Comment on Bilancio et al, page 642
A comparative analysis of isoform-specific PI3K inhibitors and cells from kinase-dead knock-in mice confirms a key role for p110δ in B-cell signaling.
Phosphoinositide 3-kinases (PI3Ks) generate lipid second messengers that are critical for diverse aspects of the immune response. Two PI3Ks (p110γ and p110δ) are preferentially expressed in leukocytes, and knock-out (KO) mice for these 2 kinases reveal unique defects in immune signaling. p110γ KO animals exhibit impaired neutrophil and macrophage chemotaxis,1,2 whereas mice lacking p110δ show defective signaling from the B- and T-cell antigen receptors,3,4 among other phenotypes. Based on these data, the pharmaceutical industry has aggressively pursued selective inhibitors of these enzymes as potential drugs for the treatment of autoimmune disease.FIG1
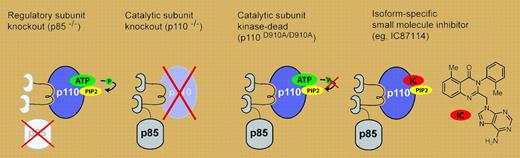
Genetic and pharmacologic approaches for inactivating the class IA PI3Ks, which are heterodimers of catalytic (p110) and regulatory (p85) subunits.
Genetic and pharmacologic approaches for inactivating the class IA PI3Ks, which are heterodimers of catalytic (p110) and regulatory (p85) subunits.
Unfortunately, the story is not quite so simple. The same studies that implicated PI3Ks in diverse immune signaling also raised questions about what a drug targeting these kinases would really do.5 One strain of p110γ KO mice, but not others, is prone to colon cancer, and the molecular basis for this difference is unknown.6 p110γ KO mice suffer from increased cardiac contractility, whereas those that express a kinase-dead (KD) p110γ (which better mimics the effects of an inhibitor) do not.7 Conversely, animals expressing p110δ KD exhibit more pronounced defects in lymphocyte signaling and development than p110δ KO mice.8 Taken together, these data have served to remind the scientific community that subtly different types of knock-outs can induce very different phenotypes, and that none of these necessarily anticipate the effect of a small molecule drug (see figure).
In this issue of Blood, Bilancio and colleagues directly address this question in murine B cells by comparing pharmacologic and genetic inactivation of PI3K isoforms. The authors use the first selective inhibitors of p110γ and p110δ to define the role of these 2 kinases in signaling from the B-cell antigen and IL-4 receptors, and then compare these results with B cells from p110δ KD mice. They report that the p110δ inhibitor IC87114 potently inhibits B-cell receptor–induced proliferation, calcium mobilization, and activation of downstream PI3K effectors such as Akt—all of which are recapitulated by cells from p110δ KD mice. By contrast, the p110γ inhibitor AS-604850 has no effect on these signaling events, excluding an essential role for p110γ in signaling from the B-cell receptor. Because small molecule inhibitors always possess imperfect specificity, the authors' use of 2 compounds with complementary selectivity helps to reinforce these conclusions.
One intriguing observation from this study is that the p110δ inhibitor IC87114 is significantly more potent in B cells (IC50 = 0.04-0.14 μM) than in most other cells (typical IC50 = 1-5 μM). This presumably reflects the fact that B-cell signaling is tuned to be more sensitive to the amount of p110δ activity than signaling in other cell types (including other leukocytes that highly express p110δ) and suggests that in the intact organism, B cells are likely to be more sensitive to pharmacologic disruption by p110δ inhibitors. As PI3K isoforms often collaborate to synthesize the same lipids within the same cell, defining these isoform and cell-type–specific thresholds represents a key challenge for understanding signaling by this family of enzymes. Pharmacologic approaches such as the one reported here by Bilancio and colleagues will be an important tool for meeting this challenge. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal