Abstract
Although the nucleoside analogs cladribine and pentostatin produce high response rates in patients with hairy cell leukemia (HCL), a significant number of patients eventually relapse. Several studies have demonstrated that patients with complete remission (CR) have a longer disease-free survival. Therefore, strategies to improve on the initial response to nucleoside analog therapy are likely to be beneficial, at least for a proportion of patients. We have treated 13 patients with newly diagnosed HCL (n = 11) or after failure of one prior chemotherapy (n = 2) with cladribine (5.6 mg/m2 given intravenously over 2 hours daily for 5 days) followed by 8 weekly doses of rituximab (375 mg/m2). All patients achieved a CR and minimal residual disease (MRD) assessed by consensus primer polymerase chain reaction (PCR) or flow cytometry was eradicated in 11 (92%) of 12 and in 12 (92%) of 13 of patients, respectively. There was no decline in the absolute CD4 and CD8 lymphocyte number after rituximab. We conclude that eradication of MRD in HCL is possible. Whether this leads to a reduced risk of relapse would need to be evaluated in a larger number of patients and with longer follow-up. Disease characteristics may potentially be used to identify patients that are more likely to benefit from such additional therapy.
Introduction
Hairy cell leukemia (HCL) is an indolent lymphoproliferative malignancy characterized by infiltration of the bone marrow, liver, spleen, and occasionally lymph nodes with neoplastic B cells with cytoplasmic hairlike projections.1 Before the availability of effective agents, splenectomy was the standard treatment option leading to significant improvements in cytopenias but of limited duration.2 More recently, interferon-α (IFN-α) and nucleoside analogs such as 2-chlorodeoxyadenosine (2-CDA or cladribine) and 2-deoxycoformycin (DCF or pentostatin) have been successfully used to treat patients with HCL.3-9
Cladribine has been highly effective in treating patients with HCL, with overall response rates ranging from 75% to 100% after a single course of the drug administered by continuous infusion daily for 7 days.10 However, responses are not universal, and a significant proportion of patients relapse.10 Saven et al11 reported the long-term follow-up of 358 patients treated at the Scripps Clinic who were followed for a median of 52 months. Twenty-six percent of patients relapsed at a median of 29 months.11 Goodman et al12 described the long-term follow-up of 209 patients treated with cladribine. Although the overall response rate was 100%, 76 patients (37%) relapsed, with a median time to relapse of 42 months.12 In a recent report from Northwestern University,13 86 consecutive patients were treated with cladribine; a complete remission (CR) rate of 79% and a partial remission (PR) rate of 21% were noted. The progression-free survival after 12 years was 54% and with a median follow-up of 9.7 years (range, 0.3-13.8 years), 31 patients (36%) relapsed. Twenty-three relapsed patients were treated with a second course of cladribine and 12 (52%) achieved CR, with 7 (30%) achieving PR. The overall survival after 12 years was 87%.13 Other studies confirm a high but not universal response rate as well as a relapse-free survival of 70% to 80% at about 4 years.14,15
Similar results have been achieved using pentostatin.16 Overall CR rates of 44% to 89% are seen with pentostatin given at a dose of 2 to 4 mg/m2 every 2 weeks.16 Dearden et al17 evaluated 165 patients treated with pentostatin and 45 patients treated with cladribine. Relapse rates were 24% for pentostatin and 29% for cladribine after a median follow-up of 71 and 45 months, respectively.17 Flinn et al9 reported their long-term follow-up of 241 patients treated with pentostatin either as initial therapy or after failure of IFN-α. The 5- and 10-year event-free survival rates were 85% and 67%, respectively.9
Therefore, there is a definite relapse rate associated with therapy of HCL with both cladribine and pentostatin, and the relapse-free survival curve does not appear to reach a plateau. Recently, a number of reports have demonstrated the efficacy of the monoclonal antibody rituximab in treating patients with relapsed HCL.18-21 Rituximab targets the pan–B-cell antigen CD20, which is expressed at high levels on the surface of hairy cells.22,23 Nieva et al19 reported a CR rate of 13% and a PR rate of 13% in 24 patients with relapsed HCL who received rituximab 375 mg/m2 once weekly for 4 weeks. Lauria et al21 treated 10 patients with relapsed or progressed HCL with a similar regimen of rituximab and noted 1 CR and 4 PR [overall response (OR), 50%]. In a study by Hagberg et al,20 11 patients with HCL (including 3 previously untreated patients) received rituximab 375 mg/m2 weekly for 4 weeks. The OR rate was 64% with 6 CRs and 1 PR (including 1 CR in a treatment-naive patient).20 Thomas et al18 used an extended dosing regimen and administered rituximab 375 mg/m2 weekly for 8 to 12 doses to 15 patients with relapsed or refractory HCL. The overall response rate was 80%, including 8 CRs (52%), 2 CRs with residual marrow disease (13%), and 2 PRs (13%).18
Rituximab has been successfully combined with nucleoside analogs in the treatment of other indolent lymphoproliferative disorders.24,25 These combinations have generally been well tolerated. We have treated 13 patients with HCL, either newly diagnosed or in relapse after one prior therapy, with cladribine followed by rituximab to eradicate residual HCL. Minimal residual disease (MRD) was evaluated using flow cytometry for HCL-specific marker expression patterns and by an immunoglobulin heavy chain (IgH) polymerase chain reaction (PCR) assay using framework-1, -2, and -3 primer sets.
Patients and methods
Eligibility
Patients were eligible if they had a new diagnosis of HCL with active disease or if they had relapsed after one prior therapy. Diagnosis of HCL was based on morphologic evaluation of peripheral blood, bone marrow aspirates, and core biopsies, in combination with a characteristic flow cytometric immunophenotype (bright positivity for CD22, CD11c, and CD103). Most cases were also stained for tartrate-resistant acid phosphatase (TRAP) on aspirate smears, and all tested cases were positive. Active disease was defined as one or more of the following: (1) hemoglobin (Hgb) level less than 100 g/L or transfusions of at least 2 units of packed red blood cells per month, absolute neutrophil count (ANC) less than 1.5 × 109/L, platelet count less than 100 × 109/L, or greater than 25% decline from baseline over 3 months in one or more cell lines; (2) circulating hairy cells at least 1 × 109/L or extramedullary HCL; and/or (3) bone marrow hairy cells at least 10% (on aspirates and/or biopsy sections); (4) recurrent infections, progressive decline in performance status, or symptomatic splenomegaly. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less and adequate organ function with a serum bilirubin level of no more than 3.5 mg/dL, aspartate aminotransferase/alanine aminotransferase (AST/ALT) less than 3 times the upper limit of normal, and creatinine no more than 2.0 mg/dL. Higher values were acceptable if they were directly related to the disease.
Patients
Between June 2004 and August 2005, 13 consecutive patients with HCL were treated. The study was approved by the institutional review board of the University of Texas M.D. Anderson Cancer Center. All patients signed an informed consent to participate in the study. Eleven patients had newly diagnosed disease and 2 had relapsed from prior therapy. One patient had received 2CDA 9 years ago and relapsed with progressive cytopenias; another patient had received chlorambucil for 8 years and had generalized adenopathy, and a variant HCL histology. The median age of the patients was 53 years (range, 31-73 years). Fluorescent in situ hybridization (FISH) was positive for the presence of a clone with p53 deletion or monosomy 17 in 2 patients. One patient with variant HCL had a complex karyotype, but no cytogenetic abnormalities were noted in the other 12 patients. Six of 10 evaluable patients had mutated immunoglobulin heavy chain variable gene (IgVH), whereas IgVH was unmutated in 4 patients. Patient characteristics are summarized in Table 1.
Patient characteristics
Patient . | Age, y/sex . | Prior Rx . | WBC/ANC, × 109/L . | Hgb level, g/dL . | Platelet count, × 109/L . | Size of spleen/nodes, cm . | IgVH homology, % . | P53 deletion/monosomy 17 by FISH . |
|---|---|---|---|---|---|---|---|---|
| 1 | 54/F | 2CDA | 2.6/0.78 | 13.1 | 105 | 0/0 | 97.9 | Negative |
| 2 | 32/M | None | 5.7/1.82 | 13.1 | 55 | 0/0 | NA | Negative |
| 3 | 54/M | None | 2.2/1.23 | 13.9 | 113 | 3/1.5 | NA | Negative |
| 4 | 47/F | None | 2.7/0.68 | 9.1 | 38 | 0/0 | 95 | Positive |
| 5* | 69/M | Chl | 72.8/0.0 | 12.6 | 140 | 0/2 | 100 | Positive |
| 6 | 53/F | None | 1.5/0.69 | 10.3 | 51 | 3/0 | NA | Negative |
| 7 | 67/F | None | 2.0/0.68 | 12.2 | 76 | 0/0 | 95.1 | Negative |
| 8* | 74/F | None | 31.6/2.53 | 13.3 | 275 | 0/0 | 100 | Negative |
| 9 | 60/M | None | 0.9/0.04 | 7.8 | 46 | 0/0 | 95.6 | Negative |
| 10 | 58/M | None | 2.4/0.65 | 10.1 | 80 | 0/0 | 98 | Negative |
| 11 | 52/F | None | 4.1/0.94 | 13.6 | 60 | 0/0 | 97.6 | Negative |
| 12 | 58/M | None | 4.1/0.98 | 12.9 | 86 | 0/0 | 98.7 | Negative |
| 13 | 47/M | None | 5.0/2.1 | 15.6 | 54 | 7/0 | 96.6 | Negative |
Patient . | Age, y/sex . | Prior Rx . | WBC/ANC, × 109/L . | Hgb level, g/dL . | Platelet count, × 109/L . | Size of spleen/nodes, cm . | IgVH homology, % . | P53 deletion/monosomy 17 by FISH . |
|---|---|---|---|---|---|---|---|---|
| 1 | 54/F | 2CDA | 2.6/0.78 | 13.1 | 105 | 0/0 | 97.9 | Negative |
| 2 | 32/M | None | 5.7/1.82 | 13.1 | 55 | 0/0 | NA | Negative |
| 3 | 54/M | None | 2.2/1.23 | 13.9 | 113 | 3/1.5 | NA | Negative |
| 4 | 47/F | None | 2.7/0.68 | 9.1 | 38 | 0/0 | 95 | Positive |
| 5* | 69/M | Chl | 72.8/0.0 | 12.6 | 140 | 0/2 | 100 | Positive |
| 6 | 53/F | None | 1.5/0.69 | 10.3 | 51 | 3/0 | NA | Negative |
| 7 | 67/F | None | 2.0/0.68 | 12.2 | 76 | 0/0 | 95.1 | Negative |
| 8* | 74/F | None | 31.6/2.53 | 13.3 | 275 | 0/0 | 100 | Negative |
| 9 | 60/M | None | 0.9/0.04 | 7.8 | 46 | 0/0 | 95.6 | Negative |
| 10 | 58/M | None | 2.4/0.65 | 10.1 | 80 | 0/0 | 98 | Negative |
| 11 | 52/F | None | 4.1/0.94 | 13.6 | 60 | 0/0 | 97.6 | Negative |
| 12 | 58/M | None | 4.1/0.98 | 12.9 | 86 | 0/0 | 98.7 | Negative |
| 13 | 47/M | None | 5.0/2.1 | 15.6 | 54 | 7/0 | 96.6 | Negative |
Rx indicates medication; WBC, white blood count; ANC, absolute neutrophil count; Hgb, hemoglobin; FISH, fluorescent in situ hybridization; 2CDA, 2-chlorodeoxyadenosine; NA, not available; Chl, chlorambucil.
Variant HCL.
Treatment plan
Cladribine 5.6 mg/m2 was administered intravenously over 2 hours daily for 5 days. Approximately 28 days after initiation of cladribine, a repeat bone marrow examination was evaluated for the presence of MRD. This was followed by 8 weekly doses of rituximab 375 mg/m2. At completion of therapy with rituximab a repeat bone marrow examination for evaluation of MRD was performed. Patients are then followed with peripheral blood evaluations every 3 months, including flow cytometry assays for MRD.
Supportive care
Prophylactic antibiotics, including levofloxacin, valacyclovir, and fluconazole (or equivalent agents), were administered at the discretion of the treating physician. Growth factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) were administered at the discretion of the treating physician. Transfusion support with irradiated and filtered packed red blood cells, platelets, or both was provided when indicated.
Criteria for response
CR was defined as the absence of hairy cells on BM aspirate smears or the presence of less than 1% atypical cells (not definitively called hairy cells) in bone marrow and blood and the disappearance of all evidence of HCL on physical examination. Achievement of CR required an ANC of at least 1.5 × 109/L; Hgb at least 120 g/L (at least 110 g/L for women); and platelet count at least 100 × 109/L without growth factor or transfusion support. CR with residual disease (CR-RD) was defined as for CR but with persistence of 1% to 5% hairy cells in the marrow (but no circulating hairy cells). PR was defined as (1) meeting the peripheral blood count criteria for CR/CR-RD but with more than 5% residual hairy cells in the marrow or (2) at least 50% improvement or correction of at least one cytopenia without decrease in any of the other cell counts, reduction in palpable abnormalities on physical examination by at least 50%, and reduction in circulating or bone marrow hairy cells by at least 50%. Responses were evaluated after completion of therapy with rituximab.
Bone marrow histology and immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded bone marrow core biopsies were stained with hematoxylin and eosin for morphologic evaluation, at all posttherapy time points. For most time points, IHC staining was performed on biopsy sections by standard techniques,26 for a pan–B-cell marker other than CD20. Antibodies used were PAX-5 (1:35; BD Biosciences Transduction Laboratories, San Jose, CA) or CD79a (1:50; Dako, Carpinteria, CA). Cases showing B-cell aggregates by IHC were scored as positive for residual disease.
MRD and immune status assessment by flow cytometry
Peripheral blood and bone marrow specimens for MRD assays were stained with a 4-color panel of antibodies with the following combinations (FITC/PE/PerCP-Cy5.5/APC): CD20/CD103/CD45/CD19, CD22/CD11c/CD45/CD19, CD20/CD25/CD45/CD19, Igκ/CD22/CD45/CD19, Igλ/CD22/CD45/CD19, and Igκ/Igλ/CD19/CD22. All antibodies were from BD Biosciences (San Diego, CA). Antibodies were added to 106 mononuclear cells per tube in 100 μL whole blood or marrow (diluted with phosphate-buffered saline [PBS] and 1% fetal bovine serum as needed), and incubated for 10 minutes at room temperature. Erythrocyte lysis was performed using BD PharmLyse (BD Biosciences), followed by a wash with PBS containing 0.1% sodium azide, using a Sorvall Cell Washer 2. Cells were resuspended for acquisition in PBS containing 1% formaldehyde. For tubes containing anti-κ or anti-λ antibodies, lysis and washing were performed prior to cell-surface staining. Data were acquired on FACSCalibur flow cytometers (BD Biosciences) using BD CellQuest Pro software. CD19+ B cells were selectively gated, with from 40 000 to 600 000 total cells acquired per tube, depending on sample quality. Data were analyzed using CellQuest Pro (BD Biosciences).
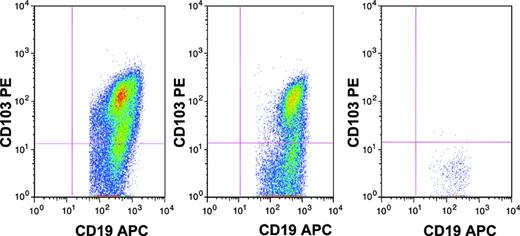
Hairy cells were identified on the basis of bright coexpression of CD11c and CD22, positivity for CD103 and CD25 (moderate to bright), and clonal expression of surface immunoglobulin light chains on CD22 bright B cells. Hairy cell variants were negative for CD25. The limit of sensitivity was established using control specimens consisting of 10 bone marrow aspirates, acquired for staging of patients with other B-lineage lymphomas, all negative for malignancy, stained with the same antibody panel. There were small subsets of CD19+ cells in most control marrows which were also positive for CD11cbright/CD22bright. Thus, this marker combination was noncontributory for MRD studies. However, the background of CD103+ cells in control marrows was low (< 0.02% of total cells), and CD25bright+ cells showed similarly low levels. Therefore, in cases with background populations of normal B cells, the limit of detection of residual HCL was 0.02%. In specimens with no normal background B cells, HCL populations with multiple phenotypic aberrancies could be detected at a level of 0.05% to 0.003% (20 aberrant cells of 40 000-600 000 collected). The MRD assessment for a representative patient is demonstrated in Figure 1.
For immune status assessment, peripheral blood lymphocytes were stained with the following antibody combinations (FITC/PE/PerCP-Cy5.5/APC): CD8/CD4/CD45/CD3 and CD3/CD56/CD45/CD19. All antibodies were from BD Biosciences. Erythrocytes were lysed using FACSLyse (BD Biosciences). Data were acquired and analyzed as described above.
MRD assessment by polymerase chain reaction
Total DNA was extracted from PB or bone marrow aspirate samples using an automated method (Autopure; Genta, Minneapolis, MN). B-cell clonality was determined using a polymerase chain reaction (PCR) method using V primers derived from the framework 1 (FR1), framework 2 (FR2), and framework 3 (FR3) regions, in combination with either a consensus JH or CH primer with detection by capillary electrophoresis.27
Assessment of hairy cells in a patient with HCL by flow cytometry performed on bone marrow aspirate specimen. CD19+ lymphocytes are shown. (A) Day 0, hairy cells are 10.7% of total cells. (B) Day 30 (after 2CDA), hairy cells are 11.0% of total cells. (C) Day 90 (after rituximab), hairy cells are undetectable, with fewer than 1/200 000 cells CD19+ with CD103 expression significantly above background.
Assessment of hairy cells in a patient with HCL by flow cytometry performed on bone marrow aspirate specimen. CD19+ lymphocytes are shown. (A) Day 0, hairy cells are 10.7% of total cells. (B) Day 30 (after 2CDA), hairy cells are 11.0% of total cells. (C) Day 90 (after rituximab), hairy cells are undetectable, with fewer than 1/200 000 cells CD19+ with CD103 expression significantly above background.
IgVH gene analysis
Total RNA was extracted from bone marrow aspirate or peripheral blood using Trizol, converted to cDNA using Superscript II (Invitrogen, Carlsbad, CA) and used to amplify the clonal immunoglobulin heavy chain gene rearrangements by a PCR method using V primers derived from the leader region with either a consensus JH or CH primer.28 The dominant IgVH clone product(s) was then sequenced by standard Sanger methods. Divergence from germ line IgVH segments29 was calculated using DNAPLOT software (VBASE Sequence Directory, I.M.; Tomlinson, MRC Center for Protein Engineering, Cambridge, United Kingdom); 2% or less changes over codons 1 to 94 of IgVH was regarded as unmutated. The VH segment used was also recorded.
Statistical considerations
A maximum of 44 patients are planned for this trial with early stopping rules to monitor futility and excess toxicity. These stopping boundaries have not been reached. The CD4 and CD8 counts during the treatment were analyzed by paired Wilcoxon and paired t tests.
Results
Response to therapy
All 13 patients (100%) have achieved CR after completion of the planned therapy. Approximately 1 month after 2CDA therapy, morphologic, IHC, or both examinations of bone marrow core biopsies showed that 8 patients had 0 or less than 1% hairy cells in their BM (CR), whereas 5 patients still had 1% to 50% hairy cells. Examination of the bone marrow core biopsies at the completion of treatment with rituximab did not show definitive evidence of HCL in any patient. MRD assessed by flow cytometry was positive in 11 patients 1 month after 2CDA therapy but became negative in 12 of 13 patients after rituximab. The flow cytometry (FC) assay appeared more sensitive than IHC for pan–B-cell markers; no specimen was positive by IHC but negative by FC, whereas several specimens showed low-level positivity by FC but indeterminate IHC results, with only rare scattered B cells. MRD by PCR assay was positive in 5 of 11 evaluable patients 1 month after 2CDA therapy and became negative in 11 of 12 evaluable patients (equivocal in 1) after rituximab. With a median follow-up of 14 months (range, 6-16 months), no patients have relapsed (median response duration, 9 months; range, 4-16 months). One patient has developed a second malignancy (pancreatic carcinoma).
Toxicity
The treatment was well tolerated with no unexpected toxicity. Nine episodes of grade 3 toxicity, including 3 episodes of grade 3 neutropenic fever thought to be related to therapy and 2 episodes of grade 3 herpes zoster possibly related to therapy, were reported. Four other grade 3 events included one episode each of cellulitis, acute gouty arthritis in a patient with history of gout, renal impairment related to an acute attack of gout in a patient with a known history of gout, and renal impairment due to antibiotic-induced interstitial nephritis thought not to be related to the treatment. Other adverse events were grade 1 and 2 and included nausea, rash, fatigue, weight gain, weight loss, and fever.
Immunosuppression
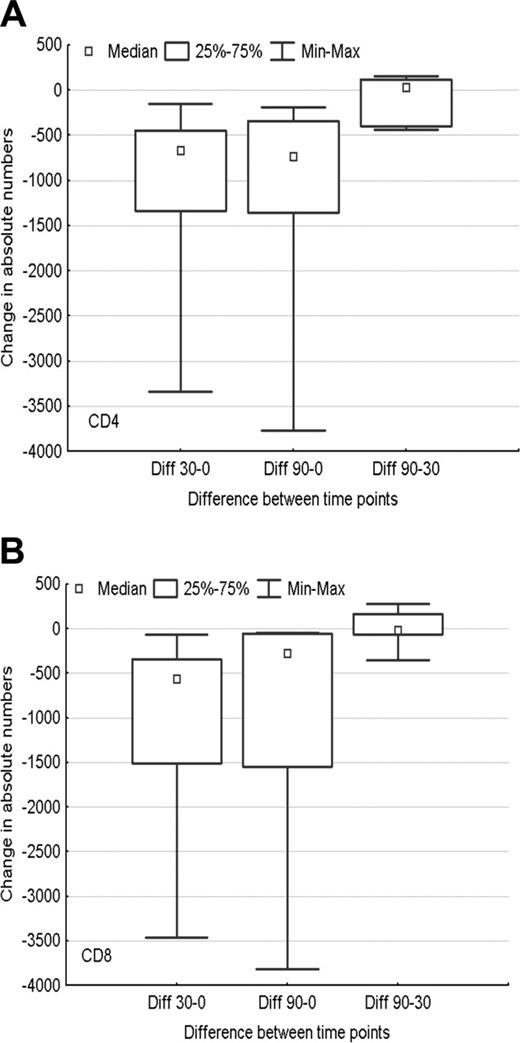
To evaluate the effect of an extended course of rituximab on immune parameters, peripheral blood CD4 and CD8 lymphocyte subsets were evaluated at various time points during the therapy. Treatment with cladribine led to a statistically significant decline in the CD4 count, which remained stable after therapy with rituximab. Both CD4 and CD8 counts dropped significantly at day 30 (after 2CDA, P = .03 and P = .03, respectively) and day 90 (after rituximab, P = .03 and P = .03, respectively), compared with day 0 (before starting therapy). However, neither CD4 (P = .84) nor CD8 (P > .999) counts dropped significantly from day 30 to day 90 (by paired Wilcoxon tests) (Figure 2).
Discussion
Before the availability of the modern chemotherapeutic agents, splenectomy was the standard therapy in patients with HCL and resulted in significant improvement in cytopenias. The introduction of IFN-α produced response rates of greater than 80%; however, most were PRs and of limited duration. The nucleoside analogs cladribine and pentostatin have been highly effective in producing lasting responses in most patients with HCL. Despite this, single-agent therapy with cladribine or pentostatin is not universally effective. Furthermore, relapses do occur in up to 40% of patients within 4 years of initial therapy. Several studies have demonstrated that the quality of the initial response is predictive of outcome, with a longer disease-free survival for those achieving CR with their initial therapy.9,17 Therefore, to realize “cure” in HCL, achievement of CR without evidence of MRD is desirable.
Changes in lymphocyte subsets during therapy. Distribution of the (A) CD4 count and (B) CD8 count changes among day 0 (before starting therapy), day 30 (after 2CDA), and day 90 (after rituximab).
Changes in lymphocyte subsets during therapy. Distribution of the (A) CD4 count and (B) CD8 count changes among day 0 (before starting therapy), day 30 (after 2CDA), and day 90 (after rituximab).
Wheaton et al30 have previously noted a shorter relapse-free survival in patients who had evidence of MRD (detected by IHC using anti-CD45RO, anti-CD20, and DBA.44 in paraffin-embedded bone marrow sections) compared with those who did not. More precise methods for detecting MRD using immunophenotyping by flow cytometry and consensus primer polymerase chain reaction (cpPCR) analysis of immunoglobulin receptors are now available.31 Whether eradication of MRD as detected by flow cytometry or cpPCR would lead to improvements in progression-free survival (PFS) and overall survival (OS) is unclear. Furthermore, any agent used for this purpose should have little additional associated toxicity. Rituximab has demonstrated efficacy in treating patients with relapsed HCL and has been used successfully to treat patients with purine analog–resistant disease.18-21 The combination of rituximab with nucleoside analogs has been associated with an increased incidence of neutropenia.24,32 However, rituximab has relatively low toxicity and is not associated with significant immunosuppression. We have demonstrated the efficacy of an extended course of rituximab in eradicating MRD in most patients with HCL treated in this study. Whether such MRD eradication could be achieved with nucleoside analog therapy alone is unclear, although previous studies have demonstrated that a significant number of patients in CR have evidence of MRD. Wheaton et al30 reported that 5 (13%) of 39 patients in CR 3 months after therapy with cladribine had evidence of disease by IHC. We used significantly more sensitive techniques and found that only 1 (8%) of 13 patients had disease detectable by flow cytometry and only 1 (8%) of 12 patients had an equivocal PCR at 3 months after therapy. As expected, treatment with rituximab was not associated with additional toxicity, and there was no significant decline in the absolute numbers of CD4 and CD8 lymphocyte subsets after extended therapy with rituximab.
Despite its limited toxicity, financial considerations in a disease highly responsive to available therapy mandate the identification of patients at risk of relapse who would be candidates for additional therapy over nucleoside analogs alone. A different approach would be to evaluate the marrow for MRD at 3 to 6 months after the completion of therapy with cladribine and administer monoclonal antibody only to those patients with evidence of MRD as has been done by Cervetti et al.33 Alternatively, pretreatment features may be identified that would predict the likelihood of relapse, thereby allowing selection of appropriate patients for additional therapy.
In most patients, the leukemic cells express mutated IgVH, suggesting their origin from a postgerminal center (GC) memory B cell.34,35 Nevertheless, we as well as other investigators have identified a number of patients with HCL with unmutated IgVH genes, suggesting a pre-GC origin.36 However, gene expression profiling experiments have not demonstrated the existence of different subsets of HCL, suggesting that HCL has a uniform phenotype.37 Furthermore, when compared with the gene expression profiles of purified normal B-cell populations, including those of pre-GC (naive), GC, and post-GC (memory) B cells, the HCL profile resembled that of memory B cells.37 The mutational status of IgVH did not influence the response to therapy in this cohort of patients. We have also identified 2 patients with deletion or mutation of p53, although they both responded to our regimen of cladribine followed by rituximab. Whether the mutational status of IgVH or p53 abnormalities can predict those patients who will relapse needs a larger cohort with longer follow-up.
Another important consideration is the number of doses of rituximab that may be necessary for achieving disease eradication. Previously reported studies of rituximab in HCL have been in the setting of relapse.18-21 Although the numbers of patients in each study are small and a definitive comparison cannot be justified, a more extended regimen (8 weekly doses versus 4) appears to be associated with an improvement in the number and quality of responses.18 This was the rationale for the selection of 8 doses of rituximab in our study. However, no definitive statements about the adequate number of doses of rituximab to achieve effective MRD eradication can be made.
In conclusion, eradication of MRD in HCL using rituximab after therapy with nucleoside analogs is safe and feasible. Longer follow-up and larger patient numbers are needed to determine whether such eradication of MRD would translate to longer disease-free survival. Identification of predictors of relapse may allow the selection of patients who are most likely to benefit from this extended regimen.
Prepublished online as Blood First Edition Paper, February 23, 2006; DOI 10.1182/blood-2005-11-4590.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal