Abstract
The nonobese diabetic/severe combined immunodeficient (NOD/SCID) assay is the current model for assessment of human normal and leukemic stem cells. We explored why 51% of 59 acute myeloid leukemia (AML) patients were unable to initiate leukemia in NOD/SCID mice. Increasing the cell dose, using more permissive recipients, and alternative tissue sources did not cause AML engraftment in most previously nonengrafting AML samples. Homing of AML cells to the marrow was the same between engrafters and nonengrafters. FLT3 internal tandem duplication (ITD) and nucleophosmin mutations occurred at a similar frequency in engrafters and nonengrafters. The only variable that was related to engraftment ability was the karyotypically defined risk stratification of individual AML cases. Of interest, follow-up of younger patients with intermediate-risk AML revealed a significant difference in overall survival between NOD/SCID engrafting and nonengrafting AMLs. Hence, the ability of AML to engraft in the NOD/SCID assay seems to be an inherent property of AML cells, independent of homing, conditioning, or cell frequency/source, which is directly related to prognosis. Our results suggest an important difference between leukemic initiating cells between engrafting and nonengrafting AML cases that correlates with treatment response.
Introduction
The nonobese diabetic/severe combined immunodeficient (NOD/SCID) xenotransplantation assay is currently the model of choice for assessment of transplantable human hematopoietic stem cells (HSCs). This approach has been crucial to our understanding of human hematopoiesis, providing reliable determination of the phenotypes of repopulating cells1 and elucidating previously undescribed HSC populations.2 More recently, a novel mouse strain has been developed by backcrossing β2 microglobulin-null (B2m-/-) mice onto the NOD/SCID background. The resulting B2m-/- NOD/SCID strain, in addition to the B-cell, T-cell, complement, and partial natural killer (NK) defects that define the NOD/SCID model, has a complete lack of NK cell activity.3 Hence, this model is reportedly even more permissive to xenotransplantation than the original NOD/SCID strain.4
Acute myeloid leukemia (AML) is characterized by a relentless accumulation of immature, abnormal hematopoietic cells in the bone marrow and peripheral blood. It has been postulated that AML is a disease maintained by leukemic stem cells and may be organized in a similar way to normal hematopoiesis. Indeed, only a subset of AML cells are capable of forming colonies in vitro and a smaller fraction can maintain colony production for 6 weeks while on feeder layers.5 Definitive proof that a small population of leukemic stem cells produce the AML blasts comes from 6-week primary and secondary engraftment experiments in NOD/SCID mice.6 Further studies have revealed that these SCID-leukemia initiating cells (SL-ICs) share many properties with normal HSCs, namely phenotype, quiescence, and in vitro CXCR-4-mediated migration.6-8
AML is an extremely heterogeneous disease, and since there are so many different known genetic abnormalities (and probably many more unknown), AML may be thought of as a collection of different diseases that have the same myeloid morphology. Indeed, for patients younger than 60 years of age the single most important prognostic factor is the karyotype.9,10 AML cases are currently divided via karyotype into the treatment groups of poor, intermediate, and favorable prognosis. The majority of patients have an intermediate-risk karyotype and the outcome of these patients is variable as well as difficult to predict using prospective tests.
Previous studies have reported that approximately 70% of AML cases will engraft in the NOD/SCID assay.11 Although many groups have used the NOD/SCID assay, the majority have only assessed the AML cases capable of engraftment.12-15 Few studies have addressed the variables that affect engraftment itself.16 Various factors affecting normal hematopoietic cell engraftment have been identified and may be applicable to AML NOD/SCID engraftment.
A complex series of interactions of adhesion molecules, cytokines, chemokines, and their receptors is responsible for the homing of transplanted human hematopoietic cells from the peripheral injection site to the bone marrow.17 A major role in hematopoietic cell homing is attributed to the interaction between the chemokine SDF-1 and its receptor CXCR-4.18 Overexpression of CXCR-4 on human CD34+ cells results in an increased ability to home to and engraft NOD/SCID marrows.19 Furthermore, antibody-blocking studies have revealed that engraftment of human hematopoietic cells in NOD/SCID mice is dependent on the interaction between CXCR-4 and SDF-1.20 In AML, although both in vitro transendothelial migration and the level of in vivo NOD/SCID bone marrow homing are dependent on CXCR-4, it is not clear whether the actual ability to engraft NOD/SCID mice is dependent on the CXCR-4/SDF-1 axis.8,21
Here, we examined 59 AML patients for their ability to initiate leukemia in NOD/SCID mice. We established via morphology, phenotype, genotype, and RNA expression that when AML engrafted, the AML produced was very similar to the patients' disease. We then investigated variables known to affect normal cell engraftment for their ability to cause AML engraftment. Increasing the cell dose, more intensive conditioning, more permissive recipients, and alternative tissue sources (bone marrow) did not cause AML engraftment in previously nonengrafting AML samples. Both the CXCR-4 expression and in vivo homing of AML cells were the same between engrafters and nonengrafters. FLT3 internal tandem duplication (ITD) and nucleophosmin (NPM) mutations occurred at a similar frequency in engrafters and nonengrafters. The only variable that did seem to be related to engraftment ability was the karyotype of individual AML cases. Of interest, follow-up of younger (< 60 years) intermediate-risk AML cases revealed a statistically significant difference in overall survival between NOD/SCID engrafting and nonengrafting cases of AML.
Hence, the NOD/SCID assay appears to reproduce an AML very similar to the patients' disease, and the ability to engraft seems to be an inherent property of AML cells that is independent of homing, conditioning, or cell dose/source but is directly related to prognosis.
Patients, materials, and methods
Primary cells
Cells were obtained from newly diagnosed and relapsed patients with AML at St Bartholomew's Hospital after informed consent. The protocol was approved by the hospital research ethics committees. Mononuclear cells (MNCs) were obtained by Ficoll-Paque density centrifugation and ammonium chloride red cell lysis.
Mice
All animal experiments were performed in compliance with Home Office and institutional guidelines. NOD/SCID mice and B2m-/- NOD/SCID mice were originally obtained from Dr Leonard Schultz (Jackson Laboratory, Bar Harbor, ME) and bred at Charles Rivers Laboratories (Margate, United Kingdom). They were kept in microisolators and fed sterile food and acidified water. Mice aged 8 to 12 weeks were irradiated at 3.75 Gy (137Caesium source) up to 24 hours before intravenous injection of cells.
CXCR-4 expression analysis
Cells were stained with either phycoerythrin (PE)-conjugated or allophycocyanin (APC)-conjugated anti-CXCR-4 antibodies with phycoerythrincyanin 5 (PE-Cy5)-conjugated anti-CD34 antibodies for 30 minutes at 4°C (all antibodies from Becton Dickinson [BD] Biosciences, Oxford, United Kingdom). Cells were washed and resuspended in phosphate-buffered saline (PBS) with 2% FCS and DAPI (4,6-diamidino-2-phenylindoiole). Cells were analyzed on a BD Life Science Research (LSR) flow cytometer (BD Biosciences). Gates were set up to exclude nonviable cells and debris. The negative fraction was determined using appropriate isotype controls.
Calcium flux measurement
Cells were labeled with 2.5 μM Indo-1 (Invitrogen, Carlsbad, CA) at 37°C for 45 minutes. Cells were then analyzed on a BD LSR-II for 30 seconds to give background levels before stimulation with 100 ng of stromal cell-derived factor 1 and analysis for a further 4 minutes. Analysis involved detection of fluorescence due to dye bound to calcium (424/44 nm filter used) and fluorescence due to unbound dye (530/30 nm filter).
Analysis of murine bone marrow
Six weeks after transplantation, mice were killed by cervical dislocation. The femurs, tibias, and pelvis were dissected and flushed with PBS. Red blood cells were lysed via ammonium chloride. Cells were stained with human-specific FITC-conjugated anti-CD19, PE-conjugated anti-CD33, and PE-Cy5-conjugated anti-CD45 antibodies. Dead cells and debris were excluded via DAPI staining. A BD LSR flow cytometer was used for analysis. More than 100 000 DAPI-negative events were collected. Engraftment of AML was said to be present if a single population of CD45+CD33+CD19- cells was present without accompanying CD45+CD33-CD19+ cells.
Assessment of engraftment potential of AML
Samples were screened to assess whether they had the potential to engraft NOD/SCID and B2m-/- NOD/SCID mice. MNCs (107) were injected into each mouse. Engraftment of AML was confirmed, where possible, with morphology and fluorescent in situ hybridization (FISH) on human cells FACSorted from engrafted murine marrows.
Fluorescence-activated cell sorting (FACS)
Murine marrow cells were suspended in PBS with 2% FCS at 3 × 107 per mL and stained with human-specific anti-CD45-PE and murine-specific anti-CD45-FITC. Cells were washed and resuspended in PBS with 2% FCS and DAPI before sorting on a MoFlo cell sorter (DakoCytomation, Fort Collins, CO). Gates were set up to exclude nonviable cells (DAPI negative) and debris.
Fluorescent in situ hybridization
Briefly, FACSorted human cells were swollen in hypotonic (0.075 M) KCl solution and fixed in Karnoy fixative (3:1 methanol-acetic acid ratio) before dropping onto clean glass slides. Nuclei were “aged” overnight before pepsin digestion, dehydration, and application of fluorescent probes. Nuclei were incubated with probes overnight at 37°C before analysis at × 1000 magnification via a 100 ×/1.4 objective lens on a Carl Zeiss Axioplan-2 microscope equipped with Axiovision software and an Axiocam MRC camera (Carl Zeiss, Thornwood, NY).
Mutation detection
Deoxyribonucleic acid was extracted using standard phenol-chloroform methodologies. Primers and precise amplification conditions are available upon request and were derived from previously published studies (FLT3 exons 14-1522 ; FLT3 exon 2023 ). Polymerase chain reaction (PCR) products were sequenced directly by use of ABI 377 and ABI Prism 3730 DNA sequencers (PE Applied Biosystems, Foster City, CA). Before direct sequencing, unincorporated primer was removed by ultra-filtration using a Centricon YM-100 000 filter device (Millipore, Bedford, MA). Sequencing data were analyzed using DNASTAR (Madison, WI). Detection of NPM mutations was performed on genomic DNA by PCR as previously described.24
Treatment for younger (less than 60 years) intermediate-risk patients
Patients were treated with 1 of 2 protocols. Patients were either treated on the Medical Research Council 15 trial or received the St Bartholomew's Hospital standard of care protocol. This comprises 3 cycles of idarubicin 30 mg/m2, cytarabine 1400 mg/m2, and etoposide 500 mg/m2, and one cycle of cytarabine 18 g/m2. Patients with an appropriate donor underwent allogeneic transplantation in first complete remission. No patients died from treatment-related causes.
Statistics
Logistic regression was used to assess the significance of factors involved in engraftment. Event-free survival (EFS) was defined as survival with no evidence of persistent or recurrent disease. Patients with primary refractory disease (defined as bone marrow blasts > 5% in the marrow) were ascribed an EFS of 0 months. The actuarial probabilities of overall survival and EFS were plotted using the methodology of Kaplan and Meier as previously described.25 No significant differences in the number of patients who underwent either treatment protocol could be found between NOD/SCID engrafting and nonengrafting AML cases (data not shown). The Student paired t test for significance of no difference was used for all other assessments.
Affymetrix array analysis
Four samples, which gave a high positive engraftment result, were processed for further microarray analysis (patients 9, 17, 19, and 37; Table 1). Details of RNA extraction, small sample cRNA target preparation, hybridization, and microarray statistical analysis are available on the Blood website (see the Supplemental Methods link at the top of the online article).
Summary of patients' details
Patient ID . | FAB . | WBC, × 109 cells/L . | Karyotype** . | Risk group . |
|---|---|---|---|---|
| Engrafters | ||||
| 1*†‡ | 1 | 151 | Normal | Intermediate |
| 2§† | 1 | 14.7 | t(6;9) | Intermediate |
| 3 | 1 | 20.3 | –5q | Poor |
| 4 | 1 | 64 | FK | Intermediate |
| 5∥ | 1 | 37 | FK | Intermediate |
| 6∥ | 1 | 5.3 | Normal | Intermediate |
| 7§ | 1 | 139 | +13 | Intermediate |
| 8* | 2 | 104 | Normal | Intermediate |
| 9§¶∥ | 2 | 66 | t(8;21) | Good |
| 10§∥ | 2 | 27 | +11+13 | Intermediate |
| 11∥ | 2 | 39.8 | Normal | Intermediate |
| 12 | 2 | 29 | t(8;9) | Intermediate |
| 13 | 2 | 40 | t(2;3) | Poor |
| 14∥ | 4 | 2.5 | Normal | Intermediate |
| 15∥ | 4 | 71 | +3+10 | Intermediate |
| 16 | 4 | 8.5 | Normal | Intermediate |
| 17¶∥ | 4 | 221 | Normal | Intermediate |
| 18†‡ | 4 | 42.9 | Normal | Intermediate |
| 19*¶∥ | 5 | 3.9 | Complex | Poor |
| 20†‡ | 5 | 115 | Normal | Intermediate |
| 21∥ | 5 | 33 | Normal | Intermediate |
| 22 | 5 | 212 | ND | Intermediate |
| 23 | 5 | 53.7 | t(9;11) | Intermediate |
| 24∥ | 5a | 42.9 | t(11;19) | Intermediate |
| 25 | 5a | 124 | +11 | Intermediate |
| 26* | tAML | 2.7 | t(11;19) | Intermediate |
| 27∥ | tAML | 25 | t(6;11) | Intermediate |
| 28*∥ | tAML | 19.5 | Complex | Poor |
| 29∥ | AML/MDS | 31 | Inv(3); –7 | Poor |
| Nonengrafters | ||||
| 30∥ | 0 | 70 | –9q +19 | Intermediate |
| 31 | 1 | 103 | +13 | Intermediate |
| 32# | 1 | 10 | –9q | Intermediate |
| 33# | 1 | 86 | +8 | Intermediate |
| 34#‡∥ | 1 | 6.1 | Normal | Intermediate |
| 35#∥ | 1 | 1.4 | Normal | Intermediate |
| 36§ | 1 | 50 | Normal | Intermediate |
| 37¶†‡ | 1 | 248 | Normal | Intermediate |
| 38∥ | 1 | 70 | t(8;21) | Good |
| 39∥ | 2 | 27.9 | t(8;21) | Good |
| 40∥ | 2 | 85 | +12+21 | Intermediate |
| 41 | 2 | 28 | t(8;21) | Good |
| 42∥ | 2 | 11.8 | Normal | Intermediate |
| 43 | 2 | 71 | Normal | Intermediate |
| 44† | 2 | 19.2 | t(6;9) | Intermediate |
| 45 | 2 | 5.7 | t(8;21) | Good |
| 46‡∥ | 2 | 5.5 | Normal | Intermediate |
| 47 | 2 | 3 | Normal | Intermediate |
| 48 | 3 | 1 | t(15;17) | Good |
| 49 | 3 | 1.3 | t(15;17) | Good |
| 50# | 3 | 1.9 | t(15;17) | Good |
| 51 | 3 | 6.1 | t(15;17) | Good |
| 52# | 3v | 35 | t(15;17) | Good |
| 53 | 4 | 61 | Inv 16 | Good |
| 54*‡ | 4 | 85 | Normal | Intermediate |
| 55 | 4 | 127 | Normal | Intermediate |
| 56 | 4 | 113 | Inv(16) | Good |
| 57 | 5a | 184 | ins(10;11) | Intermediate |
| 58# | 5a | 39 | +5; +8; +19 | Intermediate |
| 59∥ | tAML | 147 | Normal | Intermediate |
Patient ID . | FAB . | WBC, × 109 cells/L . | Karyotype** . | Risk group . |
|---|---|---|---|---|
| Engrafters | ||||
| 1*†‡ | 1 | 151 | Normal | Intermediate |
| 2§† | 1 | 14.7 | t(6;9) | Intermediate |
| 3 | 1 | 20.3 | –5q | Poor |
| 4 | 1 | 64 | FK | Intermediate |
| 5∥ | 1 | 37 | FK | Intermediate |
| 6∥ | 1 | 5.3 | Normal | Intermediate |
| 7§ | 1 | 139 | +13 | Intermediate |
| 8* | 2 | 104 | Normal | Intermediate |
| 9§¶∥ | 2 | 66 | t(8;21) | Good |
| 10§∥ | 2 | 27 | +11+13 | Intermediate |
| 11∥ | 2 | 39.8 | Normal | Intermediate |
| 12 | 2 | 29 | t(8;9) | Intermediate |
| 13 | 2 | 40 | t(2;3) | Poor |
| 14∥ | 4 | 2.5 | Normal | Intermediate |
| 15∥ | 4 | 71 | +3+10 | Intermediate |
| 16 | 4 | 8.5 | Normal | Intermediate |
| 17¶∥ | 4 | 221 | Normal | Intermediate |
| 18†‡ | 4 | 42.9 | Normal | Intermediate |
| 19*¶∥ | 5 | 3.9 | Complex | Poor |
| 20†‡ | 5 | 115 | Normal | Intermediate |
| 21∥ | 5 | 33 | Normal | Intermediate |
| 22 | 5 | 212 | ND | Intermediate |
| 23 | 5 | 53.7 | t(9;11) | Intermediate |
| 24∥ | 5a | 42.9 | t(11;19) | Intermediate |
| 25 | 5a | 124 | +11 | Intermediate |
| 26* | tAML | 2.7 | t(11;19) | Intermediate |
| 27∥ | tAML | 25 | t(6;11) | Intermediate |
| 28*∥ | tAML | 19.5 | Complex | Poor |
| 29∥ | AML/MDS | 31 | Inv(3); –7 | Poor |
| Nonengrafters | ||||
| 30∥ | 0 | 70 | –9q +19 | Intermediate |
| 31 | 1 | 103 | +13 | Intermediate |
| 32# | 1 | 10 | –9q | Intermediate |
| 33# | 1 | 86 | +8 | Intermediate |
| 34#‡∥ | 1 | 6.1 | Normal | Intermediate |
| 35#∥ | 1 | 1.4 | Normal | Intermediate |
| 36§ | 1 | 50 | Normal | Intermediate |
| 37¶†‡ | 1 | 248 | Normal | Intermediate |
| 38∥ | 1 | 70 | t(8;21) | Good |
| 39∥ | 2 | 27.9 | t(8;21) | Good |
| 40∥ | 2 | 85 | +12+21 | Intermediate |
| 41 | 2 | 28 | t(8;21) | Good |
| 42∥ | 2 | 11.8 | Normal | Intermediate |
| 43 | 2 | 71 | Normal | Intermediate |
| 44† | 2 | 19.2 | t(6;9) | Intermediate |
| 45 | 2 | 5.7 | t(8;21) | Good |
| 46‡∥ | 2 | 5.5 | Normal | Intermediate |
| 47 | 2 | 3 | Normal | Intermediate |
| 48 | 3 | 1 | t(15;17) | Good |
| 49 | 3 | 1.3 | t(15;17) | Good |
| 50# | 3 | 1.9 | t(15;17) | Good |
| 51 | 3 | 6.1 | t(15;17) | Good |
| 52# | 3v | 35 | t(15;17) | Good |
| 53 | 4 | 61 | Inv 16 | Good |
| 54*‡ | 4 | 85 | Normal | Intermediate |
| 55 | 4 | 127 | Normal | Intermediate |
| 56 | 4 | 113 | Inv(16) | Good |
| 57 | 5a | 184 | ins(10;11) | Intermediate |
| 58# | 5a | 39 | +5; +8; +19 | Intermediate |
| 59∥ | tAML | 147 | Normal | Intermediate |
Mice were injected with 107 peripheral blood nucleated cells from the peripheral blood of AML patients. Murine marrows were analyzed 6 weeks after transplantation for the presence of human hematopoietic cells. AML engraftment was defined as the presence of human CD33+/CD45+ myeloid cells without an accompanying CD19+/CD45+ B-cell population. Prognosis risk group was defined as poor, intermediate, or good via karyotype according to Grimwade et al.10 Patients in whom the WBC was less than 2 × 109/L also had their bone marrow cells tested for engraftment capacity, with identical results to the peripheral blood data. All AML cases were assessed for NOD/SCID engraftment potential before any chemotherapy. FK indicates failed karyotype at diagnosis.
Patients given supportive care only
Patients possessed an FLT3-ITD
Patients had a mutated nucleophosmin gene
Patients in relapse
Patients were tested for FLT3 mutations and were found to be negative
Patients underwent affymetrix analysis
Patients produced normal engraftment in NOD/SCID mice
Full karyotyping according to the international system for human genotype nomenclature may be found in Table S1
Results
Not all cases of AML engraft in NOD/SCID mice
Ten million nucleated cells from 59 different AML patients were injected into NOD/SCID mice (Table 1). Six weeks later, murine bone marrows were assessed via flow cytometry for the presence of human myeloid cells. As reported previously, we found that not all cases of AML can be reproduced in the NOD/SCID model.11 We detected human (CD45+) myeloid (CD33+) engraftment without any B-cell (CD19) engraftment in 49% of cases examined (29/59). Via this simultaneous assessment of both the myeloid and lymphoid lineages, we distinguished normal engraftment from leukemic engraftment. Most previous reports have only assessed the proportion of human CD45+ cells in the murine marrow. This approach may have included normal engraftment and hence may have overestimated the proportion of AML cases that engraft in the NOD/SCID model.11 Indeed, a significant proportion (∼10%) of our AML patients' cells produced normal engraftment when 107 cells were injected into NOD/SCID mice.
Engraftment in NOD/SCID mice reproduces AML
To confirm the leukemic nature of this myeloid (CD33+/CD45+/CD19-) NOD/SCID engraftment, we compared the morphologic features identified during diagnosis to the morphology of NOD/SCID engrafted cells. In all cases analyzed, the morphology of NOD/SCID engrafted cells was very similar to the original sample. A representative M2 AML is shown in Figure 1. Similar to the diagnosis smear, a high proportion of the NOD/SCID engrafted cells were myeloblasts. Pathognomic AML Auer rods were also detectable in NOD/SCID mice, confirming the leukemic nature of these cells (Figure 1B arrow). Wherever possible, we also performed FISH to detect characteristic genetic abnormalities in NOD/SCID engrafted cells (Figure 1C-F).
Confirmation of AML cell growth in NOD/SCID mice. Ten million cells were injected into NOD/SCID mice and marrows were analyzed for human myeloid cell content 6 weeks later. (A) Diagnostic peripheral blood smear from AML-M2 patient 10. Cells were stained with hematoxylin and eosin (H&E) before analysis at 400 × magnification via a 40 ×/0.75 objective lens. (B) Murine marrow that was injected with cells from the same AML-M2 patient as in panel A. Myeloblasts, featuring Auer rods (arrow), are present, indicating AML. Cells were stained with H&E before analysis at 1000 × magnification via a 100 ×/1.3 objective lens. (C) Dual fusion, dual-color fluorescent in situ hybridization of a relapsed t[8,21] AML-M2 sample. Cells positive for the rearrangement exhibit 1 green, 1 red, and 2 orange spots. (D-F) Examples of NOD/SCID engrafted, FACSorted CD33+/CD45+ cells exhibiting AML-M2 t[8,21] rearrangement. Pictures of blood smears were taken on a Leica DFC 300F camera.
Confirmation of AML cell growth in NOD/SCID mice. Ten million cells were injected into NOD/SCID mice and marrows were analyzed for human myeloid cell content 6 weeks later. (A) Diagnostic peripheral blood smear from AML-M2 patient 10. Cells were stained with hematoxylin and eosin (H&E) before analysis at 400 × magnification via a 40 ×/0.75 objective lens. (B) Murine marrow that was injected with cells from the same AML-M2 patient as in panel A. Myeloblasts, featuring Auer rods (arrow), are present, indicating AML. Cells were stained with H&E before analysis at 1000 × magnification via a 100 ×/1.3 objective lens. (C) Dual fusion, dual-color fluorescent in situ hybridization of a relapsed t[8,21] AML-M2 sample. Cells positive for the rearrangement exhibit 1 green, 1 red, and 2 orange spots. (D-F) Examples of NOD/SCID engrafted, FACSorted CD33+/CD45+ cells exhibiting AML-M2 t[8,21] rearrangement. Pictures of blood smears were taken on a Leica DFC 300F camera.
Gene expression profile is extremely similar between engrafted AML cells and the original AML sample
To ensure that we were reproducing AML correctly in the NOD/SCID model, we examined the expression profiles of 8 AMLs (4 samples before engraftment and 4 samples after) by use of the oligonucleotide U133A arrays containing approximately 22 283 unique genes. An unsupervised hierarchical cluster analysis, performed on 2476 or 2260 genes passing the variation filter, grouped samples into 4 groups. On the basis of similarity in the expression pattern, the groups corresponded to the same patient sample before and after engraftment (Figure 2). This indicates that the 2 sets of genes had expression patterns strongly associated with AML sample of origin.
The cluster dendrogram gave similar results with the 2 lists of genes, with or without the variation filter. A statistical group comparison approach was used to identify genes with statistically significant differences in expression levels between groups of samples before and after engraftment. By using a t test analysis on normalized data, we could not identify any genes differentially expressed between before and after engraftment. Indeed, gene expression profiling on these samples showed highly consistent profiles. This was the same on normalized data directly or after logging (to log 2) the normalized data.
Gene expression analysis of engrafted AML cells. A dendrogram is shown from the unsupervised hierarchical cluster analysis of the 8 chips for the 2260 genes passing the variation filter. Independent of karyotype, AML patients were grouped between before and after engraftment. This means that the AML in the original patient is very much related to the AML that has grown in the mouse. The samples corresponding to before and after engraftment were always adjacent to each other, reflecting a very close relationship between them. Pt indicates patient. Height is an arbitrary unit of association; higher numbers indicate greater degree of similarity.
Gene expression analysis of engrafted AML cells. A dendrogram is shown from the unsupervised hierarchical cluster analysis of the 8 chips for the 2260 genes passing the variation filter. Independent of karyotype, AML patients were grouped between before and after engraftment. This means that the AML in the original patient is very much related to the AML that has grown in the mouse. The samples corresponding to before and after engraftment were always adjacent to each other, reflecting a very close relationship between them. Pt indicates patient. Height is an arbitrary unit of association; higher numbers indicate greater degree of similarity.
Hence, expression profiling reveals no fundamental biologic differences in AML samples before and after engraftment. Independent of karyotype, patient AMLs were grouped between before and after engraftment. The samples corresponding to before and after engraftment were always sitting adjacent to each other, reflecting a very close relationship between them. This means that the gene expression profile of AML in the original patient is very similar to the AML that has grown in the mouse.
Increasing the cell dose and using alternative cell sources does not increase the number of engrafting AML samples
To investigate factors that affect AML NOD/SCID engraftment, we repeated certain engraftment assessments, increasing the cell numbers and using alternative tissue sources (bone marrow). Six-week NOD/SCID engraftment could not be achieved with up to 108 cells from 5 previously nonengrafting AML samples. Of interest, although in 2 patients (53 and 59) AML engraftment was not observed with 107 cells, apparently normal multilineage engraftment was seen when 108 cells were injected. Taken together, these results suggest that the NOD/SCID assay is working correctly and that the reason some AML samples are incapable of engraftment is independent of cell dose.
All previous engraftment screening was performed on peripheral blood. Occasionally, we obtained both peripheral blood (PB) samples and bone marrow (BM) samples from the same patients. The engraftment potential from either PB or BM cells was compared in paired experiments from 10 different AML patients. Six patients demonstrated engraftment from both sources (patients 6, 11, 16, 17, 18, and 42), whereas 4 patients (35, 48, 49, and 50), which did not engraft from the PB, also did not engraft when BM cells were injected.
Most cases of nonengrafting AML do not engraft in a more permissive xenotransplantation model
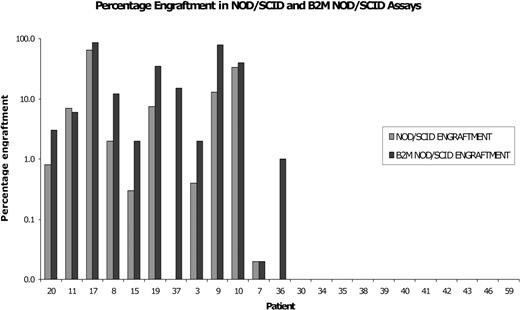
To investigate the influence of the murine microenvironment on AML engraftment, we examined engraftment in the more permissive B2m-/- NOD/SCID model. Samples were injected into both the NOD/SCID assay and the B2m-/- NOD/SCID model in paired experiments. Generally, samples that engrafted in the NOD/SCID assay engrafted at a higher level in the B2m-/- NOD/SCID model. However, the majority (10/12) of AML cases that failed to engraft in the NOD/SCID model could not be modeled in the B2m-/- NOD/SCID assay. Only 2 samples (patients 36 and 37), which failed to engraft in the NOD/SCID model, engrafted in the B2m-/- NOD/SCID assay (Figure 3). Since the major difference between the B2m-/- NOD/SCID and NOD/SCID models is probably NK cell activity, one may suggest that in most cases of AML, the reason for NOD/SCID nonengraftment is not immune mediated. 4
A lack of AML engraftment is not due to an obvious homing defect
To investigate the mechanism of homing, we first examined the expression of the CXCR-4 receptor on various NOD/SCID engrafting (n = 11) and nonengrafting (n = 10) AML cases. Although the range of CXCR-4 expressions was large, we could not identify any obvious differences in the average CXCR-4 expression between the NOD/SCID engrafting and nonengrafting AML cases (Table 2). Indeed, one sample with over 90% CXCR-4 expression did not engraft (patient 39) and conversely one of the engrafting AMLs possessed hardly any CXCR-4 expression (patient 9).
Percentage of CXCR-4 expression on CD34+ cells
Patient ID . | Percent of CXCR-4 on CD34+ . |
|---|---|
| Engrafters | |
| 9 | 2.2 |
| 29* | 4.1 |
| 26* | 19.1 |
| 8 | 21.5 |
| 27 | 22.2 |
| 28 | 24.5 |
| 10 | 63.3 |
| 24* | 80.4 |
| 5 | 95.3 |
| 20* | 96.1 |
| 14 | 98.9 |
| Mean ± SD | 48.0 ± 39.0 |
| Nonengrafters | |
| 41 | 4.1 |
| 45* | 8.4 |
| 34* | 22.5 |
| 35 | 34.9 |
| 42 | 54.2 |
| 47 | 65 |
| 44 | 69.5 |
| 46* | 75.9 |
| 43 | 73.4 |
| 39* | 98.5 |
| Mean ± SD | 50.6 ± 9.4 |
Patient ID . | Percent of CXCR-4 on CD34+ . |
|---|---|
| Engrafters | |
| 9 | 2.2 |
| 29* | 4.1 |
| 26* | 19.1 |
| 8 | 21.5 |
| 27 | 22.2 |
| 28 | 24.5 |
| 10 | 63.3 |
| 24* | 80.4 |
| 5 | 95.3 |
| 20* | 96.1 |
| 14 | 98.9 |
| Mean ± SD | 48.0 ± 39.0 |
| Nonengrafters | |
| 41 | 4.1 |
| 45* | 8.4 |
| 34* | 22.5 |
| 35 | 34.9 |
| 42 | 54.2 |
| 47 | 65 |
| 44 | 69.5 |
| 46* | 75.9 |
| 43 | 73.4 |
| 39* | 98.5 |
| Mean ± SD | 50.6 ± 9.4 |
AML cells from 21 different patients were labeled with antibodies to CD34 and CXCR-4. CXCR-4 expression is displayed as a percentage of CD34+ cells. There did not seem to be a difference in CXCR-4 expression levels between AML cases capable of NOD/SCID engraftment and those not able to do so. Of note, the highest CXCR-4 expression was observed in a nonengrafter and the lowest was seen in an engrafting AML sample.
Samples tested for intracellular calcium release from CD34+ cells when stimulated with 100 ng/mL SDF-1 as described in “Calcium flux measurement.” Student paired t test: 1.0
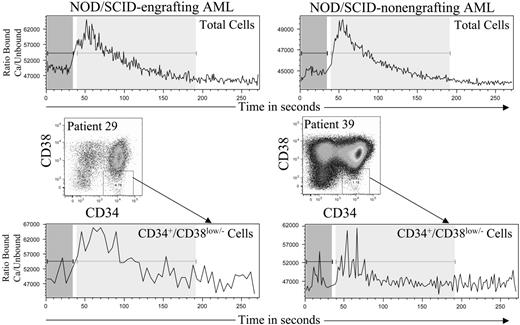
To investigate whether this detected CXCR-4 was functional, certain AML samples (Table 2) were stimulated with SDF-1 and analyzed for intracellular calcium release. All samples analyzed released significant amounts of calcium from CD34+ cells upon SDF-1 stimulation. Indeed, there was no statistically significant difference in the amount or speed of calcium release between AML samples capable of NOD/SCID engraftment and those not capable (Figure 4).
We then looked directly at the homing of PKH26-labeled AML cells to the marrows of NOD/SCID mice. Six million PKH26-positive cells from 3 NOD/SCID engrafting and 4 nonengrafting AML cases were injected into NOD/SCID mice. Sixteen hours later, murine marrows were assessed via flow cytometry for PKH26 bright cells. There was no significant difference (P = .83) between engrafting groups in the proportion of labeled cells injected that homed to the marrow (data not shown; also confirmed in B2m-/- NOD/SCID with patients 36 and 37). In addition, for one sample (a B2m-/- NOD/SCID-only engrafter, patient 37), the percentage of PKH26 bright cells that homed to the marrow was identical in B2m-/- NOD/SCID and NOD/SCID, indicating that although they are not capable of 6-week engraftment, cells still home to the NOD/SCID marrow. To confirm this result, we repeated the engraftment assessment of 4 previously nonengrafting samples but injected the cells directly into the bone marrow as previously described.26 In all 4 patients (46, 38, 43, and 35) no AML engraftment was observed. When combined, these data suggest that the SL-ICs from nonengrafting AML samples home normally to the marrow and that a lack of NOD/SCID engraftment is not due to an obvious homing defect.
Most cases of nonengrafting AML do not engraft in the B2m-/- NOD/SCID model. Ten million mononuclear cells from 23 different AML patients were injected into both NOD/SCID ( ) and B2m-/- NOD/SCID (▪) mice in paired experiments. Six weeks later, bone marrow engraftment was assessed via flow cytometry. AML engraftment was recorded if human CD33+/CD45+ myeloid cells were present without an accompanying CD19+/CD45+ B-cell population. Ten of 12 AML cases that failed to engraft in the NOD/SCID assay did not engraft in the B2m-/- NOD/SCID model.
) and B2m-/- NOD/SCID (▪) mice in paired experiments. Six weeks later, bone marrow engraftment was assessed via flow cytometry. AML engraftment was recorded if human CD33+/CD45+ myeloid cells were present without an accompanying CD19+/CD45+ B-cell population. Ten of 12 AML cases that failed to engraft in the NOD/SCID assay did not engraft in the B2m-/- NOD/SCID model.
Most cases of nonengrafting AML do not engraft in the B2m-/- NOD/SCID model. Ten million mononuclear cells from 23 different AML patients were injected into both NOD/SCID ( ) and B2m-/- NOD/SCID (▪) mice in paired experiments. Six weeks later, bone marrow engraftment was assessed via flow cytometry. AML engraftment was recorded if human CD33+/CD45+ myeloid cells were present without an accompanying CD19+/CD45+ B-cell population. Ten of 12 AML cases that failed to engraft in the NOD/SCID assay did not engraft in the B2m-/- NOD/SCID model.
) and B2m-/- NOD/SCID (▪) mice in paired experiments. Six weeks later, bone marrow engraftment was assessed via flow cytometry. AML engraftment was recorded if human CD33+/CD45+ myeloid cells were present without an accompanying CD19+/CD45+ B-cell population. Ten of 12 AML cases that failed to engraft in the NOD/SCID assay did not engraft in the B2m-/- NOD/SCID model.
Engraftment in NOD/SCID assay does not correlate with white blood cell count
The level of engraftment in NOD/SCID mice is thought to correlate with white blood cell count (WBC) within AML samples capable of NOD/SCID engraftment.27 No study to date has investigated the relationship between the actual ability to engraft and the presentation WBC count. The median WBC in our study was very similar for cases capable and incapable of NOD/SCID engraftment (39.8 × 109/L and 44.5 × 109/L, respectively) and, hence, no statistically significant difference could be detected.
Calcium flux in cells from both NOD/SCID engrafting and nonengrafting AML cases. Samples were labeled with Indo-1 dye as described in “Calcium flux measurement.” The ratio of fluorescence due to dye bound to calcium over fluorescence due to unbound dye is displayed against time. Data were collected for 30 seconds, before addition of 100 ng/mL of SDF-1 and further analysis. All samples analyzed produced detectable intracellular calcium upon SDF-1 stimulation and no differences could be detected between NOD/SCID engrafting and nonengrafting AML cases.
Calcium flux in cells from both NOD/SCID engrafting and nonengrafting AML cases. Samples were labeled with Indo-1 dye as described in “Calcium flux measurement.” The ratio of fluorescence due to dye bound to calcium over fluorescence due to unbound dye is displayed against time. Data were collected for 30 seconds, before addition of 100 ng/mL of SDF-1 and further analysis. All samples analyzed produced detectable intracellular calcium upon SDF-1 stimulation and no differences could be detected between NOD/SCID engrafting and nonengrafting AML cases.
Engraftment in NOD/SCID assay correlates with karyotypically defined prognostic group
The samples we describe here represent a broad spectrum of AML cases, including de novo and therapy-related leukemia (tAML) from French-American-British (FAB) groups 0, 1, 2, 3, 4, and 5. We excluded 8 patients from karyotypic analysis, as 5 were relapse samples, 2 as the karyotype failed at diagnosis, and 1 in which the karyotype was not performed. All of the remaining poor-prognosis patients we analyzed engrafted in the NOD/SCID assay (5/5), whereas none of the previously untreated good-prognosis patients did (0/11). Of the intermediate-risk de novo patients we analyzed, 50% (18/36) engrafted. Using logistic regression analysis, the only factor that was significantly associated with engraftment was the karyotypically defined prognosis group reported in 1998 by Grimwade et al10 (see Table 3 for a summary; WBC P = .85; FAB group P = .302; risk group P < .001).
De novo AML patients were organized into poor-, intermediate-, and good-prognosis risk groups according to karyotype definition
. | Cytogenetics prognosis group . | . | . | ||
|---|---|---|---|---|---|
| Capacity for engraftment . | Poor . | Intermediate . | Good . | ||
| Yes, no. patients | 5 | 18 | 0 | ||
| No, no. patients | 0 | 18 | 11 | ||
. | Cytogenetics prognosis group . | . | . | ||
|---|---|---|---|---|---|
| Capacity for engraftment . | Poor . | Intermediate . | Good . | ||
| Yes, no. patients | 5 | 18 | 0 | ||
| No, no. patients | 0 | 18 | 11 | ||
Four patients (nos. 7, 9, 10, 2) were excluded due to relapse, data were not collected for one patient (no. 22), and 2 karyotypes (nos. 4 and 5) failed at diagnosis. Engraftment correlates with poor prognosis; conversely, all favorable prognosis patients did not engraft.
There is no absolute correlation between 2 frequent mutations and NOD/SCID engraftment
To examine these 2 groups of AML cases further, we examined 2 genes that are frequently mutated in AML: FLT3 and nucleophosmin. We detected the FLT3-ITD mutation in 6 (of 29 tested) of our AML samples, a proportion similar to the 18% that was previously published.28 Four of these FLT3-ITD samples were capable of NOD/SCID engraftment and 2 were not (Table 1 †).
Nucleophosmin (NPM) is a nucleocytoplasmic-shuttling protein. Approximately one third of normal karyotype AML cases (35%) have an abnormality in the C-terminus of the protein that causes it to be present in the cytoplasm of affected cells rather than restricted to the nucleus.29 Although gene array analysis has revealed an up-regulation of genes associated with stem cell function,30 the NOD/SCID engraftment potential of this group remains to be determined. The most interesting aspect of abnormal nucleophosmin expression is that a subset of normal karyotype AML cases are identified. We tested 10 of our normal karyotype AML samples for the presence of the altered NPM gene. We found a higher proportion (7/10) of samples than has been previously reported (35%) containing the altered gene but this may be explained by our small number of samples (Table 1 ‡). Consistent with previous reports, we did observe the coincidence of 3 of 7 of our NPM mutations with FLT3-ITD (Table 1 †‡).30 Within the 10 patients analyzed, we cannot report a definite correlation with NOD/SCID engraftment (3/7 engraft in NOD/SCID mice).
Follow-up analysis confirms the relationship between NOD/SCID engraftment and disease behavior
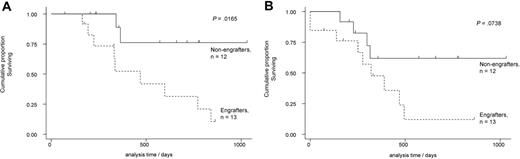
The majority of patients with AML have an intermediate-risk karyotype. Within this group are patients with AML that is refractory to treatment and who actually have a poor prognosis.10 To examine if NOD/SCID engraftment could provide prognostic information, we prospectively screened 25 consecutive samples from younger (< 60 years) patients with de novo intermediate-risk AML who underwent intensive chemotherapy. Four patients underwent an allograft in first remission and these were censored at the time of allograft. As presented in Figure 5, overall survival was significantly reduced in AML capable of NOD/SCID engraftment when compared with AML cases that were not capable of NOD/SCID engraftment. Indeed, the 2-year actuarial overall survival of younger patients (< 60 years) with intermediate-risk AML treated with intensive chemotherapy was 31% (95% confidence interval [CI] 8%-59%) and 76% (95% CI 33%-94%) for engrafting and nonengrafting AMLs, respectively (P = .02). The 2-year actuarial EFS of younger patients (< 60 years) with intermediate-risk AML treated with intensive chemotherapy was 12% (95% CI 1%-40%) and 62% (95% CI 27%-84%) for engrafting and nonengrafting AMLs, respectively (P = .07).
Discussion
This work describes the assessment of primary human AML in NOD/SCID and B2m-/- NOD/SCID mice. Via comparison to diagnostic smears, FISH, and gene expression analysis, we confirmed that both the NOD/SCID and B2m-/- NOD/SCID assays reproduce the same AML as in the original patient. Although it has been reported that the phenotype of 10 of 16 AMLs changes during engraftment, this was at a different time point of engraftment and hence may have represented cells derived from less primitive cells than those in our study.4,27
Overall survival and EFS of NOD/SCID engrafting and nonengrafting AML samples. (A) Overall survival data of NOD/SCID engrafting and nonengrafting AML samples. The overall and event-free survival data of 25 de novo intermediate-risk AML cases (< 60 years old) that received intensive multi-agent chemotherapy. Four cases were censored at allograft in first complete remission (2 in each group). NOD/SCID engrafting AML cases had a poor overall survival that was statistically lower than NOD/SCID nonengrafting AML cases. (B) Event-free data of NOD/SCID engrafting and nonengrafting AML samples. The event-free survival data of 25 de novo intermediate-risk AML cases (< 60 years old) that received intensive multi-agent chemotherapy. Four cases were censored at allograft in first complete remission (2 patients in each group). NOD/SCID engrafting AML cases had a poor event-free survival when compared with nonengrafting AML cases, though this did not reach statistical significance.
Overall survival and EFS of NOD/SCID engrafting and nonengrafting AML samples. (A) Overall survival data of NOD/SCID engrafting and nonengrafting AML samples. The overall and event-free survival data of 25 de novo intermediate-risk AML cases (< 60 years old) that received intensive multi-agent chemotherapy. Four cases were censored at allograft in first complete remission (2 in each group). NOD/SCID engrafting AML cases had a poor overall survival that was statistically lower than NOD/SCID nonengrafting AML cases. (B) Event-free data of NOD/SCID engrafting and nonengrafting AML samples. The event-free survival data of 25 de novo intermediate-risk AML cases (< 60 years old) that received intensive multi-agent chemotherapy. Four cases were censored at allograft in first complete remission (2 patients in each group). NOD/SCID engrafting AML cases had a poor event-free survival when compared with nonengrafting AML cases, though this did not reach statistical significance.
Contrary to previous studies that had investigated variables that affect the level of AML engraftment,11,27,28 here, we studied the factors that are associated with whether or not individual AML cases engraft in the NOD/SCID model. We report here that approximately 50% of AML cases examined produced leukemic engraftment.
Since our results suggest that the inability of certain AMLs to engraft in the NOD/SCID model is not due to AML SL-IC frequency, immune rejection, or tissue source, we progressed to examine the effect of homing. As mentioned in “Introduction,” the interaction between CXCR-4 and SDF-1 plays a major role in hematopoietic cell homing in NOD/SCID mice.17 We investigate here whether CXCR-4 expression and function was the same between NOD/SCID engrafting and nonengrafting AML cases. A recent study reports that within engrafting AML samples, homing to the marrow may be inhibited by anti-CXCR-4 antibodies.21 The examination of the mean percentage of CXCR-4 expression on CD34+ cells in our study is consistent with published values.27 There was no statistically significant difference in CXCR-4 expression or in calcium release upon SDF-1 stimulation between NOD/SCID engrafting and nonengrafting AML samples. Hence, when the process of homing is circumvented completely (direct BM injection), engraftment still cannot be achieved with previously nonengrafting AML samples, indicating that a homing defect is not the reason for the incapacity of some AML samples to engraft in NOD/SCID mice. Since our results suggest that the reason that some AML samples do not engraft is independent of AML SL-IC frequency, CXCR-4 expression/homing, or tissue source, we then tested for other potential correlations.
NOD/SCID engraftment correlated statistically with the karyotypically defined prognosis groups described by Grimwade et al in 1998.10 This is consistent with suggestions postulated by other authors working with AML and the NOD/SCID assay, but we can now confirm this association with a larger sample of consecutive previously untreated AML patients that were screened prospectively.16,31 For instance, Monaco et al16 included both treated and untreated patients as well as patients with variable-risk stratification in their follow-up data, whereas we studied a more homogenous group of patients that were younger than 60 years old with intermediate-risk karyotype that had not been previously treated.
This karyotypic assessment of leukemic cells is the most widely used and powerful prognostic factor in AML. Although cytogenetic analysis allows the definition of the hierarchical groups with favorable, intermediate, and poor prognosis, the intermediate-risk group contains patients with variable outcomes.10 Assessing the prognosis of this large group of patients is currently difficult. However, in this study, intermediate-risk AML cases that engrafted in the NOD/SCID assay had a poorer overall survival that was statistically significant when compared with AML cases that were incapable of NOD/SCID engraftment. Hence, the NOD/SCID assay may be used to identify poor-risk AML cases and, in conjunction with array technology, may be a useful tool to identify other pathogenic but subtle abnormalities within the intermediate-risk AML group.
Although many factors have been identified that affect the engraftment of hematopoietic cells in NOD/SCID mice, the most important factors may be the injected cells' self-renewal, proliferation, and differentiation potentials. Cells that have limited potentials (such as CD34+/CD38+ cells) cannot engraft at 6 weeks in the NOD/SCID model, whereas more primitive cells with greater cell potential (CD34+/CD38low/-) can still produce engraftment at 6 weeks.1 Leukemic engraftment in the NOD/SCID model also discriminates between cells with a primitive (CD34+/CD38low/-) and mature (CD34+/CD38+) phenotype, presumably due to the same intrinsic cellular factors.6
It is extremely interesting to note that although the NOD/SCID model assesses AML independent of the response to chemotherapy, engraftment still correlates with the response to this treatment (prognosis group). A possible explanation is that NOD/SCID engraftment reflects the stem cell nature of each individual AML case. AML cases that engraft in the NOD/SCID assay at 6 weeks may represent diseases driven by potent leukemia-initiating cells with stem cell-like self-renewal and proliferation abilities, whereas nonengrafting AML cases may involve less potent leukemia-initiating cells with more restricted progenitor-type self-renewal and proliferation abilities.
Our data may give clues as to the cellular origin of the transformation event in individual AML cases. It is possible that AML cases that engraft in the NOD/SCID assay (and have a poor prognosis) are derived from a transformation in a hematopoietic stem cell, whereas AML cases that do not engraft in the NOD/SCID assay (and have a more favorable prognosis) are derived from a progenitor-type cell. The AML-initiating cell in cases derived from normal stem cells may well inherit other biologic properties that confer an increased chemoresistance when compared with AML cases in which the initiating cell is derived from a more progenitor-type cell. Specifically, AML cases derived from normal stem cells would presumably have an increased ability to efflux and inactivate chemotherapy agents due to an increased expression of various pumps and detoxification enzymes. Further studies examining this hypothesis are currently underway.
In conclusion, engraftment of AML in the NOD/SCID assay seems to be dependent on an inherent ability of the cells, which correlates well with disease prognosis.
Prepublished online as Blood First Edition Paper, October 18, 2005; DOI 10.1182/blood-2005-06-2325.
Supported by Cancer Research UK and a National Institutes of Health grant no. HL-64856-03 (D.B.).
D.J.P. and D.T. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the patients for providing samples and Dr J Amess for providing diagnostic data. We also thank Derek Davies, Gary Warnes, Ayad Eddaoudi, and Kirsty Allen of the FACS Lab at Cancer Research UK for their invaluable expertise. This work would not have been possible without Julie Bee, Clare Millum, and Ella Smallcombe of our Biological Resource Unit. Mathew Smith kindly performed mutation analysis. We also thank Spyros Skoulakis for his statistical analysis of the patients' follow-up data. We thank Debra Lillington and her group for performing the cytogenetic analysis.

![Figure 1. Confirmation of AML cell growth in NOD/SCID mice. Ten million cells were injected into NOD/SCID mice and marrows were analyzed for human myeloid cell content 6 weeks later. (A) Diagnostic peripheral blood smear from AML-M2 patient 10. Cells were stained with hematoxylin and eosin (H&E) before analysis at 400 × magnification via a 40 ×/0.75 objective lens. (B) Murine marrow that was injected with cells from the same AML-M2 patient as in panel A. Myeloblasts, featuring Auer rods (arrow), are present, indicating AML. Cells were stained with H&E before analysis at 1000 × magnification via a 100 ×/1.3 objective lens. (C) Dual fusion, dual-color fluorescent in situ hybridization of a relapsed t[8,21] AML-M2 sample. Cells positive for the rearrangement exhibit 1 green, 1 red, and 2 orange spots. (D-F) Examples of NOD/SCID engrafted, FACSorted CD33+/CD45+ cells exhibiting AML-M2 t[8,21] rearrangement. Pictures of blood smears were taken on a Leica DFC 300F camera.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-06-2325/4/m_zh80030690030001.jpeg?Expires=1763486622&Signature=45TjJuMZ3cHB9LC3BcCQxJKtzYPaLyMs8w30bmp5qkDCu-Eh3UVxmjSSP0yg0Jz50JqbuOxiqea~KwpGDmM9FCgO-G4gE5oTB93HM9BMfQoBVTc3zoD0JYR30CTFCy19Yds90p84bHpp2FoftAUwGevFNL5V4ASE~ENRYOfsXlts0kEaPHOFuZEVVGroVOpzCRGxC-A5Rhy6rWXSxIqNA0K7Z1qoylVpabSpMan-Vzuo5WPsG9xH1GTWq9TWnAv-ww4Xt~ffRUyUCvbFDUM2zNT7A71FsxRys0Rw5M0SVsn4odBFGWohbLCoEpJVWwe~vgpIbUAFcqmewUNzG5LJcg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal