Recent data indicate that interleukin-3 (IL-3) is involved in multiple myeloma (MM) bone disease,1,2 being able either to stimulate osteoclast formation1 or to inhibit osteoblast formation.2 It has also been reported that IL-3 may act as a growth factor for MM cells, suggesting that this cytokine has a critical role in the pathophysiology of MM. Nevertheless, the potential source of IL-3 in MM patients is not known, and it is a matter of controversy. Some years ago it was reported that both MM cells and human myeloma cell lines were negative for IL-33,4 ; however, higher IL-3 levels have also been reported in the bone marrow (BM) plasma of MM patients compared with healthy subjects.1
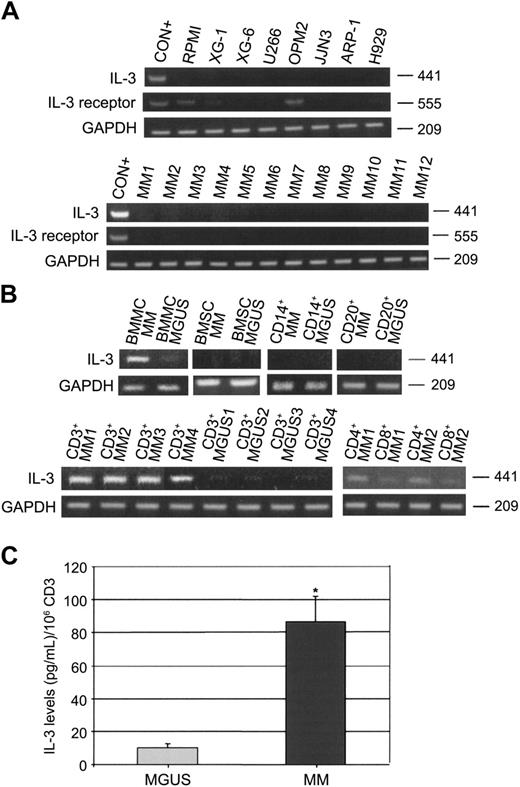
In order to clarify this issue, in the present study we have investigated IL-3 mRNA expression and IL-3 secretion by human myeloma cell lines (HMCLs), total BM mononuclear cells (BMMCs), and isolated stromal cells (BMSCs) as well as by CD138+ cells, CD14+ cells, CD20+ cells, and CD3+ (CD4+ and CD8+) cells obtained from BM aspirates of MM patients (n = 20) and MGUS subjects (n = 10). The purification of the different cell types was performed by an immunomagnetic method (magnetic activated cell sorting [MACS]; Miltenyi, Auburn, CA) using anti-CD138, anti-CD14, anti-CD20, anti-CD3, anti-CD4, and anti-CD8 mAbs coated with microbeads, and purity was checked by flow cytometry. Only samples with purity more than 90% were analyzed. Using a reverse-transcription-polymerase chain reaction (RT-PCR) as previously published2 (IL-3: primer pairs: forward, 5′-CTTCAACAACCTCAATGGGG-3′ and reverse, 5′-AATTCATCTGATGCCGCAGG-3′; IL-3 receptor: forward, 5′-TCT CCA GCG GTT CTC AAA GTT CCC ACA TCC-3′ and reverse, 5′-CCC AGA CCA CCA GCT TGT CGT TTT GGA AGC-3′), we found that RPMI-8226, XG-1, XG-6, U266, OPM-2, JJN3, ARP-1, and H929 did not express IL-3 mRNA, whereas IL-3 receptor was expressed only on RPMI and OPM2 (Figure 1A). Thereafter, we analyzed freshly purified CD138+ cells and we found that all samples tested were negative for IL-3 mRNA expression as shown for 12 representative MM patients (Figure 1A). On the other hand, we found that BMMCs of MM patients were positive for IL-3 mRNA, whereas BMMCs of MGUS subjects were negative. Among the BMMCs, we show that CD14+, CD20+ cells as well as BMSCs of both MM patients and MGUS subjects were negative for IL-3 mRNA (Figure 1B). On the contrary, we found that CD3+ cells of MM patients strongly expressed IL-3 mRNA, whereas CD3+ cells in MGUS subjects were negative as shown for 4 representative MM patients (Figure 1B). Among CD3+ cells, we found that CD4 T cells expressed IL-3 mRNA in MM patients (Figure 1B). Using flow cytometry to detect intracytoplasmic IL-3 (clone: BVD3-1F9; BD Biosciences, San Jose, CA), we confirmed that CD4+ T cells produce IL-3 in MM patients but not in MGUS subjects (data not shown). Consistently, we found significantly higher IL-3 levels, detected by enzyme-linked immunosorbent assay (ELISA; Biosource International, Camarillo, CA), in the supernatants of CD3+ (106 cells/mL) cells of MM patients compared with MGUS (mean ± SD/106 CD3+ cells: 86.6 ± 15.4 pg/mL vs 9.9 ± 2.3 pg/mL; P = .01) (Figure 1C). No detectable IL-3 levels were measured in the conditioned medium of CD138+, CD14+, CD20+, and BMSCs in both groups analyzed (data not shown).
IL-3 expression and secretion in MM patients. (A) Interleukin-3 (IL-3) and IL-3 receptor mRNA expression was evaluated by RT-PCR in human myeloma cell lines (RPMI-8226, XG-1, XG-6, U266, OPM2, JJN3, ARP-1, and H929) and in freshly purified MM cells as shown for 12 representative MM patients (CON+ = activated T cells). (B) IL-3 mRNA expression was checked in bone marrow mononuclear cells (BMMCs), BM stromal cells (BMSCs), and in purified BM CD14+, CD20+, and CD3+ (CD4+ and CD8+) cells obtained from MM patients and MGUS subjects as shown for 4 representative patients. (C) IL-3 levels were measured by ELISA in 48-hour supernatants of purified CD3+ cells obtained from MM patients (n = 20) and MGUS subjects (n = 10). Graph represents the mean ± SD IL-3 levels normalized to 106 cells (*P = .01).
IL-3 expression and secretion in MM patients. (A) Interleukin-3 (IL-3) and IL-3 receptor mRNA expression was evaluated by RT-PCR in human myeloma cell lines (RPMI-8226, XG-1, XG-6, U266, OPM2, JJN3, ARP-1, and H929) and in freshly purified MM cells as shown for 12 representative MM patients (CON+ = activated T cells). (B) IL-3 mRNA expression was checked in bone marrow mononuclear cells (BMMCs), BM stromal cells (BMSCs), and in purified BM CD14+, CD20+, and CD3+ (CD4+ and CD8+) cells obtained from MM patients and MGUS subjects as shown for 4 representative patients. (C) IL-3 levels were measured by ELISA in 48-hour supernatants of purified CD3+ cells obtained from MM patients (n = 20) and MGUS subjects (n = 10). Graph represents the mean ± SD IL-3 levels normalized to 106 cells (*P = .01).
Our data clearly indicate that CD3+ T cells, and not MM cells, are the source of IL-3 in MM patients. The involvement of T lymphocytes in MM bone disease has been recently hypothesized, showing that T cells may produce the critical osteoclastogenic factor RANKL5 and they are critical for osteoclast formation by myeloma cells.6 In line with these observations, our data indicate that T lymphocytes may also produce IL-3, a cytokine with both osteoclastogenic and antiosteoblastic effects,1,2 supporting the critical role of T cells in MM bone disease.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal