Abstract
FcγR-mediated phagocytosis of IgG-coated particles is a complex process involving the activation of multiple signaling enzymes and is regulated by the inositol phosphatases PTEN (phosphatase and tensin homolog deleted on chromosome 10) and SHIP-1 (Src homology [SH2] domain-containing inositol phosphatase). In a recent study we have demonstrated that SHIP-2, an inositol phosphatase with high-level homology to SHIP-1, is involved in FcγR signaling. However, it is not known whether SHIP-2 plays a role in modulating phagocytosis. In this study we have analyzed the role of SHIP-2 in FcγR-mediated phagocytosis using independent cell models that allow for manipulation of SHIP-2 function without influencing the highly homologous SHIP-1. We present evidence that SHIP-2 translocates to the site of phagocytosis and down-regulates FcγR-mediated phagocytosis. Our data indicate that SHIP-2 must contain both the N-terminal SH2 domain and the C-terminal proline-rich domain to mediate its inhibitory effect. The effect of SHIP-2 is independent of SHIP-1, as overexpression of dominant-negative SHIP-2 in SHIP-1-deficient primary macrophages resulted in enhanced phagocytic efficiency. Likewise, specific knockdown of SHIP-2 expression using siRNA resulted in enhanced phagocytosis. Finally, analysis of the molecular mechanism of SHIP-2 down-regulation of phagocytosis revealed that SHIP-2 down-regulates upstream activation of Rac. Thus, we conclude that SHIP-2 is a novel negative regulator of FcγR-mediated phagocytosis independent of SHIP-1. (Blood. 2006;107:813-820)
Introduction
IgG-coated particles are cleared by macrophages via FcγR by a process called phagocytosis (reviewed by Aderem and Underhill1 ). There are 3 classes of FcγR expressed on murine macrophages: FcγRI, FcγRII(b), and FcγRIII (reviewed by Daeron2 ). FcγRI and FcγRIII transduce activating signals through the immunoreceptor tyrosine-based activation motif (ITAM) within the associated γ-subunit homodimers. FcγRII(b), on the other hand, is an inhibitory receptor bearing an inhibition motif (immunoreceptor tyrosine-based inhibitory motif [ITIM]) in its cytoplasmic tail. FcγR clustering by immune complex (IC) initiates a signaling cascade that begins with the activation of the Src kinases that activate the ITAMs by phosphorylation.3 These phosphorylated ITAMs then serve as docking sites to recruit multiple cellular signaling enzymes and enzyme-adapter complexes, such as the Syk tyrosine kinase,4 the p85 subunit of phosphatidylinositol 3-kinase (PtdIns-3K),5 and the Ras adapter molecule Shc that further recruits the Grb2-Sos complex to activate the Ras-MAPK pathway.6,7 Association of PtdIns-3K with phosphorylated ITAMs places the enzyme in proximity with its lipid substrates in the plasma membrane, thus allowing for the generation of the important lipid second messenger phosphatidylinositol 3,4,5 trisphosphate (PtdIns3,4,5P3). Additionally, PtdIns3,4,5P3 recruits the adapter molecule Gab2 via the Gab2 pleckstrin homology (PH) domain.8 Association of Gab2 with the plasma membrane results in the tyrosine phosphorylation of Gab2 and the recruitment of PtdIns-3K and consequently enhancement of PtdIns3,4,5P3 in the membrane.
The importance of PtdIns-3K in phagocytosis is well established. Inhibition of PtdIns-3K results in incomplete phagocytosis.9-12 Marshall et al have demonstrated that there is an accumulation of PtdIns3,4,5P3 in the phagocytic cup.13 In addition to the recruitment of Gab2, PtdIns3,4,5P3 recruits other PH domain-containing enzymes including Vav and Akt. Vav is a guanine nucleotide exchange factor for Rac, which is necessary for actin polymerization and cytoskeletal rearrangement that are required for phagocytosis.14,15 Akt promotes FcγR-mediated phagocytosis working through the downstream molecule p70S6K.16
FcγR-mediated activation of macrophages is subject to multiple levels of regulation. It can be regulated by the inhibitory FcγRIIb that predominantly recruits negative regulatory phosphatases.17-20 Interestingly, recent studies by us and others have revealed that in addition to positive signals, the FcγR ITAMs are often capable of simultaneously activating negative regulatory proteins including the inositol phosphatase SHIP-1 (Src homology [SH2] domain-containing inositol phosphatase) so that the final biologic response is tempered.18,20,21
SHIP-1 is a hematopoietic cell-specific phosphatase that can hydrolyze PtdIns3,4,5P3 to PtdIns3,4P2 and hence down-regulate the downstream signaling pathways initiated by PH domain-containing enzymes (reviewed by Krystal22 ). The negative regulatory function of SHIP-1 in various hematopoietic cell functions has been well described (reviewed by Krystal22 and Coggeshall et al23 ). In a recent study, Cox et al have investigated the role of SHIP-1 in FcγR-mediated phagocytosis. These studies demonstrated that SHIP-1 localizes to the phagocytic cup and down-regulates phagocytosis.24
SHIP-2 is a novel inositol 5′-phosphatase that has high level of homology to SHIP-1 in its catalytic domain.25,26 The 2 molecules, however, are largely divergent in the C-terminal proline-rich domain (PRD) that also contains the phosphotyrosine-binding NPXY motif, suggesting that they likely differ in functions due to different protein-protein interactions. SHIP-2 has been recently suggested to have a role in regulating insulin receptor signaling and energy metabolism.27-30 However, the role of SHIP-2 in macrophage biology remains largely unknown. We have previously reported that SHIP-2 is activated during FcγR signaling in myeloid cells and serves to down-regulate activation of Akt and NFκB-dependent gene transcription.31 However, it is not known whether SHIP-2 influences phagocytosis. Analysis of SHIP-2 function in hematopoetic cells has lagged because transfection systems have the potential of confounding effects of the overexpressed SHIP-2 molecules on SHIP-1.
In this study, we have used independent cell models in which the function/expression of SHIP-2 is modulated without interfering with SHIP-1. We demonstrate here that SHIP-2 is a negative regulator of FcγR-mediated phagocytosis in murine macrophages. SHIP-2-mediated down-regulation of phagocytosis is dependent on an intact SH2 as well as the C-terminal proline-rich domain of SHIP-2. Our data also indicate that SHIP-2 down-regulates phagocytosis by suppressing the upstream activation of Rac. Finally, confocal microscopy experiments revealed that SHIP-2 localizes to the phagocytic cups where Rac and F-actin have been previously shown to localize during FcγR-mediated phagocytosis.32
Materials and methods
Cells, antibodies, and reagents
Raw 264.7 murine macrophage cells were obtained from ATCC (American Type Culture Collection, Manassas, VA) and maintained in RPMI with 3.5% heat-inactivated fetal bovine serum (FBS). Rabbit polyclonal anti-SHIP-2 antibody was a generous gift from Dr Bayard Clarkson (Memorial Sloan Kettering Cancer Center, New York, NY).28 Goat polyclonal anti-SHIP-2 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-SHIP-1 antibody was from Upstate Biotechnology (Lake Placid, NY). Mouse anti-Rac antibody was purchased from CHEMICON International (Temecula, CA). Alexa Fluor 594-conjugated F(ab′)2 fragment goat anti-mouse IgG was from Molecular Probes (Eugene, OR). Cy5-conjugated F(ab′)2 fragment donkey anti-goat IgG was from Jackson ImmunoResearch Labs (West Grove, PA). Hoechst nuclear stain was purchased from Molecular Probes. Antihemagglutinin (anti-HA) mAb was from Roche (Indianapolis, IN). Mouse anti-HA antibody used for confocal microscopy was from Cell Signaling (Beverly, MA). Xpress-tagged cDNAs of murine full-length, wild-type SHIP-2 (SHIP-2 WT), and catalytically inactive SHIP-2 (SHIP-2 D608A) cloned into pcDNA3 vector were provided by Dr S. Moodie (Metabolex, Hayward, CA).33 HA-tagged cDNAs of human full-length SHIP-2 (HA-SHIP-2 WT) and truncation mutations of PRD (HA-SHIP-2 ΔPRD) and SH2 domains (HA-SHIP-2 ΔSH2) cloned into the pCGN vector were a kind gift from Dr C. Mitchell (Monash University, Clayton, Australia)34 pmaxGFP was from Amaxa (Gaithersburg, MD). Constitutively active Rac (CA-Rac, Q61L), and GST-PAK1-PBD constructs were kind gifts from Dr Gary Bokoch (The Scripps Research Institute, La Jolla, CA).
Culture of murine bone marrow macrophages
SHIP-1+/- animals were generously provided by Dr G. Krystal (B.C. Cancer Agency, Vancouver, British Columbia, Canada). Heterozygotes were bred to obtain SHIP-1+/+ and SHIP-1-/- mice. Bone marrow macrophages (BMMs) were derived from these animals as previously described.35 Briefly, bone marrow cells were cultured in RPMI containing 10% FBS plus 10 μg/mL polymyxin B and supplemented with 20 ng/mL colony stimulating factor-1 (CSF-1) for 7 days.
Cell stimulation, lysis, and Western blotting
Raw 264.7 cells were activated by clustering FcγR as previously described.36 Briefly, cells were incubated with 2.4G2 mAb anti-FcγRII/III antibody for 30 minutes on ice. Unbound antibody was washed away, and cells were moved to 37°C and stimulated for varying times by adding the secondary cross-linking antibody F(ab′)2 mouse anti-rat IgG. Resting and activated cells were lysed in TN1 buffer (50 mM Tris pH 8.0, 10 mM EDTA, 10 mM Na4P2O7, 10 mM NaF, 1% Triton-X 100, 125 mM NaCl, 10 mM Na3VO4, 10 μg/mL each aprotinin and leupeptin). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose filters, probed with the antibody of interest, and developed by enhanced chemiluminescence (ECL).
In other experiments, transfected Raw 264.7 cell lysates were subjected to immunoprecipitation with anti-HA antibody and analyzed by Western blotting with the appropriate antibodies.
Western blot data quantitation
The ECL signal was quantitated using a scanner and a densitometry program (Scion Image; Scion, Frederick, MD). Background pixel values were subtracted, and the values were plotted as fold increase over unstimulated samples.
Transfection
To study the influence of SHIP-2 on phagocytosis, Raw 264.7 cells or SHIP-1-/- BMMs were transfected using the Nucleofector (Amaxa). In brief, 5 × 106 cells in 100 μL of buffer (“Kit V” for Raw 264.7 cells, “Kit T” for BMMs; Amaxa Biosystems) at room temperature were mixed with 5 μg of the SHIP-2 constructs along with 1 μg EGFP encoding plasmids. The cells were transfected using the program U-14 (for Raw 264.7 cells) or T-20 (for BMMs). After transfection the samples were transferred to 10 cm round dishes containing prewarmed media. Transfectants were cultured for 24 hours and then used in phagocytosis assays.
SHIP-2 down-regulation by siRNA
Two separate siRNA oligos (SHIP-2 siRNA1, 5′GGACUUCAUCUUUGUCAGUtt3′; SHIP-2 siRNA2, 5′GGUGUUUGACCAGCAGAGCtt3′) directed against exon 7 and 10, respectively, were purchased from Ambion (Austin, TX), along with a control siRNA, which encodes a scrambled sequence with no particular homology to any known sequence. These siRNAs were introduced at a concentration of 100 nM into Raw 264.7 cells using the Nucleofector. Cells were harvested 24 hours later, and protein-matched lysates were analyzed by Western blotting with anti-SHIP-2 antibody. Parallel lysates were probed with anti-SHIP-1 antibody.
Preparation of IgG-coated SRBCs
Sheep red blood cells (SRBCs) (Colorado Serum, Denver, CO) were washed in PBS and labeled with PKH26 Red (Sigma, St Louis, MO). Labeled cells were then washed in PBS and incubated with a subagglutinating dose of rabbit anti-SRBC IgG (Diamedix, Miami, FL) at 37°C for 1 hour. Unbound IgG was removed by washing the cells with PBS.
Phagocytosis assays
Phagocytosis assays were performed as previously described.16 Briefly, IgG-coated SRBCs were added to transfected BMMs or transfected Raw 264.7 cells. The cells were pelleted by low-speed centrifugation to increase contact between SRBCs and phagocytes. The samples were incubated for 1 hour at 37°C to study phagocytosis. Cells were then subjected to brief hypotonic lysis with water to get rid of externally bound RBCs prior to fixation in paraformaldehyde to be viewed under a fluorescence microscope. Phagocytosis analyzed was via Fc receptors as confirmed by the lack of ingested particles observed in samples incubated with fluoresceinated RBCs that were not opsonized with IgG. Phagocytosis was measured by counting the total number of RBCs ingested by 100 transfectants (GFP-positive BMMs or transfected Raw 264.7 cells). To measure binding, IgG-coated SRBCs were added to transfected Raw 264.7 cells, centrifuged briefly to increase contact, and incubated for 1 hour at 4°C. Cells were then fixed in 1% paraformaldehyde, and the “binding index” was measured by counting the total number of SRBCs bound by 100 rosetting (cells that bind 3 or more SRBC) cells. No binding was seen in samples treated with nonopsonized SRBCs. All experiments were repeated at least 3 times. Statistical analysis was performed using the Student t test, setting a P value of less than .05 as significant.
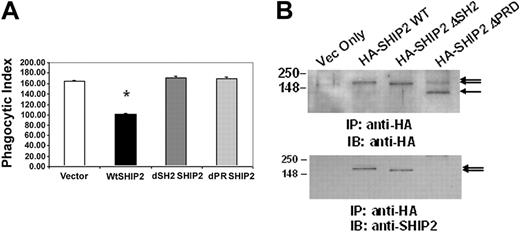
SHIP-2 down-regulation of FcγR-mediated phagocytosis is dependent on an intact SH2 domain as well as the C-terminal proline-rich domain. Raw 264.7 cells were transiently transfected with empty vector alone, HA-SHIP-2 WT, HA-SHIP-2 ΔSH2, or HA-SHIP-2 ΔPRD. GFP was cotransfected as a marker for transfection. (A) IgG-coated SRBCs were added to the transfectants 24 hours after transfection. The samples were incubated for 1 hour at 37°C to allow phagocytosis to take place. Cells were then subjected to brief hypotonic lysis prior to fixation in paraformaldehyde to be viewed under a fluorescence microscope. Phagocytosis was measured by counting the total number of SRBCs ingested by 100 transfectants (GFP-positive) each time for a total of 3 readings per sample in each experiment. *P < .05 compared with cells transfected with empty vector alone. The graph represents the mean ± SD. (B) Whole cell lysates from the transfectants were incubated with anti-HA antibody and protein G-agarose beads overnight. The precipitated proteins were next separated on 10% SDS-PAGE and transferred to nitrocellulose membrane, which was Western blotted with anti-HA antibody. The same membrane was washed and reprobed with anti-SHIP-2 antibody. These results are representative of 3 independent experiments.
SHIP-2 down-regulation of FcγR-mediated phagocytosis is dependent on an intact SH2 domain as well as the C-terminal proline-rich domain. Raw 264.7 cells were transiently transfected with empty vector alone, HA-SHIP-2 WT, HA-SHIP-2 ΔSH2, or HA-SHIP-2 ΔPRD. GFP was cotransfected as a marker for transfection. (A) IgG-coated SRBCs were added to the transfectants 24 hours after transfection. The samples were incubated for 1 hour at 37°C to allow phagocytosis to take place. Cells were then subjected to brief hypotonic lysis prior to fixation in paraformaldehyde to be viewed under a fluorescence microscope. Phagocytosis was measured by counting the total number of SRBCs ingested by 100 transfectants (GFP-positive) each time for a total of 3 readings per sample in each experiment. *P < .05 compared with cells transfected with empty vector alone. The graph represents the mean ± SD. (B) Whole cell lysates from the transfectants were incubated with anti-HA antibody and protein G-agarose beads overnight. The precipitated proteins were next separated on 10% SDS-PAGE and transferred to nitrocellulose membrane, which was Western blotted with anti-HA antibody. The same membrane was washed and reprobed with anti-SHIP-2 antibody. These results are representative of 3 independent experiments.
Rac activation assay
pGEX-2T encoding PAK1-PBD (p21 binding domain) was a kind gift from Dr Gary Bokoch. Glutathione agarose beads coated with GST-PAK1-PBD were prepared as described previously.37,38 siRNA-treated Raw 264.7 cells were activated by clustering FcγR with anti-mouse FcγRIII/II 2.4G2 antibody followed by mouse F(ab′)2 anti-rat antibody for indicated time points. Cells were lysed in TN1 buffer. Protein-matched cell lysates were incubated with GST-PAK1-PBD beads for 1 hour at 4°C. After 1 hour, beads were washed with TN1 and then boiled in 1 × SDS sample buffer for 10 minutes. Proteins were resolved by SDS-PAGE, transferred to nitrocellulose membrane, probed with anti-Rac antibody, and developed by ECL. The membranes were reprobed with anti-GST antibody to ensure equal loading of the bait protein.
Confocal microscopy
Untransfected or HA-SHIP-2-transfected Raw 264.7 cells were grown on coverslips and incubated with IgG-coated SRBCs for 5 minutes at 37°C. Cells were then fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100. After blocking in serum, untransfected cells were stained with a goat anti-SHIP-2 antibody, followed by a Cy5-conjugated donkey anti-goat F(ab′)2 fragment to detect endogenous SHIP-2. Transfectants were stained with a mouse anti-HA antibody, followed by an Alexa Fluor 594-conjugated goat anti-mouse F(ab′)2 fragment to detect HA-SHIP-2. F-actin was stained with FITC-phalloidin. Nuclei were stained with Hoechst. Coverslips were mounted on slides using mounting media (DakoCytomation, Carpinteria, CA) and read using a Zeiss LSM510 multiphoton confocal microscope (Zeiss, Oberkochen, Germany). Zeiss LSM5 Image software was used for image processing.
Results
SHIP-2 down-regulates FcγR-mediated phagocytosis in Raw 264.7 cells
We have previously reported that SHIP-2 is recruited to FcγRIIa in human monocytic cells in a manner that is dependent on an intact SH2 domain and down-regulates NFκB-driven gene transcription.31 Here, to examine the role of SHIP-2 in phagocytosis, we transiently transfected into Raw 264.7 cells plasmids encoding wild-type SHIP-2, SHIP-2 with the N-terminal SH2 domain deleted, or SHIP-2 with C-terminal proline-rich domain deleted. All transfectants also received plasmids encoding GFP. Phagocytic efficiency of these transfectants was assessed 24 hours after transfection. Phagocytosis was measured by counting the total number of SRBCs ingested by 100 GFP-positive cells. The experiments were repeated 3 times, each time analyzing phagocytosis in triplicate. The results are shown in Figure 1A. Compared with cells transfected with empty vector alone (164.67 ± 2.4), cells overexpressing wild-type SHIP-2 showed significantly lower phagocytic ability (101.22 ± 1.02) (Figure 1A, P < 0.05), indicating that SHIP-2 down-regulates phagocytosis in Raw 264.7 cells. This down-regulation is dependent on an intact SH2 domain as well as an intact PRD, because overexpression of the SHIP-2 constructs depleted of either the SH2 domain or the PRD failed to down-regulate phagocytosis (171.78 ± 3.9 and 170.33 ± 3.0, respectively) (Figure 1A). To ensure that all transfected molecules were equally expressed, aliquots of cells were lysed, and the whole cell lysates were subjected to immunoprecipitation using anti-HA antibody. The immunoprecipitated proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membrane, which was subsequently probed with anti-HA antibody (Figure 1B, upper panel). The same membrane was washed and reprobed with anti-SHIP-2 antibody (Figure 1B, lower panel). As seen in the figure, the transfected HA-SHIP-2 constructs were expressed equivalently, and they were of the expected sizes. Notably, in the lower panel, the SHIP-2 antibody does not recognize the ΔPRD-SHIP-2 because the antibody was raised against the proline-rich domain of SHIP-2. These results suggest that both the SH2 domain and the PRD of SHIP-2 are required for SHIP-2 to mediate its inhibitory effect on phagocytosis.
SHIP-2 down-regulates FcγR-mediated phagocytosis in SHIP-1-/- BMMs
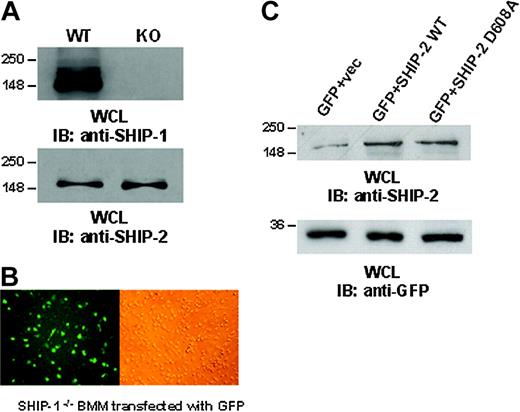
Although the above experiments demonstrate a role for SHIP-2 in down-regulating phagocytosis, there are potential problems with this interpretation. The reduction in phagocytosis observed in the cells transfected with wild-type SHIP-2 merely suggests that when SHIP-2 is overexpressed, phagocytosis is down-regulated. These experiments did not test whether there is a role for endogenous SHIP-2 in these cells. We reasoned that the use of dominant-negative constructs of SHIP-2 could resolve this issue. However, due to the high level of homology of SHIP-2 with SHIP-1 it was necessary to exclude the possibility that the dominant-negative SHIP-2 constructs interfere with endogenous SHIP-1. Therefore, to address this question, we have used 2 independent models, described below, in which expression of SHIP-2 was manipulated without influencing SHIP-1. Thus, in the first model we utilized bone marrow macrophages (BMMs) isolated from SHIP-1-/- mice to specifically study the function of SHIP-2 in FcγR-mediated phagocytosis. First, we ensured that the BMMs used were indeed deficient for SHIP-1 expression by Western blotting protein-matched lysates from SHIP-1+/+ and SHIP-1-/- BMMs (Figure 2A, upper panel). Parallel lysates were probed with anti-SHIP-2 antibody (Figure 2A, lower panel). Results indicated that both SHIP-1+/+ and SHIP-1-/- expressed equivalent levels of SHIP-2.
Ectopic expression of SHIP-2 in SHIP-1-/-BMMs. (A) BMMs from SHIP-1+/+ and SHIP-1-/- animals were tested for the expression of SHIP-1 and SHIP-2 by Western blotting. (B) Using the Nucleofector, BMMs were transfected with plasmids encoding GFP (left: immunofluorescence image; right: phase contrast image of the same field). Images were obtained using an inverted fluorescence microscope with ×40 magnification. (C) SHIP-1 knockout BMMs were transiently transfected with empty vector alone, wild-type SHIP-2 (SHIP-2 WT), or catalytically deficient SHIP-2 (SHIP-2 D608A). GFP was cotransfected as a marker for transfection. Whole cell lysates from the transfectants were separated on 10% SDS-PAGE and transferred to nitrocellulose membrane, which was blotted with anti-SHIP-2 or anti-GFP antibody. These results are representative of 3 independent experiments.
Ectopic expression of SHIP-2 in SHIP-1-/-BMMs. (A) BMMs from SHIP-1+/+ and SHIP-1-/- animals were tested for the expression of SHIP-1 and SHIP-2 by Western blotting. (B) Using the Nucleofector, BMMs were transfected with plasmids encoding GFP (left: immunofluorescence image; right: phase contrast image of the same field). Images were obtained using an inverted fluorescence microscope with ×40 magnification. (C) SHIP-1 knockout BMMs were transiently transfected with empty vector alone, wild-type SHIP-2 (SHIP-2 WT), or catalytically deficient SHIP-2 (SHIP-2 D608A). GFP was cotransfected as a marker for transfection. Whole cell lysates from the transfectants were separated on 10% SDS-PAGE and transferred to nitrocellulose membrane, which was blotted with anti-SHIP-2 or anti-GFP antibody. These results are representative of 3 independent experiments.
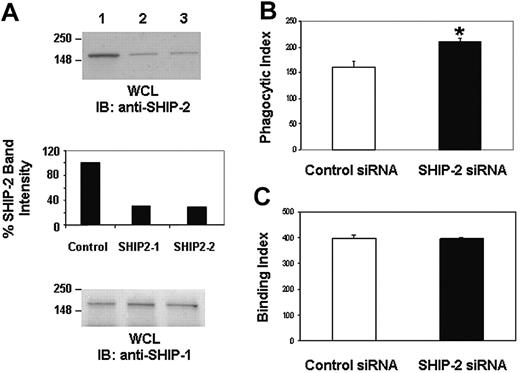
SHIP-2 knockdown by siRNA results in enhanced FcγR-function. (A) Raw 264.7 cells were transiently transfected with (1) control siRNA, (2) SHIP-2 siRNA1, and (3) SHIP-2 siRNA2. Cells were harvested 24 hours after transfection, and protein-matched lysates were analyzed by Western blotting with anti-SHIP-2 antibody (upper panel) and anti-SHIP-1 antibody (lower panel). The middle panel is a quantitative measurement of SHIP-2 band intensities. (B) Raw 264.7 cells were transiently transfected with control siRNA or SHIP-2 siRNA1. Twenty-four hours after transfection, the IgG-coated SRBCs were added. The samples were incubated for 1 hour at 37°C to study phagocytosis. Cells were then subjected to brief hypotonic lysis prior to fixation in paraformaldehyde to be viewed under a fluorescence microscope. Phagocytosis was measured by counting the total number of RBCs ingested by 100 transfectants. *P < .05. (C) Raw 264.7 cells transfected with control siRNA or SHIP-2 siRNA were incubated with IgG-coated SRBCs for 1 hour at 4°C to study binding. Cells were then subjected to brief washing prior to fixation in paraformaldehyde to be viewed under a fluorescence microscope. The binding activity was expressed as the total number of bound SRBCs on 100 rosetting Raw cells that each bound 3 or more SRBCs (binding index). Graphs represent means ± SD.
SHIP-2 knockdown by siRNA results in enhanced FcγR-function. (A) Raw 264.7 cells were transiently transfected with (1) control siRNA, (2) SHIP-2 siRNA1, and (3) SHIP-2 siRNA2. Cells were harvested 24 hours after transfection, and protein-matched lysates were analyzed by Western blotting with anti-SHIP-2 antibody (upper panel) and anti-SHIP-1 antibody (lower panel). The middle panel is a quantitative measurement of SHIP-2 band intensities. (B) Raw 264.7 cells were transiently transfected with control siRNA or SHIP-2 siRNA1. Twenty-four hours after transfection, the IgG-coated SRBCs were added. The samples were incubated for 1 hour at 37°C to study phagocytosis. Cells were then subjected to brief hypotonic lysis prior to fixation in paraformaldehyde to be viewed under a fluorescence microscope. Phagocytosis was measured by counting the total number of RBCs ingested by 100 transfectants. *P < .05. (C) Raw 264.7 cells transfected with control siRNA or SHIP-2 siRNA were incubated with IgG-coated SRBCs for 1 hour at 4°C to study binding. Cells were then subjected to brief washing prior to fixation in paraformaldehyde to be viewed under a fluorescence microscope. The binding activity was expressed as the total number of bound SRBCs on 100 rosetting Raw cells that each bound 3 or more SRBCs (binding index). Graphs represent means ± SD.
We next standardized methods to achieve efficient transfection of the SHIP-1-/- BMMs. Using the Amaxa Nucleofector, which directly delivers DNA into the nucleus while maintaining cell viability, we were able to standardize methods to achieve 20% to 30% transfection efficiency (Figure 2B, left panel: immunofluorescence image; right panel: phase contrast image). Using this method, BMMs were transiently transfected with empty vector alone, wild-type SHIP-2 (SHIP-2 WT), or catalytically inactive SHIP-2 (SHIP-2 D608A), which is a dominant negative form of SHIP-2. Plasmids encoding GFP were cotransfected as a marker for transfection. Twenty-four hours later, phagocytosis assays were performed. Phagocytosis was measured by counting the total number of SRBCs ingested by 100 GFP-positive BMMs. In parallel, aliquots of cells were lysed, and whole cell lysates were analyzed by Western blotting. Results from Western blotting confirmed overexpression of SHIP-2 constructs in the transfectants (Figure 2C, upper panel), and the transfection efficiency was equivalent as seen by the expression of GFP (Figure 2C, lower panel). Phagocytosis was measured in triplicates for each experiment, and the experiment was repeated 3 times. The data are shown in Table 1. Compared with cells transfected with empty vector alone, overexpression of wild-type SHIP-2 significantly reduced the phagocytic ability of these SHIP-1-/- BMMs (P < .05; Table 1). On the other hand, overexpression of the dominant-negative form of SHIP-2 significantly enhanced phagocytic ability (P < .05; Table 1), suggesting that SHIP-2 does indeed down-regulate phagocytosis.
SHIP-2 down-regulates FcγR-mediated phagocytosis in SHIP-1−/− BMMs
. | Phagocytic index, mean of 3 readings ± SD . | P, compared with empty vector transfectants . |
|---|---|---|
| Experiment 1 | ||
| Vector | 163.00 ± 20.66 | — |
| WT-SHIP-2 | 122.33 ± 11.24 | .018 |
| D608A-SHIP-2 | 261.00 ± 16.09 | .017 |
| Experiment 2 | ||
| Vector | 247.67 ± 23.12 | — |
| WT-SHIP-2 | 185.67 ± 8.74 | .019 |
| D608A-SHIP-2 | 355.33 ± 28.22 | <.001 |
| Experiment 3 | ||
| Vector | 202.00 ± 3.00 | — |
| WT-SHIP-2 | 151.33 ± 3.75 | .002 |
| D608A-SHIP-2 | 299.00 ± 9.97 | <.001 |
. | Phagocytic index, mean of 3 readings ± SD . | P, compared with empty vector transfectants . |
|---|---|---|
| Experiment 1 | ||
| Vector | 163.00 ± 20.66 | — |
| WT-SHIP-2 | 122.33 ± 11.24 | .018 |
| D608A-SHIP-2 | 261.00 ± 16.09 | .017 |
| Experiment 2 | ||
| Vector | 247.67 ± 23.12 | — |
| WT-SHIP-2 | 185.67 ± 8.74 | .019 |
| D608A-SHIP-2 | 355.33 ± 28.22 | <.001 |
| Experiment 3 | ||
| Vector | 202.00 ± 3.00 | — |
| WT-SHIP-2 | 151.33 ± 3.75 | .002 |
| D608A-SHIP-2 | 299.00 ± 9.97 | <.001 |
Three representative experiments are shown.
—indicates not applicable.
SHIP-2 knockdown by siRNA results in enhanced FcγR function
As an additional approach to confirm the role of SHIP-2 in down-regulating phagocytosis and to overcome any confounding effects of overexpression systems, we next specifically knocked down the expression level of SHIP-2 in Raw 264.7 cells. For this, 2 separate siRNA oligonucleotides (SHIP-2 siRNA1 and SHIP-2 siRNA2) directed against exons 7 and 10, respectively, and a control siRNA encoding a scrambled sequence were transiently transfected into Raw 264.7 cells at a concentration of 100 nM using the Nucleofector. Cells were harvested 24 hours later, and protein-matched lysates were analyzed by Western blotting with anti-SHIP-2 antibody (Figure 3A, upper panel). SHIP-2 band intensity was quantitated by laser densitometry and is shown in the middle panel. Parallel lysates were probed with anti-SHIP-1 antibody (Figure 3A, lower panel). Results indicated that both SHIP-2 siRNAs were capable of specifically down-regulating the expression of SHIP-2 with equal efficiency and did not influence the expression of the highly homologous SHIP-1. The control siRNA had no effect on either SHIP-2 or SHIP-1 expression, as expected. Time course experiments revealed that down-regulation of SHIP-2 occurs around 18 hours after introduction of siRNA and persists until around 48 hours (data not shown).
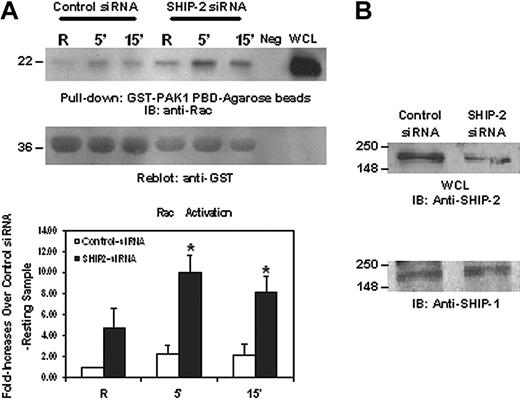
SHIP-2 down-regulates Rac activation. Raw 264.7 cells were transiently transfected with either a nonspecific control siRNA or SHIP-2 siRNA. (A) Eight hours after transfection, cells were starved in incomplete RPMI media (ie, with no FBS) for 16 hours. After starvation, cells were stimulated with 2.4G2 followed by MAR (mouse anti-rat IgG) for the indicated time. Protein-matched cell lysates were incubated with GST-PAK1-PBD-agarose beads for 1 hour at 4°C. Active Rac bound to the beads was eluted and loaded on 12% SDS-PAGE (top panel). Equal volume of lysis buffer only and whole cell lysate were loaded as negative and positive controls (neg and WCL). The membranes were reprobed with anti-GST antibody (middle panel). Rac band intensities were quantitated and are presented as fold increase over the resting control siRNA-transfected sample (bottom panel). The graph represents means ± SD. (B) Whole cell lysates from the resting samples were separated on 10% SDS-PAGE and transferred to nitrocellulose membrane, which was then probed with anti-SHIP-2 (top panel) or anti-SHIP-1 antibody (bottom panel).
SHIP-2 down-regulates Rac activation. Raw 264.7 cells were transiently transfected with either a nonspecific control siRNA or SHIP-2 siRNA. (A) Eight hours after transfection, cells were starved in incomplete RPMI media (ie, with no FBS) for 16 hours. After starvation, cells were stimulated with 2.4G2 followed by MAR (mouse anti-rat IgG) for the indicated time. Protein-matched cell lysates were incubated with GST-PAK1-PBD-agarose beads for 1 hour at 4°C. Active Rac bound to the beads was eluted and loaded on 12% SDS-PAGE (top panel). Equal volume of lysis buffer only and whole cell lysate were loaded as negative and positive controls (neg and WCL). The membranes were reprobed with anti-GST antibody (middle panel). Rac band intensities were quantitated and are presented as fold increase over the resting control siRNA-transfected sample (bottom panel). The graph represents means ± SD. (B) Whole cell lysates from the resting samples were separated on 10% SDS-PAGE and transferred to nitrocellulose membrane, which was then probed with anti-SHIP-2 (top panel) or anti-SHIP-1 antibody (bottom panel).
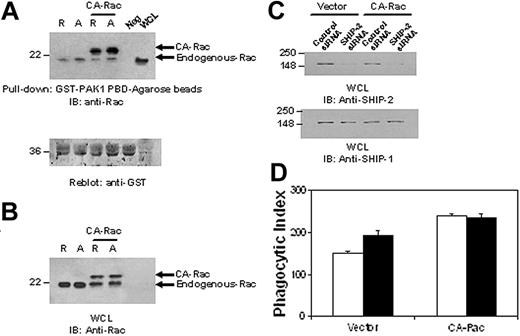
SHIP-2 down-regulates phagocytosis by suppressing upstream Rac activation. (A) Raw 264.7 cells were transiently transfected with constitutively active Rac (CA-Rac). Eight hours after transfection, cells were starved in incomplete RPMI media (ie, with no FBS) for 16 hours. After starvation, cells were stimulated with 2.4G2 followed by MAR (mouse anti-rat IgG) for 5 minutes to cluster FcγR. Protein-matched cell lysates were incubated with GST-PAK1-PBD-agarose beads for 1 hour at 4°C. Equal volume of lysis buffer was incubated with beads as a negative control (neg). Active Rac bound to the beads was eluted and loaded on 12% SDS-PAGE (top panel) and analyzed by Western blotting with anti-Rac antibody. The membrane was washed and reprobed with an anti-GST antibody (bottom panel). (B) Raw 264.7 cells were cotransfected with either a nonspecific control siRNA or SHIP-2 siRNA with CA-Rac. Twenty-four hours after transfection, whole cell lysates were made and separated on 12% SDS-PAGE and probed with an anti-Rac antibody. (C) Parallel samples were separated by 10% SDS-PAGE and probed with anti-SHIP-2 (top panel) or anti-SHIP-1 (bottom panel) antibody. (D) IgG-coated SRBCs were incubated with the transfectants for 1 hour at 37°C to assess phagocytic efficiency. Phagocytosis was measured by counting the total number of RBCs ingested by 100 phagocytosing cells. □ indicates control siRNA; ▪, SHIP-2 siRNA. The graph represents means ± SD.
SHIP-2 down-regulates phagocytosis by suppressing upstream Rac activation. (A) Raw 264.7 cells were transiently transfected with constitutively active Rac (CA-Rac). Eight hours after transfection, cells were starved in incomplete RPMI media (ie, with no FBS) for 16 hours. After starvation, cells were stimulated with 2.4G2 followed by MAR (mouse anti-rat IgG) for 5 minutes to cluster FcγR. Protein-matched cell lysates were incubated with GST-PAK1-PBD-agarose beads for 1 hour at 4°C. Equal volume of lysis buffer was incubated with beads as a negative control (neg). Active Rac bound to the beads was eluted and loaded on 12% SDS-PAGE (top panel) and analyzed by Western blotting with anti-Rac antibody. The membrane was washed and reprobed with an anti-GST antibody (bottom panel). (B) Raw 264.7 cells were cotransfected with either a nonspecific control siRNA or SHIP-2 siRNA with CA-Rac. Twenty-four hours after transfection, whole cell lysates were made and separated on 12% SDS-PAGE and probed with an anti-Rac antibody. (C) Parallel samples were separated by 10% SDS-PAGE and probed with anti-SHIP-2 (top panel) or anti-SHIP-1 (bottom panel) antibody. (D) IgG-coated SRBCs were incubated with the transfectants for 1 hour at 37°C to assess phagocytic efficiency. Phagocytosis was measured by counting the total number of RBCs ingested by 100 phagocytosing cells. □ indicates control siRNA; ▪, SHIP-2 siRNA. The graph represents means ± SD.
SHIP-2 siRNA2 was then used in experiments examining phagocytosis. Compared with cells tranfected with control siRNA (161.75 ± 10.9), SHIP-2 siRNA-transfected Raw 264.7 cells displayed significantly higher phagocytic ability (210.25 ± 6.34) (Figure 3B, P < .05). To examine whether down-regulation of SHIP-2 influences FcγR expression, binding capacity of the cells to IgG-coated SRBCs was measured. Cells transfected with either control siRNA or SHIP-2 siRNA displayed equivalent binding ability (39867 ± 10.69 versus 394.67 ± 6.03; P = .314) (Figure 3C). Taken together, these data strongly suggest that SHIP-2 is a negative regulator of phagocytosis in macrophages independent of the influence of SHIP-1.
SHIP-2 down-regulates phagocytosis by suppressing upstream Rac activation
To understand the mechanism of SHIP-2 down-regulation of phagocytosis, we set out to test whether SHIP-2 had an effect on signaling molecules involved in phagocytosis. The low molecular weight GTP-binding protein Rac has been shown to be critical for cytoskeletal remodeling necessary for phagocytosis.14,15 Rac GTP binding is facilitated by the guanine nucleotide exchange factor Vav, which in turn is reported to be activated by the binding of Vav PH domain to PtdIns3,4,5P3. Thus, we theorized that SHIP-2, with its ability to hydrolyze PtdIns3,4,5P3, would likely have a negative influence on Rac activation. To test this notion, we used SHIP-2 siRNA2 to knock down the expression of SHIP-2 in Raw 264.7 cells and then activated the cells by clustering FcγR. Active GTP-bound Rac was captured from whole cell lysates using glutathione agarose beads coated with GST-PAK1-PBD and resolved by SDS-PAGE. As seen in Figure 4A, Rac activation was prominent at 5 minutes after stimulation and went down slightly at 15 minutes in cells transfected with control siRNA (Figure 4A, top panel). The same activation pattern was observed in cells transfected with SHIP-2 siRNA, but the activation levels were significantly higher (Figure 4A). The membranes were subsequently reprobed with anti-GST antibody to ensure equal loading of the bait protein (Figure 4A, middle panel). Band intensities of Rac were quantitated and converted to the fold increases over unstimulated control sample. Data from at least 3 independent experiments are presented in Figure 4A. Compared with cells transfected with control siRNA, down-regulation of SHIP-2 expression significantly enhanced Rac activation, suggesting that SHIP-2 may negatively regulate phagocytosis by suppressing upstream Rac activation. This effect was specific for SHIP-2, because the expression of SHIP-1 was not altered by siRNA treatment (Figure 4B).
To further analyze whether the effect of SHIP-2 on phagocytosis is mediated via its influence on Rac activation, SHIP-2 expression was down-regulated in cells expressing constitutively active Rac (CA-Rac). Phagocytic efficiency of these transfectants was then assessed. Figure 5A is a Rac GTP-binding assay to ensure that CA-Rac is indeed constitutively active. Figure 5B-C is Western blots of protein-matched lysates from the transfectants indicating the presence of the transfected CA-Rac and SHIP-2 down-regulation by siRNA, respectively. Knockdown of SHIP-2 resulted in a significant enhancement of phagocytosis, as expected (151.00 ± 4.58 versus 192.67 ± 5.03; Figure 5D). In contrast, although cells expressing CA-Rac showed enhanced phagocytosis compared with cells transfected with vector alone, SHIP-2 down-regulation in these cells conferred no further enhancement in phagocytic efficiency (239.67 ± 12.22 versus 234.33 ± 9.71). These results strongly suggest that SHIP-2 influences phagocytosis by down-regulating Rac activation.
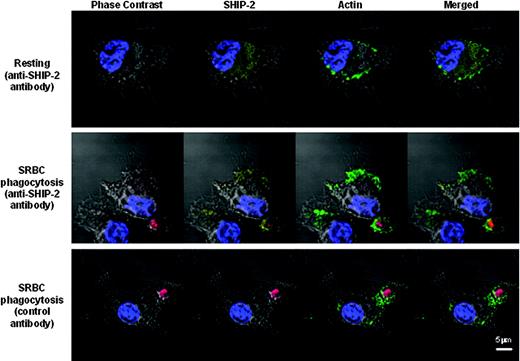
SHIP-2 is recruited to the site of phagocytosis. Raw 264.7 cells were grown on coverslips and either left untreated (resting) or incubated with IgG-coated SRBCs (red) for 5 minutes at 37°C. Cells were then fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100. After blocking, cells were stained with a goat anti-SHIP-2 antibody followed by a Cy5-conjugated donkey anti-goat F(ab′)2 fragment (yellow). F-actin was stained with FITC-phalloidin (green), and nuclei were stained with Hoechst (blue). Coverslips were mounted on slides and read using Zeiss 510 confocal microscope with ×63 magnification. SHIP-2 has a diffused cytoplasmic pattern in resting cells (top panel). SHIP-2 localizes to the site of phagocytosis (middle panel), where it colocalized with F-actin. Cells stained with normal goat IgG followed by Cy5-conjugated donkey anti-goat F(ab′)2 fragment showed clean background (bottom panel).
SHIP-2 is recruited to the site of phagocytosis. Raw 264.7 cells were grown on coverslips and either left untreated (resting) or incubated with IgG-coated SRBCs (red) for 5 minutes at 37°C. Cells were then fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100. After blocking, cells were stained with a goat anti-SHIP-2 antibody followed by a Cy5-conjugated donkey anti-goat F(ab′)2 fragment (yellow). F-actin was stained with FITC-phalloidin (green), and nuclei were stained with Hoechst (blue). Coverslips were mounted on slides and read using Zeiss 510 confocal microscope with ×63 magnification. SHIP-2 has a diffused cytoplasmic pattern in resting cells (top panel). SHIP-2 localizes to the site of phagocytosis (middle panel), where it colocalized with F-actin. Cells stained with normal goat IgG followed by Cy5-conjugated donkey anti-goat F(ab′)2 fragment showed clean background (bottom panel).
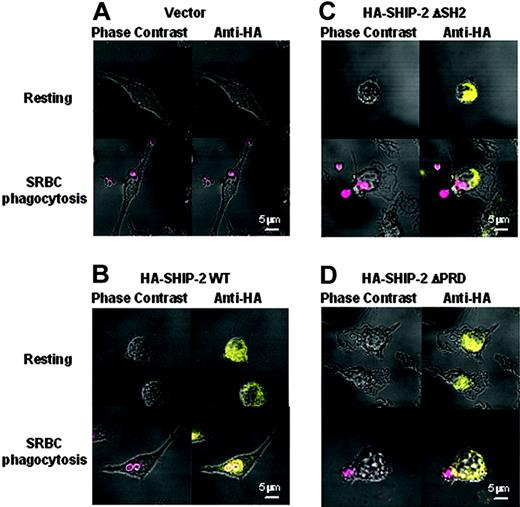
SH2 and PRD domains are both required for translocation of SHIP-2 to the site of phagocytosis. Raw 264.7 cells transfected with HA-tagged SHIP-2 constructs were grown on coverslips and either left untreated (resting) or incubated with IgG-coated SRBCs (red) for 5 minutes at 37°C. Cells were then fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100. After blocking, cells were stained with a mouse anti-HA antibody followed by an Alexa Fluor 594-conjugated goat anti-mouse F(ab′)2 fragment (yellow). Coverslips were mounted on slides and read using Zeiss 510 confocal microscope with ×63 magnification. (A) Empty vector-tranfected cells; (B) HA-SHIP-2 WT transfectants; (C) HA-SHIP-2 ΔSH2 transfectants; and (D) HA-SHIP-2 ΔPRD tranfectants.
SH2 and PRD domains are both required for translocation of SHIP-2 to the site of phagocytosis. Raw 264.7 cells transfected with HA-tagged SHIP-2 constructs were grown on coverslips and either left untreated (resting) or incubated with IgG-coated SRBCs (red) for 5 minutes at 37°C. Cells were then fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100. After blocking, cells were stained with a mouse anti-HA antibody followed by an Alexa Fluor 594-conjugated goat anti-mouse F(ab′)2 fragment (yellow). Coverslips were mounted on slides and read using Zeiss 510 confocal microscope with ×63 magnification. (A) Empty vector-tranfected cells; (B) HA-SHIP-2 WT transfectants; (C) HA-SHIP-2 ΔSH2 transfectants; and (D) HA-SHIP-2 ΔPRD tranfectants.
SHIP-2 is recruited to the phagocytic cup
Previous studies demonstrated that during FcγR-mediated phagocytosis SHIP-1 localized to the phagocytic cup.24 To examine whether SHIP-2 would likewise move to the phagocytic cup, we examined the cellular localization of SHIP-2 in Raw 264.7 cells using confocal immunofluorescence microscopy. Endogenous SHIP-2 was labeled by a goat anti-SHIP-2 primary antibody, followed by a Cy5-conjugated secondary antibody. In resting cells, SHIP-2 (yellow) was seen to localize to the cytoplasm in a diffuse pattern, with no apparent membrane staining (Figure 6, upper panel). However, 5 minutes after incubation with IgG-coated SRBCs, SHIP-2 was seen to translocate to the site of phagocytosis, around the ingested SRBCs (red) and colocalized with F-actin (green) (Figure 6, middle panel), suggesting that SHIP-2 is involved in regulating early phagocytic events including actin remodeling. The observed distribution pattern was specific for SHIP-2, because slides stained with normal goat IgG followed by the same fluorescent-labeled secondary antibody showed a clean background (Figure 6, bottom panel).
To further test whether the recruitment to the phagocytic cup has any domain requirements, we overexpressed HA-tagged SHIP-2 constructs in Raw 264.7 cells and examined their subcellular localization using confocal microscopy (Figure 7). In resting cells, WT-SHIP-2, ΔSH2-SHIP-2, and ΔPRD-SHIP-2 all have a diffuse cytoplasmic localization. The transfected WT-SHIP-2 was recruited to the phagocytic cup as expected, upon incubation with IgG-coated SRBCs (Figure 7B). However, there appeared to be no such localization for either ΔSH2-SHIP-2 or ΔPRD-SHIP-2 in phagocytosing cells (Figure 7C-D). Empty vector-transfected cells were used as a negative control (Figure 7A). This result is consistent with the finding that both the SH2 domain and the PRD are required for SHIP-2 to down-regulate phagocytosis (Figure 1).
Discussion
Macrophages play an important role in innate immune response by phagocytosing IgG-coated infectious particles. However, collateral tissue damage can occur if this process goes unchecked. Regulation of phagocytosis is rather complicated. It not only involves the opposing actions of activating versus inhibitory FcγRs, which can be affected by the microenviroment (eg, proinflammatory/anti-inflammatory cytokines),19,20,39 but also engages multiple cytoplasmic phosphatases.36,40-42 While the role of SHIP-1 in regulation of phagocytosis has been well studied,18,24 the functions of SHIP-2 in this critical immune reaction remain largely unknown.
In this study, we have demonstrated that SHIP-2 down-regulates FcγR-mediated phagocytosis using multiple cell models. We have observed similar down-regulatory effect of SHIP-2 in SHIP-2+/+ compared with SHIP-2-/- fetal liver macrophages. These latter data have not been included here because the mice used in these studies, which were initially thought to be SHIP-2 knockouts, were subsequently shown to express fragments of SHIP-2 and are also deleted in other adjacent genes.27,29
Our data showing that overexpression of wild-type SHIP-2 significantly reduced phagocytosis in Raw 264.7 cells whereas overexpression of ΔSH2-SHIP-2 or ΔPRD-SHIP-2 did not have this down-regulation effect are consistent with our previous finding that SH2 domain is required for the recruitment and activation of SHIP-2.31 Other studies have reported that SHIP-2 translocates to the cell membrane upon stimulation of macrophages with CSF-1 or fibroblasts with insulin, and this translocation depends on the interaction between its C-terminal proline-rich domain with the actin-binding protein filamin.34,43 Our current studies suggest that SHIP-2 is likely recruited to the activated FcγR via its SH2 domain, and this membrane localization is further stabilized through its PRD domain. Indeed, a similar role for the PRD of SHIP-1 has been described.44-47 Thus, mutation of either the SH2 domain or the PRD of SHIP-2 would prevent stable association of SHIP-2 with the plasma membrane, making either mutant incapable of down-regulating phagocytosis and acting in a dominant-negative fashion. Consistent with this notion, SH2 mutants and PRD mutants of SHIP-2 failed to translocate to the phagocytic cup (Figure 7).
Our results indicate that SHIP-2 down-regulates phagocytosis possibly through suppression of the upstream Rac activation. We have examined subcellular localization of SHIP-2 using confocal immunofluorescence microscopy, which demonstrated that SHIP-2 translocates from cytoplasm to the site of phagocytosis upon stimulation with IgG-coated SRBCs. Because phagocytosis requires actin polymerization and cytoskeletal rearrangement, SHIP-2 can thus be specifically recruited to the site of active actin remodeling. Once at the site of phagocytosis, SHIP-2 can hydrolyze PtdIns3,4,5P3 to PtdIns3,4P2, shutting off the downstream signaling pathways, including the vav/Rac and Akt pathways that are critical for actin polymerization. Indeed, recent studies by Hoppe and Swanson have demonstrated that Rac proteins localize to the phagocytic cup, which would put the 2 molecules (Rac and SHIP-2) in proximity.32
SHIP-1 and SHIP-2 both serve to down-regulate phagocytosis, yet they must have nonoverlapping functions as well. In addition to the divergence of the 2 molecules in their C-terminal proline-rich domain, SHIP-1 and SHIP-2 also have different numbers of tyrosine residues in the C terminus that conform to an NPXY motif shown to bind PTB (phosphotyrosine binding) domains upon phosphorylation, all of which lead to distinct protein-protein interactions. For example, the proline-rich domain of SHIP-1 associates with Grb2, whereas the proline-rich domain of SHIP-2 fails to associate with Grb2 but associates with Abl.26 These 2 molecules also differ in their expression pattern: SHIP-1 is expressed predominantly in hematopoetic cells, while SHIP-2 is much more ubiquitously expressed, suggesting that SHIP-2 has more generalized cellular functions. SHIP-2 has been reported to associate with both the phosphorylated ITIM of FcγRIIB and the ITAMs of FcγRI and FcγRIIa.31,48 Interestingly, unpublished data from our laboratory (A.M. and S.T., July 2004) showed that SHIP-2 associates with the phosphorylated ITAM of FcγRIIa with equivalent efficiency as it does with the phosphorylated ITIM of FcγRIIb. This is in contrast to the binding ability of SHIP-1, which is highly efficient for the ITIM but comparatively weaker for the ITAM. In fact, work from our group and others indicated that the association of SHIP-1 with phosphorylated ITAMs is predominantly indirect and is mediated by the Ras adapter Shc,7,49 although it can associate to some extent directly to phosphorylated ITAMs.18 These observations suggest that SHIP-2 may be the dominant regulator of phagocytosis when both SHIP-1 and SHIP-2 are present. Studies are underway to elucidate the functional differences between SHIP-1 and SHIP-2 in phagocytosis as well as other FcγR-mediated functions.
Prepublished online as Blood First Edition Paper, September 22, 2005; DOI 10.1182/blood-2005-05-1841.
Supported by National Institutes of Heath/National Cancer Institute (NIH/NCI) grants R01 AI059406 and P01 CA095426.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Stephane Schurmans for generously providing the SHIP-2 knockout animals and Dr John M. Robinson for advice on confocal microscopy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal