Abstract
Populations of regulatory T cells (Tregs) control autoimmune and allergic immunopathology induced by self or foreign antigens. Several types of CD4+ MHC class II–restricted Treg populations have been characterized, but the biology of CD8+, MHC class I–restricted Tregs is less understood. We show here that CD8+ Tregs are rapidly generated in the presence of IL-4 and IL-12, produce IL-10, and exhibit a unique cell-surface phenotype with coexpression of activation and naive cell-associated markers. They block activation of naive or effector T cells and suppress IgG/IgE antibody responses and graft-versus-host disease in vivo. Suppression is dependent on cell contact and mediated by direct T-cell–T-cell interaction that antagonizes T-cell–receptor (TCR) signals. The data establish the existence of a CD8 T-cell suppressor effector subset distinct in both phenotype and function from T cytotoxic 1 (Tc1) and Tc2 cells. Production of such CD8 Tregs has potential for cell-based therapy of CD4 or CD8 T-cell–mediated disease.
Introduction
Acquired immunity is controlled by regulatory T cells (Tregs) that suppress responses of effector T cells. CD4+, MHC class II–restricted Tregs are best characterized and consist of thymically generated CD4+CD25+ cells with self-antigen specificities1 and related cells generated in secondary lymphoid tissue in response to foreign antigen.2 Both types of CD4 Tregs express a memory T-cell phenotype, and their absence results in uncontrolled autoimmunity.3 CD4+CD25+ Tregs act via a cell contact–dependent mechanism to block T-cell proliferation or secrete suppressive cytokines, including TGF-β and IL-10.4-6 IL-107 or TGF-β–secreting8 Tregs can emerge from peripheral CD4 populations after antigenic challenge.
The existence of regulatory CD8 T-cell subsets has received less attention despite earlier studies that identified CD8 populations as effectors of suppression in models of cellular and humoral immunity.9,10 Those reports proposed suppressive mechanisms which became discredited or dismissed. Recently, however, CD8 Tregs have been generated by stimulation with plasmacytoid dendritic cells (DCs)11 or immature DCs12 or in the presence of TGF-β13 and shown to act via IL-10 or by influencing the phenotype of antigen-presenting cells (APCs). Furthermore, suppressive CD8s that prevent secondary autoimmune responses have been demonstrated using Qa-1–deficient mice.14 These cells have unconventional Qa-1 restriction, and, although their mechanism of action is uncertain, they may kill autoreactive CD4 cells expressing Qa-1–presented peptides.15 It is not clear whether conventional antigen-specific CD8 cells mediate contact-dependent suppressor functions or contain a distinct Treg population equivalent to CD4+CD25+ cells. Here, we show that regulatory function can be rapidly induced in class I–restricted CD8 cells, resulting in potent contact-dependent suppression combined with IL-10 production. Transfer of CD8 Tregs into mice blocked antigen-specific antibody responses and graft-versus-host disease (GVHD). Such cells possess a unique cell-surface phenotype and represent a T-cell lineage distinct from T cytotoxic 1 (Tc1) and Tc2 subsets. They are induced by cytokines in vitro and by immunization in vivo, suggesting a role in regulation of acute or established inflammation.
Materials and methods
Mice and reagents
C57BL/6, BALB/c and CB6F1 [C57BL/6 × BALB/c]F1 mice (5-8 weeks old) were obtained from Harlan (Bicester, United Kingdom). OT-1 TCR transgenic mice (OVA257-264/H-2Kb–restricted; a gift from Dr M. Merkenschlager, Imperial College, London, United Kingdom) and OT-2 TCR transgenics (OVA323-339/I-Ab–restricted; Iffa Credo, Lyon, France) were bred in our facility. Experiments complied with United Kingdom Home Office regulations and were approved by the King's College London institutional Animal Welfare Committee. OVA257-264 (OVA257) and OVA323-339 (OVA323) peptides were synthesized by Sheffield University, United Kingdom. Antibodies were from BD Bioscience, Oxford, United Kingdom, unless otherwise indicated; cytokines were from PeproTech, London, United Kingdom. CpG 1826 was from VHBio, Gateshead, United Kingdom. Concentrations of neutralizing anti–IL-10 (JES5-16E3) and TGF-βR-Fc (R&D Systems, Abingdon, United Kingdom) were optimized by in vitro assay. Anti–TGF-β-biotin was from R&D. Biotinylated OVA was prepared using a Sulfo-NHS biotinylation kit (Perbio Science, Tattenhall, United Kingdom); alum-precipitated OVA was as described.16 Cell cultures were in DMEM (Invitrogen, Paisley, United Kingdom) + 10% FCS (Sera-Lab, Loughborough, United Kingdom) + l-glutamine (2 mM), nonessential amino acids (1 mM each), gentamicin (50 μg/mL), and 2-mercaptoethanol (50 μM). Cell fractionations and staining were performed in PBS + 1% FCS. CD152 staining was performed at 37°C. GITR labeling was performed using 1 μL anti-GITR antiserum followed by 1 μL anti–goat IgG-FITC. Flow cytometric analysis was performed using a FACScalibur (BD), with gating on live cells determined by forward versus side scatter. Other reagents were from Sigma, Poole, United Kingdom.
Cell fractionations
Dendritic cells (DCs) were purified from C57BL/6 splenocytes by labeling with anti–CD11c-biotin (0.5 μg/106 cells) followed by antibiotin microbeads (Miltenyi Biotec, Bisley, United Kingdom). CD11c+ cells were selected by 2 rounds of magnetic selection (autoMACS; Miltenyi Biotec) and were greater than 80% CD11c+ MHC class IIhi CD14–. CD4/CD8 T cells (> 98% purity) were isolated from spleen/lymph node using anti-CD4/CD8 microbeads. High-purity, APC-depleted CD8s were from OT-1 cells using high stringency autoMACS depletion for CD11c/CD11b/CD4/CD22 followed by CD8 positive selection. They were less than 0.1% positive for these markers and greater than 99% CD8+. CD44hi and CD44lo cells were separated from OT-1 CD8 cells prepared by negative selection and labeled with 0.001 μg anti–CD44-biotin per 106 cells. CD4+CD25+ cells were prepared by labeling with anti–CD25-PE/anti-PE microbeads and 2 rounds of selection (> 90% CD4+CD25+).
T-cell differentiation
Tregs were generated from OT-1 spleen/lymph node cells (106/mL) by addition of OVA257 (1 μg/mL) + IL-4 (10 ng/mL) + IL-12 (10 ng/mL) + dexamethasone (DEX; 10 nM), along with conventional Tc1 cells (peptide alone) as a control population. After 4 days cells consisted of greater than 98% CD8+ T-effector cells. For generation of stable cell lines, OT-1 Tc1 cell/Tregs were restimulated at weekly intervals with C57BL/6 DCs (1:20 DC/T cells) + OVA257 + anti-CD28 (1 μg/mL) + IL-15 (50 ng/mL) + IL-7 (10 ng/mL) ± IL-4/IL-12/DEX as described. Mixed lymphocyte reactions (MLRs) were induced using 2 × 106 CD4/CD8 BALB/c T cells + 105 C57BL/6 DCs ± IL-4/IL-12/DEX over 7 days. Cytokine profiles were determined by restimulation with immobilized anti-CD3/anti-CD28 (1 μg/mL each) + 3 μM monensin for 5 hours followed by intracellular cytokine staining as described.17 OT-2 T helper 1 (Th1)/Th2 effector cells were generated as described.18 Th1 or Th2 cells (106) were restimulated ± OT-1 Tc1 cells/Tregs + OVA323/OVA257 (1 μg/mL each) + 105 C57BL/6 DCs + 3 μM monensin for 5 hours.
CFSE suppression assays
OT-1/OT-2 spleen/lymph node cells were washed twice in PBS and labeled with CFSE (1 μM; Cambridge Bioscience, Cambridge, United Kingdom) for 10 minutes at 37°C in PBS, washed, and cultured with 1 μg/mL OVA257 (OT-1 assay) or 1 μg/mL OVA257 + 1 μg/mL OVA323 (OT-2 assay), ± OT-1 Tc1 cells/Tregs. For MHC pentamer-induced suppression, highly purified OT-1 CD8 T cells were labeled with CFSE then OVA257/H-2Kb Pro5 Pentamer (ProImmune, Oxford, United Kingdom), washed, and cultured ± unlabeled Tc1 cells or Tregs. For transwell experiments, 0.4-μm cell-culture inserts (BD Bioscience) were used with targets placed above and below the membrane. Tc1 cells or Tregs were placed below the membrane. After 2 days cells were stained with anti–CD25-PE + anti–CD4/CD8-APC before flow cytometry. Divided cells have reduced CFSE fluorescence. For the in vivo assay 1.5 × 107 CFSE+ OT-2 cells ± 5 × 106 OT-1 Tc1 cells or Tregs were injected intravenously into C57BL/6 mice, which were then challenged intranasally with 50 μg OVA in 50 μL PBS. After 2 days mediastinal lymph node cells were stained for CD4 and CD25.
Proliferation and cytotoxicity
For MLR proliferation assays BALB/c CD8 cells (106/mL) were stimulated with C57BL/6 DCs (5 × 104/mL) in microtiter plates with or without addition of cultured Tc1 cells/Tregs from MLRs. After 3 days wells were pulsed with 0.5 μCi (0.0185 MBq) 3H-thymidine, and incorporation was measured by scintillation counting after cell harvesting. Cytotoxicity was measured using the just another method (JAM) assay. OVA-transfected EL4 (EG7) cell targets labeled overnight with 3H-thymidine (2.5 μCi [0.0925 MBq] per mL of cells in exponential growth) were washed and cultured at 5 × 104/well in U-bottomed wells ± Tc1 cells/Tregs. Plates were centrifuged for 2 minutes and incubated for 4 hours before harvesting. Solubilization of labeled DNA in dying target cells was measured as reduction in cpm compared with control. Apoptosis/necrosis was measured by staining with annexin V-FITC + propidium iodide (0.5 μg/mL) in annexin-binding buffer (BD) before flow cytometry.
Enzyme-linked immunosorbent assay (ELISA) assays
Antibody pairs for IL-10 and IL-2 (all used at 1 μg/mL) were from BD. Serum IgE was determined using anti-IgE capture mAb (LOME-3, 3 μg/mL; Serotec, Oxford, United Kingdom) and either anti–κ-biotin (OX20, 3 μg/mL; Serotec) for total IgE or biotinylated OVA (3 μg/mL) for OVA-specific IgE. Anti-DNA autoantibodies were assayed as in Noble et al.19 For OVA-specific IgG1 assays, plates were coated with 50 μg/mL OVA. Standard curves were constructed using positive serum given a value in arbitrary units (AU/mL). Sera diluted in PBS + 0.5% FBS + 0.5% Tween 20 or culture supernatants were added overnight. Plates were incubated for 90 minutes with detecting mAbs. For OVA-specific IgE, biotinylated OVA was diluted in PBS/0.5% FCS/0.05% Tween 20. Plates were incubated with Extravidin-alkaline phosphatase (1/2000) for 60 minutes. p-Nitro-phenyl phosphate in 1 M diethanolamine was added and OD405nm was measured.
RT-PCR analysis of Foxp3 expression
RNA was prepared from 5 × 106 OT-1 Tc1 cells and Tregs from day 3 cultures or freshly isolated CD4+CD25+ and CD4+CD25– cells as controls. Reverse transcriptase–polymerase chain reaction (RT-PCR) protocol and primer sequences for Foxp3 and HPRT control were as described by Chen et al.20 Forty cycles of amplification were used.
In vivo assessment of Treg activity
Antibody responses to OVA were induced by intraperitoneal immunization with alum-precipitated OVA + Tc1 cells or Tregs, followed by tail bleeds. Bronchoalveolar lavage was performed by flushing lungs of killed mice with 1 mL PBS via the trachea using syringe and cannula. GVHD was induced by intraperitoneal injection of 7 × 107 BALB/c spleen/lymph node cells into CB6F1 recipients, with a second injection of cultured Tc1 cells/Tregs or PBS intraperitoneally. This protocol results in the chronic form of GVHD, and mice showed no signs of ill-health at any stage. Mice were killed after 7 weeks, and splenomegaly was measured by weighing spleens. Kidneys were snap-frozen in liquid nitrogen for assessment of glomerulonephritis by immunohistology. Cryostat sections (5 μM) were collected on polylysine-coated slides, air-dried, fixed in acetone, dried, and incubated with anti–mouse IgG1-FITC (1/100 in PBS/FCS; Serotec) before washing in PBS/FCS. Images were visualized using an Olympus BX40 microscope at 400 × magnification (40 ×/0.70 numeric aperture no oil objective; Olympus, Southall, United Kingdom), were acquired using a Nikon DXM1200 camera and Nikon ACT1 software version 2.20 (Nikon, Kingston, United Kingdom) and were saved as unprocessed JPEG files. Spleen cells were stained with anti–H-2Kb-FITC + anti–CD4/CD8-APC to assess the engraftment of donor (H-2Kb-negative) T cells by flow cytometry.
Statistical analysis
Differences between experimental groups were analyzed using the Mann-Whitney test.
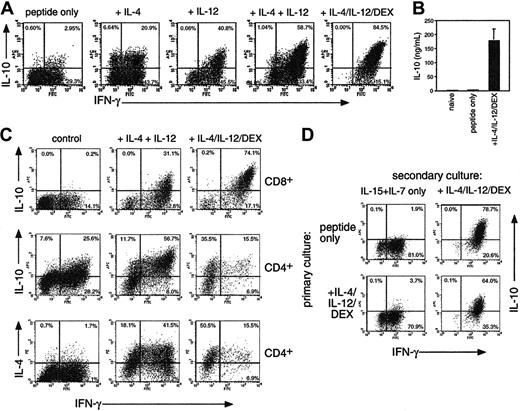
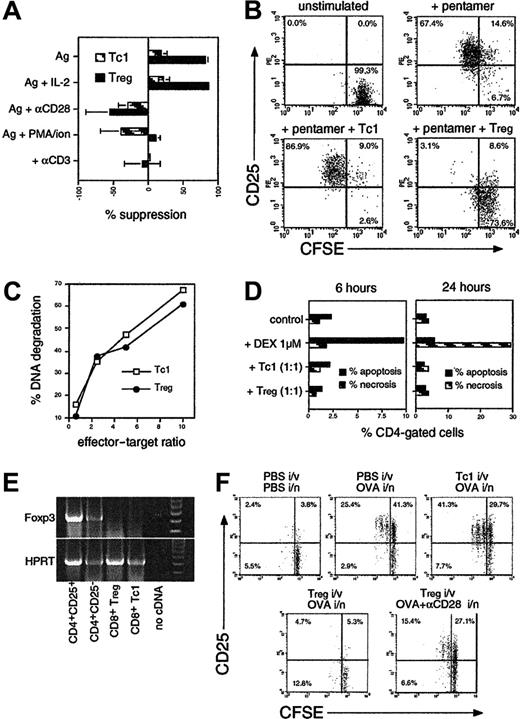
Regulation of IL-10 production in CD8 T cells. (A) OT-1 cells were stimulated with OVA257 alone or with IL-4, IL-12, and 10 nM DEX. After 4 days cells were restimulated before intracellular cytokine staining for IFN-γ, IL-4, and IL-10. The percentage of cytokine-producing cells is shown. IL-4 staining was less than 1% in all CD8 populations (not shown). (B) IL-10 secretion of naive OT-1 cells or those primed for 4 days with peptide ± IL-4/IL-12/DEX after restimulation with splenic APC + OVA257. (C) Comparison of CD8 (top row) with CD4 (bottom 2 rows) differentiation in MLRs using BALB/c CD4 or CD8 cells + C57BL/6 DCs. Cells were analyzed after 7 days as in panel A. IL-4 staining was less than 1% in all CD8 populations. (D) Generation of IL-10–producing cells from differentiated Tc1 effectors. OT-1 Tc1 cells were generated by peptide stimulation for 4 days, washed, and put into secondary culture with IL-15 + IL-7 ± IL-4/IL12/DEX. Four days later, cells were analyzed as in panel A. All data are representative of 4 independent experiments with similar results; in panel B, means ± SEMs from 4 independent experiments are shown.
Regulation of IL-10 production in CD8 T cells. (A) OT-1 cells were stimulated with OVA257 alone or with IL-4, IL-12, and 10 nM DEX. After 4 days cells were restimulated before intracellular cytokine staining for IFN-γ, IL-4, and IL-10. The percentage of cytokine-producing cells is shown. IL-4 staining was less than 1% in all CD8 populations (not shown). (B) IL-10 secretion of naive OT-1 cells or those primed for 4 days with peptide ± IL-4/IL-12/DEX after restimulation with splenic APC + OVA257. (C) Comparison of CD8 (top row) with CD4 (bottom 2 rows) differentiation in MLRs using BALB/c CD4 or CD8 cells + C57BL/6 DCs. Cells were analyzed after 7 days as in panel A. IL-4 staining was less than 1% in all CD8 populations. (D) Generation of IL-10–producing cells from differentiated Tc1 effectors. OT-1 Tc1 cells were generated by peptide stimulation for 4 days, washed, and put into secondary culture with IL-15 + IL-7 ± IL-4/IL12/DEX. Four days later, cells were analyzed as in panel A. All data are representative of 4 independent experiments with similar results; in panel B, means ± SEMs from 4 independent experiments are shown.
Results
Regulation of IL-10 production in CD8 T cells
In mice, Tr1 populations producing predominantly IL-10 can be induced by using repeated stimulation with IL-107 or treatment with vitamin D3 and DEX in the presence of neutralizing anti–IL-4 and anti–IL-12.21 We found strikingly different results when examining the generation of IL-10–producing CD8 T cells (Figure 1A). Stimulation of OT-1 CD8 cells with OVA257 resulted in a homogeneous population of Tc1-type effectors after 4 days, which produced IFN-γ, no IL-4, and little IL-10 when restimulated. Addition of IL-4 or IL-12 induced substantial IL-10 production, whereas a combination of IL-4 and IL-12 induced highly polarized IL-10+ IFN-γ+ populations. The level of IL-10 produced was further enhanced by the addition of DEX. Vitamin D3 had no effect on IL-10 in combination with DEX (not shown). The effects of IL-4 and IL-12 were not due to their ability to enhance growth or development of CD8 cells, because IL-15, which enhances growth to a similar extent, had no effect on cytokine profile (not shown). Intracellular staining for IL-10 after anti-CD3/CD28 restimulation correlated with the secretion of IL-10 after restimulation of similar cultures with irradiated APCs and peptide (Figure 1B). CD8 cells primed with IL-4, IL-12, and DEX secreted very high levels of IL-10 similar to that reported for Tr1 CD4 cells. Because TCR/peptide/MHC interactions exert a strong influence over T-cell differentiation,17 we determined the effects of the addition of IL-4/IL-12/DEX in cultures of nontransgenic CD8 T cells from BALB/c CD8 cells stimulated in MLRs with C57BL/6 DCs (Figure 1C) or with superantigen (not shown). Similar results were obtained, showing that induction of IL-10 by IL-4/IL-12/DEX was a general phenomenon. We used MLRs to compare CD4 and CD8 differentiation in the presence of IL-4 and IL-12 ± DEX (Figure 1C). Although CD8 cells failed to produce IL-4 under any conditions, IL-4 + IL-12 induced a Th0 phenotype in CD4 cells, with substantial numbers of IL-4, IFN-γ, and IL-10–positive cells. Unlike CD8 populations, DEX selectively blocked IFN-γ+ CD4 cells, resulting in Th2-type effectors. These data underline the distinct regulation of IL-10 in CD8 T cells.
We then determined whether induction of IL-10+ CD8 cells occurred only from naive T cells or could be initiated in differentiated CD8 cells. We found that an identical IL-10+ phenotype could be induced in differentiated Tc1 effectors if IL-4/IL-12/DEX was added after 4 days and cells were cultured for a further 4-day period (Figure 1D, top). Furthermore, IL-4/IL-12/DEX induced an equivalent IL-10+ phenotype from freshly isolated CD44hi memory or CD44lo naive OT-1 populations (not shown). Conversely, removal of IL-4/IL-12/DEX from Tregs and culture for a further 4 days resulted in a loss of IL-10–producing capacity (Figure 1D, bottom).
IL-10+ CD8 T cells exert potent contact-dependent suppressive activity
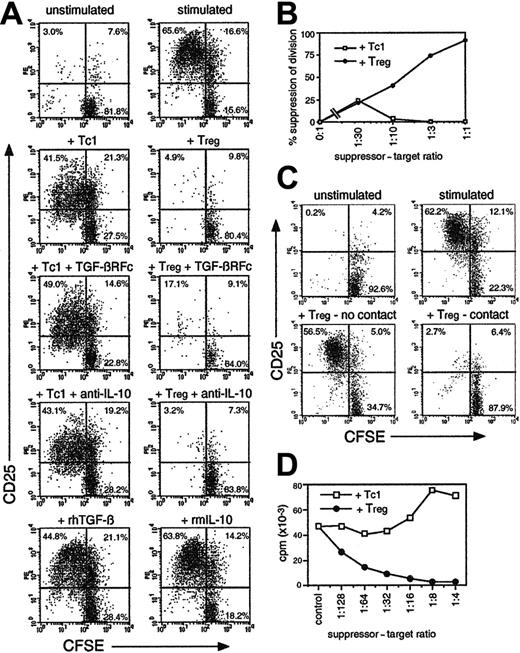
CD8 cells cultured with IL-4/IL-12/DEX were shown to potently suppress the proliferative response of other T cells (Figure 2) and are henceforth referred to as CD8 Tregs. Cells cultured without IL-4/IL-12/DEX exerted negligible suppressive activity and are termed Tc1 cells. Figure 2A shows the effect of OT-1 Tregs on OT-2 CD4 cells labeled with CFSE to determine proliferation, and stimulated with OVA323. Tregs inhibited both division of OT-2 cells (CFSE dilution) and their activation (appearance of CD25). Suppression was not dependent on IL-10 or TGF-β secretion, because the addition of neutralizing anti–IL-10 or TGF-β-receptor-Fc fusion protein failed to prevent suppression. Furthermore, addition of IL-10/TGF-β to OT-2 cells failed to inhibit growth (Figure 2A). OT-1 Tregs suppressed the response of both OT-2 CD4 cells and OT-1 CD8 T cells (Figure 2B). Maximum suppression was observed using a ratio of 1:2 Tregs to target T cells (Figure 2B). Transwell experiments were used to determine whether suppression was mediated by cytokines or cell contact (Figure 2C). Suppression was entirely contact dependent, because separation of CFSE+ target cells from Tregs by a permeable membrane completely abrogated suppression. IL-10–producing cells generated in CD8 T-cell MLRs + IL-4/IL-12/DEX (as in Figure 1C) were also tested for suppressive activity (Figure 2D). Tc1 cells or Tregs from MLRs (after 7 days) were added to conventional secondary MLRs, and proliferation was determined by thymidine incorporation. Tregs, but not Tc1 cells, completely blocked the MLRs, with suppression observed with as few as 1 Treg per 100 targets. Because this assay did not differentiate between target and effector proliferation, these data also indicate the anergic phenotype of Tregs. MLR-induced Tregs were enriched for alloantigen-specific suppressive activity, because they were less potent suppressors of an MLR against a third-party (C3H strain) APC (not shown).
IL-10–secreting CD8 cells suppress CD4 and CD8 T-cell growth and activation in a contact-dependent fashion. (A) Fresh CFSE-labeled OT-2 cells were stimulated with OVA257 + OVA323 ± TGF-βR-Fc neutralizing reagent (10 μg/mL), anti–IL-10 (5 μg/mL), TGF-β (10 ng/mL), or IL-10 (50 ng/mL). OT-1 cells primed for 4 days with OVA257 (Tc1 cells) or with IL-4/IL-12/DEX (Tregs; as in Figure 1) were added at a ratio of 1 Tc1/Treg to 2 targets. After 3 days, cells were stained for CD4/CD25. Gated CD4+ events (OT-2 targets) are shown. Top left quadrants indicate activated CD25+ CD4 cells which have divided. Total numbers of undivided targets recovered from each sample were 32 900 ± 4920 in unstimulated controls and 20 800 ± 7510 in the presence of Tregs. (B) Dose response of suppressive activity using OT-1 Tc1 cells or Tregs added to fresh CFSE-labeled OT-1 cultures at various doses. Experiment was analyzed as in panel A, but targets were distinguished by CFSE positivity alone. The percentage of suppression of the number of divided targets in a fixed volume of culture is shown. (C) Suppression by Tregs is contact dependent. CFSE+ OT-2 targets were cultured as in panel A but placed above and below a transwell membrane. OT-1 Tregs were added below the membrane. Cells were harvested after 3 days from above (no contact) and below (contact) the membrane and analyzed as in panel A. Total numbers of undivided targets recovered were 24 900 ± 8040 in unstimulated controls and 24 800 ± 6990 in the presence of Tregs. (D) Alloantigen-stimulated CD8 Tregs exhibit an anergic/suppressive phenotype. Tc1 cells/Tregs, generated in MLRs as in Figure 1C, were added to fresh BALB/c CD8 T cells + C57BL/6 DCs. Proliferation was measured by thymidine incorporation after 3 days. All data are representative of 3 independent experiments with similar results.
IL-10–secreting CD8 cells suppress CD4 and CD8 T-cell growth and activation in a contact-dependent fashion. (A) Fresh CFSE-labeled OT-2 cells were stimulated with OVA257 + OVA323 ± TGF-βR-Fc neutralizing reagent (10 μg/mL), anti–IL-10 (5 μg/mL), TGF-β (10 ng/mL), or IL-10 (50 ng/mL). OT-1 cells primed for 4 days with OVA257 (Tc1 cells) or with IL-4/IL-12/DEX (Tregs; as in Figure 1) were added at a ratio of 1 Tc1/Treg to 2 targets. After 3 days, cells were stained for CD4/CD25. Gated CD4+ events (OT-2 targets) are shown. Top left quadrants indicate activated CD25+ CD4 cells which have divided. Total numbers of undivided targets recovered from each sample were 32 900 ± 4920 in unstimulated controls and 20 800 ± 7510 in the presence of Tregs. (B) Dose response of suppressive activity using OT-1 Tc1 cells or Tregs added to fresh CFSE-labeled OT-1 cultures at various doses. Experiment was analyzed as in panel A, but targets were distinguished by CFSE positivity alone. The percentage of suppression of the number of divided targets in a fixed volume of culture is shown. (C) Suppression by Tregs is contact dependent. CFSE+ OT-2 targets were cultured as in panel A but placed above and below a transwell membrane. OT-1 Tregs were added below the membrane. Cells were harvested after 3 days from above (no contact) and below (contact) the membrane and analyzed as in panel A. Total numbers of undivided targets recovered were 24 900 ± 8040 in unstimulated controls and 24 800 ± 6990 in the presence of Tregs. (D) Alloantigen-stimulated CD8 Tregs exhibit an anergic/suppressive phenotype. Tc1 cells/Tregs, generated in MLRs as in Figure 1C, were added to fresh BALB/c CD8 T cells + C57BL/6 DCs. Proliferation was measured by thymidine incorporation after 3 days. All data are representative of 3 independent experiments with similar results.
CD8 Tregs inhibit cytokine production in naive and effector CD4 T cells
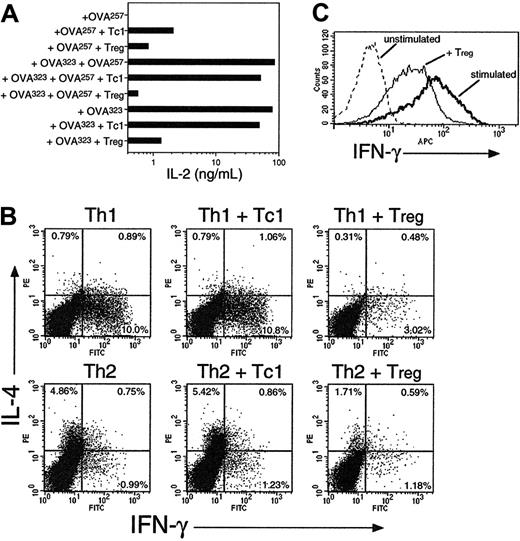
We assessed the cytokine responses of both naive and effector T cells in the presence of Tregs or control Tc1 cells. Freshly isolated OT-2 cells were stimulated in the presence or absence of OT-1 Tc1 cells or Tregs (Figure 3A). When stimulated with only OVA257, little IL-2 secretion was observed, because CD8 cells are poor IL-2 producers. In cultures stimulated with both class I– and class II–restricted peptides to activate both CD4 targets and CD8 effectors, high-level IL-2 secretion was observed which was inhibited 100-fold in the presence of Tregs. Thus, CD8 Tregs block IL-2 synthesis in CD4 targets, as reported for CD4+CD25+ cells. In contrast, Tc1 cells inhibited IL-2 secretion by only 40%, an effect that might be attributed to IL-2 consumption. Interestingly, suppression of IL-2 was also observed when cells were restimulated with OVA323 only. Thus, Treg effectors can exert suppression without the need for restimulation, if they are in a sufficiently active state. This is consistent with the notion of contact-dependent rather than cytokine-mediated suppression.
The restimulation of Th1 and Th2 effector CD4 cells was inhibited in the presence of CD8 Tregs (Figure 3B). In vitro–generated OT-2 Th1 and Th2 cells were restimulated with peptide + DCs + monensin and OT-1 Tc1 cells or Tregs. Accumulation of both intracellular IFN-γ from CD4 Th1 cells and IL-4 from Th2 cells was inhibited by Tregs but not Tc1 cells. This suppression was markedly less potent than observed with naive target cells (70% at 1:1 ratio). Tregs also partially inhibited the high levels of IFN-γ production in activated Tc1 effector cells (Figure 3C). Because these assays were performed in the presence of a protein transport inhibitor, they confirm that Treg cytokine secretion is not required for regulatory function. They also indicate the potential of these cells to control both primary and secondary T-cell–mediated immune responses.
CD8 Tregs have a unique “naive effector” cell-surface phenotype
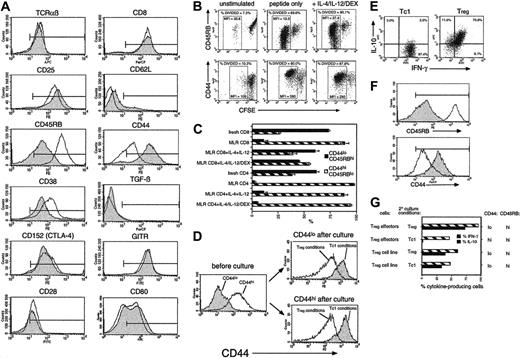
Characterization of OT-1 Tc1 cells and Tregs using a panel of antibodies is shown in Figure 4A. Tc1 cells and Tregs expressed equivalent levels of TCR, CD8, CD25, and CD28. Neither population expressed the lymph node homing receptor CD62L, indicating a memory/effector phenotype. Surprisingly, Tregs expressed a high level of CD45RB and a low level of CD44, whereas Tc1 cells displayed the conventional effector/memory phenotype (CD45RBlo CD44hi). Treg expression of CD38, a molecule associated with CD4+CD25+ cells, was slightly higher than in Tc1 cells. However, CD152/CTLA-4 and glucocorticoid-induced TNF receptor family-related gene (GITR), also associated with CD4+CD25+ cells,1 were expressed at equally high levels on Tc1 cells and Tregs. Both populations failed to express detectable cell-surface TGF-β, which has been implicated in CD4 Treg function.5 Thus, Tregs express an unusual phenotype, not previously described, with coexpression of both naive T-cell markers and an activated/effector phenotype. Analysis of CD45RB and CD44 in proliferating CFSE-labeled cells (Figure 4B) showed that cells developing into Tregs failed to convert to a memory phenotype despite equivalent cell division to Tc1 cultures. This also occurred in alloantigen-stimulated CD8 T cells but not CD4 cells treated with IL-4/IL-12/DEX (Figure 4C). Furthermore, freshly purified CD44hi memory cells from OT-1 mice developed into CD44lo Tregs when cultured in IL-4/IL-12/DEX (Figure 4D), indicating that appearance of the CD44lo phenotype is linked to Treg development and not an artifact of the culture conditions.
CD8 Tregs inhibit cytokine production from naive and effector CD4 and CD8 T cells. (A) OT-2 cells were stimulated with OVA257, OVA323, or both, with or without addition of OT-1 Tc1 cells or Tregs (generated as in Figure 1) at a ratio of 1 Tc1/Treg to 2 OT-2 CD4 cells. After 24 hours, IL-2 secretion was measured by ELISA. (B) CD8 Tregs inhibit cytokine production of Th1 and Th2 effectors. OT-2 Th1 or Th2 effector cells were restimulated for 5 hours with DCs + OVA257 + OVA323 + monensin, with or without OT-1 Tc1 cells or Tregs at a 1:1 ratio. The percentage of IL-4 and IFN-γ–producing cells in gated OT-2 targets are shown. (C) CD8 Tregs inhibit cytokine production of Tc1 CD8 effectors. OT-1 Tc1 effector cells were restimulated for 5 hours with DCs ± OVA257 + monensin, ± CFSE-labeled OT-1 Tregs (1:1). IFN-γ staining in CFSE-negative Tc1 targets is shown. All data are representative of 3 independent experiments with similar results.
CD8 Tregs inhibit cytokine production from naive and effector CD4 and CD8 T cells. (A) OT-2 cells were stimulated with OVA257, OVA323, or both, with or without addition of OT-1 Tc1 cells or Tregs (generated as in Figure 1) at a ratio of 1 Tc1/Treg to 2 OT-2 CD4 cells. After 24 hours, IL-2 secretion was measured by ELISA. (B) CD8 Tregs inhibit cytokine production of Th1 and Th2 effectors. OT-2 Th1 or Th2 effector cells were restimulated for 5 hours with DCs + OVA257 + OVA323 + monensin, with or without OT-1 Tc1 cells or Tregs at a 1:1 ratio. The percentage of IL-4 and IFN-γ–producing cells in gated OT-2 targets are shown. (C) CD8 Tregs inhibit cytokine production of Tc1 CD8 effectors. OT-1 Tc1 effector cells were restimulated for 5 hours with DCs ± OVA257 + monensin, ± CFSE-labeled OT-1 Tregs (1:1). IFN-γ staining in CFSE-negative Tc1 targets is shown. All data are representative of 3 independent experiments with similar results.
CD8 Tregs display a unique cell-surface phenotype which becomes stable after extensive culture. (A) OT-1 Tc1 (filled histograms) and Treg (open histograms) effectors were stained with a panel of antibodies. Positive staining relative to negative control is indicated by the markers. Similar results were obtained in 6 independent experiments. CD86 was barely detectable on either subset (not shown). (B) OT-1 cells fail to down-regulate CD45RB and up-regulate CD44 during culture in Treg-inducing conditions. CFSE-labeled OT-1 cells cultured in Tc1 or Treg conditions were analyzed after 3 days. CD8-gated events are shown. Boxed regions (% divided) indicate the similar extent of cell division observed under Tc1 and Treg conditions. Mean fluorescence intensities (MFIs) in these regions are indicated. Similar results were obtained in 3 independent experiments. (C) Conversion of naive to memory phenotype is inhibited by IL-4 + IL-12 in CD8 but not CD4 populations. The percentage of CD45RBhi CD44lo(naive phenotype) and CD45RBlo CD44hi (memory phenotype) cells was determined in CD8/CD4 BALB/c populations and after 7 days stimulation with C57BL/6 DC (MLR CD4/CD8) ± IL-4/IL-12/DEX. All cultured cells were CD62L negative (not shown). Means ± SEMs from 3 experiments are shown. (D) Both naive and memory CD8 T cells segregate into CD44hi and CD44lo populations after culture in Tc1- or Treg-inducing conditions, respectively. OT-1 CD8 cells were separated into CD44lo (naive) and CD44hi (memory) populations (left), mixed with DCs (20:1 ratio) and stimulated in Tc1- or Treg-inducing conditions as in panel B. CD44 expression of cells that were initially naive (top right) or memory (bottom right) phenotype was determined after 4 days. OT-1 Tc1/Treg cells were maintained in culture for 4 weeks using repeated restimulation with DCs, OVA257, and cytokines ± IL-4/IL-12/DEX. Intracellular cytokine analysis (E; as in Figure 1), or surface marker staining (F; as in Figure 4A) was performed. Filled histograms indicate Tc1 cell line; open histograms, Treg cell line. (G) Tregs were generated over a period of 4 days as in Figure 1 (Treg effectors) or 4 weeks as in panel E (Treg cell line). Washed cells were split and placed in secondary cultures with IL-15 + IL-7 + IL-4/IL-12/DEX (Treg conditions) or with IL-15 + IL-7 only (Tc1 conditions) for a further 4 days (effectors) or 7 days (cell lines). Treg cell lines also received DCs + OVA257-264 for the final 7 days. Finally, cells were reanalyzed for cytokine profile and phenotype. The percentage of cells positive for IFN-γ and IL-10 are shown (central histogram), and levels of CD44/CD45RB are indicated on the right. Data are representative of 3 independent experiments.
CD8 Tregs display a unique cell-surface phenotype which becomes stable after extensive culture. (A) OT-1 Tc1 (filled histograms) and Treg (open histograms) effectors were stained with a panel of antibodies. Positive staining relative to negative control is indicated by the markers. Similar results were obtained in 6 independent experiments. CD86 was barely detectable on either subset (not shown). (B) OT-1 cells fail to down-regulate CD45RB and up-regulate CD44 during culture in Treg-inducing conditions. CFSE-labeled OT-1 cells cultured in Tc1 or Treg conditions were analyzed after 3 days. CD8-gated events are shown. Boxed regions (% divided) indicate the similar extent of cell division observed under Tc1 and Treg conditions. Mean fluorescence intensities (MFIs) in these regions are indicated. Similar results were obtained in 3 independent experiments. (C) Conversion of naive to memory phenotype is inhibited by IL-4 + IL-12 in CD8 but not CD4 populations. The percentage of CD45RBhi CD44lo(naive phenotype) and CD45RBlo CD44hi (memory phenotype) cells was determined in CD8/CD4 BALB/c populations and after 7 days stimulation with C57BL/6 DC (MLR CD4/CD8) ± IL-4/IL-12/DEX. All cultured cells were CD62L negative (not shown). Means ± SEMs from 3 experiments are shown. (D) Both naive and memory CD8 T cells segregate into CD44hi and CD44lo populations after culture in Tc1- or Treg-inducing conditions, respectively. OT-1 CD8 cells were separated into CD44lo (naive) and CD44hi (memory) populations (left), mixed with DCs (20:1 ratio) and stimulated in Tc1- or Treg-inducing conditions as in panel B. CD44 expression of cells that were initially naive (top right) or memory (bottom right) phenotype was determined after 4 days. OT-1 Tc1/Treg cells were maintained in culture for 4 weeks using repeated restimulation with DCs, OVA257, and cytokines ± IL-4/IL-12/DEX. Intracellular cytokine analysis (E; as in Figure 1), or surface marker staining (F; as in Figure 4A) was performed. Filled histograms indicate Tc1 cell line; open histograms, Treg cell line. (G) Tregs were generated over a period of 4 days as in Figure 1 (Treg effectors) or 4 weeks as in panel E (Treg cell line). Washed cells were split and placed in secondary cultures with IL-15 + IL-7 + IL-4/IL-12/DEX (Treg conditions) or with IL-15 + IL-7 only (Tc1 conditions) for a further 4 days (effectors) or 7 days (cell lines). Treg cell lines also received DCs + OVA257-264 for the final 7 days. Finally, cells were reanalyzed for cytokine profile and phenotype. The percentage of cells positive for IFN-γ and IL-10 are shown (central histogram), and levels of CD44/CD45RB are indicated on the right. Data are representative of 3 independent experiments.
CD8 Tregs represent a stable T-cell lineage after repeated division
Development of Th1/Th2 and Tc1/Tc2 subsets is characterized by divergent but unstable phenotypes in primary effectors (4-5 days after stimulation), followed by irreversible, stable phenotypes after multiple cell divisions (> 3 weeks of culture with repeated stimulation).22 We sought to generate stable Treg cell lines to establish such cells as a genuine T-cell subset distinct from Tc1 and Tc2 cells. OT-1 cells were repeatedly stimulated in Tc1- or Treg-inducing conditions over a period of 4 weeks. IL-15 and IL-7 were added to both Tc1 cells and Tregs to maintain viability. Resulting Tregs expressed the expected characteristics of high IL-10/IFN-γ production (Figure 4E) and naive CD45RBhiCD44lo phenotype (Figure 4F). Stability of Treg effectors and long-term cell lines was compared (Figure 4G). Treg effectors rapidly lost their ability to produce IL-10 and CD44lo phenotype if IL-4/IL-12/DEX was removed from the culture, although CD45RBhi expression was retained. Conversely, Treg cell lines retained all phenotypic characteristics after a final round of stimulation in Tc1-inducing culture conditions. Thus, Tregs represent a distinct lineage distinguishable from their Tc1 and Tc2 counterparts by cytokine profile, cell-surface phenotype, and functional properties.
CD8 Tregs antagonize TCR-dependent activation signals and act via direct T-cell–T-cell interaction
CFSE suppression assays were used to investigate the mechanism of contact-dependent Treg suppression. The addition of IL-2 was unable to reverse the suppression mediated by Tregs, but anti-CD28 costimulation completely restored the response (Figure 5A). Addition of PMA and ionomycin, which directly activate TCR-linked signaling pathways, also abrogated suppression, as did replacing the peptide stimulus with immobilized anti-CD3. These results suggested that Tregs antagonized TCR signaling in target T cells, because only stimuli that amplified or induced TCR-mediated signals were effective. To determine whether this was mediated via direct T-cell–T-cell interaction or via APCs, we used highly purified, APC-depleted OT-1 cells stimulated directly with OVA257–H-2Kb pentamers (Figure 5B). Addition of Tregs effectively blocked pentamer-induced activation in the absence of APCs. Cytotoxicity in Tc1 cells and Tregs was compared, because it was possible that Tregs could mediate suppression by killing their targets (Figure 5C). However, Tregs and Tc1 cells were equally cytotoxic. Furthermore, direct measurement of apoptosis and necrosis in OT-2 targets in suppression cultures showed that target T cells were not killed (Figure 5D). The mechanism of CD8 Treg function appeared similar to that reported for CD4+CD25+ Tregs, although such suppression is typically reversed by IL-2. However the pathway of CD8 Treg development is distinct, because day 3 Tregs did not express the Foxp323 transcription factor (Figure 5E). Foxp3 mRNA was highly expressed in CD4+CD25+ cells but undetectable in CD8 Tc1 cells or Tregs. To test whether the same suppressive mechanism operates in vivo, we transferred CFSE+ targets and Tc1 cells or Tregs intravenously into naive mice and delivered OVA via the lung (Figure 5F). This showed that Tregs also block target CD25 up-regulation and proliferation in draining lymph nodes in vivo. Furthermore, delivery of anti-CD28 along with OVA reversed suppression, suggesting the same mechanism was operative as seen in vitro.
Suppression of the immune response to exogenous Ag and GVHD by CD8 Tregs
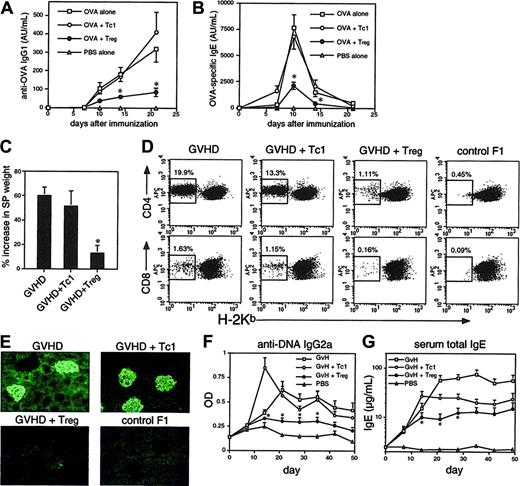
Transfer of OT-1 Tregs, but not Tc1 cells, into C57BL/6 mice suppressed a conventional antibody response induced by OVA/alum immunization (Figure 6A-B). We previously showed that Tc1 cells inhibit IgE, but not predominant IgG1 responses to OVA/alum.16 Here, Tc1 cells failed to inhibit both IgG1 and IgE responses at the dose used, whereas Treg cells significantly inhibited both IgG and IgE. Likewise, alloantigen-primed, in vitro–generated CD8 Tregs were potent suppressors of GVHD in vivo (Figure 6C-G). GVHD was induced in nonlymphopenic CB6F1 mice by injection of BALB/c parental cells, resulting in chronic disease with both Th1 and Th2 components. Alloantigen-primed Tc1 had little effect on splenomegaly, donor T-cell engraftment, or deposition of immune complexes in the kidney induced during GVHD (Figures 6C-E). These responses were almost completely inhibited in recipients of Tregs. Anti-DNA IgG2a autoantibodies (Th1 associated) were more rapidly induced in Tc1-cell recipients but inhibited by Tregs (Figure 6F). Development of hyper-IgE syndrome, a Th2-dependent characteristic of chronic GVHD, was inhibited by approximately 50% in Tc1-cell recipients but by greater than 80% in Treg recipients (Figure 6G).
Mechanism of suppression used by CD8 Tregs. (A) Suppression is reversed by TCR or CD28-related signals but not IL-2. Suppression assays were performed as in Figure 2A by using CFSE-labeled OT-2 cells ± OT-1 Tc1/Treg, stimulated with OVA257 + OVA323 (Ag) ± IL-2 (10 ng/mL), anti-CD28 (1 μg/mL), PMA (10 ng/mL) + ionomycin (400 ng/mL), or immobilized anti-CD3 alone (1 μg/mL). The percentage of suppression of divided targets generated in a fixed volume of culture (means ± SEMs from 3 independent experiments) is shown. (B) Direct T/T suppression in the absence of APCs. CFSE+ OT-1 CD8 cells depleted of all APCs (< 0.1%) were labeled with OVA257–H-2Kb Pentamer. OT-1 Tc1 cells/Tregs were added at a 1:2 suppressor-to-target ratio, and CD25 was analyzed in CFSE+-gated targets after 2 days. One of 3 independent experiments with similar results is shown. (C) Tregs possess equivalent cytotoxicity to Tc1 cells. OT-1 Tc1cells/Tregs were mixed with 3H-thymidine–labeled EG-7 cells, and CTL activity was measured after 4 hours. The percentage of DNA degradation in labeled targets is shown. (D) Tc1 cells/Tregs do not kill OT-2 targets in a suppression assay. OT-2 cells were stimulated with OVA257 + OVA323 ± OT-1 Tc1/Treg (1:1) for 6 or 24 hours, then stained with anti-CD4, annexin V, and propidium iodide (PI). Levels of apoptotic (annexin V+ PI–) and necrotic (annexin V+ PI+) CD4 cells are shown. Similar results were obtained in 3 independent experiments. (E) CD8 Tregs do not express Foxp3. RT-PCR for Foxp3 (top) and HPRT control (bottom) were performed on OT-1 Tc1 cells/Tregs after 3 days and from CD4+CD25– or CD4+CD25+ cells stimulated with anti-CD3/anti-CD28/IL-2 as a positive control. Molecular weight markers (right-hand lane) confirmed correct size of PCR products. Data are representative of 3 independent experiments. (F) In vivo suppression of T-cell activation is abrogated by costimulation. CFSE+ OT-2 cells were transferred into C57BL/6 mice with or without unlabeled OT-1 Tc1 cells or Tregs (3:1), and mice were challenged with OVA ± anti-CD28 intranasally as indicated. Target cell activation in draining lymph nodes (CD4+ CFSE+ gated events) is shown after 2 days.
Mechanism of suppression used by CD8 Tregs. (A) Suppression is reversed by TCR or CD28-related signals but not IL-2. Suppression assays were performed as in Figure 2A by using CFSE-labeled OT-2 cells ± OT-1 Tc1/Treg, stimulated with OVA257 + OVA323 (Ag) ± IL-2 (10 ng/mL), anti-CD28 (1 μg/mL), PMA (10 ng/mL) + ionomycin (400 ng/mL), or immobilized anti-CD3 alone (1 μg/mL). The percentage of suppression of divided targets generated in a fixed volume of culture (means ± SEMs from 3 independent experiments) is shown. (B) Direct T/T suppression in the absence of APCs. CFSE+ OT-1 CD8 cells depleted of all APCs (< 0.1%) were labeled with OVA257–H-2Kb Pentamer. OT-1 Tc1 cells/Tregs were added at a 1:2 suppressor-to-target ratio, and CD25 was analyzed in CFSE+-gated targets after 2 days. One of 3 independent experiments with similar results is shown. (C) Tregs possess equivalent cytotoxicity to Tc1 cells. OT-1 Tc1cells/Tregs were mixed with 3H-thymidine–labeled EG-7 cells, and CTL activity was measured after 4 hours. The percentage of DNA degradation in labeled targets is shown. (D) Tc1 cells/Tregs do not kill OT-2 targets in a suppression assay. OT-2 cells were stimulated with OVA257 + OVA323 ± OT-1 Tc1/Treg (1:1) for 6 or 24 hours, then stained with anti-CD4, annexin V, and propidium iodide (PI). Levels of apoptotic (annexin V+ PI–) and necrotic (annexin V+ PI+) CD4 cells are shown. Similar results were obtained in 3 independent experiments. (E) CD8 Tregs do not express Foxp3. RT-PCR for Foxp3 (top) and HPRT control (bottom) were performed on OT-1 Tc1 cells/Tregs after 3 days and from CD4+CD25– or CD4+CD25+ cells stimulated with anti-CD3/anti-CD28/IL-2 as a positive control. Molecular weight markers (right-hand lane) confirmed correct size of PCR products. Data are representative of 3 independent experiments. (F) In vivo suppression of T-cell activation is abrogated by costimulation. CFSE+ OT-2 cells were transferred into C57BL/6 mice with or without unlabeled OT-1 Tc1 cells or Tregs (3:1), and mice were challenged with OVA ± anti-CD28 intranasally as indicated. Target cell activation in draining lymph nodes (CD4+ CFSE+ gated events) is shown after 2 days.
Induction of CD8 Treg in vivo
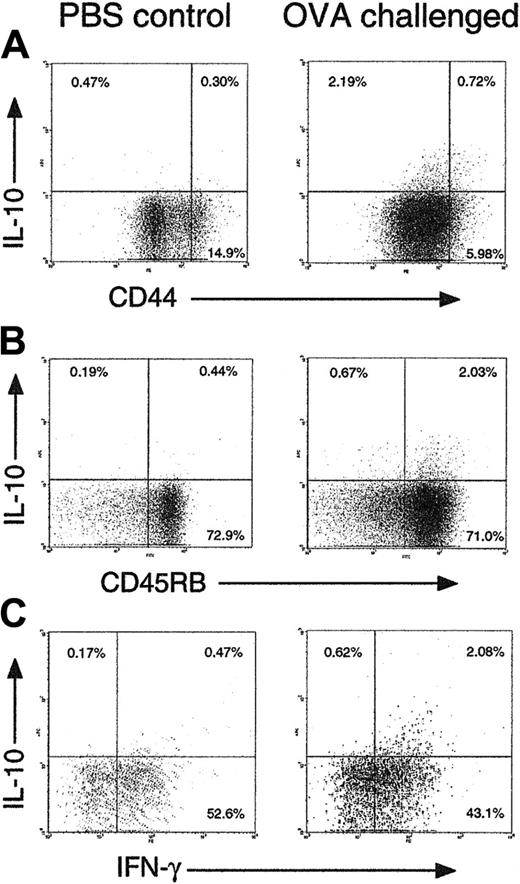
We hypothesized that the Treg subset we could induce in vitro would be generated at inflammatory sites in vivo, in response to IL-4 and IL-12. To test this hypothesis, OT-1 CD8 cells and OT-2 CD4 T cells from untreated mice were adoptively transferred into C57BL/6 recipients. These mice were then immunized via the intranasal route with OVA + adjuvant to induce an inflammatory response in the lung (Figure 7). Analysis of lung T cells after 6 days by intracellular staining showed that IL-10–producing CD8 cells were induced in the lung after sensitization. The majority of the IL-10+ CD8 cells were CD44lo, CD45RBhi, and IFN-γ+, suggesting that a population of CD8 Tregs similar to those identified in vitro were indeed present in inflammation. Such cells were rare in draining lymph nodes (not shown). They were only detected approximately 6 to 8 days after sensitization, a time course that is similar to in vivo Tc1-cell development.
CD8 Tregs suppress Th1- and Th2-associated immunity in vivo. (A-B) C57BL/6 mice were immunized with 400 μg OVA/alum + PBS (OVA alone) or 2.5 × 106 OT-1 Tc1 and Treg cells intraperitoneally. Serum anti-OVA IgG1 (A) and IgE (B) antibodies were monitored by serial bleeding. Antibody titers are shown in arbitrary units. (C-G) Alloantigen-primed CD8 Tregs prevent development of chronic GVHD. Tc1 cells or Tregs were generated in MLRs as in Figure 2D. GVHD was induced by injection of CB6F1 mice with 7 × 107 BALB/c splenocytes, ± Tc1 cells or Tregs (3 × 106). Control F1 mice received PBS only. After 7 weeks spleens were harvested and weighed to assess splenomegaly (C) and engraftment of donor BALB/c cells in each spleen measured by staining for H-2Kb (D). Donor cells lack H-2Kb; the percentage of donor engraftment in CD4 and CD8 populations is indicated. (E) Kidneys harvested at 7 weeks were sectioned and stained with anti–IgG1-FITC before fluorescence microscopy. Deposition of IgG1 immune complexes in glomeruli is indicated by the green fluorescent structures. (F-G) Suppression of autoantibody production (F) and hyper-IgE syndrome (G) by CD8 Treg cells. Mice as in panel C were monitored for anti-DNA IgG2a autoantibodies (F) and total serum IgE (G). Data shown are means ± SEMs from groups of 4 mice (A-C and F-G) or are representative of groups of 4 mice (D-E). Similar data were obtained in a further experiment with groups of 3 mice. *P < .05 when comparing Tregs with the Tc1 control group.
CD8 Tregs suppress Th1- and Th2-associated immunity in vivo. (A-B) C57BL/6 mice were immunized with 400 μg OVA/alum + PBS (OVA alone) or 2.5 × 106 OT-1 Tc1 and Treg cells intraperitoneally. Serum anti-OVA IgG1 (A) and IgE (B) antibodies were monitored by serial bleeding. Antibody titers are shown in arbitrary units. (C-G) Alloantigen-primed CD8 Tregs prevent development of chronic GVHD. Tc1 cells or Tregs were generated in MLRs as in Figure 2D. GVHD was induced by injection of CB6F1 mice with 7 × 107 BALB/c splenocytes, ± Tc1 cells or Tregs (3 × 106). Control F1 mice received PBS only. After 7 weeks spleens were harvested and weighed to assess splenomegaly (C) and engraftment of donor BALB/c cells in each spleen measured by staining for H-2Kb (D). Donor cells lack H-2Kb; the percentage of donor engraftment in CD4 and CD8 populations is indicated. (E) Kidneys harvested at 7 weeks were sectioned and stained with anti–IgG1-FITC before fluorescence microscopy. Deposition of IgG1 immune complexes in glomeruli is indicated by the green fluorescent structures. (F-G) Suppression of autoantibody production (F) and hyper-IgE syndrome (G) by CD8 Treg cells. Mice as in panel C were monitored for anti-DNA IgG2a autoantibodies (F) and total serum IgE (G). Data shown are means ± SEMs from groups of 4 mice (A-C and F-G) or are representative of groups of 4 mice (D-E). Similar data were obtained in a further experiment with groups of 3 mice. *P < .05 when comparing Tregs with the Tc1 control group.
Discussion
Many experimental models have been used to define regulatory/suppressor T cells, which have been identified as CD4+, CD8+, or CD4/CD8 double negative and shown to use a myriad of mechanisms from anti-idiotypic recognition and cytotoxicity to immune deviation, cytokine secretion, and “prohibition” of APCs.6,8,24-26 This has made it difficult to provide a unifying model for induction of suppressive T cells from T cells of any antigen specificity. CD4 Tr1 cells can be induced in vitro to a number of antigens using IL-10 or vitamin D3 + DEX treatments.7,21 These protocols require repeated rounds of stimulation and absence of IL-4 and IL-12. This contrasts with our data using CD8 precursors, indicating that such growth-promoting cytokines induce Tregs, and suggests that CD8 Tregs might be induced during periods of active inflammation when such cytokines are secreted. CD8 Tregs are induced using a variety of primary stimuli, indicating that their development is directly induced via STAT-4 (IL-12) and STAT-6 (IL-4) signaling pathways irrespective of TCR signals, which are critical for Tc1/Tc2 regulation.17 The ability of DEX to enhance development of these Tregs provides another pathway to account for the potent immunosuppressive effects of corticosteroids. Circulating levels of natural corticosterone are known to be elevated during episodes of acute inflammation, for example, during superantigen responses.27,28 It can be envisaged that excessive T-cell–APC stimulation would lead to simultaneous release of IL-4, IL-12, and circulating corticosterone, allowing development of CD8 Tregs required to bring the inflammatory reaction under control. Indeed, intranasal challenge of mice with OVA elicited IL-10+ CD8 T cells with a Treg phenotype in the lung. Similarly, exposure to superantigen is known to switch CD8, as well as CD4 T cells, into an IL-10–secreting phenotype.29
CD44lo CD45RBhi IL-10+ CD8 T cells appear in an inflammatory site in vivo. C57BL/6 mice were injected with 107 OT-1 CD8 and 107 OT-2 CD4 cells intravenously on day 0. On days 0 and 3 they were challenged intranasally with 50 μg OVA + 10 μg CpG 1826 oligonucleotide in 50 μL PBS (right) or PBS alone (left). On day 6, mice were killed, and T cells were extracted from lung tissue by collagenase digestion. T cells (106) were labeled with anti-CD44 + anti-CD8 (A), anti-CD45RB + anti-CD8 (B), or anti-CD8 alone (C), then restimulated with anti-CD3 + anti-CD28 for 5 hours followed by intracellular cytokine staining. Gated CD8+ cells are shown in all plots. Representative results from groups of 4 mice are shown. Similar data were obtained in 3 independent experiments.
CD44lo CD45RBhi IL-10+ CD8 T cells appear in an inflammatory site in vivo. C57BL/6 mice were injected with 107 OT-1 CD8 and 107 OT-2 CD4 cells intravenously on day 0. On days 0 and 3 they were challenged intranasally with 50 μg OVA + 10 μg CpG 1826 oligonucleotide in 50 μL PBS (right) or PBS alone (left). On day 6, mice were killed, and T cells were extracted from lung tissue by collagenase digestion. T cells (106) were labeled with anti-CD44 + anti-CD8 (A), anti-CD45RB + anti-CD8 (B), or anti-CD8 alone (C), then restimulated with anti-CD3 + anti-CD28 for 5 hours followed by intracellular cytokine staining. Gated CD8+ cells are shown in all plots. Representative results from groups of 4 mice are shown. Similar data were obtained in 3 independent experiments.
Suppressive activities of Tr1 cells have been attributed to their secretion of IL-10. By contrast, CD4+CD25+ T cells produce less IL-10 and suppress via IL-10–independent and IL-10–dependent mechanisms.30,31 The CD8 subset described here is unusual in that its suppressive mechanism is clearly contact dependent, but it produces copious IL-10 in addition to IFN-γ. Although Tr1 cells are reported to secrete low levels of IFN-γ,21 other studies describe Tregs producing both IL-10 and IFN-γ,32 and in certain models IFN-γ is identified as part of a suppressive pathway.33 Thus, despite its proinflammatory functions, this cytokine may contribute to the regulation of T-cell responses.34,35 Another unusual feature of CD8 Tregs is their naive effector phenotype. Previous data show that activation of naive T cells results in a rapid loss of CD45RB expression and up-regulation of CD44, and that the CD45RBloCD44hi phenotype is stable in memory T-cell populations. However, our data show that in Treg-inducing conditions naive CD8 T cells fail to switch to the memory phenotype despite developing into fully activated blasts. Remarkably, memory CD8 cells also adopted the CD44lo, naive phenotype after differentiation into Tregs. Although in vivo reversion of CD45 isoforms has been described,36 this is the first evidence to our knowledge that naive T cells can retain their phenotype during differentiation to effector cells. Whether this has functional significance is unknown.
The mechanism of action of CD8 Tregs bears many similarities to that of CD4+CD25+ Tregs, including inhibition of IL-2 production in target cells, dependence on cell contact, and abrogation of suppression with PMA/ionomycin. It differs in that CD4+CD25+-mediated suppression is reversed by exogenous IL-2, but CD8 Treg-mediated inhibition was reversed by ligation of CD28 or TCR-mediated stimuli, but not IL-2. The lack of response to IL-2 is presumably due to the observed blockade of CD25 up-regulation, which provides a high-affinity IL-2 receptor. Studies of CD4+CD25+ cells, Tr1 cells, and human CD8 Tregs suggest that suppression is mediated through APCs via down-regulation of costimulatory molecules or up-regulation of inhibitory receptors. CD4+CD25+ Tregs also suppress via direct T-T contact37 ; an analogous mechanism appears to predominate in our system. Together our data suggest that CD8 Tregs directly antagonize TCR signaling in target cells. Because CD28 ligation acts mainly to amplify TCR signals via induction of lipid raft formation,38 this model explains why anti-CD28, immobilized anti-CD3, and PMA/ionomycin are able to abrogate suppression but not IL-2. A number of molecules have been identified which interfere with TCR-dependent activation.39
Peripheral tolerance to self-antigens is thought to be maintained by presentation of tissue antigens from dying cells by immature DCs. This results in partial deletion and/or an anergic/regulatory phenotype in responding T cells. However, DC activation by inflammatory stimuli can prevent or reverse this type of tolerance,40 and unresponsiveness can be broken by the provision of IL-2 to tolerized cells.41 Tregs are also induced by proinflammatory challenges to the immune system. These Tregs might develop to control existing inflammation triggered by a persistent antigen or to prevent sterilizing immunity.30 Cytokine-induced Tregs, such as those described here, would provide such a negative feedback mechanism. A role for cytokines in Treg induction might have been predicted by the observation of Gershon42(p170) in 1975 that “feedback signals from target cells are of crucial importance in determining and maintaining the activity of suppressor T cells.” More recently, in vivo development of CD8 Tregs which act primarily on secondary CD4 immune responses has been shown to control experimental autoimmune encephalomyelitis.14 These cells recognize Qa-1 nonclassic MHC, which allows them to eliminate pathogenic CD4 cells. Our data indicate that CD8 T cells with conventional antigenic specificity can progress along a differentiative pathway associated with specialized suppressive function and independent of the Foxp3-initiated program.23
Rapid in vitro generation of Tregs is of potential value in the treatment of immune-mediated disease. Although tolerance-inducing protocols can generate Tregs in vivo, they are less successful when used to treat established immune reactivity. The approach described here would rapidly generate Tregs ex vivo, and use of CD8 Tregs has the advantage that antigen-specific CD8 T precursors can be readily purified using MHC multimers. Because CD8 Tregs inhibit restimulation of Th1, Th2, and Tc1 effector cells, they would provide a useful tool for established disease. One example is GVHD, which remains a major barrier to hemopoietic stem cell transplantation. Our model indicates that generation of allospecific CD8 Tregs could be used as an improved method for delayed donor T-cell transfusion in GVHD. Likewise, the ability of CD8 Tregs to block Th2 activation and IgE antibody responses would be valuable in immunotherapy of allergic disease. A defect in IL-10–associated regulation is implicated in severe atopic asthma refractive to steroid therapy.43 Dust mite allergen–specific CD8 T cells produce IFN-γ and IL-10 in sensitized but asymptomatic individuals, but IFN-γ without IL-10 in severe atopic dermatitis.44 These data implicate IL-10–secreting CD8 cells as potential regulators of allergic immunopathology. Our finding that suppressive function can be induced in differentiated Tc1 effectors or memory cells is consistent with the idea that immune deviation toward IL-10–associated tolerance is achievable in established disease.
It has previously been shown that CD8 Tregs are induced during induction of mucosal tolerance.45 Our data suggest that they are also a component of productive immune responses, but their effect is counteracted by costimulation. Regulation of the class I–restricted compartment is crucial because all tissues can present self-antigen to activated autoreactive CD8 T cells, and cross-presentation is important for balanced responses to environmental antigens.16 Furthermore, not all CD8 T-cell responses require CD4 help46 and some might escape regulation maintained via the CD4 compartment alone. Self-reactive CD8 Treg cell lines with a Tc2 phenotype have recently been generated from patients with ankylosing spondylitis.47 Suppressive activity has also been described in endogenous FoxP3-expressing rat T cells.48 Given the emerging diversity of CD8 Treg subsets, the original concept of the CD8 population as a suppressor/cytotoxic subset might have been discarded prematurely.
Prepublished online as Blood First Edition Paper, February 7, 2006; DOI 10.1182/blood-2005-10-3994.
Supported by the MRC (grant G9803622) (A.N.) and the Biotechnology and Biological Sciences Research Council UK (grant 29/C16755) (A.N.).
A.N., A.G., and J.A.L. performed the research; A.N. designed the research and wrote the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Joanna Galea-Lauri for EG-7 cells, Matthias Merkenschlager for OT-1 mice, Mike Kemeny for OT-2 mice, Ela Sawicka and Christophe Walker for antibodies, Kasia Hawrylowicz for helpful discussions, and Ray Thatcher and Ted Davies for technical advice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal