Abstract
Emerging evidence suggests the insulin-like growth factor-1 receptor (IGF-1R) to be an important mediator of tumor-cell survival and resistance to cytotoxic therapy in multiple myeloma (MM). Recently, members of the cyclolignan family have been shown to selectively inhibit the receptor tyrosine kinase (RTK) activity of the IGF-1R β-chain. The effects of the cyclolignan picropodophyllin (PPP) were studied in vitro using a panel of 13 MM cell lines and freshly purified tumor cells from 10 patients with MM. PPP clearly inhibited growth in all MM cell lines and primary MM samples cultured in the presence or absence of bone marrow stromal cells. PPP induced a profound accumulation of cells in the G2/M-phase and an increased apoptosis. Importantly, IGF-1, IGF-2, insulin, or IL-6 did not reduce the inhibitory effects of PPP. As demonstrated by in vitro kinase assays, PPP down-regulated the IGF-1 RTK activity without inhibiting the insulin RTK activity. This conferred decreased phosphorylation of Erk1/2 and reduced cyclin dependent kinase (CDK1) activity. In addition, the expression of mcl-1 and survivin was reduced. Taken together, we suggest that interfering with the IGF-1 RTK by using the cyclolignan PPP offers a novel and selective therapeutic strategy for MM.
Introduction
The insulin-like growth factor-1 receptor (IGF-1R) is strongly suggested to play key roles in malignant transformation and in promoting survival of tumor cells from cancers of, for example, breast, prostate, and colon.1,2 Also in multiple myeloma (MM) cells the IGF-1R has been shown by us and others to stimulate growth and potently mediate survival.3-11
Some characteristic features of the MM disease include growth of tumor cells almost exclusively restricted to the bone marrow, complex genetic aberrations, the presence at a high frequency of illegitimate translocations of a few identified partner genes (ie, 11q13 [cyclin D1], 6p21 [cyclin D3], 4p16 [FGFR3/MMSET], 16q23 [c-maf], and 20q11 [mafB]) to the immunoglobulin heavy chain locus, and the altered expression of c-Myc and Bcl-2 family genes.12 So far, genetic alterations of the IGF-1R in malignant cells, including MM, have not been reported.13 However, in the bone marrow environment, the IGF-1R in MM cells may become hyperactive as a result of autocrine and/or paracrine stimulation, making molecules of the IGF-1R signaling pathway equally important targets for intervention as mutated oncogenes. Supporting the notion of the hyperactivated IGF-1R in MM is the recent finding that IGF-1 serum level is a prognostic factor in MM.14 The fact that IGF-1R signaling seems not to be an absolute requirement for maintenance of normal cell homeostasis13 would encourage the development of IGF-1R inhibitors for clinical use in MM, as they may not be associated with the severe side effects of conventional cytotoxic drugs.
On ligand interaction with the IGF-1R α-subunit, tyrosine residues in the intracellular, membrane-bound β-subunit become autophosphorylated.15 This enables docking and phosphorylation of the insulin receptor substrate (IRS) and Shc, thereby activating 2 important pathways mediating proliferation and survival, that is, the phosphatidylinositol 3-kinase (PI3K)/Akt and the mitogen-activated protein kinase (MAPK) pathways.16 Different approaches have been used to inhibit the IGF-1R function in malignant cells, including neutralizing antibodies, IGF-1 mimetics, dominant-negative IGF-1R, and IGF-1R antisense/siRNA.17 Another possibility is represented by directly targeting the receptor tyrosine kinase (RTK). However, selective inhibition of the IGF-1R activity has been difficult to achieve, because the IGF-1 RTK shows 84% homology with the insulin RTK.15 Inhibition of the insulin RTK inhibition could lead to therapy-resistant diabetes and thus be fatal.18 Information from crystallographic studies revealing the conformational differences of IGF-1R and insulin R kinases has been the basis for the development of selective inhibitors.19-21 Recently, members of the cyclolignan family were shown to potently inhibit the activity of the IGF-1 RTK.22 In particular the inhibitory effect of picropodophyllin (PPP) seemed promising, because it displayed selectivity for the IGF-1R and did not coinhibit tyrosine phosphorylation of the insulin R or other, less related receptors like FGF-R, PDGF-R, and EGF-R.22 Inhibition of the IGF-1 RTK with PPP was shown to be noncompetitive with respect to ATP, suggesting interference with the IGF-1R at the substrate level.22 A more recent study has shown that PPP specifically blocks phosphorylation of the residue Tyr1136 in the activation loop of the IGF-1R kinase.23 Phosphorylation of Tyr1136 is important for the IGF-1R activity because it stabilizes the catalytically optimized conformation of the receptor.19
We here demonstrate the capacity of the selective IGF-1 RTK inhibitor PPP to induce cell-cycle accumulation and apoptosis in MM cells. These findings further emphasize a pivotal role of IGF-1R signaling in maintaining the vitality of the malignant cells in MM and suggest that IGF-1R inhibition is a potentially important therapeutic principle in MM.
Materials and methods
MM cell lines
The MM cell lines used were EJM,24 JJN3,25 Karpas 707,26 L363,27 LP-1,28 OPM-2,29 U-1957, U-1958, U-1996,30 U-266-1970,31 RPMI 8226,32 and its subclones RPMI 8226/Dox4033 and RPMI 8226/LR534 showing increased resistance to doxorubicin and melphalan, respectively. All cell lines were maintained in RPMI 1640 (Flow, Irvine, United Kingdom) supplemented with 10% fetal bovine serum (FBS; Sigma, St Louis, MO), glutamine (2 mM), and antibiotics (penicillin 100 U/mL and streptomycin 50 μg/mL) at 37°C in a humidified 5% CO2 in-air atmosphere. The interleukin-6 (IL-6)–dependent U-1957, U-1958, and U-266-1970 cell lines were routinely cultured on normal human skin fibroblasts AG1523 or SK1069 (Human Cell Repository, Camden, NJ). This was also true for the IL-6–independent U-1996 cell line responding to IL-6 with increased proliferation.35 In the experimental settings, recombinant IL-6, 10 ng/mL for U-1957, U-1958, and U-1996 and 4 ng/mL for U-266-1970, was used instead of fibroblasts to facilitate end point analyses. Exponentially growing cells were seeded into 24-, 48-, or 96-well flat-bottomed tissue culture plates (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ) at 4 × 105 cells/mL (EJM, JJN3, Karpas 707, L363, LP-1, OPM-2, U-1957, U-1958, U-1996, and U-266-1970) or 2 × 105 cells/mL (RPMI 8226, RPMI 8226/Dox40, and RPMI 8226/LR5) and incubated overnight before the addition of reagents. The IGF-1R–negative mouse MM cell line 5T33MMvt36 was cultured in DMEM (Sigma) medium supplemented with 10% FBS, antibiotics, glutamine, and MEM (Sigma).
Purified plasma cells from patients with MM
Heparinized bone marrow samples were obtained from patients with newly diagnosed MM (Patients 1-4), stable disease (Patient 5), and relapse (Patients 6-10). Mononuclear cells separated by Ficoll-Paque Plus density sedimentation (Amersham Biosciences, Uppsala, Sweden) were subjected to CD138 immunomagnetic purification (Miltenyi Biotech, Paris, France). The enriched fraction showed a purity of greater than 95% plasma cells as determined by May-Grünwald-Giemsa staining and morphology. The experiments were performed using round-bottomed 96-well tissue culture plates (Falcon) where the cells were seeded at 0.5 × 106 cells/mL. The study was conducted in accordance with the Helsinki protocol and approved by ethical committees of the involved universities. All bone marrow samples were obtained after informed consent.
Bone marrow stromal-cell (BMSC) and fibroblast culture
BMSC culture was established from healthy donors and patients with MM essentially as described previously.37,38 BMSCs and SK1069 fibroblasts were harvested by trypsinization and seeded at 1 × 104 cells/well into 96-well flat-bottomed tissue culture plates (Falcon) and grown until confluency before PPP treatment was initiated.
Chemicals and reagents
The cyclolignan PPP was prepared as described and dissolved in ethanol to a concentration of 0.5 mM.22 In the experiments the amount of ethanol never exceeded 0.4%, a concentration not affecting growth or survival of the MM cells (data not shown). Dexamethasone, doxorubicin, and melphalan (Sigma) were dissolved according to the manufacturer. Recombinant IGF-1, IGF-2, insulin (R&D Systems, Minneapolis, MN), IL-6 (PeproTech, Rocky Hill, NJ), and the IGF-1 analog LongR3-IGF-1 (Sigma) were used at indicated concentrations. The selective CDK1 inhibitor CGP74512A,39 the mTOR inhibitor rapamycin, the p38 inhibitor SB203580 (Merck Biosciences, Darmstadt, Germany), and the pan-caspase inhibitor Z-VAD-fmk (R&D Systems) were dissolved in DMSO and used at indicated concentrations. The amount of DMSO in the experiments never exceeded 0.1%, a concentration not altering growth or survival of the MM cells (data not shown). The agonistic Fas-antibody CH11 (Immunotech, Marseilles, France) was used at indicated concentrations. Purified polyclonal IgM antibodies (R&D Systems) served as control antibodies.
Analysis of cell growth using the resazurin assay
At harvest of the MM cell line experiments, the cells were washed twice in 200 μL PBS containing 20 mM HEPES (pH 6.8) before 100 μL serum-free RPMI 1640 containing 40 mM HEPES (pH 6.8) and 10% of resazurin (Sigma) at a concentration of 440 μM was added to each well followed by incubation in the dark for 1 hour at room temperature. At harvest of the experiments with freshly purified MM cells, BMSCs, or SK1069 fibroblasts the washing step was omitted, and the resazurin was added directly at a concentration of 10%, followed by incubation for 3 hours at 37°C in a humidified 5% CO2 in-air atmosphere. Analysis of fluorescence by using a Wallac Victor Multilabel Counter (Wallac, Turku, Finland) was followed by calculation of the relative number of viable cells expressed as a percentage of untreated cells. Resazurin is the active compound of the commercially available growth indicator dye Alamar Blue.40 In response to metabolically active cells resazurin becomes increasingly fluorescent,41-43 and separate experiments show a linear correlation between the number of viable MM cells and the emitted light (data not shown).
Analysis of DNA synthesis in cocultures
The high yield of plasma cells from 4 bone marrow samples (Patients 5, 8, 9, and 10) allowed assessment of the effect of PPP using cocultures containing primary MM cells cultured on top of patient-derived long-term BMSCs. BMSCs characteristically exhibit a fibroblastlike morphology and effectively sustain viability of primary MM cells when cultured ex vivo.37 BMSCs were harvested by trypsinization and seeded at 5 × 104 cells/well into 96-well flat-bottomed tissue culture plates (Falcon) and incubated overnight. The medium was exchanged and the purified, primary MM cells were seeded at 1 × 106 cells/well on top of the confluent, nonproliferative monolayer of BMSCs and allowed to adhere for 12 hours. The cultures were then treated with 1 μM PPP for 72 hours, including the addition of 1 μCi/well (0.037 MBq/well)3H-thymidine (3H-TdR; Amersham Biosciences) for the final 20 hours of incubation. Following trypsinization and harvest on filters (Wallac) radioactivity was quantified using a 1450 Microbeta Liquid Counter (Wallac). The number of counts was corrected for the counts generated by the BMSCs alone, and results are expressed as a percentage of untreated cells.
Analyses of cell-cycle phase distribution and apoptosis
Analysis of cell-cycle distribution was performed according to Vindelöv,44 whereby propidium iodide (PI)–stained nuclei were analyzed by flow cytometry (FACSort, BD Biosciences, San Jose, CA), and cell-cycle phases were calculated using ModFit LT 3.0 Analysis Software (Verity Software House, Topsham, ME). Apoptosis was quantified by staining with annexin V (AV)–fluorescein isothiocyanate (FITC) and PI using TACS Annexin V–FITC Apoptosis Detection Kit (R&D Systems). The samples were treated as instructed by the manufacturer followed by analysis using flow cytometry. Apoptosis was also quantified by the terminal transferase-mediated nick end labeling (TUNEL) assay using Fluorescein-FragEL DNA Fragmentation Detection Kit (Merck Biosciences) or FlowTACS Apoptosis Detection Kit (R&D Systems) according to the manufacturer.
Analysis of IGF-1 RTK and insulin RTK autophosphorylation in vitro
Autophosphorylation of the IGF-1 RTK and insulin RTK, respectively, was analyzed by a sandwich enzyme-linked immunosorbent assay (ELISA) essentially as described previously22 using a tyrosine kinase assay kit (Sigma). Briefly, 96-well plates (Immunolon; Nunc, Roskilde, Denmark) were coated with 5 μg/well of the H-60 antibody specific for the extracellular part of IGF-1R β-subunit or C-19 specific for the insulin R β-chain (Santa Cruz Biotechnology, Santa Cruz, CA) and incubated overnight at 4°C. The plates were blocked with 1% bovine serum albumin in PBS/Tween for 1 hour followed by addition of 150 μg/well total protein lysate from the RPMI 8226 and Karpas 707 cell line, respectively. PPP was added at indicated concentrations in tyrosine kinase buffer without ATP at room temperature for 30 minutes before the kinase was activated by the addition of ATP. As a negative control ATP-free reaction was used.
Western blotting
To analyze the expression of protein kinases and other signaling components downstream of the IGF-1R, RPMI 8226 and 5T33MMvt cells were serum-starved overnight before the addition of PPP at 1 μM. At indicated time points the cells were then treated for 5 minutes with 6.7 nM IGF-1 followed by immediate lysis and analysis by Western blotting as described previously.45 Primary antibodies used were specific for Akt (total), Akt (Ser473), GSK-3α/β (Ser21/Ser9), p70S6K (total), p70S6K (Thr389), p70S6K (Thr421/Ser424), caspase-3 (Asp175), caspase-9 (9502), and caspase-8 (1C12) (Cell Signaling Technology, Beverly, MA), GSK-3β (total), Erk1/2 (total), Erk1/2 (Thr202/Tyr204), CDK1 (total), CDK1 (Thr14/Tyr15), cyclin B (H-20), cyclin D2 (C-17), cyclin E (HE12), bcl-xL (H-62), bcl-2 (C-21), mcl-1 (S-19), actin (I-19), and survivin (N-18) (Santa Cruz Biotechnology), hsp60, hsp70, and hsp90 (BD Biosciences, Franklin Lakes, NJ), and p-Tyr (PY-99) (Santa Cruz Biotechnology). The level of actin was used as a loading control.
In vitro kinase assay
To analyze CDK1 kinase activity, RPMI 8226 cells were serum-starved overnight, and PPP was then added at 1 μM. At indicated time points the cells were treated for 5 minutes with 6.7 nM IGF-1 and lysed using 150 mM NaCl, 0.5% NP-40, 50 mM Tris pH 6.8, and 1 mM DTT lysis buffer. Equal amounts of protein were immunoprecipitated with 2 μg CDK1 antibody (Santa Cruz Biotechnology) for 2 hours at 4°C. Protein G–Sepharose was added, and incubation continued for another 1 hour at 4°C. The immunoprecipitates were washed 3 times in lysis buffer and 3 times in kinase buffer (10 mM Tris pH 7.5, 4 mM MgCl2, 1 mM DTT). The beads were suspended in 10 μL reaction buffer containing 20 mM Tris pH 7.5, 8 mM MgCl2, 1 mM DTT, 5 μg Histone H1, and 5 μCi (0.185 MBq)32P-γ-ATP (Amersham Biosciences), and incubated for 30 minutes at 37°C. After addition of reducing sample buffer and boiling at 95°C, the samples were loaded on 4% to 12% NuPAGE gels. The amount of labeled Histone H1 was quantified by using a Fuji BAS 2000 Imaging System (Fuji, Tokyo, Japan).
Statistical analysis
Statistical analysis was done by analysis of variance (ANOVA) followed by multiple comparison by the Fisher method using StatView software (SAS, Cary, NC).
Results
Cyclolignan PPP inhibits growth of MM cell lines and primary MM cells
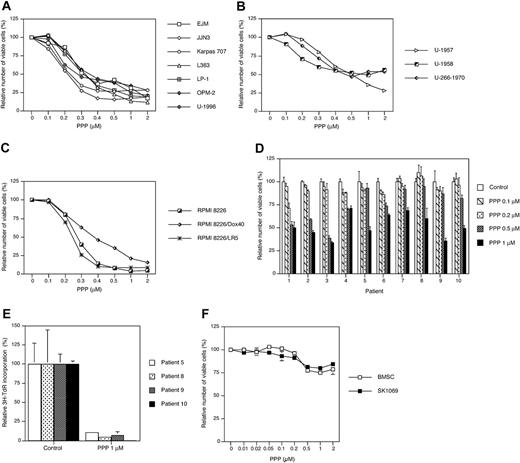
We first examined the effect of PPP on the growth of 13 selected MM cell lines. After 48 hours of exposure to PPP, the relative number of viable cells was determined by using the resazurin assay. All MM cell lines responded to PPP with growth inhibition exhibiting IC50s ranging from 0.2 μM as for the JJN3 cell line to 0.5 to 1 μM as for the U-1958 and U-266-1970 cell lines (Figure 1A-C). A slightly higher sensitivity to PPP was observed among the IL-6–independent cell lines (Figure 1A,C) as compared with the IL-6–dependent cell lines (Figure 1B). The RPMI 8226 cell line and its drug-resistant subclones showed variable responses to PPP (Figure 1C). Both the melphalan-resistant RPMI 8226/LR5 subclone and the wild-type RPMI 8226 cell line were highly sensitive to inhibition by PPP. Although the doxorubicin-resistant RPMI 8226/Dox40 cell line was the least sensitive of the RPMI 8226 clones (Figure 1C), it was still more sensitive to PPP than the IL-6–dependent cell lines (Figure 1B).
Purified tumor cells from 10 patients with MM responded variably to 72 hours of PPP treatment with 30% to 65% decrease in the number of viable cells (Figure 1D). Although none of the samples could be regarded as nonresponding, the primary MM cells were in general less sensitive to PPP as compared with the MM cell lines. To assess the potency of PPP during more in vivo–like conditions, purified MM cells from 4 patients with MM were preincubated on top of confluent BMSCs before addition of PPP. Treatment of these cocultures with PPP for 72 hours greatly down-regulated DNA synthesis in all tumor-cell samples as measured by 3H-TdR incorporation (Figure 1E). For the sake of comparison, the effect of increasing concentrations of PPP on BMSC and SK1069 fibroblasts was monitored by the resazurin assay. At 1 μM PPP the amount of viable cells was only reduced by 20% to 25% at 72 hours (Figure 1F).
PPP induces G2/M-phase accumulation and apoptosis
To dissect the growth inhibitory effects of PPP, we analyzed the cell lines for cell-cycle phase distribution and apoptosis. Exposure of LP-1 cells to 1 μM PPP for 24 hours increased the fraction of cells in the G2/M phase from 10% to 78% with a corresponding decrease of the fraction of cells in G1 and S phases (Figure 2A). Induction of G2/M-phase accumulation and decrease in the G1 and S phases by PPP was consistently found in all other MM cell lines of the panel as well (data not shown). Furthermore, analysis of cell death revealed that treatment with 1 μM PPP for 24 hours increased the amount of apoptosis in the LP-1 cell line more than 6 times as compared with control (Figure 2B). Similarly, exposure of Karpas 707 cells to 1 μM PPP for 48 hours drastically increased the amount of apoptosis as analyzed by TUNEL (Figure 2C). Apoptosis induced by PPP was consistently demonstrated in all MM cell lines studied (data not shown).
PPP suppresses IGF-1R autophosphorylation in the absence of coinhibition of the insulin RTK activity, inhibits Erk1/2 and CDK1 phosphorylation, and down-regulates mcl-1 and survivin
Because the capacity of PPP to inhibit the IGF-1 RTK activity has previously been shown in nonhematopoietic cell lines (sarcoma, melanoma, and carcinoma),22 we assessed whether this could be demonstrated also in MM cells. Thus, we used an ELISA-based approach in which the IGF-1R autophosphorylation was quantified in vitro. The autophosphorylation of IGF-1R extracted from RPMI 8226 cells could be inhibited by 60% in response to PPP with an IC50 of approximately 0.5 μM (Figure 3A). This was also true for the Karpas 707 cells, in which the autophosphorylation was inhibited by 90% at the highest PPP concentration (Figure 3A). In contrast, the autophosphorylation of the insulin R was not inhibited by PPP treatment (Figure 3B). In addition, IGF-1R autophosphorylation in vivo was quantified in lysates prepared from RPMI 8226 cells cultured in the presence of serum. As expected, IGF-1 increases autophosphorylation of the IGF-1R (Supplemental Figure S1A, available at the Blood website; click on the Supplemental Figures link at the top of the online article). PPP was shown to clearly down-regulate the basal IGF-1R autophosphorylation (Supplemental Figure S1B). Furthermore, PPP inhibited IGF-1–induced phosphorylation of IRS-1 without affecting insulin-induced IRS-1 phosphorylation (Supplemental Figure S2). PPP did not affect the growth of IGF-1R–negative fibroblasts (Supplemental Figure S3).
The effect of cyclolignan PPP on growth in MM cell lines and primary MM cells. A panel of 13 MM cell lines was treated with PPP at indicated concentrations for 48 hours followed by analysis using the resazurin assay. The cell lines were divided into 3 groups showing (A) IL-6–independent EJM, JJN3, Karpas 707, L363, LP-1, OPM-2, and U-1996 cell lines; (B) IL-6–dependent U-1957, U-1958, and U-266-1970 cell lines; and (C) the IL-6–independent RPMI 8226 cell line and its subclones RPMI 8226/Dox40 and RPMI 8226/LR5 with increased resistance to doxorubicin and melphalan, respectively. IL-6 was added to U-1957, U-1958, U-1996, and U-266-1970. Four experiments were performed and 1 representative is shown. (D) Plasma cells were purified from bone marrow samples of 10 patients with MM and treated for 72 hours with indicated concentrations of PPP. At harvest the relative number of viable cells was analyzed using the resazurin assay. (E) Alternatively, primary MM cells from 4 patients were allowed to adhere to BMSCs before treatment with PPP for 72 hours. By using 3H-TdR the amount of DNA synthesis was quantified, whereby 3H-TdR incorporation of the MM cells was calculated as described in “Materials and methods.” The experiments were performed in triplicate, and data are presented as means ± SD. Error bars not visible are included within the symbols. (F) BMSC and SK1069 fibroblasts were treated with PPP at 1 μM for 72 hours. At harvest the relative number of viable cells was analyzed using the resazurin assay. Three experiments were performed and 1 representative is shown whereby data are presented as mean ± SD.
The effect of cyclolignan PPP on growth in MM cell lines and primary MM cells. A panel of 13 MM cell lines was treated with PPP at indicated concentrations for 48 hours followed by analysis using the resazurin assay. The cell lines were divided into 3 groups showing (A) IL-6–independent EJM, JJN3, Karpas 707, L363, LP-1, OPM-2, and U-1996 cell lines; (B) IL-6–dependent U-1957, U-1958, and U-266-1970 cell lines; and (C) the IL-6–independent RPMI 8226 cell line and its subclones RPMI 8226/Dox40 and RPMI 8226/LR5 with increased resistance to doxorubicin and melphalan, respectively. IL-6 was added to U-1957, U-1958, U-1996, and U-266-1970. Four experiments were performed and 1 representative is shown. (D) Plasma cells were purified from bone marrow samples of 10 patients with MM and treated for 72 hours with indicated concentrations of PPP. At harvest the relative number of viable cells was analyzed using the resazurin assay. (E) Alternatively, primary MM cells from 4 patients were allowed to adhere to BMSCs before treatment with PPP for 72 hours. By using 3H-TdR the amount of DNA synthesis was quantified, whereby 3H-TdR incorporation of the MM cells was calculated as described in “Materials and methods.” The experiments were performed in triplicate, and data are presented as means ± SD. Error bars not visible are included within the symbols. (F) BMSC and SK1069 fibroblasts were treated with PPP at 1 μM for 72 hours. At harvest the relative number of viable cells was analyzed using the resazurin assay. Three experiments were performed and 1 representative is shown whereby data are presented as mean ± SD.
The downstream consequences of inhibition of the IGF-1 RTK were investigated using the RPMI 8226 cell line. The expression of Akt, GSK-3β, p70S6K, Erk1/2, and their corresponding phosphorylated forms were quantified by Western blotting. As expected, treatment with IGF-1 clearly increased the phosphorylation of Erk1/2, Akt, GSK-3β, and p70S6K (Figure 3C,E). The ligand-stimulated phosphorylation of Erk1/2 was significantly down-regulated at 2 and 4 hours of PPP treatment (Figure 3C). Phosphorylation of CDK1 at Thr14/Tyr15 by IGF-1 at 4 hours was totally abrogated by PPP and significantly reduced at 24 hours (Figure 3C). This confirms our findings from mass spectrometric analysis of potential targets for PPP (data not shown). Notably, in the mouse 5TMMvt cell line lacking the expression of IGF-1R,36 CDK1 phosphorylation was not affected by treatment with PPP and/or IGF-1 (Figure 3D). The expression of cyclin B1, the major cyclin associating with CDK1, was analyzed together with cyclin D2 and E. The expression of cyclin E was down-regulated at 24 hours of PPP treatment (Figure 3C), whereas cyclin B1 and cyclin D2 remained unaffected. Treatment with IGF-1 did not affect the expression level of any of the cyclins. In contrast to the reduced phosphorylation of Erk1/2, the ligand-induced phosphorylation of Akt or phosphorylation of GSK-3β and p70S6K was not suppressed during the initial 2 hours of PPP treatment (Figure 3E). However, at 24 hours of PPP treatment the IGF-1–induced phosphorylation of GSK-3β at Ser9 and p70S6K at Thr389 was clearly suppressed, whereas phosphorylation of p70S6K at Thr421/Ser424 was not affected even at this late time point (Figure 3E).
The effects of PPP on cell-cycle phase distribution and apoptosis. (A) MM cell lines were treated with PPP for 24 hours followed by analysis of cell-cycle phase distribution,44 whereby one representative experiment using the LP-1 cell line is demonstrated. (B) In a parallel experiment apoptosis was analyzed using AV/PI staining. MM cells were categorized as living cells (AV-negative/PI-negative), apoptotic cells (AV-positive/PI-negative), apoptotic/necrotic cells (AV-positive/PI-positive), and necrotic cells (AV-negative/PI-positive), whereby the relative cell numbers are presented as percentage of 10 000 cells. (C) Apoptosis was also quantified in the Karpas 707 cell line at 48 hours of PPP treatment by using TUNEL, whereby data are presented as percentage of 10 000 cells.
The effects of PPP on cell-cycle phase distribution and apoptosis. (A) MM cell lines were treated with PPP for 24 hours followed by analysis of cell-cycle phase distribution,44 whereby one representative experiment using the LP-1 cell line is demonstrated. (B) In a parallel experiment apoptosis was analyzed using AV/PI staining. MM cells were categorized as living cells (AV-negative/PI-negative), apoptotic cells (AV-positive/PI-negative), apoptotic/necrotic cells (AV-positive/PI-positive), and necrotic cells (AV-negative/PI-positive), whereby the relative cell numbers are presented as percentage of 10 000 cells. (C) Apoptosis was also quantified in the Karpas 707 cell line at 48 hours of PPP treatment by using TUNEL, whereby data are presented as percentage of 10 000 cells.
The effects of PPP on IGF-1R RTK autophosphorylation and downstream signaling proteins. (A) IGF-1R was extracted from total cell lysates of the RPMI 8226 and the Karpas 707 cell lines and incubated with PPP at indicated concentrations before the kinase reaction was initiated by the addition of ATP. The autophosphorylation of the IGF-1 RTK was quantified spectrophotometrically. ♦ indicates RPMI 8226; ▪, Karpas 707. (B) Insulin R was extracted from total cell lysates of the RPMI 8226 and Karpas 707 cell lines and incubated with PPP at indicated concentrations before kinase reaction was initiated by the addition of ATP. The autophosphorylation of the insulin R was quantified spectrophotometrically. ♦ indicates RPMI 8226; ▪, Karpas 707. (C) Expression of p-Erk1/2, tot-Erk1/2, and the cell-cycle–associated p-CDK1, tot-CDK1, cyclin B1, cyclin D2, and cyclin E was analyzed by Western blotting of serum-starved RPMI 8226 cells incubated for 2, 4, and 24 hours with or without 1 μM PPP. (D) Similarly, expression of p-CDK1 and tot-CDK1 was analyzed in the mouse MM cell line 5T33MMvt lacking the IGF-1R. (E) Expression of p-Akt, tot-Akt, and the downstream signaling proteins GSK-3β p70S6K and their corresponding phosphorylated forms were analyzed according to the same procedure. Protein expression of (F) bcl-xL, mcl-1, survivin, and (G) hsp60, hsp70, and hsp90 was analyzed by Western blotting of serum-starved RPMI 8226 cells incubated for 2, 4, and 24 hours with or without 1 μM PPP. In all experiments the cells were stimulated with 6.7 nM IGF-1 for 5 minutes at each time point.
The effects of PPP on IGF-1R RTK autophosphorylation and downstream signaling proteins. (A) IGF-1R was extracted from total cell lysates of the RPMI 8226 and the Karpas 707 cell lines and incubated with PPP at indicated concentrations before the kinase reaction was initiated by the addition of ATP. The autophosphorylation of the IGF-1 RTK was quantified spectrophotometrically. ♦ indicates RPMI 8226; ▪, Karpas 707. (B) Insulin R was extracted from total cell lysates of the RPMI 8226 and Karpas 707 cell lines and incubated with PPP at indicated concentrations before kinase reaction was initiated by the addition of ATP. The autophosphorylation of the insulin R was quantified spectrophotometrically. ♦ indicates RPMI 8226; ▪, Karpas 707. (C) Expression of p-Erk1/2, tot-Erk1/2, and the cell-cycle–associated p-CDK1, tot-CDK1, cyclin B1, cyclin D2, and cyclin E was analyzed by Western blotting of serum-starved RPMI 8226 cells incubated for 2, 4, and 24 hours with or without 1 μM PPP. (D) Similarly, expression of p-CDK1 and tot-CDK1 was analyzed in the mouse MM cell line 5T33MMvt lacking the IGF-1R. (E) Expression of p-Akt, tot-Akt, and the downstream signaling proteins GSK-3β p70S6K and their corresponding phosphorylated forms were analyzed according to the same procedure. Protein expression of (F) bcl-xL, mcl-1, survivin, and (G) hsp60, hsp70, and hsp90 was analyzed by Western blotting of serum-starved RPMI 8226 cells incubated for 2, 4, and 24 hours with or without 1 μM PPP. In all experiments the cells were stimulated with 6.7 nM IGF-1 for 5 minutes at each time point.
To assess whether treatment with PPP and/or IGF-1 affected the expression of apoptosis-related molecules, the protein levels of bcl-xL, mcl-1, survivin, and hsp60, hsp70, and hsp90 were analyzed in the RPMI 8226 cell line by Western blotting. At 4 hours of PPP treatment the expression of mcl-1 and survivin was clearly but transiently reduced (Figure 3E). Although 1 μM PPP induced extensive cell death, no clear down-regulation of the antiapoptotic protein bcl-xL could be detected even at 24 hours (Figure 3E). This was true also for the heat-shock proteins hsp60, hsp70, and hsp90 (Figure 3F). Treatment with IGF-1 did not affect the expression level of any of the bcl-xL, mcl-1, survivin, and hsp proteins.
PPP suppresses IGF-1–induced CDK1 activity
To assess the consequences of the altered CDK1 phosphorylation, an in vitro kinase assay of CDK1 was performed. PPP was added at 1 μM to the RPMI 8226 cells. At indicated time points the cells were treated for 5 minutes with 6.7 nM IGF-1. The CDK1 immunoprecipitates were then subjected to the kinase assay using Histone H1 as a substrate. The amount of labeled Histone H1 was quantified and displayed as fold reduction relative to the IGF-1–treated control at each time point (Figure 4A). At all time points, the CDK1 activity was reduced in response to PPP.
To further functionally validate the role of CDK1, 13 MM cell lines were treated with the selective CDK1 inhibitor CGP74512A39 at indicated concentrations for 48 hours followed by analysis of cell growth using the resazurin assay. All MM cell lines responded to CGP74512A with reduced growth (Figure 4). The RPMI 8226 cell line was highly sensitive to CGP74512 with an IC50 at 2 μM, whereas the U-1958 cell line was least sensitive exhibiting an IC50 slightly above 4 μM (Figure 4C-D).
The effect of PPP on CDK1 activity and the effect of CDK1 inhibition on growth. (A) Serum-starved RPMI 8226 cells were treated with PPP at 1 μM. At indicated time points the cells were treated for 5 minutes with 6.7 nM IGF-1. Cell lysates were subjected to CDK1 immunoprecipitation and subsequent in vitro kinase assay using Histone H1 as a substrate. The amount of labeled H1 was quantified and expressed as fold reduction as compared with relevant IGF-1–treated time controls. Each of the 13 MM cell lines used previously were treated with CGP74512A at indicated concentrations for 48 hours followed by analysis using the resazurin assay. As in Figure 1 the cell lines were divided into 3 categories: (B) IL-6–independent, (C) IL-6–dependent, and (D) the RPMI 8226 cell line and its subclones. Four experiments were performed in triplicate, and 1 representative is shown whereby data are expressed as mean percentage of control ± SD. Error bars not visible are included within the symbols.
The effect of PPP on CDK1 activity and the effect of CDK1 inhibition on growth. (A) Serum-starved RPMI 8226 cells were treated with PPP at 1 μM. At indicated time points the cells were treated for 5 minutes with 6.7 nM IGF-1. Cell lysates were subjected to CDK1 immunoprecipitation and subsequent in vitro kinase assay using Histone H1 as a substrate. The amount of labeled H1 was quantified and expressed as fold reduction as compared with relevant IGF-1–treated time controls. Each of the 13 MM cell lines used previously were treated with CGP74512A at indicated concentrations for 48 hours followed by analysis using the resazurin assay. As in Figure 1 the cell lines were divided into 3 categories: (B) IL-6–independent, (C) IL-6–dependent, and (D) the RPMI 8226 cell line and its subclones. Four experiments were performed in triplicate, and 1 representative is shown whereby data are expressed as mean percentage of control ± SD. Error bars not visible are included within the symbols.
Neither IGFs, insulin, nor IL-6 counteract the effects of PPP
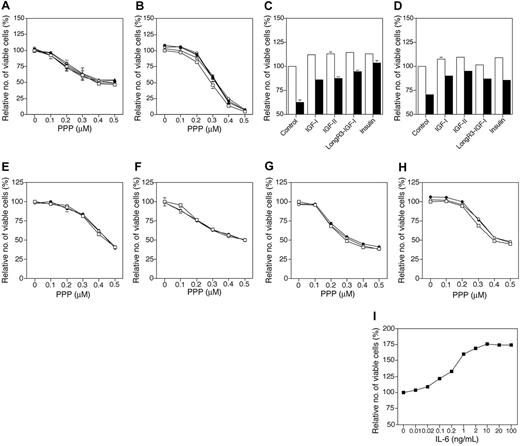
To clarify whether high concentrations of growth-promoting factors could quench the inhibitory effects of PPP, MM cell lines were treated for 1 hour with these factors before the addition of PPP at indicated concentrations. Thus, Karpas 707 and RPMI 8226 cell lines were preincubated with 100 nM IGF-1, IGF-2, LongR3-IGF-1, or insulin, whereas the IL-6–dependent/responsive U-1957, U-1958, U-1996, and U-266-1970 cell lines were preincubated with 10 or 100 ng/mL IL-6. In the Karpas 707 and RPMI 8226 cell lines none of the factors, despite pretreatment, substantially counteracted the inhibitory effects of PPP (Figure 5A-B). In contrast, these ligands clearly reduced the previously described dexamethasone-induced growth inhibition (Figure 5C-D).5,7,10 Similar results were obtained by pretreating the U-1957, U-1958, U-1996, and U-266-1970 cell lines with IL-6 before addition of PPP (Figure 5E-H). Also in the IL-6–independent Karpas 707 and RPMI 8226 cell lines IL-6 did not block PPP-induced growth inhibition (data not shown). In control cultures using the U-1958 cell line, IL-6 clearly stimulated growth (Figure 5I) in concordance with previous findings.35
Pan-caspase inhibitor Z-VAD-fmk does not diminish the response to PPP
Because the caspase-cascade has been suggested to be crucial for execution of apoptotic cell death induced by, for example, Fasligation and diverse cytotoxic stimuli, we examined as to what extent caspases were involved in apoptosis induced by PPP. Cleavage, and thus activation, of caspase 8, 9, and 3 in response to PPP was analyzed by Western blotting in parallel with experiments in which the effect of the pan-caspase inhibitor Z-VAD-fmk on PPP-induced apoptosis was examined. Treatment of RPMI 8226 cells with PPP induced cleavage of all caspases studied (Figure 6A). However, this effect was evident only after relatively long exposure to PPP, that is, 24 hours. Preincubation of RPMI 8226 cells with Z-VAD-fmk at 30 μM for 1 hour only slightly reduced the apoptotic response to PPP (Figure 6B). In contrast, Z-VAD-fmk at the same concentration completely inhibited Fas-induced apoptosis in RPMI 8226 cells (Figure 6B). Isotype control antibodies, added at the same concentrations as CH11, were without effect (data not shown).
PPP sensitizes MM cells to cytotoxic drugs and pharmacologic inhibitors
Previous reports have demonstrated sensitization of MM cells to cytotoxic stimuli/compounds when IGF-1R is inhibited,4,7,10,21 or when distinct intracellular signaling components are targeted, that is, mTOR45,46 and p38.47 To assess the potential of PPP in this aspect, RPMI 8226 cells were treated with PPP in combination with cytotoxic drugs or the pharmacologic inhibitors rapamycin and SB203580 that target mTOR and p38, respectively. Pretreatment of RPMI 8226 cells for 24 hours with 0.3 μM PPP followed by addition of dexamethasone, doxorubicin, or melphalan for another 24 hours of incubation resulted in significantly (P < .001) increased effects as compared with any of the compounds used alone (Figure 7A). PPP also significantly (P < .001) sensitized RPMI 8226 cells to rapamycin and SB203580 (Figure 7B).
Discussion
Substantial evidence suggests an important role for IGF/IGF-1R signaling in MM. IGF-1 has been shown to have pleiotropic effects in human MM cells and in the mouse 5TMM model of MM, including stimulation of proliferation, survival, chemotaxis, and angiogenesis.3,4,10,11,21,36,48-55 In this study we describe the effects of the selective IGF-1 RTK inhibitor cyclolignan PPP on growth and survival of MM cell lines and primary MM cells in vitro.
The 13 MM cell lines used in the study included IL-6–dependent and –independent cell lines as well as the drug-resistant subclones of the RPMI 8226 cell line. Notably, all MM cell lines of the panel responded to PPP with growth inhibition. The IL-6–independent JJN3, RPMI 8226, and RPMI 8226/LR5 cell lines were highly sensitive to PPP, whereas the 2 IL-6–dependent U-1958 and U-266-1970 cell lines were least sensitive. These results are in concordance with previous observations in which MM cell lines during standard, serum-containing conditions were growth inhibited when treated with the blocking IGF-1R antibody αIR3.4,7 However, in comparison with the αIR3 antibody, PPP induces a more prominent growth inhibition probably due to its direct effects on the kinase region of the IGF-1R. Further emphasizing the potency of PPP, pretreatment of the MM cell lines with high concentrations of IGF-1, IGF-2, LongR3-IGF-1, insulin, or IL-6 did not substantially reduce the inhibitory effects of PPP. Moreover, PPP prominently inhibited DNA synthesis in primary MM cells cultured on top of survival-promoting, confluent monolayers of BMSCs. Although PPP clearly has direct effects on the viability of MM cell lines and primary cells, in vivo PPP may also act on the BMSCs supporting MM growth and thus possibly be favorable for clinical outcome. Supporting this notion may be the discrete inhibitory effect of PPP in cultures of BMSCs and fibroblast as demonstrated in this study. In comparison with cell lines derived from human nonhematopoietic tumors22 the MM cell lines of this study seemed to be less responsive to PPP. In this respect, the effects of PPP differed slightly from the effects of the selective IGF-1 RTK inhibitor NVP-ADW742 as described by Mitsiades et al,21 whereby MM cell lines are suggested to be more sensitive to inhibition of IGF-1R signaling than cell lines established from solid tumors.
Effects of IGFs, insulin, and IL-6 on growth inhibition induced by PPP. (A) Karpas 707 and (B) RPMI 8226 cells were treated for 48 hours with PPP alone (□) or in the presence of IGF-1 (•), IGF-2 (○), LongR3-IGF-1 (▴), or insulin (▵). In control cultures (C) Karpas 707 and (D) RPMI 8226, cells were treated for 72 hours with the indicated ligands alone (□) or with the addition of 1 μM dexamethasone (▪). Each ligand was added at the concentration 100 nM and always 1 hour before treatment with PPP/dexamethasone was initiated. The 4 IL-6–dependent/responsive MM cell lines (E) U-1957, (F) U-1958, (G) U-1996, and (H) U-266-1970 were treated for 48 hours with PPP alone (□) or in the presence of IL-6 at 10 ng/mL (○) or 100 ng/mL (•). IL-6 was added 1 hour before treatment with PPP, at indicated concentrations, was initiated. (I) As a control experiment the IL-6–dependent U-1958 cell line was treated with increasing concentrations of IL-6. Three independent experiments were performed in triplicate and 1 representative is shown. Data are presented as means ± SD. Error bars not visible are included within the symbols.
Effects of IGFs, insulin, and IL-6 on growth inhibition induced by PPP. (A) Karpas 707 and (B) RPMI 8226 cells were treated for 48 hours with PPP alone (□) or in the presence of IGF-1 (•), IGF-2 (○), LongR3-IGF-1 (▴), or insulin (▵). In control cultures (C) Karpas 707 and (D) RPMI 8226, cells were treated for 72 hours with the indicated ligands alone (□) or with the addition of 1 μM dexamethasone (▪). Each ligand was added at the concentration 100 nM and always 1 hour before treatment with PPP/dexamethasone was initiated. The 4 IL-6–dependent/responsive MM cell lines (E) U-1957, (F) U-1958, (G) U-1996, and (H) U-266-1970 were treated for 48 hours with PPP alone (□) or in the presence of IL-6 at 10 ng/mL (○) or 100 ng/mL (•). IL-6 was added 1 hour before treatment with PPP, at indicated concentrations, was initiated. (I) As a control experiment the IL-6–dependent U-1958 cell line was treated with increasing concentrations of IL-6. Three independent experiments were performed in triplicate and 1 representative is shown. Data are presented as means ± SD. Error bars not visible are included within the symbols.
In general, the primary MM cells seemed to be less responsive to PPP than the MM cell lines. This may be explained by the fact that purified MM cells in vitro are essentially nondividing and thus unable to respond to the antiproliferative effects induced by PPP. However, because PPP exhibits almost no toxicity in animals (LD50 > 500 mg/kg in rodents),22 the relative resistance of primary MM cells to PPP may be overcome in vivo by increasing the concentrations of PPP. In line with this, PPP significantly reduced the growth of MM cells in the immunocompetent 5TMM mouse model without negatively affecting glucose and albumin levels, indicating that PPP, at therapeutic concentrations, does not negatively influence insulin receptor signaling and metabolism.54 As demonstrated by using an in vitro kinase assay, PPP greatly down-regulates autophosphorylation of the IGF-1R in human MM cells without inhibiting the autophosphorylation of the insulin R. Thus, the obvious advantage of PPP over the NVP-AFW742 inhibitor is the complete absence of coinhibitory activity of the insulin R.
Effects of PPP on caspases. (A) Cleavage of caspase 8, 9, and 3 was analyzed by Western blotting of lysates obtained from serum-starved RPMI 8226 cells treated with 1 μM PPP for 2, 4, and 24 hours and 6.7 nM IGF-1 for 5 minutes at each time point. (B) RPMI 8226 cells were left untreated or were treated with PPP at 0.5 μM or the agonistic Fas-antibody CH11 (200 ng/mL) alone (filled area), or in the presence of the pan-caspase inhibitor Z-VAD-fmk at 30 μM (open area), for 48 hours. Two independent experiments were performed and one representative is shown. The bars indicate the apoptotic TUNEL-positive fraction.
Effects of PPP on caspases. (A) Cleavage of caspase 8, 9, and 3 was analyzed by Western blotting of lysates obtained from serum-starved RPMI 8226 cells treated with 1 μM PPP for 2, 4, and 24 hours and 6.7 nM IGF-1 for 5 minutes at each time point. (B) RPMI 8226 cells were left untreated or were treated with PPP at 0.5 μM or the agonistic Fas-antibody CH11 (200 ng/mL) alone (filled area), or in the presence of the pan-caspase inhibitor Z-VAD-fmk at 30 μM (open area), for 48 hours. Two independent experiments were performed and one representative is shown. The bars indicate the apoptotic TUNEL-positive fraction.
PPP drastically increased the fraction of cells in the G2/M phase, which is consistent with previous findings showing the need for signaling via the IGF-1R for propagation through the late cell-cycle phases.56-58 In addition, PPP potently induced apoptosis, and at late time points, also activated caspase 8, 9, and 3. The pan-caspase inhibitor Z-VAD-fmk only slightly decreased the sensitivity to PPP. It is therefore conceivable that both caspase-dependent and -independent pathways59 are activated by PPP in MM cells.
Although the autophosphorylation of the IGF-1 RTK was clearly down-regulated in vitro, as also demonstrated in MM tumor cells of the 5TMM model,54 we were unable to completely inhibit autophosphorylation similar to that which has been demonstrated in tumor cells of epithelial origin.22 A probable explanation could be that PPP is not an ATP inhibitor but blocks phosphorylation of a specific tyrosine residue in the activation loop of the IGF-1 RTK.23 As expected, the ligand-induced phosphorylation of Erk1/2 was down-regulated by treatment with PPP, which is in concordance with the effect of PPP in MM cells of the 5TMM mouse model.54 We could not detect any immediate changes in phosphorylation of the downstream kinases Akt, GSK-3β, and p70S6K. However, at 24 hours of incubation with PPP the ligand-induced phosphorylation of GSK-3β at Ser9 and p70S6K at Thr389 was suppressed. These late changes suggest that the inhibitory effect of PPP may not primarily result from a perturbed signaling via these kinases. Importantly, the ligand-activated phosphorylation and kinase activity of CDK1 were clearly down-regulated at early time points. The CDK1 was selected following mass spectrometric analysis of captured tyrosine phosphorylated proteins potentially being regulated by PPP (data not shown). The significance of CDK1 as a target for PPP was further established by using the in vitro–propagated mouse MM cell line 5T33MMvt, which does not express the IGF-1R36 and consequently does not respond to PPP with inhibition of growth.54 Connections between the MAPK/Erk, PI3K/Akt, and 14-3-3 pathways and the action of CDK1/cyclin B1 are well known,60,61 and it is reasonable to suggest that the G2/M-phase accumulation induced by PPP is due to interference with the function of the CDK1/cyclin B1 complex. This conclusion is supported by our finding that the CDK1 activity in MM cells was decreased in association with the PPP-induced G2/M arrest. The reduced CDK-1 phosphorylation (Tyr15) is indicative of the inhibited IGF-1R signal (Figure 3C) but does not predict the activity of the CDK1/cyclin B1 complex. Therefore, the reduced phosphorylation of CDK1 is likely to be a consequence of dissociation of CDK1 from the CDK1/cyclin B1 complex. This is also indicated by the kinetics of the kinase assay whereby the reduced activity of CDK1 precedes the reduced phosphorylation of CDK1. An important role for CDK1 in IGF-1R signaling is further supported by the finding that the in vivo use of the IGF-1R–blocking antibody αIR3 in a rhabdomyosarcoma mouse model, besides inhibiting tumor growth, was also capable of down-regulating CDK1.62 Furthermore, all MM cell lines responded to the selective CDK1 inhibitor CGP74512A by growth inhibition. This CDK1 inhibitor has previously been used in leukemic cell lines with comparable results.63,64 The expression of a panel of bcl-2 family–related apoptosis-regulating proteins was also examined in response to PPP. Both mcl-1 and survivin were down-regulated, although transiently, whereas the level of bcl-xL expression was unaltered. The importance of mcl-1 and survivin in MM survival has previously been suggested by us and others.45,65-69 Also, the expression of heat-shock proteins hsp60, hsp70, and hsp90 was found not to be affected by PPP and/or IGF-1.
Effects of PPP in combination with cytotoxic drugs and pharmacologic inhibitors. (A) RPMI 8226 cells were incubated for 24 hours with 0.3 μM PPP before treatment with dexamethasone, doxorubicin, and melphalan for another 24 hours followed by analysis using the resazurin assay. (B) RPMI 8226 cells were treated simultaneously with PPP and rapamycin or SB203580 for 48 hours followed by analysis using the resazurin assay. Three independent experiments were performed in triplicate and 1 representative is shown. Data are presented as means ± SD. Error bars not visible are included within the symbols (P < .001).
Effects of PPP in combination with cytotoxic drugs and pharmacologic inhibitors. (A) RPMI 8226 cells were incubated for 24 hours with 0.3 μM PPP before treatment with dexamethasone, doxorubicin, and melphalan for another 24 hours followed by analysis using the resazurin assay. (B) RPMI 8226 cells were treated simultaneously with PPP and rapamycin or SB203580 for 48 hours followed by analysis using the resazurin assay. Three independent experiments were performed in triplicate and 1 representative is shown. Data are presented as means ± SD. Error bars not visible are included within the symbols (P < .001).
Previous reports have demonstrated sensitization of MM cells to cytotoxic drugs when IGF-1R is inhibited7,10,21 or when IGF-1R downstream components, for example, mTOR45,46 and p38,47 are targeted. Pretreatment with PPP for 24 hours significantly sensitized RPMI 8226 cells to dexamethasone, doxorubicin, and melphalan. The sensitization of MM cells to cytotoxic drugs by IGF-1 RTK inhibition has previously been demonstrated by others, although the drugs in these experiments were added 24 or 48 hours before treatment with the IGF-1 RTK inhibitor was initiated.21 However, preincubation of MM cell lines with doxorubicin or melphalan for 24 or 48 hours before PPP treatment did not add to the effect of PPP (data not shown). Unexpectedly, when adding doxorubicin and melphalan together with PPP, the potency of PPP diminished. Interestingly, both doxorubicin and melphalan, like PPP, induce accumulation of cells in the G2/M phase.70,71 It is therefore tempting to speculate that PPP and these cytotoxic drugs affect common signaling molecules/substrates downstream of the IGF-1R, for example, CDK1.72 PPP also sensitized MM cells to mTOR inhibitor rapamycin and the p38 inhibitor SB205380. However, in contrast to doxorubicin and melphalan, both rapamycin and SB203580 could be added together with or before PPP and still increase the potency of PPP. This was also true for dexamethasone (data not shown). Notably, both dexamethasone and rapamycin, in contrast to PPP, induce accumulation of cells in the G1 phase of the cell cycle,7,45,73,74 which might explain their inability to negatively influence the PPP effect.
In conclusion, we show that the highly selective IGF-1 RTK inhibitor PPP of the cyclolignan family potently decreases both proliferation and survival of MM cells possibly via down-regulated phosphorylation of Erk1/2 and reduced CDK1 activity in conjunction with lowered expression of mcl-1 and survivin. These results in combination with low toxicity in vivo suggest the cyclolignan PPP to be a candidate compound for improved treatment of MM.
Prepublished online as Blood First Edition Paper, September 15, 2005; DOI 10.1182/blood-2005-01-0306.
Supported by grants from the Swedish Cancer Society, Göran Gustafssons Stiftelse, the Multiple Myeloma Research Foundation (MMRF), Hans von Kantzows and Magnus Bergwalls Stiftelse, Selanders Stiftelse, and the Swedish Research Council. K.V. is a postdoctoral fellow of the FWO-Vlaanderen, Belgium.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Pernilla Martinsson and Charlotta Sandberg for excellent skillful technical assistance. We also thank the staff at University Hospital, Uppsala, and at the Department of Hematology, Karolinska Hospital, Stockholm, for providing the MM tumor samples.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal