Abstract
Friend virus is an acutely oncogenic retrovirus that causes erythroblastosis and polycythemia in mice. Previous studies suggested that the Friend virus oncoprotein, gp55, constitutively activates the erythropoietin receptor (EPOR), causing uncontrolled erythroid proliferation. Those studies showed that gp55 confers growth factor independence on an interleukin-3 (IL-3)-dependent cell line (Ba/F3) when the EPOR is coexpressed. Subsequently, we showed that a truncated form of the stem-cell kinase receptor (sf-STK) is required for susceptibility to Friend disease. Given the requirement for sf-STK, we sought to establish the in vivo significance of gp55-mediated activation of the EPOR. We found that the cytoplasmic tyrosine residues of the EPOR, and signal transducer and activator of transcription-5 (STAT5), which acts through these sites, are not required for Friend virus-induced erythroblastosis. The EPOR itself was required for the development of erythroblastosis but not for gp55-mediated erythroid proliferation. Interestingly, the murine EPOR, which is required for gp55-mediated Ba/F3-cell proliferation, was dispensable for erythroblastosis in vivo. Finally, gp55-mediated activation of the EPOR and STAT5 are required for Friend virus-induced polycythemia. These results suggest that Friend virus activates both sf-STK and the EPOR to cause deregulated erythroid proliferation and differentiation.
Introduction
Friend disease is a multistage viral disease in mice.1-3 In the initial stage of Friend disease, expression of the viral oncoprotein, gp55, causes uncontrolled erythroid proliferation and erythroblastosis. Erythroblastosis is a condition characterized by the rapid accumulation of immature erythroid cells leading to acute splenic enlargement. In the later stages of Friend disease, retroviral integrations in Sfpi1, p53, and Nfe2 cause progression to erythroleukemia. Friend virus is a complex of 2 viruses, Friend murine leukemia virus and spleen focus-forming virus (SFFV).4,5 SFFV encodes a mutant envelope protein, gp55, which is necessary and sufficient for the erythroblastosis stage of the disease.6 There are 2 strains of Friend virus, an anemia-inducing strain (FVA) and a polycythemia-inducing strain (FVP).1,7 The amino acids responsible for this phenotypic difference have been localized to the transmembrane domain of gp55 (gp55A and gp55P).8 Previously, it was shown that gp55P could interact with the erythropoietin receptor (EPOR) and support proliferation of the interleukin-3 (IL-3)-dependent cell line Ba/F3.9 Based on this observation, it was proposed that Friend virus causes erythroblastosis through constitutive activation of the EPOR.
Additional insights into the mechanism of action of Friend virus have been provided by host factors that confer resistance or susceptibility to Friend disease. Fv1 and Fv4 confer resistance to Friend disease through interference with the virus life cycle or interference with virus binding to the ecotropic receptor, respectively.10,11 Fv2 confers susceptibility through its effect on gp55-mediated erythroid proliferation.12,13 Strains of mice that are Fv2 resistant (Fv2r/r) fail to develop either the initial erythroblastosis or late erythroleukemic phases of Friend disease. Fv2 has been identified as the stem-cell kinase receptor (STK).14 Strains of mice that are Fv2 susceptible (Fv2s/s) express a truncated form of STK (sf-STK). sf-STK encodes the transmembrane and tyrosine kinase domains of STK but not the extracellular domain.15 Targeted disruption of Stk confers resistance to Friend disease, and enforced expression of sf-STK confers susceptibility to Friend disease in Fv2r/r strains of mice.14 Thus, sf-STK is both necessary and sufficient for susceptibility to Friend disease at the Fv2 locus.
The essential role of sf-STK in gp55-mediated erythroid proliferation contrasts with the uncertain significance of gp55-mediated activation of the EPOR. One possibility is that gp55 facilitates an interaction between sf-STK and the EPOR. The receptor tyrosine kinase KIT interacts with the EPOR at the erythroid colony-forming unit (CFU-E) stage of erythroid development.16,17 By analogy to KIT, an interaction between sf-STK and the EPOR could cause phosphorylation of the EPOR and support erythroid proliferation and differentiation. Alternatively, gp55 could activate sf-STK and the EPOR independent of one another. In that case, gp55-mediated activation of the EPOR might primarily affect erythroid-cell survival and differentiation. Consistent with the latter hypothesis, our studies show that the EPOR is not essential for Friend virus-induced erythroid proliferation but is required for the development of polycythemia.
Materials and methods
Mice
Stat5a-/-;Stat5b-/-, EporH, EporHM, Tg(GFP), and Epor-/-;Tg(EPOR) mouse strains were previously described.18-21 EporH and EporHM are targeted strains that express truncated forms of the EPOR lacking the distal 7, or all 8, cytoplasmic tyrosine residues, respectively.19 Stat5a-/-; Stat5b-/- mice contain targeted mutations of the Stat5a and Stat5b genes.18 Stat5a-/-;Stat5b-/-, EporH, and EporHM mice were backcrossed to a Friend virus sensitive background (Balb/cByJ). Stat5a-/-;Stat5b-/- mice were maintained on a RAG1-deficient background to prevent development of autoimmune disease associated with signal transducer and activator of transcription-5 (STAT5) deficiency.22,23 Epor-/-;Tg(EPOR) mice contain an 80-kb human EPOR transgene, which complements targeted mutation of the murine Epor gene.21 To obtain Epor-/-;Tg(EPOR) mice on a Friend virus-susceptible background, we mated them with Balb/cByJ Epor+/- mice. Incidentally, the Epor mutation in the Epor+/- mice was from a different strain.24,25 Tg(GFP) mice contain a green fluorescent protein (GFP) transgene, linked to the H-2Kb promoter, which is widely expressed in hematopoietic cells.20 C57BL6/J (Fv2r/r) mice were backcrossed to a Balb/cByJ background through the use of marker-assisted accelerated crossing (MAX-BAX; Charles River Laboratories, Wilmington, MA) to generate a new congenic mouse strain C.B6-Fv2r/r. All studies were performed under an animal protocol approved by the Institutional Animal Care and Use Committee of Saint Jude Children's Research Hospital, Memphis, TN.
Friend virus
FVA (gift from M. Bondurant) was passaged once in Balb/cByJ mice and the plasma frozen in small aliquots. We diluted the plasma 1:15 in Iscove modified Dulbecco medium (IMDM) and injected 0.2 mL into the tail vein. FVP was harvested from the supernatant of FP63 producer cells (gift from A. Bernstein). We diluted the supernatant 1:5 in IMDM and injected 0.2 mL into the tail vein. Two weeks after infection with FVA or FVP, we humanely killed the mice and harvested the spleens for analysis. Spun hematocrits were determined 3 to 4 weeks after infection with FVP. The results were analyzed by Student t test through the use of Microsoft Excel 2003 software (Redmond, WA).
Fetal liver transplantation
We intercrossed Balb/cByJ Epor+/-;Tg(GFP) mice to obtain embryonic day 12.5 Epor-/-;Tg(GFP) embryos. Epor-/-;Tg(GFP) embryos were identified by their pale appearance and fluorescence with a GFP flashlight (BLS, Budapest, Hungary). We harvested fetal livers and prepared a single-cell suspension. Fetal liver cells (5 × 105 to 2 × 106 cells) were injected into the tail vein of lethally irradiated (9 Gy [900 rad]) adult C.B6-Fv2r/r mice. Four weeks after transplantation, we analyzed GFP expression in circulating leukocytes, platelets, and erythrocytes by flow cytometry. Transplant recipients were T-cell depleted prior to Friend virus infection through administration of 2 intraperitoneal injections of anti-CD4 (rat monoclonal GK1.5) and anti-CD8 (rat monoclonal 2.43) antibody. T-cell depletion has been reported to increase the susceptibility of mice to Friend disease.26
Immunohistochemistry
For GFP, gp55, TER119, and GATA1 immunohistochemistry, we used rabbit anti-GFP antibody (Molecular Probes, Eugene, OR), goat anti-Rauscher leukemia virus gp69/71 antibody (ViroMed, Camden, NJ), rat anti-mouse TER119 antibody (BD Pharmingen, San Jose, CA), and goat anti-mouse GATA1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Following blocking for endogenous peroxidase, endogenous biotin, and nonspecific sera, slides were sequentially incubated with primary antibody (rabbit anti-GFP, 1:200; goat anti-Rauscher gp69/71, 1:8000; rat anti-mouse TER119, 1:500; or goat anti-mouse GATA1, 1:75) for 30 minutes (60 minutes for GATA1), biotinylated secondary antibody (1:200) for 10 minutes (30 minutes for GATA1), streptavidin conjugated to horseradish peroxidase for 10 minutes, and diaminobenzidine for 5 minutes. Tris-buffered saline was used for washes in between each step. For GATA1, heat-induced epitope retrieval was performed at more than 95°C for 30 minutes prior to staining. Slides were counterstained with hematoxylin and examined with a Nikon E600 microscope with Nikon Plan Apo 2×/0.10, 10 ×/0.45, and 40 ×/0.95 objective lenses (Nikon, Tokyo, Japan). Photomicrographs were taken with a Nikon DXM1200 digital camera. ACT-1 version 2.63 (Nikon) software was used to acquire images, and image contrast was adjusted with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA).
Immunoprecipitation
For immunoprecipitation and Western blotting, anti-STAT5A, anti-STAT5B, and anti-pTyr 4G10 antibody were purchased (Upstate, Lake Placid, NY). Anti-Janus kinase-2 (anti-JAK2) antibody was previously described.27 Friend virus-infected erythroblasts were isolated28 and cultured in complete Friend virus medium (30% fetal bovine serum, 1% deionized bovine serum albumin, 0.001% monothioglycerol, 2 mM glutamine, and penicillin-streptomycin in IMDM) without EPO for 8 hours. Erythroblasts were stimulated with EPO (epoetin alfa, 4 U/mL; Amgen, Thousand Oaks, CA) for 15 minutes. Whole-cell lysates were prepared from 2 × 107 cells in 0.5 mL RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris, pH 7.5) with protease inhibitors (aprotinin 5 μg/mL, leupeptin 5 μg/mL, pepstatin 1 μg/mL, 1 mM DTT, and 0.5 mM PMSF). Immunoprecipitation and Western blotting were performed in accordance with standard procedures.29
Results
The distal EPOR and STAT5 are not required for Friend virus-induced erythroblastosis
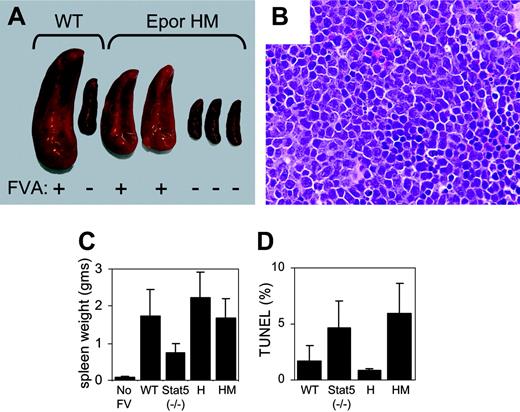
First, we considered the possibility that an interaction between sf-STK and the EPOR causes phosphorylation of cytoplasmic tyrosine residues of the EPOR and the activation of downstream signal transduction pathways. To test this hypothesis, we infected 3 mutant strains of mice with Friend virus. The EporH strain expresses a truncated EPOR, which lacks the distal 108 amino acids of the EPOR and all but the most proximal tyrosine residue (Y343). This tyrosine residue is sufficient for STAT5 activation.19 The EporHM strain is identical to the EporH strain except that Y343 has been mutated to phenylalanine. Due to the lack of cytoplasmic tyrosine residues, the EporHM strain is unable to activate STAT5 in response to EPO stimulation.19 The STAT5-deficient strain contains targeted mutations of the 2 isoforms of STAT5 (Stat5a-/-; Stat5b-/-).18 EporH/H, EporHM/HM, and Stat5a-/-;Stat5b-/- mice were susceptible to Friend virus-induced erythroblastosis (Figure 1A-C). Stat5a-/-;Stat5b-/- mice showed less splenic enlargement than wild-type mice, but their spleens were still significantly larger than those of uninfected mice (P < .001). Histologic sections from all 3 strains showed that splenic tissue was replaced with immature erythroblasts. The immature erythroid phenotype was confirmed by immunohistochemistry, which showed that most of the cells expressed GATA1 but not the late erythroid marker TER119 (Supplemental Figure S1, available at the Blood website; see the Supplemental Figure link at the top of the online article). Compared with wild-type and the EporH strain, splenic erythroblasts from the EporHM and STAT5-deficient strains had a slightly elevated rate of apoptosis (Figure 1D).
The distal EPOR and STAT5 are not required for Friend virus-induced erythroblastosis. (A) Photograph of spleens from FVA-infected mice. Mouse strains are indicated at the top. Infection with Friend virus is indicated at the bottom (FVA). (B) Photomicrograph of spleen from Friend virus-infected EporHM mouse, stained with hematoxylin and eosin. Original magnification × 400. (C) Spleen weights of FVA-infected mice in grams. The mouse strains are indicated at the bottom. Stat5 (-/-) denotes the Stat5a-/-;Stat5b-/- strain. The spleen weights of uninfected mice are provided as a control (no FV). Error bars represent standard deviation (Stat5 (-/-), EporH, EporHM, P < .001). (D) Percent transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL)-positive cells in the spleens of FVA-infected mice (Stat5 (-/-), P = .07; EporH, P > .10; EporHM, P = .10).
The distal EPOR and STAT5 are not required for Friend virus-induced erythroblastosis. (A) Photograph of spleens from FVA-infected mice. Mouse strains are indicated at the top. Infection with Friend virus is indicated at the bottom (FVA). (B) Photomicrograph of spleen from Friend virus-infected EporHM mouse, stained with hematoxylin and eosin. Original magnification × 400. (C) Spleen weights of FVA-infected mice in grams. The mouse strains are indicated at the bottom. Stat5 (-/-) denotes the Stat5a-/-;Stat5b-/- strain. The spleen weights of uninfected mice are provided as a control (no FV). Error bars represent standard deviation (Stat5 (-/-), EporH, EporHM, P < .001). (D) Percent transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL)-positive cells in the spleens of FVA-infected mice (Stat5 (-/-), P = .07; EporH, P > .10; EporHM, P = .10).
The EPOR is required for Friend virus-induced erythroblastosis, but not for gp55-mediated erythroid proliferation
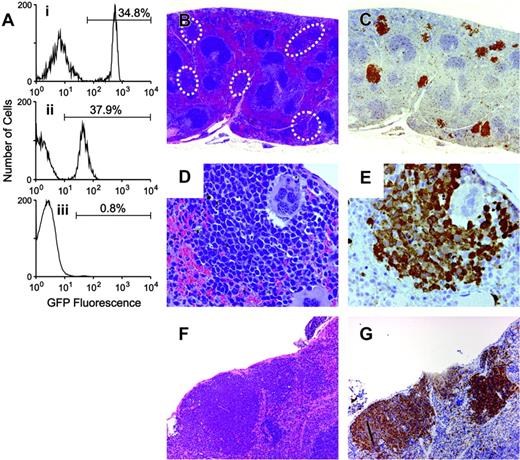
Next, we considered whether the EPOR might be entirely dispensable for susceptibility to Friend virus-induced erythroblastosis. To test this possibility, we transplanted Epor-/- fetal liver cells into lethally irradiated adult mice. To provide transplant recipients, we backcrossed Fv2r/r mice to a Balb/cByJ background to create a new congenic strain (C.B6-Fv2r/r). C.B6-Fv2r/r mice are histocompatible with donor fetal liver cells and resistant to Friend disease at the Fv2 locus. Mice containing a targeted mutation of the Epor were crossed with (H-2Kb)-GFP transgenic mice to allow identification of donor-derived cells in transplant recipients.20 Epor-/-;Fv2s/s; Tg(GFP) fetal liver cells were transplanted into lethally irradiated C.B6-Fv2r/r mice and bone marrow reconstitution assessed by the percentage of GFP-positive leukocytes and platelets (Figure 2A). As expected, the percentage of GFP-positive erythrocytes was very low (less than 1%). The mice were T-cell depleted to increase their susceptibility to Friend disease26 and infected with Friend virus. The infected mice failed to develop enlarged spleens; however, microscopic examination showed that foci of immature erythroblasts were present (Figure 2B-G; Supplemental Figure S1). The cells in these foci stained with antibody against GFP, indicating they were donor-derived Epor-/-;Fv2s/s;Tg(GFP) erythroid cells. They also stained with antibody against Rauscher gp69/71 antigen, which recognizes gp55,30 indicating that they expressed the virally encoded oncoprotein. These results show that gp55 can support erythroid proliferation in the absence of the EPOR, but the EPOR is required for erythroblastosis to develop.
The murine EPOR is not required for Friend virus-induced erythroblastosis
To further assess the role of the EPOR in Friend virus-induced erythroblastosis, we exploited the existence of a species-specific interaction between gp55P and the EPOR. gp55P supports factor-independent Ba/F3-cell proliferation but only in the presence of the murine EPOR. The human EPOR is ineffective in this context.31 We studied mice with a targeted mutation of the murine Epor that were transgenic for the human EPOR (Epor-/-;Tg(EPOR)).21
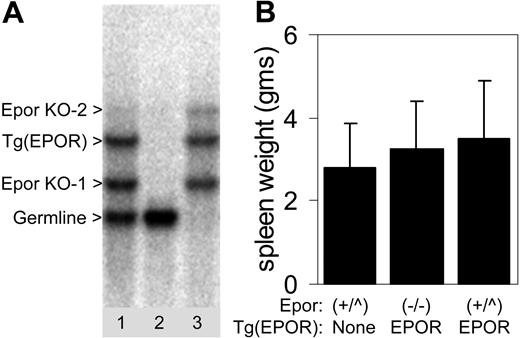
Murine EPOR deficiency in these mice is rescued by the human EPOR transgene, and EPOR signaling is essentially normal. Epor-/-;Tg(EPOR) mice were bred for one generation to a Friend virus-susceptible background (Balb/cByJ). An incidental consequence of our breeding strategy was the Epor-/-;Tg(EPOR) mice used in our studies contained 2 different targeted mutations of the Epor.24,25 To ensure a proper genotype, we identified mice that lacked a germ-line murine Epor band by Southern blotting (Figure 3A). Epor-/-;Tg(EPOR) mice were fully susceptible to Friend virus-induced erythroblastosis (Figure 3B). Thus, the murine EPOR, which is essential for gp55-mediated Ba/F3-cell proliferation, is not required for Friend virus-induced erythroblastosis.
The EPOR is not required for gp55-mediated erythroid proliferation. GFP-positive Fv2s/s;Epor-/- fetal liver cells were transplanted into Fv2r/r recipients. (A) Flow cytometry was performed 4 weeks after transplantation to assess bone marrow reconstitution. Three subpanels correspond to granulocytes (i), platelets (ii), and erythrocytes (iii). The percentage of GFP-positive cells is indicated by the number above the line. (B-G) Photomicrographs of FVA-infected spleens. The sections in panels B, D, and F were stained with hematoxylin and eosin. The sections in panels C and E were stained with anti-GFP antibody and counterstained with hematoxylin. The section in panel G was stained with Rauscher anti-gp69/71 antibody. Original magnifications × 20 (B-C), × 400 (D-E), and × 100 (F-G).
The EPOR is not required for gp55-mediated erythroid proliferation. GFP-positive Fv2s/s;Epor-/- fetal liver cells were transplanted into Fv2r/r recipients. (A) Flow cytometry was performed 4 weeks after transplantation to assess bone marrow reconstitution. Three subpanels correspond to granulocytes (i), platelets (ii), and erythrocytes (iii). The percentage of GFP-positive cells is indicated by the number above the line. (B-G) Photomicrographs of FVA-infected spleens. The sections in panels B, D, and F were stained with hematoxylin and eosin. The sections in panels C and E were stained with anti-GFP antibody and counterstained with hematoxylin. The section in panel G was stained with Rauscher anti-gp69/71 antibody. Original magnifications × 20 (B-C), × 400 (D-E), and × 100 (F-G).
The murine EPOR is not required for Friend virus-induced erythroblastosis. (A) Southern blot of genomic DNA from the progeny of Epor-/-;Tg(EPOR) × Balb/cByJ Epor+/- mice. The parents contained different targeted mutations of the Epor,24,25 which are indicated by the arrowheads (Epor KO-1 and Epor KO-2). Bands corresponding to the germ-line band and the human EPOR transgene (Tg(EPOR)), are indicated. Genotypes: lane 1, Epor+/-;Tg(EPOR); lane 2, wild type; lane 3, Epor-/-;Tg(EPOR). (B) Spleen weights of FVA-infected mice in grams. Genotypes are indicated at the bottom. Epor+/+ and Epor+/- genotypes are grouped together as (+/^). Error bars represent standard deviations.
The murine EPOR is not required for Friend virus-induced erythroblastosis. (A) Southern blot of genomic DNA from the progeny of Epor-/-;Tg(EPOR) × Balb/cByJ Epor+/- mice. The parents contained different targeted mutations of the Epor,24,25 which are indicated by the arrowheads (Epor KO-1 and Epor KO-2). Bands corresponding to the germ-line band and the human EPOR transgene (Tg(EPOR)), are indicated. Genotypes: lane 1, Epor+/-;Tg(EPOR); lane 2, wild type; lane 3, Epor-/-;Tg(EPOR). (B) Spleen weights of FVA-infected mice in grams. Genotypes are indicated at the bottom. Epor+/+ and Epor+/- genotypes are grouped together as (+/^). Error bars represent standard deviations.
STAT5 activation is required for Friend virus-induced polycythemia
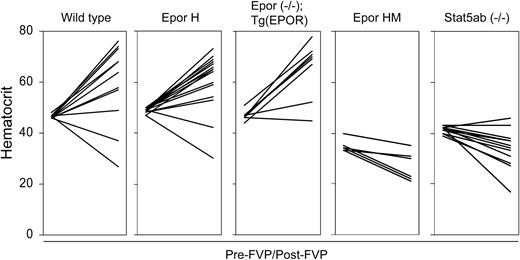
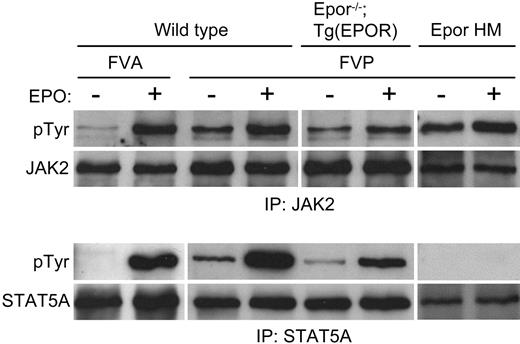
The underlying cause of FVP-induced polycythemia is the ability of gp55P to support EPO-independent terminal erythroid differentiation.32 To see if this effect is dependent on gp55P-mediated activation of the EPOR, we examined the susceptibility of our mutant strains of mice to FVP-induced polycythemia. Following infection with FVP, wild-type, EporH/H, and Epor-/-;Tg(EPOR) mice developed polycythemia, whereas EporHM/HM and Stat5a-/-; Stat5b-/- mice showed minimal change or developed anemia (Figure 4). Thus, STAT5, which is inactive or absent in EporHM/HM and Stat5a-/-;Stat5b-/- mice, respectively, has a role in FVP-induced polycythemia. To determine the effect of FVA and FVP on downstream targets of EPOR signaling, Friend virus-infected erythroblasts were purified by gravity sedimentation.28 In FVA-infected erythroblasts, in the absence of EPO there was faint JAK2 and no STAT5A tyrosine phosphorylation (Figure 5). EPO induced JAK2 and STAT5A phosphorylation. In FVP-infected erythroblasts, in the absence of EPO there was constitutive tyrosine phosphorylation of JAK2, STAT5A, and STAT5B (Figure 5 and data not shown). EPO induced an increase in JAK2 phosphorylation and a greater increase in STAT5A phosphorylation. We obtained similar results with erythroblasts isolated from Epor-/-; Tg(EPOR) mice. In FVP-infected erythroblasts from EporHM/HM mice there was constitutive JAK2 activation, but no STAT5A activation, in the absence or presence of EPO. Together, these results indicate that FVP causes polycythemia by constitutively activating the EPOR and STAT5.
STAT5 activation is required for Friend virus-induced polycythemia. Hematocrits of different strains of mice are shown at baseline (pre-FVP) and 3 to 4 weeks after infection with FVP (post-FVP). The mouse strains and genotypes are indicated at the top. In each panel, the pre-FVP hematocrits are shown on the left and the post-FVP hematocrits on the right with a line connecting the values. Low hematocrit values were occasionally caused by internal bleeding.
STAT5 activation is required for Friend virus-induced polycythemia. Hematocrits of different strains of mice are shown at baseline (pre-FVP) and 3 to 4 weeks after infection with FVP (post-FVP). The mouse strains and genotypes are indicated at the top. In each panel, the pre-FVP hematocrits are shown on the left and the post-FVP hematocrits on the right with a line connecting the values. Low hematocrit values were occasionally caused by internal bleeding.
FVP constitutively activates JAK2 and STAT5. Whole-cell extracts of Friend virus-infected erythroblasts were immunoprecipitated with anti-JAK2 (top group) or anti-STAT5A (bottom group) antibody and Western blotted. The antibody used for the Western blot is indicated to the left of the panels. The strain of mice, strain of Friend virus, and stimulation with erythropoietin are indicated at the top.
FVP constitutively activates JAK2 and STAT5. Whole-cell extracts of Friend virus-infected erythroblasts were immunoprecipitated with anti-JAK2 (top group) or anti-STAT5A (bottom group) antibody and Western blotted. The antibody used for the Western blot is indicated to the left of the panels. The strain of mice, strain of Friend virus, and stimulation with erythropoietin are indicated at the top.
Discussion
Friend virus differs from most acutely oncogenic retroviruses in that it lacks a mutated cellular protooncogene. Instead, the transforming gene in Friend virus encodes a mutated retroviral envelope protein, gp55. Previous studies suggested that the mechanism of Friend virus-induced erythroblastosis involves constitutive activation of the EPOR. One study showed that gp55-mediated activation of the EPOR supports Ba/F3-cell proliferation.9 Another showed that recombinant SFFV, engineered to express a constitutively active mutant of the EPOR, causes Friend-like disease and erythroleukemia.33 On the other hand, our laboratory has shown that susceptibility to Friend virus-induced erythroblastosis depends on expression of a truncated receptor tyrosine kinase, sf-STK.14 Because the previous studies utilized cell lines9 or enforced expression of a mutated receptor,33 we sought to establish the in vivo significance of gp55-mediated activation of the EPOR.
Through the use of mutant strains of mice, we showed that Friend virus-induced erythroblastosis does not depend on an interaction between sf-STK and the distal EPOR; nor does it require STAT5 activation. The diminished splenomegaly in the STAT5-deficient mice suggests that STAT5 may contribute to the development of erythroblastosis, but STAT5 is not essential, because it is not activated in the susceptible EporHM strain. Also, our results do not exclude the possibility that an interaction between the EPOR and sf-STK could result in the phosphorylation of sf-STK by JAK2. Recently, it was reported that phosphorylation of full-length STK by JAK2 can support the proliferation of erythroid cells.34
To determine whether the EPOR itself was required for susceptibility to Friend virus-induced erythroblastosis, we transplanted Epor-/-;Fv2s/s;Tg(GFP) fetal liver cells into Fv2r/r mice. Following infection, the transplant recipients did not develop erythroblastosis; however, because the target cell for Friend virus is EPO responsive35 and therefore potentially underrepresented, the failure of these mice to develop overt disease was not unexpected. Also, their spleens contained foci of EPOR-deficient erythroblasts, which expressed gp55, demonstrating that the EPOR is not essential for gp55-mediated erythroid proliferation. Another approach we took was to exploit a species-specific interaction between gp55 and the EPOR. The murine EPOR, but not the human EPOR, is able to support gp55P-mediated Ba/F3-cell proliferation.31 Indeed, the structural basis for this difference has been localized to a single amino acid in the transmembrane domain of the EPOR.36 We found that mice expressing the human EPOR instead of the murine EPOR were fully susceptible to Friend virus-induced erythroblastosis. Surprisingly, infection of these mice with FVP also caused constitutive phosphorylation of JAK2 and STAT5 and the development of polycythemia. Thus, the human EPOR is activated by gp55P in these mice and supports terminal differentiation. Differences in the mechanism of human EPOR activation between Ba/F3 cells and primary erythroid cells remain to be resolved.
There is evidence that constitutive activation of the EPOR can support erythroid proliferation and transformation. In addition to constitutively active mutants of the EPOR that cause erythroleukemia, there are deletion mutants of gp55P that circumvent Fv2 restriction.37 These mutants activate the EPOR in Ba/F3 cells and cause an attenuated form of Friend disease in Fv2s/s and Fv2r/r mice. However, they fail to interact with sf-STK and are poor inducers of EPO-independent erythroid bursts in vitro.38 We showed that gp55P causes constitutive phosphorylation of JAK2 and STAT5, which raises the possibility that activation of the EPOR could contribute to gp55P-mediated erythroid proliferation. Any effect of gp55P on EPOR signaling, however, is insufficient to cause Friend disease in the absence of sf-STK (ie, in Fv2r/r mice). Furthermore, gp55A, which minimally activates JAK2 and does not activate STAT5, is comparably effective at inducing erythroblastosis. Given the requirement for sf-STK, we conclude that Friend virus-induced erythroblastosis is primarily caused by gp55-mediated activation of sf-STK. Consistent with that interpretation, gp55 constitutively activates sf-STK,38 and gp55-mediated erythroid colony formation depends on sf-STK signaling.39
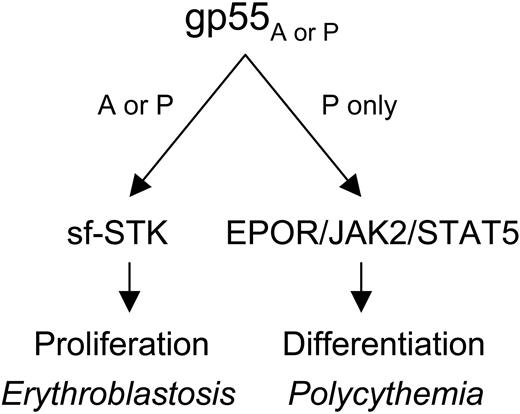
Mechanism of Friend virus-induced erythroblastosis and polycythemia. gp55 of the anemia-(gp55A) or polycythemia-inducing (gp55P) strains of Friend virus activates sf-STK, causing uncontrolled erythroid proliferation and erythroblastosis. gp55P activates the EPOR, JAK2, and STAT5, causing deregulated erythroid differentiation and polycythemia.
Mechanism of Friend virus-induced erythroblastosis and polycythemia. gp55 of the anemia-(gp55A) or polycythemia-inducing (gp55P) strains of Friend virus activates sf-STK, causing uncontrolled erythroid proliferation and erythroblastosis. gp55P activates the EPOR, JAK2, and STAT5, causing deregulated erythroid differentiation and polycythemia.
We found that gp55P-mediated activation of the EPOR and STAT5 are required for Friend virus-induced polycythemia. JAK2 and STAT5 were constitutively phosphorylated in FVP-infected, wild-type erythroid cells. JAK2 but not STAT5 was phosphorylated in FVP-infected, EporHM erythroid cells, and loss of STAT5 activity effectively converted FVP from a polycythemia- to an anemia-inducing strain. Also, STAT5 deficiency impairs EPO-dependent differentiation of FVA-infected erythroblasts (P.A.N., unpublished data, April 18, 2002). Thus, EPOR signaling, and specifically STAT5 activation, has a role in promoting the differentiation of Friend virus-infected erythroblasts. In summary, our results show that gp55 can activate sf-STK and the EPOR with distinct biologic effects (Figure 6). Activation of sf-STK by gp55A or gp55P causes uncontrolled erythroid proliferation and erythroblastosis, whereas activation of the EPOR by gp55P causes EPO-independent terminal differentiation and polycythemia.
Prepublished online as Blood First Edition Paper, September 20, 2005; DOI 10.1182/blood-2005-05-1784.
Supported by National Institutes of Health grant RO1 CA084214 (P.A.N.), National Institutes of Health Cancer Center support grant P30 CA21765, and the American, Lebanese, and Syrian Associated Charities.
J.Z. and M.S.R. contributed equally to this study.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We appreciate the efforts of the Flow Cytometry Laboratory and the Animal Resources Center of St Jude Children's Research Hospital. We thank Len Zon and Frank Costantini for providing Epor knock-out mice and Maurice Bondurant and Alan Bernstein for providing FVA and FVP. We thank Janet Partridge for helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal