Abstract
We demonstrated that B-cell–dendritic cell (DC) interactions via transmembrane activator and calcium modulator and cyclophilin ligand (CAML) interactor (TACI) and B-lymphocyte stimulator (BLyS) provide an early signal critical to generate adequate numbers of mature antigen presenting cells (APCs) to prime naive CD8+ T cells (CTLs) in vivo. Evidence that B cells are required for efficient CTL generation in mice and that reconstitution with wild-type but not TACI-knockout B cells restored normal CTL responses support our conclusion. Moreover, low doses of a TACI fusion protein (TACI-Fc) that express the extracellular domain of TACI (amino acid [aa] 1-126) restored CTL priming in B-cell–deficient mice in vivo and induced DC maturation in vitro. In fact, following interactions with B cells, splenic DCs rapidly express the CD86 costimulatory molecule, to an extent comparable to the exposure to antigenic stimuli. BLyShigh peptide-pulsed bone marrow–derived DCs, used as vaccines in vivo, cannot generate CTLs in B-cell–deficient and TACI-deficient mice, strongly supporting a need for B-cell–DC cooperation through TACI-BLyS during CTL first encounter with antigens in vivo.
Introduction
Immune responses are initiated in the T-cell areas of secondary lymphoid organs where naive T lymphocytes encounter dendritic cells (DCs) that present antigens derived from peripheral tissues or from locally synthesized endogenous molecules.1-3 Antigen presentation to naive CD4+ T cells in the lymph nodes has been elegantly described in previous studies.4 Antigen-primed CD8+ T cells (CTLs) have been shown to expand and differentiate into memory T cells faster than CD4+ T-helper cells (reviewed in Seder and Ahmed5 ), suggesting that CD4+ and CD8+ T cells may differ in their requirements for primary expansion in vivo. Indeed, while CTL development after priming has been well characterized,6-8 the events and the antigen-presenting cell (APC) type(s) involved during naive CD8+ T cell priming have yet to be fully elucidated.
Our previous results suggested that the affinity of the epitope for class I major histocompatibility complex (MHC) molecules determines the requirements for T-cell help during primary CTL expansion. However, depending upon the epitope, either a class II MHC–restricted T-helper (Th) cell peptide or the administration of antibody to CD40 was required to obtain significant CTL priming.9 To define the reason of these unexpected differences, we decided to study the role of B lymphocytes during primary CTL expansion: the expansion of Th cells and the engagement of the CD40 molecules are in fact critical requirements for B-cell biology being involved in B-cell activation and antibody production.
Here we explored the possibility that B-cell–specific costimulatory molecules provide critical signals required for CTL expansion during their first encounter with antigens in vivo. This hypothesis turned out to be correct because the transmembrane activator and calcium modulator and cyclophilin ligand (CAML) interactor (TACI)10 expressed on B cells is required to bind the B-lymphocyte stimulator molecule BLyS (also known as BAFF, TALL-1, THANK, or zTNF4) on DCs (reviewed in Locksley et al11 ) for a productive CTL generation. In fact, the results of the current study indicate that the timely interaction between B cells and DCs through TACI-BLyS during CTL priming induces rapid DC maturation, essential to provide an adequate number of professional APCs to expand naive CD8+ T cells.
Materials and methods
Mice
Six- to 12-week-old male and female C57BL/6 mice and mice knocked out for β2 microglobulin, CD40, interleukin-4 (IL-4), and IL2 gene expression on the C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME). Male and female B-cell–deficient mice (μMT–/–/μMT–/–) generated by the targeted disruption of the immunoglobulin M (IgM) H-chain12 and back-crossed on the C57BL/6 background were also purchased from the Jackson Laboratory. TACI knockout mice13 were backcrossed onto an H-2b background (C57BL/6). In some in vitro experiments, CD8+ T cells were derived from mice that were transgenic for the OT-1 TcR that recognize the Kb-restricted chicken ovalbumin (OVA) immunodominant sequence 257-264.14 These mice were bred at the Mayo Clinic, Rochester, MN.
Peptides and proteins
The peptides used in this study were synthesized by Fmoc chemistry using a multiple peptide synthesizer (Symphony/Multiplex; Protein Technologies, Tucson, AZ). Peptides were 97% pure as assessed by C18 reverse-phase high-performance liquid chromatography (HPLC), and the identity of the peptides was verified by mass spectroscopy. Four CTL peptides have been used in this study: the immunodominant Th cell–independent sequence from the vesicular stomatitis virus (VSV) NP 52-59 (RGYVYQGL); the immunodominant Th cell–independent sequence from the Sendai virus (SEV) NP 324-332 (FAPGNYPAL); the chicken OVA subdominant Th cell–dependent sequence 55-62 (KVVRFDKL); and the subdominant Th cell–dependent sequence from the lymphochoriomeningitis virus (LCMV) NP 205-212 (YTKVYPNL). These 4 peptides have been chosen on the basis of their MHC class I binding affinity, which was previously shown to correlate with their requirement for T-cell help for CTL induction.9
Two H-2 IAb–restricted helper determinants have been also used in this study: the core sequence 128-140 from the hepatitis B virus (HBV; TPPAYRPPNAPIL) and the immunodominant OVA sequence 323-336 (ISQAVHAAHAEINE). The latter peptide was found to be a better source of T-cell help for generating CTLs against OVA 55-62. The whole OVA protein derived from chicken egg white (Sigma, St Louis, MO) was also used as an immunogen in some experiments.
Monoclonal antibodies and fusion proteins
TACI and BLyS expression on B cells, DCs and CD8+ T cells were analyzed by fluorescence-activated cell-sorting (FACS) using rat monoclonal antibodies (MoAbs) specific for murine TACI (clone 8F10) and BLyS (clone 5A8) (Apotech, Bern, Switzerland). Other MoAbs used for analyzing cell markers were purchased from BD Pharmingen (San Diego, CA). This panel included MoAbs to the following specificities: CD8α (clone 53-6.7), B220 (clone RA3-6B2), CD19 (clone 1D3), CD69 (clone H1.2F3), CD11c (clone HL3), and CD86 (clone GL1). In some experiments, CD11c+ cells were labeled with 5 μM 5,6-carboxy-fluorescein diacetate succinimidil ester (CFSE; Molecular Probes, Eugene, OR).
Fusion proteins used in this study included the TACI-fusion protein (TACI-Fc), which was previously generated by cloning the extracellular domain of murine TACI (residues 1-126) into the signal pIg-plus vector, which encodes the Fc portion of human IgG1.15 As control, the B7RP-1–Fc fusion protein, kindly provided by Amgen (Thousand Oaks, CA), was used. A DNA fragment encoding the human B7RP-1 extracellular domain (1-246) was fused in-frame upstream of DNA encoding the C-terminal 235 amino acids of human IgG1 in the pDSRα vector. The DNAs were stably transfected in Chinese hamster ovary (CHO) cells as previously described.16,17 These compounds, used for in vivo administration, have been found endotoxin free (sensitivity of the assay ≤ 18 EU). To test for the presence of a coreceptor for TACI on T cells, TACI-Fc was used for staining together with a secondary fluoroscein isothiocyanate (FITC)–conjugated goat anti–human IgG antibody (Jackson ImmunoResearch Labs, West Grove, PA). Human IgG protein, namely ctrl IgG (Jackson ImmunoResearch Labs), was used as a control for the latter experiments.
Cell populations were sorted or analyzed by multiparameter flow cytometry using FACSAria, FACSCalibur, and FACScan instruments (Becton Dickinson Immunocytometry Systems, Mountain View, CA).
Immunization protocols
Peptide immunizations included (1) injection of CTL peptide(s) (50 μg/mouse) in incomplete Freund adjuvant (IFA; Sigma) or in complete Freund adjuvant (CFA; Difco Laboratories, Detroit, MI). Peptides were injected subcutaneously at the base of the tail in 200 μL volume; (2) co-injection of CTL and a Th cell peptide (50 μg and 140 μg/mouse, respectively), subcutaneously; (3) injection of OVA in IFA or in CFA subcutaneously at the base of the tail (100 μg/mouse in 200 μL); (4) TACI-Fc or B7RP-1–Fc administered intraperitoneally 24 hours prior and 48 hours after peptide priming; and (5) intravenous injection of in vitro generated and terminally matured, bone marrow–derived DCs18 that had been pulsed with a CTL-inducing peptide (VSV NP 52-59) for 2 hours before injection.
B-cell reconstitution
Freshly isolated B cells from wild-type or mutant knockout mice were positively selected using anti-CD19 microbeads and magnetic-activated cell-sorting (MACS) columns (Miltenyi Biotec, Auburn, CA) after depletion of CD43+ cells by negative selection.19 The resultant cell preparations were more than 98% B220+ cells as determined by FACS analysis. After purification, B cells were washed extensively and used to reconstitute B-cell–deficient mice by transferring 5 × 107 purified B cells/mouse, intravenously, into recipients 24 hours prior to peptide immunization.
CTL measurement
Seven days after antigen priming, spleen from immunized mice were harvested and homogenized into single-cell suspensions. Two types of analyses for CTL induction were performed: (1) an ex vivo interferon-gamma (IFN-γ) enzyme-linked immunospot (ELISPOT) assay20 ; and (2) the 51Cr release assay to measure cytotoxicity after culturing in vivo–primed splenocytes with antigens for 7 days. For the ex vivo analyses, 2 different protocols were optimized depending upon the immunogen. In experiments using mice primed with peptides, CD8+ T effector cells were enriched by positive selection (MACS system) and plated with irradiated syngeneic B-cell blasts pulsed with peptides as an APC source (1:1 ratio; 2.5 × 105 cells/well). B-cell blasts were activated in vitro for 48 hours with lipopolysaccharide (LPS; from Salmonella typhosa: Sigma) and dextran sulfate (Pharmacia Biotech AB, Uppsala, Sweden). This method was optimized in our laboratory to maximize the read-out by keeping the nonspecific background low. In experiments investigating the CTL response to the whole OVA protein that requires antigen processing, CD4-depleted splenocytes (≤ 3% contaminating cells) were used as APCs.
Culture medium consisted of RPMI-1640 supplemented with 20 mM glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 50 μM 2-ME, and 10% heat-inactivated fetal calf serum (FCS) (all reagents from Life Technologies, Gaithersburg, MD). After 5 days, cells were collected, purified using Ficoll gradients, and T-cell blasts were cultured in complete RPMI medium conditioned with supernatant from Concanavalin A (ConA)–activated splenocytes as an IL-2 source. Two days later (day 7 of culture), T cells were tested for specific CTL activity. Peptide specificity was determined in a standard 51Cr release assay using EL-4 (H-2b) thymoma cells as targets, at the indicated effector-to-target (E/T) ratios, with and without antigen. Nonspecific lysis was calculated by subtracting the cytolytic response to EL-4 cells in the absence of peptide (background). Data were calculated as % cytotoxicity = [(sample release – spontaneous release)/(maximum release – spontaneous release)] × 100.
Induction of apoptosis and necrosis
EL-4 cells grown in complete RPMI-1640 medium were used as a source of necrotic and apoptotic cells to study CD86 and BLyS expression in splenic CD11c+ cells after coculture with these antigenic stimuli. EL-4 cells were harvested, washed, and plated in 15-mL V-bottom Falcon tubes (Becton Dickinson, Franklin Lakes, NJ) at a final concentration of 5 × 106/mL prior to exposure to necrotic and apoptotic stimuli. Primary necrosis was induced by repeated freezing at –80°C and thawing at 37°C. One cycle of freezing and thawing resulted in cells that incorporated trypan blue; after 3 freeze-thaw cycles, EL-4 cells were completely disrupted into fragments, as previously described.21 UV-triggered apoptosis was induced using a 60-mJ UVB lamp, calibrated to provide 2 mj/cm2/sample (5 × 106 cells) as described.21
Preparation of bone marrow–derived and spleen-derived dendritic cells
The bone marrow cells were gently flushed from femurs of C57BL/6 mice. Spleens were homogenized in a single-cell suspension. Both preparations were depleted of red cells with acetate kinase (ACK) buffer and extensively washed. B lymphocytes, T lymphocytes, and macrophages were depleted by negative selection using biotinylated anti-CD4+, anti-CD8+, anti-CD19, anti-IAb, and anti-CD14 MoAbs, and streptavidin microbeads through MACS columns. CD11c+ cells were further positively selected to ensure purity (contaminants ≤ 3%). In some experiments CD11c+ cells were further purified by FACS sorting.
Bone marrow–derived DCs for CTL priming in vivo were terminally matured by tumor necrosis factor alpha (TNFα) added to cultures 24 hours prior to harvest18 after 10-day culture in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF). CD11c+ cells were then tested for purity and suitable preparations pulsed with 50 μg/mL VSV NP 52-59 peptide at the cell concentration of 107/mL for 2 hours prior to intravenous transfer (5 × 106 cells/mouse).
Results
Lack of CTL expansion in B-cell–deficient mice
We examined CTL expansion in B-cell–deficient mice (μMT–/–/μMT–/–) by using 2 immunodominant Th cell–independent peptides, VSV NP 52-59 and SEV NP 324-332, and 2 subdominant Th cell–dependent peptides with lower affinity for MHC class I molecules OVA 55-62 and LCMV NP 205-212.9 We also tested CTL responses to the OVA protein, which is capable of inducing CTLs following antigen processing and presentation by MHC class I molecules.22,23 Three immunization protocols were used: (1) CTL peptide in IFA (50 μg), administered subcutaneously; (2) co-injection of CTL peptide and helper T cell–inducing peptide (140 μg) in IFA, subcutaneously; and (3) OVA protein in CFA (100 μg), subcutaneously.
Seven days later, splenocytes from primed animals were stimulated in vitro with the peptide immunogen. Seven days after in vitro restimulation, splenocyte cultures were tested for CTL activity using a standard 51Cr release assay. In some experiments, CTL responses ex vivo were also analyzed by an IFNγ ELISPOT assay.
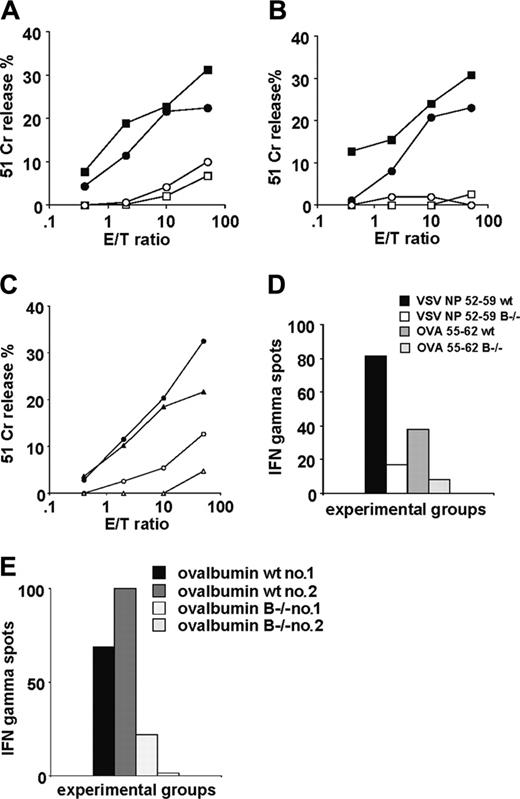
Surprisingly, the results indicated that cytolytic activity in response to immunization with VSV NP 52-59 or SEV NP 324-332 was extremely low in B-cell–deficient mice compared with wild-type mice, despite the immunodominance of both Th-independent peptides (Figure 1A). T-cell help during priming provided by co-injection with the helper peptide HBc 128-1409,24 did not improve the response (Figure 1B).
Similarly, CTL responses in mice coimmunized with subdominant, Th-dependent CTL peptides OVA 55-62 and LCMV NP 205-212,9 co-injected with a Th cell–inducing peptide were very poor (Figure 1C). This defective responsiveness of B-cell–deficient mice to CTL peptide immunization was also reflected by the lower number of IFNγ-producing cells measured ex vivo by the ELISPOT assay (Figure 1D). In some experiments peptides were emulsified in CFA, as for the OVA protein later described, to ensure complete DC maturation in vivo. The results were highly comparable to IFA priming showing the same discrepancy between wild-type and B-cell–deficient mice as reported with peptides emulsified in IFA (not shown).
When the whole OVA protein emulsified in CFA was used as the immunogen, a profound difference between wild-type and B-cell–deficient mice was also observed (Figure 1E). Collectively, these results support a critical role for B cells in expansion of naive CTLs in models where antigens with differing in vivo antigen processing requirements were tested.
CTL priming in B-cell–deficient mice. Four CTL peptides of different MHC class I binding affinity were used as immunogens in B-cell–deficient mice and compared with wild-type C57BL/6 mice. Open symbols represent the cytolytic response in B-cell–deficient mice, and solid symbols represent responses in wild-type mice in all panels. Two experiments have been averaged (SD ≤ 6% lysis for all E/T ratios). (A) Specific lysis measured by 51Cr release using as immunogens 2 immunodominant and Th cell–independent viral sequences emulsified in IFA. (B) Immunization with the same CTL epitopes where T-cell help is provided by co-injection with a helper peptide, the sequence derived from the hepatitis B virus (HBV) HBc 128-140. (C) CTL induction following priming with 2 subdominant Th-dependent peptides co-injected with Th (HBc 128-140 for the CTL determinant LCMV NP 205-212 and OVA 323-336 for the CTL determinant OVA 55-62). (D) Representative ex vivo IFNγ measurement by the ELISPOT assay in response to the T helper–independent VSV NP 52-59 and T helper–dependent OVA 55-62 peptides, co-injected with the helper OVA 323-336 determinant (SD ≤ 2%). (E) Ex vivo IFNγ measurement by ELISPOT assay in CD4+ T-cell–depleted spleen cells in response to the whole OVA protein used as immunogen in CFA: representative CTL responses in 2 wild-type C57BL/6 mice and 2 B-cell–deficient mice from the same experiment.
CTL priming in B-cell–deficient mice. Four CTL peptides of different MHC class I binding affinity were used as immunogens in B-cell–deficient mice and compared with wild-type C57BL/6 mice. Open symbols represent the cytolytic response in B-cell–deficient mice, and solid symbols represent responses in wild-type mice in all panels. Two experiments have been averaged (SD ≤ 6% lysis for all E/T ratios). (A) Specific lysis measured by 51Cr release using as immunogens 2 immunodominant and Th cell–independent viral sequences emulsified in IFA. (B) Immunization with the same CTL epitopes where T-cell help is provided by co-injection with a helper peptide, the sequence derived from the hepatitis B virus (HBV) HBc 128-140. (C) CTL induction following priming with 2 subdominant Th-dependent peptides co-injected with Th (HBc 128-140 for the CTL determinant LCMV NP 205-212 and OVA 323-336 for the CTL determinant OVA 55-62). (D) Representative ex vivo IFNγ measurement by the ELISPOT assay in response to the T helper–independent VSV NP 52-59 and T helper–dependent OVA 55-62 peptides, co-injected with the helper OVA 323-336 determinant (SD ≤ 2%). (E) Ex vivo IFNγ measurement by ELISPOT assay in CD4+ T-cell–depleted spleen cells in response to the whole OVA protein used as immunogen in CFA: representative CTL responses in 2 wild-type C57BL/6 mice and 2 B-cell–deficient mice from the same experiment.
TACI-deficient B cells cannot restore CTL generation in B-cell–deficient mice
Next, we performed B-cell reconstitution experiments in B-cell–deficient mice to further define their role during CTL priming, focusing on the relevance of antigen presentation, costimulatory molecules of the TNF family expressed on B cells,11 and B-cell–mediated lymphokines.25 VSV NP 52-59 was chosen as the CTL determinant in these experiments since the in vivo immunogenicity of this peptide epitope is not dependent on T-cell help.9 B cells purified from wild-type or knockout mice with targeted disruption of the genes encoding β2 microglobulin, CD40, TACI, IL-2, or IL-4, were injected intravenously (5 × 107/mouse) into B-cell–deficient mice 24 hours prior to priming with the VSV peptide. Splenocytes were isolated 7 days after priming and restimulated in vitro for 7 additional days before CTL specificity was measured by the 51Cr release assay. Untreated wild-type mice immunized with the VSV NP 52-59 peptide in IFA were also included in each experiment as a comparator control. CTL responses from B-cell–reconstituted mice measured in 3 independent experiments were expressed as a response ratio compared to the wild-type control group, with the latter being assigned a ratio of 1.
The suboptimal CTL response observed in B-cell–deficient mice following immunization with VSV NP 52-59 was effectively reversed by reconstitution with wild-type B cells as indicated by an increase in the wild-type response ratio from 0.1 to 0.8 (Table 1). Similarly, transfer of B cells from β2 microglobulin–deficient or CD40-deficient mice almost completely reconstituted the generation of VSV NP 52-59–specific CTL in B-cell–deficient mice (wild-type response ratio of 0.9), indicating that neither antigen presentation nor CD40 expression on B cells are essential for CTL expansion in vivo. In contrast, mice reconstituted with TACI-deficient B cells showed very poor VSV NP 52-59–specific CTL expansion (response ratio, 0.2), indicating a critical role for TACI-mediated B-cell interactions in primary CTL expansion following in vivo immunization. Last, IL-4– and IL-2–deficient B cells were capable of reconstituting the CTL repertoire in B-cell–deficient mice (both treatment groups with wild-type response ratios of 0.9), suggesting that B-cell–mediated lymphokines are not involved in primary CTL expansion.
TAC1-deficient B cells cannot restore CTL generation in B-cell–deficient mice
B-cell source* . | Response ratio to wild type† . |
|---|---|
| None (controls) | 0.1 |
| Wild-type C57/BL6 B cells | 0.8 |
| β2-/- B cells | 0.9 |
| CD40-/- B cells | 0.9 |
| TAC1-/- B cells | 0.2 |
| IL-2-/- B cells | 0.9 |
| IL-4-/- B cells | 0.9 |
B-cell source* . | Response ratio to wild type† . |
|---|---|
| None (controls) | 0.1 |
| Wild-type C57/BL6 B cells | 0.8 |
| β2-/- B cells | 0.9 |
| CD40-/- B cells | 0.9 |
| TAC1-/- B cells | 0.2 |
| IL-2-/- B cells | 0.9 |
| IL-4-/- B cells | 0.9 |
Purified B cells from different knockout mouse strains were injected intravenously into B-cell—deficient mice 24 hours before CTL priming with the VSV NP 52-59 peptide in IFA.
The wild-type response ratio was calculated by dividing the percent specific lysis at a 10:1 E/T ratio of the VSV NP 52-59—specific CTL response in B-cell—deficient recipient mice to the percent specific lysis observed in untreated wild-type C57/BL6 mice immunized with the same peptide. Data represent the average of 3 independent experiments (SD ≤ 0.01 in all of the groups tested).
B cells provide survival/costimulatory signals through TACI during CTL priming
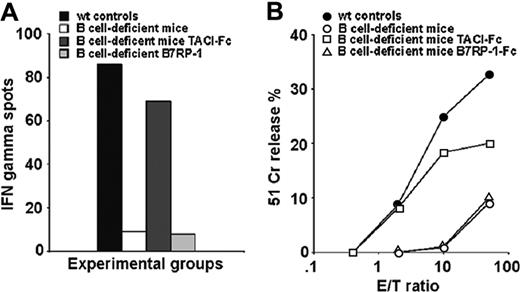
To further investigate the role of TACI expression on B cells during CTL priming, we determined if CTL expansion following priming with the VSV NP peptide could be restored in B-cell–deficient mice by intraperitoneal treatment with a recombinant murine TACI-Fc protein that contained the extracellular domain of TACI (residues 1-126) fused to the Fc fragment of human IgG1. As a control, we used an unrelated costimulatory fusion protein that binds ICOS, B7RP-1-Fc.16,17 B7RP-I has been chosen as a control molecule in these experiments because it, like TACI, is predominantly expressed on B lymphocytes.16,17 Both fusion protein preparations tested free of endotoxin (< 18 EU). Three independent experimental groups of B-cell–deficient mice were treated following different protocols: (1) immunization with 50 μg peptide in IFA; (2) intraperitoneal injection of 1 μg TACI-Fc 24 hours prior to and 48 hours after peptide priming; and (3) intraperitoneal injection of 1 μg B7RP-1-Fc following the same treatment schedule as TACI-Fc. As indicated in Figure 2A and B, B-cell–deficient mice primed with the VSV NP 52-59 peptide again did not respond to the immunization, however treatment with TACI-Fc did result in CTL induction as indicated by the comparable magnitude of IFNγ spot-forming cell responses in TACI-Fc–treated mice versus wild-type control mice (Figure 2A). In contrast, treatment with the control B7RP-1–Fc protein failed to reconstitute CTL induction in B-cell–deficient mice primed with the VSV peptide since the low level of IFNγ spot-forming CTL responses were comparable to the poor response observed in untreated B-cell–deficient mice immunized with the VSV NP 52-59 peptide. In support of these results, the cytolytic activity measured in splenocyte cultures from the TACI-Fc–treated B-cell–deficient experimental groups were similar to that observed in the wild-type controls (Figure 2B). Together, these experiments indicate that the requirement for B cells in CTL expansion can be replaced by a fusion protein bearing the TACI extracellular domain (aa 1-126).
CD8+ T cells do not express a ligand for TACI
Next, we addressed the role of cognate interactions between B cells and CD8+ T cells during priming. The specific TACI ligand BLyS is expressed in BLyS-transgenic T cells26-28 but not in human or murine wild-type T cells.29,30
We explored the possibility that a different interacting partner for TACI might be expressed by activated CD8+ T cells. In these experiments, we studied CD8+ T cells from mice that were transgenic for the OT-1 TcR that recognizes the Kb-restricted chicken OVA immunodominant sequence 257-264, SIINFEKL.14 Briefly, splenocytes prepared from OT-1 mice were stimulated in vitro with varying concentrations (10-1000 ng/mL) of the SIINFEKL peptide for 24 hours then analyzed for TACI ligand expression by FACS following treatment with TACI-Fc and double-staining with fluorochrome-conjugated anti–human IgG and anti-CD8 antibodies. Human IgG protein (ctrl IgG) was used as control.
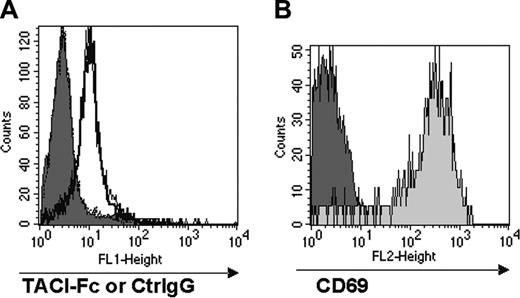
No appreciable staining with TACI-Fc was detectable on either resting or peptide-stimulated OT-1 CD8+ T cells compared with controls (Figure 3A). However, significant CD69 expression in the peptide-stimulated OT-1 population was observed compared with resting OT-1 (Figure 3B), demonstrating that T-cell activation had occurred. In contrast, TACI-Fc efficiently stained monocytes used as positive control in the assay (data not shown). These results indicate that CD8+ T cells do not express a TACI ligand at detectable levels, and therefore suggest that B cells do not directly engage CD8+ T cells through TACI.
TACI-Fc treatment restores CTL expansion in B-cell–deficient mice. B-cell–deficient mice (2 per group) were pretreated with either TACI-Fc or B7RP-1–Fc fusion proteins (1 μg/mouse, intraperitoneally, 24 hours before and 48 hours after peptide priming) then immunized with the VSV NP 52-59 peptide in IFA. Untreated wild-type C57/BL6 mice were also immunized as a control. Mice were killed 7 days after immunization, their spleens were harvested and homogenized into a single-cell suspension, then analyzed ex vivo for peptide-specific IFNγ-producing cells by the ELISPOT assay (A) and for cytolytic activity following additional in vitro stimulation of primed splenocytes with peptide (B). An average of 2 experiments is shown with SD ranging from ≤ 3 (A) and ≤ 4 (B) at all E/T ratios tested.
TACI-Fc treatment restores CTL expansion in B-cell–deficient mice. B-cell–deficient mice (2 per group) were pretreated with either TACI-Fc or B7RP-1–Fc fusion proteins (1 μg/mouse, intraperitoneally, 24 hours before and 48 hours after peptide priming) then immunized with the VSV NP 52-59 peptide in IFA. Untreated wild-type C57/BL6 mice were also immunized as a control. Mice were killed 7 days after immunization, their spleens were harvested and homogenized into a single-cell suspension, then analyzed ex vivo for peptide-specific IFNγ-producing cells by the ELISPOT assay (A) and for cytolytic activity following additional in vitro stimulation of primed splenocytes with peptide (B). An average of 2 experiments is shown with SD ranging from ≤ 3 (A) and ≤ 4 (B) at all E/T ratios tested.
CD8+ T cells do not express a coreceptor for TACI. Total splenocytes from OT-1 mice were stimulated with the SIINFEKL peptide (0.1μg/mL) for 24 hours, and stained for FACS analysis with TACI-Fc followed by FITC-conjugated goat anti–human IgG. Human IgG (Ctrl IgG) was used as a control for nonspecific binding in the FACS analysis. Panel A shows the staining with TACI-Fc of unstimulated and peptide-stimulated CD8+ T cells; panel B shows CD69 expression in unstimulated and peptide-stimulated CD8+ T cells. Substantial binding of TACI-Fc to either unstimulated (A, filled histogram) or peptide-stimulated (A, empty histogram) CD8+ T cells was not detectable. The staining with TACI-Fc is in fact highly comparable with the Ctrl IgG control in both cell preparations. T-cell activation has been proven by the selective CD69 expression in the peptide-stimulated T cells (B, light gray histogram).
CD8+ T cells do not express a coreceptor for TACI. Total splenocytes from OT-1 mice were stimulated with the SIINFEKL peptide (0.1μg/mL) for 24 hours, and stained for FACS analysis with TACI-Fc followed by FITC-conjugated goat anti–human IgG. Human IgG (Ctrl IgG) was used as a control for nonspecific binding in the FACS analysis. Panel A shows the staining with TACI-Fc of unstimulated and peptide-stimulated CD8+ T cells; panel B shows CD69 expression in unstimulated and peptide-stimulated CD8+ T cells. Substantial binding of TACI-Fc to either unstimulated (A, filled histogram) or peptide-stimulated (A, empty histogram) CD8+ T cells was not detectable. The staining with TACI-Fc is in fact highly comparable with the Ctrl IgG control in both cell preparations. T-cell activation has been proven by the selective CD69 expression in the peptide-stimulated T cells (B, light gray histogram).
B-cell–DC cocultures promotes DC maturation as a variety of antigenic stimuli
Because of the lack of a coreceptor for TACI on T cells, we asked whether B cells interact with dendritic cells through TACI inducing their maturation during CTL priming in vivo. In fact, DCs are known to constitutively express BLyS that TACI binds with high affinity in mice and humans. BLyS is up-regulated in inflammatory conditions.29,30
DC differentiation is reached within 7 to 10 days in culture in the presence of GM-CSF18 and is physiologically triggered in vivo by multiple stimuli, including LPS and necrotic cells.21 Apoptotic cells are also thought to induce DC maturation; however, this conclusion remains somewhat controversial.21,31
To assess the relationship between TACI signaling through BLyS and DC maturation we compared CD86 and BLyS expression on CD11c+ cells after exposure to a variety of antigenic stimuli or B-cell cocultures.
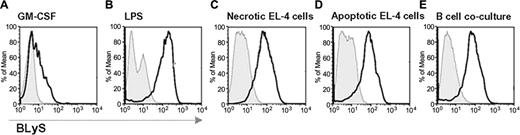
FACS-sorted CD11c+ cells, derived from the spleen of wild-type C57BL/6 mice, were cultured in different conditions: (1) GM-CSF control; (2) LPS (2.5 μg/mL); (3) necrotic EL-4 cells (5 × 106 cell/well, 1:1 ratio); (4) apoptotic EL-4 cells (5 × 106 cell/well, 1:1 ratio); or (5) splenic naive B cells (5 × 106 cell/well, 1:1 ratio) FACS-sorted with anti-CD19 MoAb. After 48 hours in culture, CD11c+ cells were analyzed by FACS for CD86 and BLyS expression.
BLyS up-regulation correlates with CD86 expression in maturing DCs exposed to a variety of antigenic stimuli or in B-cell cocultures. FACS-sorted splenic CD11c+ cells were cultured under different conditions to define a correlation between BLyS up-regulation and CD86 expression after exposure to a variety of antigenic stimuli or B-DC cocultures. Open bold histograms show the expression of BLyS in CD86+ gated cells compared with isotype controls (solid histograms). The culture conditions tested were: (A) GM-CSF alone; (B) 2.5 μg/mL LPS; (C) 5 × 106 necrotic EL-4 cells; (D) 5 × 106 apoptotic EL-4 cells; and (E) 5 × 106 splenic B cells.
BLyS up-regulation correlates with CD86 expression in maturing DCs exposed to a variety of antigenic stimuli or in B-cell cocultures. FACS-sorted splenic CD11c+ cells were cultured under different conditions to define a correlation between BLyS up-regulation and CD86 expression after exposure to a variety of antigenic stimuli or B-DC cocultures. Open bold histograms show the expression of BLyS in CD86+ gated cells compared with isotype controls (solid histograms). The culture conditions tested were: (A) GM-CSF alone; (B) 2.5 μg/mL LPS; (C) 5 × 106 necrotic EL-4 cells; (D) 5 × 106 apoptotic EL-4 cells; and (E) 5 × 106 splenic B cells.
In these experiments CD11c+ cells cultured in GM-CSF alone served as controls (Figure 4A). As expected, in the presence of LPS, 70% of the CD11c+ cells were CD86+ and BLyShigh (Figure 4B). Similar results were obtained in cocultures with necrotic EL-4 cells (Figure 4C) and apoptotic cells (Figure 4D), supporting a correlation between antigen-induced maturation and BLyS up-regulation. Despite the absence of antigenic stimuli, the presence of B cells in DC cultures had a profound effect on CD11c+ cell maturation and BLyS expression (Figure 4E) compared with controls (Figure 4A). Together, these results support a role for B cells in inducing DC maturation through TACI-BLyS interaction, possibly via a reverse-signaling event through cell-surface BLyS.
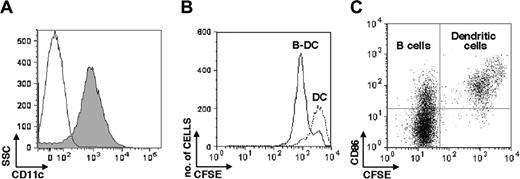
DCs mature and divide in the presence of B cells
Driven by previous results, we next asked whether the role of B lymphocytes during CTL priming involves dendritic cell maturation or expansion. Spleen-derived DCs were sorted for CD11c+ cells (Figure 5A) and labeled with 5 μM CFSE32 prior to culture with purified splenic B cells (isolated by sorting for CD19-expressing cells) at a 1:1 ratio at the cell concentration of 5 × 106 cells/well. B-cell–DC cocultures and DC cultures were harvested 72 hours later and analyzed by FACS for CFSE dilutions and CD86 expression. CD11c+ cells underwent 1 cell division in the presence of B cells, while no cell division was detectable in the CD11c+ cell population cultured alone (Figure 5B). DC division occurred rapidly, as suggested by the distribution of CFSE intensities in cells. Moreover, the large majority of the CFSE-labeled CD11c+ cells in the B-cell cocultures expressed CD86 (Figure 5C, top right panel). It is worth noticing that B cells in the cocultures also up-regulated CD86 (Figure 5C, CFSE-negative cells, top left panel), supporting a critical role for TACI-mediated signaling in B-cell activation.13
Unsuccessful DC priming in B-cell– and TACI-deficient mice
To further investigate the requirement for B-cell–DC interaction to prime naive CD8+ cells in vivo, we studied CTL expansion in B-cell–deficient and TACI-deficient mice immunized with BLyShigh mature bone marrow–derived DCs, obtained by culturing bone marrow precursor cells as described.18 Mature DCs were pulsed for 2 hours with VSV NP 52-59 peptide prior to intravenous transfer into wild-type, B-cell–deficient, or TACI-deficient mice at a dose of 2 × 106 cells/mouse. Mice were killed 7 days after DC priming to examine the VSV NP 52-59–specific CTL response ex vivo as determined by IFNγ production and cytotoxicity after in vitro expansion with specific peptide.
B-cell–DC cocultures induce CD11c+ cell maturation and increase cell division. Splenic CD11c+ cells were purified by FACS sorting and CFSE-labeled prior cocultures with B cells. CD11c+ cells alone have been used as control in these experiments. After 72 hours cells were collected and analyzed for cell division and expression of the CD86 maturation marker. Splenic CD11c+ were analyzed for purity after FACS sorting (A). SSC indicates side scatter. CFSE dilutions are shown in panel B, where B-cell–DC cocultures are represented by solid histograms and DCs alone by dotted histograms. Confirming previous results, CD86 was expressed in the large majority of the CD11c+ cells cocultured with B cells (C, top right quadrant). As expected, DC induced B-cell maturation through BLyS (C, top left quadrant).
B-cell–DC cocultures induce CD11c+ cell maturation and increase cell division. Splenic CD11c+ cells were purified by FACS sorting and CFSE-labeled prior cocultures with B cells. CD11c+ cells alone have been used as control in these experiments. After 72 hours cells were collected and analyzed for cell division and expression of the CD86 maturation marker. Splenic CD11c+ were analyzed for purity after FACS sorting (A). SSC indicates side scatter. CFSE dilutions are shown in panel B, where B-cell–DC cocultures are represented by solid histograms and DCs alone by dotted histograms. Confirming previous results, CD86 was expressed in the large majority of the CD11c+ cells cocultured with B cells (C, top right quadrant). As expected, DC induced B-cell maturation through BLyS (C, top left quadrant).
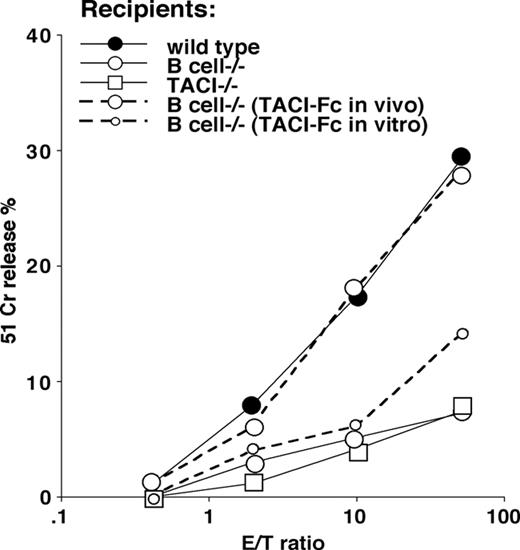
The specific cytolytic activity measured after 7 days in culture showed profound differences between wild-type controls and B-cell–deficient or TACI-deficient mice (Figure 6).
To address the role of TACI in the lack of primary CTL expansion in B-cell–deficient mice immunized with mature peptide-pulsed DCs, we introduced 2 experimental groups: (1) B-cell–deficient recipients that received 1 μg of a recombinant murine TACI-Fc protein containing the extracellular domain of TACI (residues 1-126) fused to the Fc fragment of human IgG1 (intraperitoneally) 24 hours prior and 48 hours after peptide-pulsed DC transfer; (2) B-cell–deficient recipients that received peptide-pulsed mature DCs treated the last 48 hours in culture with 1 μg/mL TACI-Fc.
The results confirm that TACI-Fc restored VSV NP 52-59–specific CTL expansion in B-cell–deficient mice when administered in vivo after DC transfer, as previously demonstrated for peptide priming, but not when used in vitro during DC cultures (Figure 6, dotted lines). These results were obtained regardless from the evidence that TACI-Fc–treated DCs were rapidly differentiating and expressed high levels of CD86 prior to peptide pulsing and in vivo transfer.
Requirement for B cells or TACI for DC priming in mice. VSV NP 52-59–specific cytolytic activity in mice primed with 2 × 106 mature, BLyShigh bone marrow–derived DCs pulsed with the VSV NP 52-59 peptide after terminal differentiation with TNFα. Three experiments, each containing 2 mice per group, have been averaged (SD ≤ 4 at all the E/T ratios studied). Three recipient mouse strains (wild-type C5BL7/6, and B-cell–deficient and TACI-deficient knockout strains) were used in these experiments, as indicated. To address the importance of TACI signaling in this priming protocol, 2 independent groups of B-cell–deficient mice have been treated either with TACI-Fc administered intraperitoneally 24 hours prior to and 48 hours after CTL priming, or TACI-Fc administered in vitro, the last 48 hours in DC cultures prior to peptide pulsing and in vivo transfer. The results of these experiments, shown with dotted lines, support the need for an ongoing TACI-BlyS signal during priming, as proven by the restored CTL induction in B-cell–deficient mice that received TACI-Fc in vivo but not in vitro (dotted lines).
Requirement for B cells or TACI for DC priming in mice. VSV NP 52-59–specific cytolytic activity in mice primed with 2 × 106 mature, BLyShigh bone marrow–derived DCs pulsed with the VSV NP 52-59 peptide after terminal differentiation with TNFα. Three experiments, each containing 2 mice per group, have been averaged (SD ≤ 4 at all the E/T ratios studied). Three recipient mouse strains (wild-type C5BL7/6, and B-cell–deficient and TACI-deficient knockout strains) were used in these experiments, as indicated. To address the importance of TACI signaling in this priming protocol, 2 independent groups of B-cell–deficient mice have been treated either with TACI-Fc administered intraperitoneally 24 hours prior to and 48 hours after CTL priming, or TACI-Fc administered in vitro, the last 48 hours in DC cultures prior to peptide pulsing and in vivo transfer. The results of these experiments, shown with dotted lines, support the need for an ongoing TACI-BlyS signal during priming, as proven by the restored CTL induction in B-cell–deficient mice that received TACI-Fc in vivo but not in vitro (dotted lines).
Three independent experiments each using 2 mice per group were performed and gave similar results. Together, these findings suggest that BLyShigh mature DCs interact with B cells through TACI to allow CTL priming, and that this interaction should be ongoing during the first encounter with antigens in vivo. In fact, TACI engages a larger number of DCs through BLyS and induces their maturation, expanding consistently the number of professional APCs available to prime naive CD8+ T cells.
Discussion
The BLyS receptor is a TNF family member critical for B-cell survival, proliferation, and differentiation through BLyS-mediated B-cell–DC interaction.33 BLyS is predominantly expressed on myeloid cells such as neutrophils, macrophages, monocytes, and mature DCs and is up-regulated during inflammatory conditions.29,30 BLyS binds with high affinity to TACI,27,34 a type III transmembrane protein with an extracellular NH2-terminus lacking a cleaved signal sequence.10 TACI is constitutively expressed on naive B cells and up-regulated on activated B cells. T cells also express TACI early after activation.10
Here we define a novel, reciprocal relationship by which DCs mature through BLyS binding with TACI expressed on B cells. Evidently via a process of reverse signaling, this mechanism leads to amplification of the mature APC population critical to prime naive CD8+ T cells in vivo.
Signaling induced by BLyS binding to the extracellular domains of BAFF-R, TACI, and B-cell maturation antigen (BCMA) has been implicated in numerous B-cell functions. However, there has been no indication, to date, that reverse signaling might occur in cells expressing membrane-bound BLyS. Like the other members of the TNF family, BLyS is a type II transmembrane protein, with a small intracellular domain at its N-terminus. Although the molecular events that mediate reverse signaling through TNF homologs are not yet completely understood, it is clear that this type of signaling can be activated by cross-linking various family members, including TNF-related activation-induced cytokine (TRANCE),35 LIGHT,36 OX40L,37 FasL,38 CD30L,39 CD40L,40 and TNF itself.41
Here we have presented data indicating that the requirements for B cells in inducing DC maturation can be replaced by a recombinant form of the extracellular domain of the TACI receptor. This effect occurs at TACI-Fc concentrations far below what would be required to serve as a decoy receptor, suggesting that cell-surface BLyS on DCs can be stimulated by TACI-Fc binding. In addition, we show that TACI-Fc induces DC division and maturation, further supporting this hypothesis.
The other known BLyS receptors do not play a role in this model: TACI-deficient B cells express BAFF-receptor (BAFF-R) and BCMA,13 and yet cannot restore CTL priming in B-cell–deficient mice. On the other hand, the APRIL ligand, which is able to bind to TACI with lower affinity,42 was detectable only on the cell surface of immature DCs and not in DCs induced to mature by antigenic stimuli. In addition, the secreted form of APRIL43 was not detected in DC culture supernatants (not shown); thus, we do not attribute a significant role to this ligand in DC-mediated CTL expansion.
CTLs divide within a few days of antigen exposure,6-8 which is significantly faster than CD4+ T-helper cells (reviewed in Seder and Ahmed5 ). The involvement of TACI and BLyS in inducing dendritic cell maturation and expansion during CTL priming may explain this difference.
It seems likely that TACI-BLyS reverse signaling occurs when a certain threshold of BLyS and TACI expression is reached. It is well known that BLyS is up-regulated under inflammatory conditions.29,30 Also, the effector molecules produced by CD8+ T cells after antigen recognition, IFNγ and TNFα, up-regulate BLyS expression,30 increasing the probablity for productive TACI-BLyS interactions.
The data presented here suggest that circulating mature DCs following the initial uptake of antigens reach secondary lymphoid organs where they activate B cells through BLyS that is up-regulated (Craxton et al44 and data in this study). The expression of TACI increases on activated B cells allowing signals to neighboring immature DCs that progressively up-regulate BLyS during T-cell–antigen priming. Moreover, activated B cells can efficiently present antigens to naive T cells, increasing the number of professional APCs available.
This novel role for B cells supports several models where B cells are required for virus-specific immunity, independent of antibody responses.45-53
Because of the possible expression of TACI on activated T cells, exceptions from an absolute requirement for B cells to prime naive CD8+ T cells are possible. In fact, CTL priming using intact cells as antigen involving a large antigen-specific and by-standard T-cell activation does not impair anti-HY CTL responses.54 Moreover, it has recently been reported that either CD4+ T cells or B cells prolonged the life span of transferred antiviral CTLs in vivo,51 raising the possibility that B-cell signaling through TACI could be compensated by TACI expression on activated CD4+ T-helper cells. By the same token, up-regulation of TACI and B-cell activation may shape the requirement for CD4+ T-cell help in secondary CTL responses.55-58 It is also possible that the extent of TACI-BLyS interactions regulate T-cell expansion independently from regulatory T cells, as shown in an elegant autoimmune model.59
All together, these results elucidate a novel role for TACI and BLyS in providing the correct antigen presentation for CTL-based immune responses, bridging innate and native immunity.
Prepublished online as Blood First Edition Paper, September 29, 2005; DOI 10.1182/blood-2004-12-4708.
Supported by National Institutes of Health (NIH) grant no. CA78657, Department of Defense grant no. BC010002, Aging and Alzheimer Research Center grants (A.F.), and NIH grant no. 1CA76274 (R.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Christopher Benedict for helpful suggestions; Dr Chrystelle Assemann for help provided in the in vivo experiments; Yoav Altman and Kurt Van Gunft for FACS analysis; and Christine N. Auciello for excellent secretarial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal