Thus, MCK-2 likely provides a strong evolutionary benefit by increasing the level of virus shedding, thereby maximizing viral spread within a population.
Somewhat surprisingly, we failed to detect any contribution of CCR2-mediated monocyte/macrophage migration to either dissemination or control of MCMV infection. Macrophages have long been implicated in the innate phase of viral clearance,20,26 however, their role is less well understood here than in clearance of intracellular bacterial infections, where CCR2-mediated recruitment of PB monocytes into tissue macrophages is crucial to immune control during both the innate and adaptive phases of the immune response.59 MCMV infection of macrophages has been reported to be differentiation dependent and to potentially alter the innate or adaptive immune response.3,23,28 Interferon-γ has been implicated in control of MCMV replication,3 particularly in clearance from SGs, however, our results suggest that control of infection through day 35 after inoculation in this organ is independent of the activated macrophage response. Although the role of the MCP-1/CCR2 system in the adaptive immune response has not been subjected to detailed study, our failure to observe any differences in viral replication levels in combined MCP-1- and CCR2-deficient mice suggests a subtle, if any, impact on MCMV clearance. MCK-2-recruited LMPs exhibit a phenotype ascribed to myeloid suppressors61 ; however, clearance of mck-expressing and mck-mutant viruses is comparable in all tissues examined, even when there is a dramatic difference in the levels of LMPs and inflammation.14 Thus, recruitment of these PB MNCs into tissues has no quantitative impact on viral clearance.
By producing a chemokine-like gene product, MCMV increases the recruitment of a subset of LMPs. The cells that are enhanced by MCK-2 are mobilized from BM less efficiently in the absence of MCK-2, suggesting that LMP mobilization is a common characteristic of the host response to infection. Once mobilized from the BM, LMPs transit the bloodstream and arrive at initial sites of infection. MCK-2 induces a stronger and more durable mobilization that contributes to greater numbers of immature myeloid cells in PB as well as at initial sites of infection. Myeloid progenitor cells have been shown to be mobilized from BM in many immunomodulatory settings such as cancer,61 irradiation,62 and administration of immunosuppressants such as cyclophosphamide63,64 or cytokines such as G-CSF, M-CSF, and GM-CSF.18,65 In all of these settings, an immature (CD31+/Mac-1+/Gr-1+) myeloid cell similar to that characterized here has been detected.18,61,66,67 This cell population may be related to preimmunocytes,18 which have been suggested to be a progenitor of monocytes, DCs, or other MNCs and shown to give rise to interferon-producing cells. Our investigation extends the mobilization of BM-derived myeloid progenitors to a herpesvirus infection and identifies MCK-2 mobilization of these cells as a viral means of enhancing a natural response that provides potential vehicles for viral dissemination. These results have implications well beyond control and dissemination of MCMV and suggest a means of hematogenous spread for other intracellular pathogens. In addition to seeding SGs, CD31+/Mac-1+/Gr-1int cells may mediate the dissemination of virus to BM25,45 or other sites, particularly under the low-dose conditions that would be encountered during natural infection. The initial attraction of this cell type may assure that a latent reservoir is established in myeloid cells.68
Virus-encoded MCK-2 appears to systemically mobilize a natural population of CD31+/Mac-1+/Gr-1int cells that may mature into macrophages or DCs through normal host pathways.69 Maturation to macrophages appears to be expendable, which leaves the possibility that the virus relies on immature cells or a DC. While MCK-2 and LMPs have to be evaluated further to understand the role of myeloid-cell differentiation in viral dissemination, cells that are recruited in response to MCK-2 do not appear to be stem cells18,70 or otherwise characterized immature myeloid-cell types.60 The peak in PB mobilization we have identified corresponds to times after infection when primary viremia occurs15,17 and when virus reaches maximal levels in SP, lungs, and liver.14 During secondary viremia, virus-positive MNCs are present in the PB by day 5 of infection and reach higher levels in the presence of MCK-2,11,16 suggesting that the major benefit of mobilization is the increased availability of vehicles for viral dissemination. Reduced mobilization of LMPs in the absence of MCK-2 results in reduced levels of peak viremia as well as in reduced dissemination to SGs detected as early as day 7 after infection and continuing for several weeks. The preferential recruitment and infection of a CD31+/Mac-1+/Gr-1int population represents a unique viral strategy for attracting myelomonocytic cells from bone marrow that may retain recirculation capabilities that enable cells to become infected at a portal of entry and transit via the bloodstream to SGs.
Prepublished online as Blood First Edition Paper, July 26, 2005; DOI 10.1182/blood-2005-05-1833.
Supported by a Japan Herpesvirus Infection Forum Scholarship Award in Herpesvirus Infection Research (S.N.) and by grants from the U.S. Public Health Service National Institutes of Health (KO8 AI016838 [S.A.A.], RO1 AI30363 and RO1 AI33852 [E.S.M.]).
S.N. performed analyses shown in Figures 2, 4, 5, 6, and 7, as well as the FCM profile in Figure 8, and wrote the first draft of the manuscript. S.A.A. completed all of the first runs and design of the experiments shown and did the majority of the work shown in Figure 3. She also revised the manuscript. A.B. assisted S.N. with the FCM. J.M.B. supervised A.B., provided expertise on myeloid progenitors, and revised the manuscript. J.H. performed mouse work, assembled the data in Figure 8, and assisted S.N. and S.A.A. with the manipulations of mice and viruses. T.S. performed experiments on bone marrow cells and provided experimental advice. E.S.M. oversaw the project, conceptualized the problem, guided the experiments, and wrote the final draft as well as the revision of the manuscript.
S.N. and S.A.A. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Thomas Schall and Mark Penfold, ChemoCentryx, Inc, for providing N-Met-MCK-2, IL-8, vCXCL1, and C10 and for many discussions; Dr Izzi Charo, Gladstone Institute, Univerity of California San Francisco, for providing CCR2-deficient mice; and Dr Barrett Rollins, Harvard Medical School, for providing MCP-1-deficient mice. We thank Yin Dong for cell culture; Dr Donna Bouley, Dr Hamish Smith, and lab members for discussion; and Dr Yasuhiro Koga and Dr Kazuo Tanaka for encouragement. The authors have no conflicting financial interests.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal