Abstract
CD163 mediates the internalization of hemoglobin-haptoglobin (Hb-Hp) complexes by macrophages. Because Hp binding capacity is exhausted during severe hemolysis, an Hp-independent Hb-clearance pathway is presumed to exist. We demonstrate that Hb interacts efficiently with CD163 in the absence of Hp. Not only is Hb internalized into an endosomal compartment by CD163 as a result of active receptor-dependent endocytosis; it also inhibits the uptake of Hb-Hp complexes, suggesting a common receptor-binding site. Free Hb further induces heme oxygenase mRNA expression in CD163+ HEK293 cells, but not in CD163- cells. Additional evidence for Hp-independent Hb-CD163 interaction is provided by the demonstration that CD163 mediates the uptake of αα-DBBF crosslinked Hb, a chemically modified Hb that forms minimal Hp complexes. Moreover, certain modifications to Hb, such as polymerization or the attachment of specific functional groups (3 lysyl residues) to the β-Cys93 can reduce or enhance this pathway of uptake. In human macrophages, Hp-complex formation critically enhances Hb uptake at low (1 μg/mL), but not at high (greater than 100 μg/mL), ligand concentrations, lending support for a concentration-dependent biphasic model of macrophage Hb-clearance. These results identify CD163 as a scavenger receptor for native Hb and small-molecular-weight Hb-based blood substitutes after Hp depletion.
Introduction
Heme, which is mainly derived from hemoglobin (Hb), is a strong oxidant and has potent pro-inflammatory properties. These properties become apparent if the intricate intra-erythrocytic compartmentalization of heme is compromised after the destruction of erythrocytes.1-4 Large quantities of free hemoglobin can be found in the circulation of patients who have undergone transfusion with cell-free hemoglobin products as a blood substitute.5 Macrophages are the primary scavengers of Hb after systemic hemolysis and during wound healing. These cells also play a key role in the clearance of exogenously administered blood substitutes.6
CD163 is a member of the cysteine-rich scavenger receptor family and is exclusively expressed by cells of monocyte/macrophage lineage.7 Resident tissue macrophages contain the highest levels of CD163, most notably Kupffer cells in the liver and macrophages within the bone marrow and spleen red pulp.8-10 To date, the Hb-haptoglobin (Hp) complex is the only known ligand of CD163,11,12 and neither Hp alone nor free Hb has been found to display high-affinity binding to the receptor. Because the Hb-Hp complex binds to CD163 with high affinity and the receptor system has a high endocytotic capacity, CD163 is thought to mediate the clearance of Hb-Hp complexes from the blood.13
Several lines of evidence indicate that CD163 plays a key role in the anti-inflammatory and wound-healing process. First, there is a high level of CD163 expression by macrophages during the down-regulatory phase of inflammatory reactions.8,14 Second, CD163 expression is strongly induced by glucocorticoids15,16 and by the prototypic anti-inflammatory cytokine interleukin-10.17 Accordingly, we have recently demonstrated that glucocorticoid-induced CD163 expression in macrophages enhances their capacity to bind and internalize Hb-Hp complexes.16 Removal of prooxidant and pro-inflammatory Hb may, in fact, be an important function of macrophages during intravascular hemolysis and on the local release of large quantities of free Hb at sites of injury. However, the rapid decline that occurs in Hp binding capacity during hemolytic anemia,18-20 malaria,21,22 and local extravascular Hb release suggests the existence of a highly efficient, but Hp-independent, Hb clearance pathway. Neither Hp knockout mice23,24 nor humans with anhaptoglobinemia25 display compromised plasma Hb clearance, and the cellular distribution of Hb uptake in liver and spleen is not changed during hemolysis in haptoglobin deficiency,26 providing additional evidence of such a pathway.
The current study examined the CD163-dependent uptake of Hb in greater detail. Our results are consistent with a biphasic model of macrophage-mediated Hb clearance. In this model, small amounts of free Hb are removed by the well-characterized, high-affinity interaction of CD163 with the Hb-Hp complex.11,12 However, once plasma Hp is depleted, CD163-mediated uptake of free Hb is maintained through an apparently lower-affinity, Hp-independent interaction between free Hb and the macrophage scavenger receptor CD163.
Materials and methods
Cell culture
Human embryonic kidney cells (HEK293; Invitrogen, Basel, Switzerland) were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen) containing 10% fetal calf serum (FCS; Invitrogen). The total Hp concentration in the complete culture medium was determined with a recently described bovine-Hp-specific enzyme-linked immunosorbent assay (ELISA)27 and was 0.29 μg/mL. The total free Hb concentration was estimated by spectrophotometry (A540) and was approximately 5 μg/mL. Therefore, no free Hp-binding capacity is expected to be present under these conditions. Cells that had been stably transduced with lentiviral vectors (control or CD163) were propagated in the presence of 5 μg/mL blasticidin (Invitrogen). Blasticidin was removed from cultures at least one cell passage before use in experiments.
Human macrophages were prepared from buffy coats of healthy blood donors (blood donation program; Swiss Red Cross, Zurich, Switzerland), as described previously.9 Cells were cultured in Iscove modified Dulbecco medium (IMDM; Invitrogen) supplemented with 20% heat-inactivated, pooled human serum and 20 μg/mL gentamicin (Sigma, Buchs, Switzerland). The medium was replaced on days 1 and 4. On day 8, the medium was changed to IMDM containing 10% FCS. During this time, the differentiated macrophages took on a uniform, egg-shaped morphology. Experiments were performed on day 10. Alternatively, monocytes were incubated for 36 hours with dexamethasone (Sigma) at a concentration of 2.5 × 10-7 M to achieve maximal CD163 expression.16
Generation of a CD163-expressing lentiviral vector and establishment of the CD163-expressing HEK293 cell line
The full-length open reading frame of human CD163 was amplified from a cDNA clone (OriGene Technologies, Rockville, MD) using a standard polymerase chain reaction (PCR) and the following primers: forward, 5′-CACCTCTTTGGAATGAGCAAACTCAG-3′; reverse, 5′-TATTCCTCTGCATGGTTCTTTC-3′. The amplification product was cloned into the pLenti6/V5-DEST lentiviral vector (Invitrogen), and vector integrity was verified by nucleotide sequencing. Replication-incompetent lentivirus was obtained by cotransfection of the 293FT producer cell line with the CD163-pLenti6/V5-DEST and the Lentiviral Support Kit (Invitrogen). A CD163-expressing HEK293 cell line was established by transduction with viral supernatant and subsequent selection with blasticidin (Invitrogen). To obtain a uniform population of CD163-expressing cells, cells were labeled with fluorescent Hb-Hp for 60 minutes and separated by fluorescence-activated cell sorting (FACS; FACSAria; Becton Dickinson, Basel, Switzerland). After cell sorting, the cells were passaged at least 5 times before experiments were performed. CD163 expression was confirmed by quantitative real-time reverse transcription PCR (RT-PCR) and immuofluorescence staining with the monoclonal antibody clone 5C6-FAT (BMA, Augst, Switzerland), as previously described.16,28
Quantification of Hb-Hp uptake by macrophages and HEK293 cells
HbA0 (Sigma) or Hp phenotype 2-2 (Sigma) were labeled with the Alexa-488, Alexa-633, or Alexa-647 protein labeling kit (Molecular Probes, Eugene, OR), as described previously.16 To quantify Hb or Hb-Hp uptake, cells were incubated with the respective ligands in an incubator at 37°C, 5% CO2, and 95% humidity. All uptake assays were performed in cell culture medium without serum. Hb-Hp complexes were generated by combining Hb and Hp at a 1:1 ratio (wt/wt) 10 minutes before experimentation. Molar concentrations of tetrameric Hb species are indicated as the respective amount of Hb dimers, and concentrations of Hb-Hp complexes are given with respect to the Hb dimer concentration. Ca2+ concentrations of less than 0.2 mM led to Hb-Hp dissociation from CD163.12 Therefore, after incubation with ligand, the cells were trypsinized and washed 3 times with cold Ca2+-free phosphate-buffered saline (PBS) supplemented with EDTA (ethylenediaminetetraacetic acid) to remove noningested Hb-Hp complexes. Uptake of the fluorescent ligand was then quantified by flow cytometry using a FACScalibur (Becton Dickinson) equipped with a 488-nm argon laser and a 633-nm He-Ne laser. The flow cytometer was calibrated daily according to the manufacturer's instructions. Nonspecific fluorescence was determined by adding a 1000-fold excess of unlabeled ligand, and the values obtained were subtracted from experimental samples. Polyclonal rabbit anti-human CD163 IgG, which has previously been demonstrated to specifically inhibit the CD163 Hb-Hp interaction, was kindly provided by Soren K. Moestrup (Aarhus, Denmark).11 The nonblocking anti-CD163 antibodies RM3/1 and 5C6-FAT were obtained from BMA. Immunofluorescence staining of nontagged Hb was performed with a rabbit polyclonal antibody against human Hb (IgG fraction; Abcam, Cambridge, United Kingdom) and Alexa-586 goat-antirabbit secondary antibody (Molecular Probes). For fluorescence microscopy, z-staples were recorded using a motorized Axioskop 2 epifluorescence microscope equipped with an oil-immersion 100×/1.3 objective lens, an AxioCam MR digital camera, and AxioVision software (Zeiss, Feldbach, Switzerland). Z-staples were deconvoluted using a constrained iterative algorithm and were visualized with AxioVision Inside4D software (Zeiss). Some samples were analyzed as 0.2-μm-thick optical sections using a confocal laser-scanning microscope (CLSM; Leica, Heidelberg, Germany) and Imaris 3.0 software (Bitplane AG, Zürich, Switzerland). Final images were prepared using Photoshop software (Adobe Systems, San Jose, CA).
RNA isolation and quantitative real-time reverse transcription PCR
Total cellular RNA was isolated using the QIAgen RNAeasy Mini Kit (Qiagen, Basel, Switzerland) which included a DNAse digest. Total RNA was quantified spectrophotometrically, and equal amounts of RNA (5 μg) were reverse transcribed into cDNA with oligo(d)T primers and M-MuLV Reverse Transcriptase using the ProSTAR First Strand Kit (Stratagene, Rotkreuz, Switzerland). Duplicates of cDNA were amplified by RT-PCR with gene-specific primers using the LightCycler System (Roche Diagnostics, Basel, Switzerland) and the Fast Start DNA Master SYBR Green I (Roche Diagnostics), as described previously.9 Sequence-specific primers were selected using PrimerDesign software (Roche Diagnostics), taking into account the specific chemical conditions in the LightCycler System so as to prevent primer-dimer formation during amplification. The following primers were used for heme oxygenase (HO-1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH): HO-1 forward, 5′-AGGGTGATAGAAGAGGCCAAGACT-3′; HO-1 reverse, 5′-TTCCACCGGACAAAGTTCATGGC-3′; GAPDH forward, 5′-AACAGCGACACCCACTCCTC-3′; GAPDH reverse, 5′-GGAGGGGAGATTCAGTGTGGT-3′. PCR was carried out with an initial denaturation step (10 minutes, 95°C) followed by 42 cycles of denaturation (15 seconds, 95°C), annealing (10 seconds, 67°C-55°C), and extension (12 seconds, 72°C). Fluorescence was measured at the end of each extension. Relative mRNA levels were quantified from a standard curve that was included in each assay. Data were analyzed using LightCycler (Roche Diagnostics) analysis software version 3.5. The expression level of heme oxygenase (HO-1) mRNA was normalized to GAPDH levels in each experimental sample. Final data were expressed as mRNA expression in treated cells relative to the expression level in untreated cells. A melting curve analysis was performed for each amplicon to determine the specificity of each amplification, and the amplification products were sequenced to determine target specificity (Microsynth, Switzerland).
Modified hemoglobin solutions
Highly purified human Hb (HbA0) was obtained from Hemosol (Mississauga, Ontario, Canada). Bis(3,5-dibromosalicyl) fumarate αα crosslinked human Hb (αα-DBBF Hb) was a kind gift from the United States Army. The covalent fumaryl link between the α-globin chains at the lysine 99 position prevents dissociation of the Hb tetramer into αβ-dimers. Oxyglobin (Biopure, Cambridge, MA) is a Food and Drug Administration (FDA)-approved blood substitute for veterinary use in canine anemia. It is produced by reacting purified bovine Hb (HbBv) with glutaraldehyde. This nonspecific crosslinking and polymerization reaction generates a wide distribution of Hb species with molecular weights (MWs) from approximately 87 kDa to 500 kDa. Purity of all Hb preparations was confirmed by analysis on a Bioanalyzer 2100 using protein LabChips (Agilent Technologies, Basel, Switzerland).
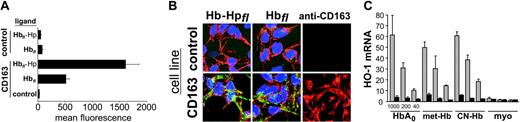
CD163 mediates Hp-independent uptake of free Hb in HEK293 cells. (A) CD163+ (CD163) and CD163- (control) HEK293 cells were incubated with 10 μg/mL Alexa-488-labeled Hbfl or Hbfl-Hp complexes for 30 minutes (fl indicates the fluorescence protein). After washing and trypsinization, cell-associated fluorescence was determined by FACS analysis. Although only minimal Alexa-488 fluorescence was detected in the CD163- cell line (control), significant fluorescence, above background levels, was detected in the CD163+ cells. Data represent mean ± SD of 3 independent experiments. (B) Confocal fluorescence microscopy images (original magnification, 400 ×)of CD163+ (CD163) and CD163- (control) cells were acquired after 30-minute incubation of cells with 10 μg/mL Hbfl or Hb-Hpfl. The pattern of green Alexa-488 fluorescence demonstrates that free Hbfl and Hb-Hpfl complexes are taken up into an intracellular endosomal compartment, and this is mediated by CD163 (green, Alexa-488 Hb; blue, DAPI nuclear staining; red, actin filaments). Immunofluorescent staining of CD163 with anti-CD163 (clone 5C6-FAT) and Alexa-594 (red) goat antimouse antibody was performed to confirm receptor expression in CD163-transformed HEK293 cells (right panels). (C) CD163+ and CD163- HEK293 cells were incubated for 8 hours with the indicated CD163 ligands in DMEM without added serum (to avoid nonspecific protein-protein interactions). Each ligand was applied at 3 different concentrations: 1000, 200, and 40 μg/mL. HO-1 mRNA induction relative to nontreated samples was determined by quantitative real-time RT-PCR and was corrected for differences in glyceraldehyde-3-phosphate dehydrogenase mRNA. Results are expressed as mean ± SD of 3 independent experiments. HbA0 indicates native human hemoglobin; CN-Hb, hemoglobin blocked with cyanide; myo, myoglobin.
CD163 mediates Hp-independent uptake of free Hb in HEK293 cells. (A) CD163+ (CD163) and CD163- (control) HEK293 cells were incubated with 10 μg/mL Alexa-488-labeled Hbfl or Hbfl-Hp complexes for 30 minutes (fl indicates the fluorescence protein). After washing and trypsinization, cell-associated fluorescence was determined by FACS analysis. Although only minimal Alexa-488 fluorescence was detected in the CD163- cell line (control), significant fluorescence, above background levels, was detected in the CD163+ cells. Data represent mean ± SD of 3 independent experiments. (B) Confocal fluorescence microscopy images (original magnification, 400 ×)of CD163+ (CD163) and CD163- (control) cells were acquired after 30-minute incubation of cells with 10 μg/mL Hbfl or Hb-Hpfl. The pattern of green Alexa-488 fluorescence demonstrates that free Hbfl and Hb-Hpfl complexes are taken up into an intracellular endosomal compartment, and this is mediated by CD163 (green, Alexa-488 Hb; blue, DAPI nuclear staining; red, actin filaments). Immunofluorescent staining of CD163 with anti-CD163 (clone 5C6-FAT) and Alexa-594 (red) goat antimouse antibody was performed to confirm receptor expression in CD163-transformed HEK293 cells (right panels). (C) CD163+ and CD163- HEK293 cells were incubated for 8 hours with the indicated CD163 ligands in DMEM without added serum (to avoid nonspecific protein-protein interactions). Each ligand was applied at 3 different concentrations: 1000, 200, and 40 μg/mL. HO-1 mRNA induction relative to nontreated samples was determined by quantitative real-time RT-PCR and was corrected for differences in glyceraldehyde-3-phosphate dehydrogenase mRNA. Results are expressed as mean ± SD of 3 independent experiments. HbA0 indicates native human hemoglobin; CN-Hb, hemoglobin blocked with cyanide; myo, myoglobin.
Preparation of βCys93 αα-DBBF Hb multiple peptide conjugate (αα-DBBF Hb-MPC)
Modification of the βCys93 of αα-DBBF Hb was performed according to Boykins et al.29 The purified di-amino bromoacetyl peptide (H-Lys(Lys)2 (COCH2Br)2) was analyzed by LC-ESI-tandem mass spectrometry (LCQ Deca; Thermo-Finnigan, San Jose, CA); this gave a monoisotopic mass consistent with the expected product mass ([MH]1+, m/z = 644.17). H-Lys(Lys)2 (COCH2Br)2 was then reacted with αα-DBBF Hb in a ratio of 5:1 (H-Lys(Lys)2 (COCH2Br)2:αα-DBBF Hb) for 45 minutes followed by quenching and purification. This reaction is highly specific for cysteine residues; in the case of αα-DBBF Hb, the highly accessible surface area of βCys93 lends itself to modification. Using matrix-assisted laser desorptionionization mass spectrometry (MALDI-MS; Applied Biosystems, Framingham, MA) modifications to the β-globin chains by 484 mass units were confirmed (ie, loss of 2 bromine atoms from H-Lys(Lys)2 (COCH2Br)2). The structures of each component are shown in Figure 3A.
Purification of Oxyglobin fractions
The heterogeneous mixture that comprises Oxyglobin (Biopure) was separated into 4 distinct and homogeneous fractions using a Bio-Sil-TSK-250 (600 mm × 7.5 mm) exclusion column (Bio-Rad Laboratories, Hercules, CA) attached to a Waters 626 pump and a Waters 2487 dual wavelength detector, all controlled by a Waters 600s controller (Waters, Milford, MA) using Millenium32 software. The running buffer, 0.1 M NaH2PO4 (pH 6.5), was pumped at a rate of 0.5 mL/min; absorbance was monitored at 214 and 280 nm. Fractions were collected on a Spectra/Chrom CF-1 fraction collector (Spectrum Laboratories, Rancho Dominguez, CA). Each fraction collected was buffer-exchanged with five 10-mL volumes of 0.9% NaCl and concentrated using 30-kDa cut-off centrifuge tubes (Centricon YM30; Millipore, Bedford, MA). Fraction homogeneity was analyzed initially by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then, more specifically, multiangle laser light scattering size-exclusion chromatography (SEC-MALLS) was used to analyze the average MW number (Mn), weighted average MW, and polydispersity (Mw/Mn). SEC-MALLS analysis showed that fractions 1 to 4 have MWs of 502, 272, 189, 87 kDa, respectively and that HbBv has an MW of 62 kDa. MW/Mn values indicated nearly monodispersed MW distributions (MW/Mn, approximately 1.0) within each fraction. These values were 1.022, 1.007, 1.008, 1.008, and 1.005 for fractions 1 to 4 and HbBv, respectively.
Preparation and separation of hemoglobin CNBr digest fragments
HbA0 was digested with cyanogen bromide (CNBr) (Sigma-Aldrich Chemical, St Louis, MO). Briefly, CNBr was prepared in 70% formic acid and added to HbA0 in sufficient quantity to make up a 50-fold molar excess of the methionine content within HbA0. The mixture was allowed to react at ambient temperature for 16 hours, after which the mixture was frozen at -80°C and lyophilized. The lyophilized HbA0 peptide mixture was dissolved in 0.1% trifluoroacetyl acid (TFA) and separated using a Vydac protein/peptide (250 mm × 4.6 mm) analytic C18 column (Vydac, Columbia, MD). The Waters HPLC system, described previously, was used to pump a gradient of 0.1% TFA and 100% acetonitrile at a rate of 1 mL/min, increasing acetonitrile from 0% to 70% over 50 minutes. Peptide separation was monitored at a wavelength of 214 nm, and like peaks were pooled over several 1-column injections. Collected peptides were identified by MALDI-MS with comparison of experimental and theoretic MS data to confirm the identity of peptides.
Statistical analysis
Results are expressed as mean plus or minus SD and are derived from multiple independent experiments, as indicated in the figure legends. Error bars were omitted if SDs were too small to be properly displayed. Data were analyzed by one-way analysis of variance (ANOVA), and appropriate posttests were carried out using GraphPad Prism 4.0 software. P values of less than .05 were accepted as statistically significant.
Results
CD163 mediates endocytosis of free Hb in the absence of Hp
We used CD163-transduced HEK293 cells and fluorescence ligand assays to study the interaction of CD163 with purified hemoglobin. In accordance with earlier studies, CD163-expressing cells, but not CD163- parental or mock-transduced cells, avidly ingested fluorescently labeled Hb complexed with Hp. A lower, but significant, uptake of Hb was also observed in the absence of Hp. No uptake of free Hb was observed in the CD163- cell line (Figure 1A). Confocal laser-scanning microscopy confirmed that both ligands, Hb and Hb-Hp, not only are bound to the surfaces of the CD163+ cells, they are also subsequently taken up into an intracellular compartment (Figure 1B). Fluorescent Hp and high-density lipoprotein (Alexa-488 HDL) were used as probes to evaluate any CD163-related changes in non-specific, fluid-phase pinocytosis. No significant uptake of these proteins was detected in either CD163+ or CD163- HEK293 cells after 60 minutes of incubation at a concentration of 50 μg/mL (data not shown).
CD163-dependent, but Hp-independent, induction of HO-1 by free Hb
Transcriptional induction of the inducible heme-metabolizing enzyme HO-1 was used as an indirect measure of Hp-independent Hb uptake by CD163. Both Hb and Hb-Hp increased HO-1 mRNA levels in CD163+, but not in CD163-, cell lines. CD163-dependent induction of HO-1 mRNA was significantly higher in cultures treated with 1 mg/mL free Hb than in those treated with 1 mg/mL Hb-Hp (25.9 ± 6.2-fold vs 10.2 ± 2.4-fold induction at 8-hour incubation; P < .01).
Given that auto-oxidation of native Hb and subsequent heme release could occur during prolonged incubation in cell culture medium, we compared the level of HO-1 expression induced by native Hb, met-Hb, and CN-met-Hb (Figure 1C). All these hemoglobins induced comparable levels of HO-1 in CD163+, but not in CD163-, cells. Expression of HO-1 was dose dependent in all Hb solutions. Because the heme in CN-met-Hb is irreversibly blocked by a cyanide atom and thus cannot be released, nor can it engage in oxidative reactions, extracellular heme release and nonspecific oxidative reactions do not appear to play a role in the HO-1 induction observed in CD163+ cells. HO-1 expression was not induced by the smaller, structurally related hemoprotein myoglobin. By contrast, HO-1 mRNA expression is strongly induced in CD163+ and CD163- cells after incubation with free heme, which crosses cell membranes by a receptor-independent mechanism (data not shown). These results imply that conformational and molecular size requirements exist for the interactions between CD163 ligands and the subsequent internalization and transcriptional activation of the HO-1 gene.
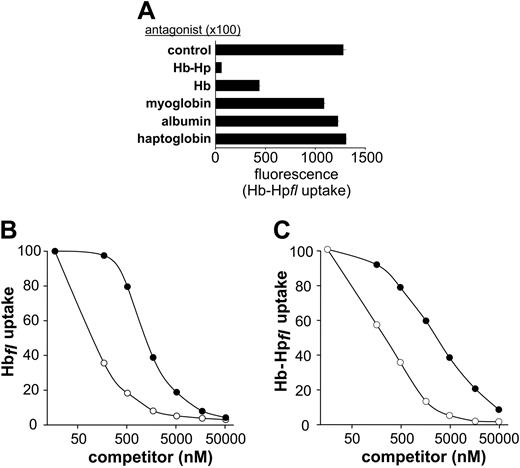
We have excluded endogenous synthesis and secretion of Hp by the HEK293 cell lines used throughout these studies by analyzing cell lysates and cell culture supernatant by Western blot with a polyclonal antibody against human Hp. The sensitivity of Western blot analysis was determined to be between 0.1 ng and 1 ng (Figure S1A; see the Supplemental Figures link at the top of the online article, at the Blood website). The possibility that Hb preparations were inadvertently contaminated with Hp was discounted by analyzing each Hb preparation using a biochip protein assay. The analytic sensitivity for the detection of Hp was determined to be well below 1% of the total protein (see Figure S1B). Furthermore, no free Hp-binding capacity existed in our culture medium containing 10% FCS. The minute amount of bovine Hp (0.29 μg/mL) was irreversibly blocked by the free Hb in FCS, which exceeded the Hp concentration by more than 10-fold. Substantially lower concentrations of these contaminants were present during the endocytosis assays, which were all performed in serum-free medium after thorough washing of cells with PBS. In addition, to exclude the possibility that nonspecific CD163-Hb interactions occurred because of structural disruptions induced by covalent Alexa-dye labeling, we performed identical experiments using the Hp moiety of the Hb-Hp complex as the fluorescent indicator ligand. When added in excess, nonlabeled Hb-Hp and free Hb competitively inhibited the uptake of labeled Hb-Hp complexes in CD163+ cells (Figure 2A). To exclude the possibility that suppression of the fluorescence signal is the result of physical fluorescence quenching by Hb absorbance, instead of competitive receptor binding, we compared the effect of Hb with the effect of equal concentrations of the structurally related hemoprotein myoglobin, which displays comparable spectral characteristics. Minimal inhibition of fluorescence was observed in the presence of myoglobin (Figure 2A), and no quenching of fluorescence signal was observed in the presence of free heme at concentrations up to 1 mM (data not shown). Similarly, the addition of albumin and free Hp, at the same competitor concentrations, did not inhibit CD163-mediated Hb-Hp endocytosis (Figure 2A).
Hp complex formation enhances CD163-Hb interactions. (A) CD163+ HEK293 cells were incubated for 30 minutes with Hb-Hpfl (fl indicates the fluorescence protein) in the presence or absence (control) of potential competitor proteins. Whereas both Hb and Hb-Hp significantly inhibited the uptake of fluorescent Hb-Hpfl, none of the control proteins (myoglobin, albumin, and free haptoglobin) significantly inhibited fluorescent-ligand uptake when added in a 100-fold excess concentration. Data shown represent the mean ± SD of 3 replicate values from one representative experiment. (B-C) Native Hb (•) and Hb-Hp (○) competitively inhibited the uptake of fluorescent Hbfl (B) and Hb-Hpfl complexes (C). CD163+ HEK293 cells were incubated for 30 minutes with fluorescent ligand (50 nM) and the unlabeled competitor at the concentrations indicated. Irrespective of the fluorescent ligand used, the Hb-Hp complexes inhibited CD163-mediated ligand uptake approximately 10 times more efficiently than free Hb. Data shown are the means of 2 independent experiments performed in duplicate. Molar concentrations are indicated with respect to the amount of Hb dimer.
Hp complex formation enhances CD163-Hb interactions. (A) CD163+ HEK293 cells were incubated for 30 minutes with Hb-Hpfl (fl indicates the fluorescence protein) in the presence or absence (control) of potential competitor proteins. Whereas both Hb and Hb-Hp significantly inhibited the uptake of fluorescent Hb-Hpfl, none of the control proteins (myoglobin, albumin, and free haptoglobin) significantly inhibited fluorescent-ligand uptake when added in a 100-fold excess concentration. Data shown represent the mean ± SD of 3 replicate values from one representative experiment. (B-C) Native Hb (•) and Hb-Hp (○) competitively inhibited the uptake of fluorescent Hbfl (B) and Hb-Hpfl complexes (C). CD163+ HEK293 cells were incubated for 30 minutes with fluorescent ligand (50 nM) and the unlabeled competitor at the concentrations indicated. Irrespective of the fluorescent ligand used, the Hb-Hp complexes inhibited CD163-mediated ligand uptake approximately 10 times more efficiently than free Hb. Data shown are the means of 2 independent experiments performed in duplicate. Molar concentrations are indicated with respect to the amount of Hb dimer.
Free Hb competitively inhibits endocytosis of Hb-Hp complexes by CD163
We performed competitive uptake experiments involving incubation of either fluorescence-labeled Hb (Figure 2B) or fluorescence-labeled Hb-Hp complexes (Figure 2C) in the presence of increasing concentrations of unlabeled competitor ligands. Results revealed that Hb-Hp complexes inhibit fluorescent ligand uptake by CD163-HEK293 cells approximately 10 times more efficiently than free Hb does.
Endocytosis of αα-crosslinked Hb by CD163
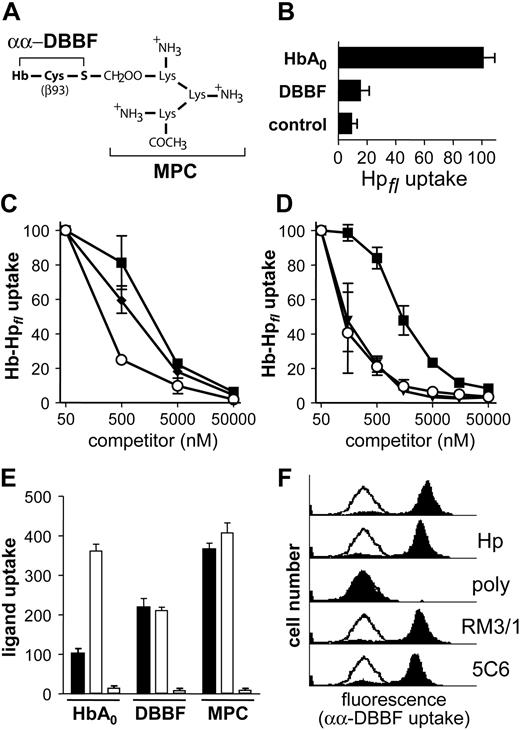
To further confirm Hp independence of CD163-mediated Hb endocytosis, we performed experiments using αα-crosslinked Hb (αα-DBBF Hb) and αα-DBBF Hb to which a site-specific multiple peptide conjugate (MPC) template of 3 lysine residues was attached on βCys93 (αα-DBBF Hb-MPC; Figure 3A). Unlike native Hb, which rapidly dissociates into αβ-dimers, αα-DBBF Hb retains its tetrameric molecular conformation as a result of covalent crosslinking between the Hb α-chains. Because Hp preferentially binds to Hb dimers,30 tetrameric αα-DBBF Hb forms limited complexes with Hp. We confirmed this observation of Panter et al31 by demonstrating that, in contrast to native HbA0, αα-DBBF Hb does not support substantial uptake of fluorescent Hp by CD163-expressing HEK293 cells (Figure 3B). Therefore, αα-DBBF Hb is an appropriate model ligand with which to study Hp-independent Hb endocytosis. As with native HbA0, αα-DBBF Hb induces strong HO-1 mRNA expression in CD163+, but not in CD163-, cells (HO-1 induction in CD163+ cells, 38.4 ± 1.6-fold; in CD163- cells, 2.2 ± 0.2-fold induction; P < .01). Similarly, αα-DBBF Hb competitively inhibits the uptake of fluorescent Hb-Hp complexes (Figure 3C). The slightly greater efficiency of αα-DBBF Hb when competing for Hb-Hp uptake, in comparison to native Hb, may be explained by the stable tetrameric conformation of the molecule. However, the much stronger inhibition of Hb-Hp uptake by αα-DBBF Hb-MPC was unexpected (Figure 3C). Quantitatively, the competitive inhibition of Hb-Hp uptake exerted by αα-DBBF Hb-MPC equals that exhibited by the Hb-Hp complex (Figure 3D). Alexa-633-labeled αα-DBBF Hb and its derivative were endocytosed by CD163+, but not by CD163-, cells; uptake was completely inhibited by a blocking polyclonal rabbit anti-CD163 IgG, but not by equal concentrations of the nonblocking CD163 antibodies RM3/1 and 5C6-FAT. This indicates that endocytosis of these hemoglobins results from direct ligand-receptor interactions (Figure 3E-F). Thereby, as expected from the competitive inhibition experiments, CD163-mediated endocytosis of αα-DBBF Hb-MPC appeared to be as efficient as the uptake of Hb-Hp complex (Figure 3E). These data indicate that the nature and potentially the site(s) of chemical modifications to Hb-based blood substitutes may directly influence their distribution, metabolism, and elimination.
Hp independence of CD163-mediated Hb endocytosis revealed by αα-DBBF-Hb. (A) Molecular schematic of the β-Cys93 modification in αα-DBBF-Hb MPC. (B) CD163-HEK293 cells were incubated with 10 μg/mL Alexa-488 Hp in the presence or absence (control) of equimolar concentrations of HbA0 or αα-DBBF Hb. Unlike HbA0, αα-DBBF-Hb did not support the uptake of fluorescent Hp in CD163-HEK293 cells. (C) Uptake of Hb-Hpfl complexes (50 nM) by CD163-HEK293 cells was determined in the presence of increasing competitor concentrations of unlabeled human HbA0 (▪), αα-DBBF Hb (♦), and αα-DBBF Hb-MPC (○). (D) Identical experiments were performed with human HbA0 (▪), αα-DBBF Hb-MPC (○), and Hb-Hp complexes (▾) (Hp phenotype 2-2) as competitors. Although αα-DBBF-Hb has a slightly higher capacity to compete for fluorescent Hb-Hp uptake by CD163+ cells than native HbA0, the αα-DBBF-Hb MPC variant displayed ligand properties equal to those displayed by the Hb-Hp complexes. Data represent mean ± SD of 3 independent experiments. (E) HbA0, αα-DBBF-Hb (DBBF), and αα-DBBF-Hb MPCs (MPC) were all labeled with Alexa-633, and uptake by CD163-HEK293 was determined after incubation for 30 minutes at a concentration of 5 μg/mL (▪). Parallel samples were incubated in the presence of 20 μg/mL human Hp 2-2 (□) or 50 μg/mL polyclonal rabbit anti-human CD163 IgG (right bar of each group). Data represent mean ± SD of triplicate well samples from one representative experiment. (F) Uptake of Alexa-633-labeled αα-DBBF Hb (5 μg/mL) by CD163-HEK293 cells was determined after 30-minute incubation with or without added Hp 2-2 (10 μg/mL; Hp), polyclonal rabbit anti-human CD163 IgG (50 μg/mL; poly), or mouse anti-human CD163 monoclonal antibodies RM3/1 or 5C6-FAT (each at 50 μg/mL; RM3/1 and 5C6, respectively; filled curves). Open curves represent the fluorescence of a control sample obtained after concurrent incubation with a 300-fold excess of unlabeled HbA0.
Hp independence of CD163-mediated Hb endocytosis revealed by αα-DBBF-Hb. (A) Molecular schematic of the β-Cys93 modification in αα-DBBF-Hb MPC. (B) CD163-HEK293 cells were incubated with 10 μg/mL Alexa-488 Hp in the presence or absence (control) of equimolar concentrations of HbA0 or αα-DBBF Hb. Unlike HbA0, αα-DBBF-Hb did not support the uptake of fluorescent Hp in CD163-HEK293 cells. (C) Uptake of Hb-Hpfl complexes (50 nM) by CD163-HEK293 cells was determined in the presence of increasing competitor concentrations of unlabeled human HbA0 (▪), αα-DBBF Hb (♦), and αα-DBBF Hb-MPC (○). (D) Identical experiments were performed with human HbA0 (▪), αα-DBBF Hb-MPC (○), and Hb-Hp complexes (▾) (Hp phenotype 2-2) as competitors. Although αα-DBBF-Hb has a slightly higher capacity to compete for fluorescent Hb-Hp uptake by CD163+ cells than native HbA0, the αα-DBBF-Hb MPC variant displayed ligand properties equal to those displayed by the Hb-Hp complexes. Data represent mean ± SD of 3 independent experiments. (E) HbA0, αα-DBBF-Hb (DBBF), and αα-DBBF-Hb MPCs (MPC) were all labeled with Alexa-633, and uptake by CD163-HEK293 was determined after incubation for 30 minutes at a concentration of 5 μg/mL (▪). Parallel samples were incubated in the presence of 20 μg/mL human Hp 2-2 (□) or 50 μg/mL polyclonal rabbit anti-human CD163 IgG (right bar of each group). Data represent mean ± SD of triplicate well samples from one representative experiment. (F) Uptake of Alexa-633-labeled αα-DBBF Hb (5 μg/mL) by CD163-HEK293 cells was determined after 30-minute incubation with or without added Hp 2-2 (10 μg/mL; Hp), polyclonal rabbit anti-human CD163 IgG (50 μg/mL; poly), or mouse anti-human CD163 monoclonal antibodies RM3/1 or 5C6-FAT (each at 50 μg/mL; RM3/1 and 5C6, respectively; filled curves). Open curves represent the fluorescence of a control sample obtained after concurrent incubation with a 300-fold excess of unlabeled HbA0.
Hb polymerization impairs uptake by CD163
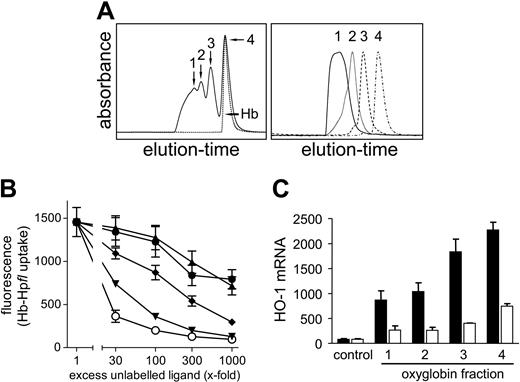
Next, we assessed the impact of Hb polymerization on its interaction with CD163. Oxyglobin (Biopure) is an Hb-based oxygen carrier that is composed of polymerized bovine Hb.32 We chromatographically purified the 4 main fractions and assessed their effect on CD163-mediated Hb-Hp uptake and HO-1 mRNA induction. The 4 fractions had a mean MW of 87 kDa, 185 kDa, 272 kDa, and 502 kDa, respectively, and represent tetrameric and multitetrameric Hb species (Figure 4A). In contrast to native bovine Hb and the smallest Oxyglobin (Biopure) fraction, which roughly corresponded to the tetrameric Hb, a progressive loss of CD163 binding (Figure 4B) and HO-1 mRNA induction (Figure 4C) was observed with increasing Hb polymer size.
Molecular size of polymerized Hb negatively correlates with CD163 binding and Hb-induced HO-1 mRNA induction. (A) Four Oxyglobin fractions (fraction 1, 502 kDa; fraction 2, 272 kDa; fraction 3, 185 kDa; and fraction 4, 87 kDa) were isolated and purified using size-exclusion chromatography (left panel). Reanalysis of the purified fractions is shown in the right panel. (B) Each of the 4 oxyglobin fractions and native bovine Hb were tested for their ability to inhibit Hb-Hpfl (50 nM) uptake by CD163-HEK293 cells when applied in excess. Excess competitor concentrations were calculated by means of the absolute MW of the respective fraction. The increasing molecular size of each respective fraction is associated with diminished receptor interaction, as indicated by a decreased capacity to inhibit Hb-Hpfl uptake, even at high excess concentrations. ▴, fraction 1. •, fraction 2. (♦) fraction 3. ▾, fraction 4. ○, bovine Hb. (C) Heme oxygenase (HO-1) mRNA was measured after 8-hour incubation with each of the Oxyglobin fractions at concentrations of 1.5 μM (□) and 15 μM (▪). In accordance with limited CD163 interaction, HO-1 mRNA induction by the large (1 and 2) fractions of 502 kDa and 272 kDa was diminished. Data represent mean ± SD of 3 (B) and 2 (C) independent experiments.
Molecular size of polymerized Hb negatively correlates with CD163 binding and Hb-induced HO-1 mRNA induction. (A) Four Oxyglobin fractions (fraction 1, 502 kDa; fraction 2, 272 kDa; fraction 3, 185 kDa; and fraction 4, 87 kDa) were isolated and purified using size-exclusion chromatography (left panel). Reanalysis of the purified fractions is shown in the right panel. (B) Each of the 4 oxyglobin fractions and native bovine Hb were tested for their ability to inhibit Hb-Hpfl (50 nM) uptake by CD163-HEK293 cells when applied in excess. Excess competitor concentrations were calculated by means of the absolute MW of the respective fraction. The increasing molecular size of each respective fraction is associated with diminished receptor interaction, as indicated by a decreased capacity to inhibit Hb-Hpfl uptake, even at high excess concentrations. ▴, fraction 1. •, fraction 2. (♦) fraction 3. ▾, fraction 4. ○, bovine Hb. (C) Heme oxygenase (HO-1) mRNA was measured after 8-hour incubation with each of the Oxyglobin fractions at concentrations of 1.5 μM (□) and 15 μM (▪). In accordance with limited CD163 interaction, HO-1 mRNA induction by the large (1 and 2) fractions of 502 kDa and 272 kDa was diminished. Data represent mean ± SD of 3 (B) and 2 (C) independent experiments.
Role of the C-terminal Hb β-chain in CD163-Hb interaction
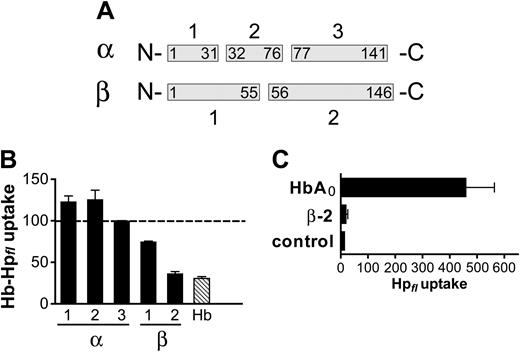
To characterize the peptide region of Hb involved in the CD163-Hb interaction, we examined CNBr digest fragments of HbA0 for their interactions with CD163-mediated uptake of fluorescent Hb-Hp. The 5 peptides derived from CNBr digest of human HbA0 represent amino acids 1-31, 32-76, and 77-141 of the Hb α-chain and amino acids 1-55 and 56-146 of the Hb β-chain, respectively (Figure 5A). When compared with intact HbA0, only the peptide representing the C-terminal Hb β-chain (β-2) exerted quantitatively comparable properties with an approximately 70% inhibition of fluorescent Hb-Hp uptake at a 100-fold excess molar competitor concentration (Figure 5B). Beyond implying a critical role of the C-terminal Hb β-chain in the interaction with CD163, these experiments lend additional evidence for Hp-independent endocytosis of free Hb. The Hb α-chain contains the high-affinity Hp-binding site,33 and removal of the Hb α-chain by means of proteolytic cleavage is thus expected to abrogate Hb-Hp complex formation. Accordingly, unlike native Hb, the isolated β 56-146 fragment (β-2) does not support the uptake of fluorescent Hp by CD163-expressing cells (Figure 5C). These data suggest that independent regions in Hb are responsible for Hp and CD163 interaction, and the presence of a specific binding region on Hb strongly indicates the presence of an Hb-binding region within the CD163 domains.
Human macrophages endocytose native and αα crosslinked Hb by the CD163 pathway
Finally, we examined CD163-Hb interactions in cultured human macrophages. Antibody labeling and FACS analysis revealed low levels of CD163 cell-surface expression on freshly isolated monocytes (Figure 6A); the density of CD163 increased more than 20-fold during a culture period of 8 days. Accordingly, the uptake of free Hb paralleled this increase in CD163 cell surface expression. Addition of a polyclonal rabbit IgG directed against human CD163 almost completely inhibited free Hb endocytosis; however, this was not the case when nonimmune rabbit serum was added (data not shown) (Figure 6A). As was demonstrated with HEK293 cells, Hb-Hp complexes and free Hb (but not free Hp) significantly inhibited the uptake of fluorescent Hb-Hp. The uptake characteristics of free Hb in macrophages are, therefore, comparable to those observed in CD163-HEK293 cells; approximately 70% inhibition of Hb-Hp uptake was observed with a 100-fold excess of unlabeled Hb (Figure S2). The dose-response curves shown in Figure 6B demonstrate the critical impact of Hp-complex formation on Hb uptake by macrophages at low ligand concentrations (1 μg/mL). However, at higher ligand concentrations (100 μg/mL), there is no difference between the uptake of Hb-Hp and free Hb.
Hb β-chain contains the putative CD163 binding site. Human HbA0 was cleaved by CNBr, and the 5 resultant fragments (A) were purified and analyzed for their ability to compete with Hb-Hpfl (50 nM) endocytosis by CD163. When added at a 100-fold molar-excess concentration, the peptide corresponding to the C-terminal Hb β-chain (β-2) had a quantitatively similar potency to antagonize Hb-Hpfl uptake, as does complete HbA0 (B). Unlike native HbA0, the β-2 peptide does not form complexes with Hp, as evidenced by its inability to support the uptake of fluorescent Hp. CD163-HEK293 cells were incubated for 30 minutes with Alexa 488-Hp (Hpfl; 5μg/mL) with or without (control) HbA0 (10 μg/mL) or β-2 peptide (10 μg/mL), respectively (C).
Hb β-chain contains the putative CD163 binding site. Human HbA0 was cleaved by CNBr, and the 5 resultant fragments (A) were purified and analyzed for their ability to compete with Hb-Hpfl (50 nM) endocytosis by CD163. When added at a 100-fold molar-excess concentration, the peptide corresponding to the C-terminal Hb β-chain (β-2) had a quantitatively similar potency to antagonize Hb-Hpfl uptake, as does complete HbA0 (B). Unlike native HbA0, the β-2 peptide does not form complexes with Hp, as evidenced by its inability to support the uptake of fluorescent Hp. CD163-HEK293 cells were incubated for 30 minutes with Alexa 488-Hp (Hpfl; 5μg/mL) with or without (control) HbA0 (10 μg/mL) or β-2 peptide (10 μg/mL), respectively (C).
CD163 is the macrophage Hb scavenger receptor: the crucial role of Hp at low ligand concentrations. (A) CD163 is involved in Hb uptake in human macrophages. Human monocytes purified by plastic adherence and cultured for 1 day show low expression levels of CD163, and, as expected, these cells have a low Hbfl-uptake capacity. After 8-day culture in the presence of human serum, an increase occurs in CD163 expression (as measured by staining with FITC anti-CD163 [clone 5C6-FAT] and subsequent FACS analysis) and in Hbfl uptake. Polyclonal rabbit IgG directed against CD163 (which is known to inhibit CD163/Hb-Hp interaction) almost completely inhibits macrophage uptake of Hbfl. Data represent mean ± SD of 3 experiments performed with monocytes obtained from 3 different donors. (B) Hp increases Hb uptake of macrophages at low (1 μg/mL), but not at high (100 μg/mL), ligand concentrations. Human macrophages were incubated with increasing concentrations of either free Hbfl or Hbfl-Hp for 30 minutes. Cell-associated fluorescence was determined by FACS. Data represent the mean ± SD of triplicate values obtained from a representative experiment.
CD163 is the macrophage Hb scavenger receptor: the crucial role of Hp at low ligand concentrations. (A) CD163 is involved in Hb uptake in human macrophages. Human monocytes purified by plastic adherence and cultured for 1 day show low expression levels of CD163, and, as expected, these cells have a low Hbfl-uptake capacity. After 8-day culture in the presence of human serum, an increase occurs in CD163 expression (as measured by staining with FITC anti-CD163 [clone 5C6-FAT] and subsequent FACS analysis) and in Hbfl uptake. Polyclonal rabbit IgG directed against CD163 (which is known to inhibit CD163/Hb-Hp interaction) almost completely inhibits macrophage uptake of Hbfl. Data represent mean ± SD of 3 experiments performed with monocytes obtained from 3 different donors. (B) Hp increases Hb uptake of macrophages at low (1 μg/mL), but not at high (100 μg/mL), ligand concentrations. Human macrophages were incubated with increasing concentrations of either free Hbfl or Hbfl-Hp for 30 minutes. Cell-associated fluorescence was determined by FACS. Data represent the mean ± SD of triplicate values obtained from a representative experiment.
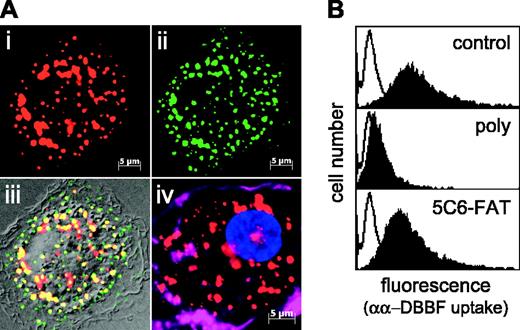
Before uptake studies, performed in serum-free media, primary human monocyte-derived macrophages must be cultured in the presence of human serum containing Hp. Thus, to prove Hp independence of Hb endocytosis in macrophages, we determined the uptake of non-Hp-binding αα crosslinked Hb. As seen in Figure 7, macrophages endocytose αα crosslinked Hb into an endosomal compartment, demonstrated by colocalization with the early endosomal marker transferrin. A comparable intracellular distribution can also be observed with the use of a polyclonal Hb antibody after the incubation of macrophages with non-labeled HbA0 or αα-DDBF Hb (Figure 7A). According to the results obtained in the heterologous expression model, uptake of αα crosslinked Hb is inhibited by excess nonlabeled Hb and by a blocking antibody directed against CD163; however, uptake is not inhibited by the nonblocking monoclonal anti-CD163 antibody (Figure 7B).
CD163 mediates Hp-independent macrophage uptake of αα crosslinked Hb into an endosomal compartment. (A) Internalization of Alexa-647 αα-DBBF Hb in macrophages was confirmed by deconvoluted fluorescence microscopy. A high degree of intracellular colocalization (panel iii, yellow) was found after coincubation with αα-DBBF Hb (panel i, Alexa-647) and the early endosomal marker transferrin (panel ii, Alexa-488) for 15 minutes at a concentration of 20 μg/mL. Alternatively, the same endosomal distribution was revealed by anti-Hb immunofluorescence staining of macrophages after incubation with nonlabeled αα-DBBF Hb (panel iv; red, Hb; magenta, Alexa-647 phalloidin stain of actin cytoskeleton; blue, DAPI nuclear stain) (original magnification, 1000 ×). (B) Macrophage-associated fluorescence was determined after 30-minute incubation with 10 μg/mL Alexa-633-labeled αα-DBBF Hb (control). Nonspecific binding was determined by the addition of a 300-fold excess of unlabeled Hb (open curves). Uptake of αα-DBBF Hb was inhibited by a blocking rabbit polyclonal IgG (poly) but not by an equal concentration of a nonblocking monoclonal anti-CD163 antibody (5C6-FAT).
CD163 mediates Hp-independent macrophage uptake of αα crosslinked Hb into an endosomal compartment. (A) Internalization of Alexa-647 αα-DBBF Hb in macrophages was confirmed by deconvoluted fluorescence microscopy. A high degree of intracellular colocalization (panel iii, yellow) was found after coincubation with αα-DBBF Hb (panel i, Alexa-647) and the early endosomal marker transferrin (panel ii, Alexa-488) for 15 minutes at a concentration of 20 μg/mL. Alternatively, the same endosomal distribution was revealed by anti-Hb immunofluorescence staining of macrophages after incubation with nonlabeled αα-DBBF Hb (panel iv; red, Hb; magenta, Alexa-647 phalloidin stain of actin cytoskeleton; blue, DAPI nuclear stain) (original magnification, 1000 ×). (B) Macrophage-associated fluorescence was determined after 30-minute incubation with 10 μg/mL Alexa-633-labeled αα-DBBF Hb (control). Nonspecific binding was determined by the addition of a 300-fold excess of unlabeled Hb (open curves). Uptake of αα-DBBF Hb was inhibited by a blocking rabbit polyclonal IgG (poly) but not by an equal concentration of a nonblocking monoclonal anti-CD163 antibody (5C6-FAT).
Discussion
In this study, we show for the first time that CD163-mediated Hb uptake by macrophages is not dependent on the formation of Hb-Hp complexes. This is supported by several lines of evidence. First, Hb uptake is observed in the absence of Hp in human macrophages and in CD163-transduced HEK293 cells but not in CD163- cells. Second, highly purified Hb inhibits CD163-mediated uptake of labeled Hb-Hp complexes or free Hb. Competitive inhibition of labeled Hb-Hp uptake by nonlabeled Hb not only implies a common receptor-binding site of free Hb and Hb-Hp, it rules out the possibility that CD163-Hb interactions are affected by structural alterations induced through the covalent labeling process. Third, free Hb induces transcriptional induction of HO-1, an indirect measure of hemoprotein internalization and degradation, in CD163-expressing cells in a dose-dependent manner. No significant HO-1 mRNA induction was observed in CD163- cells. CD163-mediated HO-1 induction by CN-met-Hb excludes the possibility that Hb oxidative side reactions and extracellular heme release are involved in the observed transcriptional response. Finally, disruption of the Hb interaction with Hp by chemical crosslinking of Hb between its α-chains or, alternatively, by proteolytic cleavage does not significantly affect the CD163-Hb interaction. The latter observations exclude the possibility that any traces of Hp in cell culture medium before the uptake studies could have affected the results. Inhibition of αα-crosslinked Hb uptake in CD163-expressing HEK cells and human macrophages by the previously characterized blocking antibodies against CD16312 further implies that the Hp-independent Hb uptake is a direct result of the interaction of Hb with CD163.
Our results apparently contradict previous studies (Kristiansen et al11 and Asleh et al34 ) that describe CD163 as a specific receptor for Hb-Hp complexes but not for free Hb. The experiments presented in our study focused on the biologic end points of CD163-mediated Hb endocytosis and induction of the heme breakdown pathway. Kristiansen et al11 and Asleh et al,34 however, attempted to determine putative high-affinity physical interactions between free Hb and CD163 in binding studies that used either CD163-expressing cell lines34 or immobilized receptor.11 Consistent with these studies, we also failed to detect high-affinity binding of Hb to CD163-expressing cells when using classic binding assays that measured receptor-associated ligand after prolonged incubation of ligand with receptor-expressing cells at 4°C, followed by extensive washing to remove unbound ligand.
However, the apparent lack of significant binding observed in these experiments does not exclude low-affinity, reversible interactions between free Hb and CD163. The induction of HO-1 observed on the uptake of free Hb was stronger than that observed for Hb-Hp complexes and may, in fact, further indicate low-affinity binding because a weak CD163-Hb interaction may facilitate endosomal receptor-ligand dissociation and subsequent trafficking of Hb to the lysosomal compartment. Regardless of the precise characteristics of the Hb-CD163 interaction, our studies demonstrated that, under physiologic conditions, Hb is effectively endocytosed by CD163 in transfected cell lines and in human macrophages. Thereby, CD163-mediated free Hb endocytosis appears to be a structurally constrained process because even the closely related hemoprotein myoglobin neither affects the endocytosis of Hb-Hp nor induces HO-1 expression in CD163+ cells.
The uptake of free Hb by CD163 described here may become relevant once Hp-binding capacity has been depleted by massive hemolysis or on extravasation of erythrocytes. Indeed, we found that macrophage Hb uptake was significantly enhanced by Hp at low Hb concentrations of 1 μg/mL but not at concentrations greater than 100 μg/mL. These higher concentrations can be observed after intravascular hemolysis25,35 ; even higher concentrations of free Hb accumulate at sites of tissue injury because of erythrocyte extravasation.
In light of the results presented here, a biphasic model of free Hb clearance by macrophages is proposed. When concentrations of free Hb are low, such as during mild intravascular hemolysis, Hb is rapidly sequestered by circulating Hp. The resultant Hb-Hp complexes are cleared from the circulation by high-affinity binding to, and uptake by, CD163-expressing macrophages in the liver, spleen, and bone marrow. This high-affinity pathway is operative as long as the Hp-binding capacity is higher than the total amount of Hb released from bursting erythrocytes. However, after more pronounced systemic hemolysis, plasma Hp is rapidly depleted, in some cases rendering it undetectable. Low to undetectable plasma Hp levels are, therefore, a sensitive marker of hemolysis in clinical practice.18 We propose that free Hb clearance by macrophages under these circumstances is mediated by an Hp-independent CD163-Hb interaction. This Hb clearance mechanism is even more applicable at sites of tissue injury, where large amounts of Hb must be removed by macrophages during the wound-healing process. The quantitative role of other low-affinity Hb receptors in systemic Hb clearance, such as megalin and cubilin—2 epithelial, multipurpose receptors with particularly high expression within the renal proximal tubule—remains to be established.36
The proposed model of an Hp-independent, CD163-mediated Hb clearance pathway may account for the observation that Hb clearance from plasma is unimpaired in Hp-knockout mice23,24 and that humans with a haptoglobin gene null mutation (Hp0-Hp0 genotype) do not display obvious alterations in hemoglobin catabolism. Interestingly, a high prevalence of the Hp0 allele has been observed in West African populations, indicating that the evolutionary advantage imposed by an as yet undefined genotype linked to this allele may exceed the potential disadvantage imposed by Hp deficiency, even in a population that is exposed to an endemic hemolytic disease such as malaria.25,37-39
Our studies with modified Hb shed light on the basic structural requirements of the CD163-Hb interaction. Increasing the molecular mass of polymerized Hb reduces uptake by CD163. Therefore, the conformational changes imparted by Hb polymerization probably limit access to the Hb-CD163 binding site. In contrast, the disruption of Hb-Hp complex formation by site-specific αα tetrameric crosslinking does not significantly affect CD163-mediated Hb uptake. These data suggest that the surface of the Hb tetramer, rather than its dimer interface, may play a role in the putative CD163 binding site. The distinct properties of Hb cleavage fragments imply a prominent role of the C-terminal part of the Hb β-chain in this interaction. The importance of this part of the Hb molecule in the CD163-Hb interaction is further supported by the finding that attachment of a multiple peptide conjugate (MPC) of 3 lysine residues to βCys93, which displays a high surface accessibility and locates within the putative Hb-binding domain, dramatically enhances CD163-mediated uptake of cell-free Hb.
These findings demonstrate for the first time that Hb-based therapeutics may be tailored toward low or high macrophage clearance by specific chemical modifications. Our data show competitive and quantitatively indistinguishable CD163-mediated uptake of DBBF-Hb-MPC and Hb-Hp complexes. This may suggest that a structurally related binding site is involved in the interaction of CD163 with these 2 ligands. Although speculative, one could suggest that the conformational changes of the Hb molecule, which occur on Hp complex formation, could be mimicked by chemical modification of the Hb molecule. Either Hp complex formation or specific chemical modification, as shown with αα-DBBF Hb-MPC, might thereby enhance binding-site accessibility and thus strengthen the Hb-CD163 interaction. This idea is also compatible with the model initially proposed by Kristiansen et al,11 which implies exposure of an as yet undefined neo-epitope when Hb forms a complex with Hp.
Our findings related to these interactions have important implications for the development of Hb-based oxygen carriers and related drugs. It is likely that macrophages play a significant role in the systemic clearance of low-molecular-weight blood substitutes (eg, tetrameric species within heterogeneous mixtures). Conversely, high-molecular-weight blood substitutes (eg, polymeric species within heterogeneous mixtures) are less likely to be cleared by this mechanism, as demonstrated by the present study. Further study of the macrophage Hb scavenger receptor in the clearance of Hb-based blood substitutes will help to optimize the safety, efficacy, and dosing of these potential therapeutic agents.
In conclusion, we propose that Hp-independent CD163-Hb interactions form the basis for an alternative Hb clearance pathway by human macrophages. This pathway is operative under conditions of systemic or local depletion of Hp, such as those commonly observed in patients with hemolytic anemia or after tissue destruction and erythrocyte extravasation.
Prepublished online as Blood First Edition Paper, September 27, 2005; DOI 10.1182/blood-2005-03-1014.
Supported by the Foundation for Research at the Medical Faculty, University of Zurich (D.J.S.), and by the Hartmann-Muller Foundation (D.J.S.).
D.J.S. designed and performed research and wrote the paper; C.A.S. performed research; P.W.B. performed research; R.A.B. provided vital materials; G.S. performed research; A.I.A. provided vital materials and wrote the paper; and A.S. wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Stephanie Hiss (University of Bonn, Germany) for bovine Hp determination.

![Figure 6. CD163 is the macrophage Hb scavenger receptor: the crucial role of Hp at low ligand concentrations. (A) CD163 is involved in Hb uptake in human macrophages. Human monocytes purified by plastic adherence and cultured for 1 day show low expression levels of CD163, and, as expected, these cells have a low Hbfl-uptake capacity. After 8-day culture in the presence of human serum, an increase occurs in CD163 expression (as measured by staining with FITC anti-CD163 [clone 5C6-FAT] and subsequent FACS analysis) and in Hbfl uptake. Polyclonal rabbit IgG directed against CD163 (which is known to inhibit CD163/Hb-Hp interaction) almost completely inhibits macrophage uptake of Hbfl. Data represent mean ± SD of 3 experiments performed with monocytes obtained from 3 different donors. (B) Hp increases Hb uptake of macrophages at low (1 μg/mL), but not at high (100 μg/mL), ligand concentrations. Human macrophages were incubated with increasing concentrations of either free Hbfl or Hbfl-Hp for 30 minutes. Cell-associated fluorescence was determined by FACS. Data represent the mean ± SD of triplicate values obtained from a representative experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/1/10.1182_blood-2005-03-1014/4/m_zh80010688840006.jpeg?Expires=1767699660&Signature=C~qyzt94cla8J5cz57cQrJdDtyyewNTYYWKJgn3pClE5z~MMr6kTC4~2MbbWmLPjBJTlhBvoSvJvhcwrv~AP3J4dPN6hWn1suNIuH~r01PNOqbMBMr38SBLn9EDKDjj69FpnUx0fLi8RTpncmc4Nh82Rdv5XaXniqRTR0QCFnwQxI-vvuNRJyEWGqQjE4~DBnzNzsX9sxrAO~VdmzoL4U1Qyeav5pj5yzAOLkgVGl7vBUjNXGmNKemmu5pP9i9bX7RPeQ77axrXz89Aol031ixPRShYD7VXdQn1B2~tT99mWPumfBSykJg4Cj58kVjcJwWVRoRlcCyeoMS6Y2WLmKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal