Abstract
Elevated levels of plasma homocysteine (Hcy) correlate with increased risk of cardiovascular and Alzheimer diseases. We studied the effect of elevated Hcy on the blood-brain barrier (BBB) to explore the possibility of a vascular link between the 2 diseases. On a hyperhomocysteinemic diet, cystathionine beta-synthase (Cbs)–heterozygous mice develop hyperhomocysteinemia. Intravital microscopy analysis of the mesenteric venules showed that leukocyte rolling velocity was markedly decreased and numbers of adherent cells were increased in the mutant mice. This was due at least in part to increased expression of P-selectin. BBB permeability was measured by Evans blue dye permeation and was found to be 25% greater in the Cbs+/– cortex compared with wild-type controls. Our study suggests an important toxic effect of elevated Hcy on brain microvessels and implicates Hcy in the disruption of the BBB.
Introduction
Elevated levels of plasma homocysteine (Hcy) correlate with an increased risk of cardiovascular disease.1,2 Moreover, a plasma Hcy level greater than 14 μM increases the risk of Alzheimer disease (AD) by almost 2-fold.3 The pathologic effects of Hcy in these diseases and the possible role of Hcy in blood-brain barrier (BBB) degradation have yet to be explored.
Mutations in genes involved in Hcy metabolism, including cystathionine beta-synthase (Cbs), can result in severe hyperhomocysteinemia (HHcy). Cbs-deficient (–/–) mice have 40-fold higher total plasma Hcy levels and exhibit severe growth retardation, liver disease, and dislocation of the lens. These problems do not occur in Cbs-heterozygous (+/–) mice, with 2-fold elevated plasma Hcy, making them a suitable model to study mild HHcy.4 The present study explores endothelial function in these animals, with particular emphasis on the effects of elevated Hcy on the BBB.
Study design
Mice
Wild-type (WT) and Cbs+/– mice on C57BL/6J background were obtained from The Jackson Laboratory (Bar Harbor, ME). Procedures were approved by the Animal Care and Use Committee of the CBR Institute for Biomedical Research.
Induction and measurement of HHcy
Mice were fed a methionine-enriched, low-folate (0.08 mg/kg), vitamin B6 (0.01 mg/kg), B12 (10.4 μg/kg), and choline bitartrate (2.5 g/kg) diet (TD 97345; Harlan Teklad, Madison, WI) for 8 weeks. Normal chow was from Purina Farmer's Exchange (Framingham, MA). Total plasma Hcy levels were measured using a BAS total Hcy assay kit and BAS 200A high-performance liquid chromatography (HPLC) apparatus with an electrochemical detector; data were analyzed with ChromGraph software (BAS, West Lafeyette, IN).
Analysis of leukocyte rolling by intravital microscopy
BBB permeability
Evans blue (EB) was injected intraperitoneally (50 μg/g body weight) and mice were killed 3 hours later.7 Brain and tissues were harvested and placed in formamide for 72 hours (Sigma, St Louis, MO). EB was expressed as optical density per gram tissue normalized for plasma concentration.
Statistical analysis
Data are reported as mean plus or minus standard error of mean. Analysis of variance (ANOVA) followed by Kruskal-Wallis post-test were used.
Results and discussion
Animal and human studies have demonstrated that HHcy affects endothelial function.8 HHcy impairs endothelium-derived nitric oxide (NO) production and vasodilation.8 Acute HHcy produced by infusion9 or superfusion10 of high concentrations of Hcy in rats leads to increased leukocyte adhesion in blood vessels. The mild HHcy of Cbs+/– mice also causes a decrease in bioavailable NO.11 Inhibition of NO production in mice increases spontaneous secretion of Weibel-Palade bodies as NO inhibits granule exocytosis,12 and this could lead to elevated expression of P-selectin. Therefore, we wished to see whether we could detect systemic endothelial activation/increased leukocyte adherence in the Cbs+/– mice as an indicator of inflammation. Inflammation can lead to BBB breakdown.13
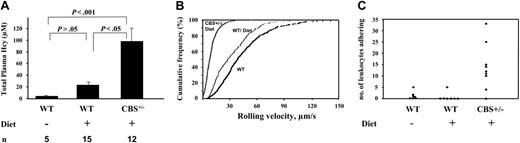
Effect of hyperhomocysteinemia on leukocyte adhesion. (A) Plasma Hcy levels. n indicates number of mice; Diet, hyperhomocysteinemic diet. Data are presented as mean ± SEM. (B-C) Seven venules of 200 to 300 μm in diameter from 4 WT mice on chow, 7 venules from 4 WT, and 9 venules from 5 Cbs+/– on Diet were analyzed. (B) Leukocyte rolling velocity. Cumulative histogram of rolling velocities allows direct comparison of distribution among groups; number of leukocytes analyzed: WT (538), WT/Diet (339), and Cbs+/–/Diet (497). (C) Number of leukocytes adhering to the endothelium (> 20 seconds) over a 5-minute period. Each point represents a single venule. Leukocyte rolling velocities and number of adhering leukocytes were significantly different between Cbs+/–/Diet and WT, as well as Cbs+/–/Diet and WT/Diet (P < .05).
Effect of hyperhomocysteinemia on leukocyte adhesion. (A) Plasma Hcy levels. n indicates number of mice; Diet, hyperhomocysteinemic diet. Data are presented as mean ± SEM. (B-C) Seven venules of 200 to 300 μm in diameter from 4 WT mice on chow, 7 venules from 4 WT, and 9 venules from 5 Cbs+/– on Diet were analyzed. (B) Leukocyte rolling velocity. Cumulative histogram of rolling velocities allows direct comparison of distribution among groups; number of leukocytes analyzed: WT (538), WT/Diet (339), and Cbs+/–/Diet (497). (C) Number of leukocytes adhering to the endothelium (> 20 seconds) over a 5-minute period. Each point represents a single venule. Leukocyte rolling velocities and number of adhering leukocytes were significantly different between Cbs+/–/Diet and WT, as well as Cbs+/–/Diet and WT/Diet (P < .05).
To evaluate the extent of HHcy induced by the 8-week diet in our animals, we determined plasma Hcy concentration. These were 23.5 ± 5 μM for WT and 98.4 ± 22 μM for Cbs+/– on a hyperhomocysteinemic diet and 4.1 ± 0.20 μM in WT on chow (Figure 1A). The elevation observed in Cbs+/– mice is within the range observed in human pathology and the range seen in normal aging.3 Many humans also reach abnormally high Hcy levels after a large meal.14
We observed mesenteric venules (200-300 μm) in WT and Cbs+/– mice on the diet for 8 weeks as a means of Hcy induction, and these observations were compared with WT on normal chow. Shear rates among different animal groups were similar: 98.14 ± 15.90 s–1 in WT/Diet, 126.44 ± 14.05 s–1 in Cbs+/–/Diet (P = .1), and 102 ± 13.57 s–1 (P > .05) in WT on chow. Leukocyte rolling velocity was slower in Cbs+/– than in WT (Figure 1B), indicating an increase in the density of adhesion molecules on the endothelium. In Cbs+/– mice, 60% of leukocytes rolled at a velocity of less than 10 μm/s compared with only 8% in WT mice on diet (P < .002) and 3% WT on chow (Figure 1B). The decrease in leukocyte rolling velocity was likely due to increased expression of P-selectin. Infusion of fluorescent beads coated with antibody to P-selectin showed a several-fold increase in their binding to venules of Cbs+/– mice with HHcy compared with WT (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We also observed increased leukocyte adherence in Cbs+/– mice (Figure 1C). Since P-selectin mainly mediates leukocyte rolling, P-selectin was probably not the only molecule up-regulated on the endothelium of the Cbs+/– mice. Aortas from rats subjected to a diet rich in methionine show expression of MCP-1, E-selectin, and VCAM-1.15
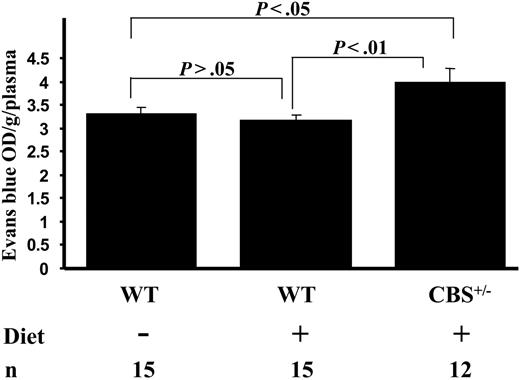
We compared BBB function after the 8-week hyperhomocysteinemic diet. EB binds to albumin and does not readily cross the BBB. Optical density (OD) in blood samples was similar: 0.17 and 0.14 for WT and Cbs+/– mice, respectively (P > .05). The Cbs+/– mice exhibited 25% greater EB in cortex compared with WT (Figure 2). EB leakage in WT mice on the diet was 3.19 ± 0.09 OD/g/plasma and 3.99 ± 0.29 OD/g/plasma in Cbs+/– (P < .01). WT mice on the diet showed higher Hcy levels than on chow, but this increase was not sufficient to break the BBB (Figure 1A). Perhaps higher threshold levels are necessary to compromise BBB function. Recently, a threshold effect (ie, nonlinear response) of endothelial cell oxygen species generation with Hcy incubation was observed in vitro.16
No differences were observed in vascular permeability in the peripheral tissues. Skin leakage of diet-fed mice was 3.64 ± 0.38 for WT, compared with 5.59 ± 1.65 OD/g/plasma for Cbs+/– (n = 12-15; P = .27). EB accumulation in liver and spleen was similar. Therefore, the permeability defect appears selective to brain vessels. A recent human study showed that patients with mild cognitive impairment and HHcy who were supplemented with vitamin B12-B6-folate not only reduced their Hcy levels but also improved their BBB function.17 Several groups have demonstrated that apolipoprotein E–deficient mice have compromised BBB function.7,18,19 Independent clinical associations with AD have been linked to both the effects of Hcy and apolipoprotein E isoform ϵ4,3 and, interestingly, the ϵ4 isoform also predisposes to atherosclerosis.20
BBB endothelium is different from that of the peripheral vasculature, including tight junctions and low vesicular transport.21 Because permeability is more stringently controlled in the cortex, the microcirculation of the brain may be more susceptible to disruption by Hcy than that of the periphery. Superoxide may be a key mediator of Hcy-induced endothelial dysfunction in the murine cerebral circulation.22 It has been reported that Hcy causes cell detachment and apoptotic death.23,24 Hcy induces endoplasmic reticulum (ER) stress and the unfolded protein response (UPR).25 The UPR, along with prolonged or severe ER stress, may contribute to the pathogenesis of a number of human diseases, including AD and Parkinson disease.26 Since the Cbs+/– mice have a relatively mild phenotype even on the hyperhomocysteinemic diet and the peripheral vascular permeability appeared normal, we hypothesize that cerebrovascular regulatory processes influencing BBB function were affected, rather than a loss of endothelium by apoptosis. In addition to causing endothelial dysfunction, Hcy has direct effects on other components of the BBB, such as astrocytes.27 Astrocytes mediate signaling between endothelium and neurons.28 Perhaps Hcy-induced endothelial and astrocytic dysfunction could alter neuronal function. Permeability of vessels induced by Hcy may also fit a model of toxin-mediated injury to surrounding neurons or glial cells.29
Evans blue dye accumulation in the cortices of mice. n indicates number of mice analyzed; Diet, hyperhomocysteinemic diet. Data are presented as mean ± SEM.
Evans blue dye accumulation in the cortices of mice. n indicates number of mice analyzed; Diet, hyperhomocysteinemic diet. Data are presented as mean ± SEM.
A direct causal relationship between HHcy and accelerated atherosclerosis was reported,30-32 and a connection between atherosclerosis and neurodegenerative disease postulated.20 We now suggest that vascular dysfunction could be the common denominator for these conditions promoted by HHcy. Thus, the injurious effect of Hcy on blood vessels may not only be central in atherosclerosis but may also contribute to neurodegenerative diseases such as sporadic AD.
Prepublished online as Blood First Edition Paper, September 27, 2005; DOI 10.1182/blood-2005-06-2506.
Supported by grants from the National Institutes of Health, National Heart, Lung and Blood Institute R37 HL41002 to D.D.W. and HL58976 to J.L.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lesley Cowan for preparation of the manuscript, Kenneth Thomas and Sarah Eichenberger for technical assistance, and Ali Hafezi-Moghadam for discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal