Abstract
Extensive cardiac amyloid deposition in systemic AL amyloidosis is associated with a grave prognosis. Heart transplantation is rarely performed because of the systemic and progressive nature of the disease. Patients with severe cardiac amyloid infiltration are ineligible for the preferred treatment of melphalan chemotherapy with stem cell transplantation (SCT) rescue because of the high risk for treatment-related mortality. Heart transplantation followed by SCT was performed in 5 patients with AL amyloidosis and predominant cardiomyopathy. Patients were followed up for a median of 95 months (range, 37-118 months) from diagnosis. At censor, 3 of 5 patients were well without evidence of intracardiac or extracardiac amyloid accumulation, and median overall survival by Kaplan-Meier estimate was not reached. Two patients died of progressive amyloidosis 33 and 90 months after heart transplantation after relapse of their underlying plasma cell dyscrasia. Heart transplantation followed by SCT is feasible in selected patients with cardiac AL amyloidosis and may confer substantial survival benefit.
Introduction
Systemic AL amyloidosis is a severe progressive disease with a median survival time from diagnosis of 24 to 36 months.1 Symptomatic cardiac involvement in AL amyloidosis is associated with a particularly poor prognosis, typically 9 months.2-4 Treatment consists of chemotherapy aimed at suppressing the underlying monoclonal B-cell dyscrasia.5
Only 20% to 30% of patients respond to oral melphalan and prednisolone.3,6 Response rates and overall survival are improved with high-dose melphalan and autologous stem cell transplantation (SCT), although this therapy incurs substantial treatment-related mortality (TRM).7-10 Cardiac amyloidosis is an important risk factor for TRM,9 and patients with advanced cardiomyopathy are ineligible for SCT.11
Heart transplantation in patients with AL amyloidosis, first reported in 1988,12 remains controversial because amyloid deposition can be expected to recur in the graft and to accumulate in other organs with fatal consequences within 3 to 5 years.13
We present here the outcome of heart transplantation followed by SCT in 5 patients with systemic AL amyloidosis who were evaluated at the United Kingdom National Amyloidosis Centre (NAC), highlighting the feasibility of this approach in selected patients with systemic amyloidosis and predominant cardiomyopathy.
Study design
Between 1992 and 2005, 5 patients evaluated at the NAC with advanced AL amyloid cardiomyopathy and limited extracardiac amyloid were selected to undergo cardiac transplantation followed by SCT.
The diagnosis of AL amyloidosis was established histologically and was supported by the demonstration of a plasma cell dyscrasia in each patient. The extent and course of extracardiac visceral organ involvement by amyloid was evaluated by serial whole body iodine 123 (123I)-labeled serum amyloid P (SAP) component scintigraphy.14-16
Evaluation, performed every 6 to 12 months at the NAC, included clinical assessment, electrocardiography, echocardiography, bone marrow aspirate and trephine, and full biochemical analysis of serum and urine. After 2003, serum-free light chain (FLC) concentration was determined in all patients and, where necessary, was performed retrospectively on stored sera.17 The novel medical care described here was performed with informed consent from each patient in accordance with the Declaration of Helsinki.
Results and discussion
Patients
Patient characteristics at presentation are shown in Table 1. A substantial monoclonal FLC excess was present in each patient, but no patient had evidence of lytic bone lesions.
Patient characteristics at presentation and during heart transplantation
. | Age at diagnosis of amyloid, y . | Time from amyloid diagnosis to heart transplantation, mo . | ECHO IVS LVPW thickness, mm . | Extracardiac amyloid by SAP scan, total load/organs . | Serum creatinine, μmol/L (proteinuria, d) . | Plasma cell dyscrasia . | . | . | . | . | No. rejection episodes (time after transplantation, wk) . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | . | . | . | . | . | PCs, % . | FLC(κ),*mg/L . | FLC(λ),*mg/L . | κ/λ,*ratio . | LOS after transplantation, d . | . | ISHLT rejection grade . | |||
| 1 | 56 | 4 | 18/17 | Small/spleen, kidneys | 97/0.1 | 7 | 349 | 44.3 | 7.9 | 14 | 2 (8, 20) | 2, 3A | |||
| 2† | 59 | 9 | 19/17 | Small/spleen, kidneys | 89/0.17 | 7 | 5.1 | 129 | 0.04 | 22 | 1 (25) | 3A | |||
| 3 | 44 | 2 | 17/18 | Small/none | 108/0.6 | 7 | 4.0 | 342 | 0.01 | 24 | 1 (5) | 3A | |||
| 4 | 38 | 4 | 15/15 | Small/spleen | 113/0.3 | 15 | 1.4 | 479 | 0.00 | 28 | 0 | – | |||
| 5 | 53 | 8 | 17/16 | Small/spleen, kidneys | 119/0.6 | <5 | 1040 | 23.8 | 43.7 | 30 | 0 | – | |||
. | Age at diagnosis of amyloid, y . | Time from amyloid diagnosis to heart transplantation, mo . | ECHO IVS LVPW thickness, mm . | Extracardiac amyloid by SAP scan, total load/organs . | Serum creatinine, μmol/L (proteinuria, d) . | Plasma cell dyscrasia . | . | . | . | . | No. rejection episodes (time after transplantation, wk) . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | . | . | . | . | . | PCs, % . | FLC(κ),*mg/L . | FLC(λ),*mg/L . | κ/λ,*ratio . | LOS after transplantation, d . | . | ISHLT rejection grade . | |||
| 1 | 56 | 4 | 18/17 | Small/spleen, kidneys | 97/0.1 | 7 | 349 | 44.3 | 7.9 | 14 | 2 (8, 20) | 2, 3A | |||
| 2† | 59 | 9 | 19/17 | Small/spleen, kidneys | 89/0.17 | 7 | 5.1 | 129 | 0.04 | 22 | 1 (25) | 3A | |||
| 3 | 44 | 2 | 17/18 | Small/none | 108/0.6 | 7 | 4.0 | 342 | 0.01 | 24 | 1 (5) | 3A | |||
| 4 | 38 | 4 | 15/15 | Small/spleen | 113/0.3 | 15 | 1.4 | 479 | 0.00 | 28 | 0 | – | |||
| 5 | 53 | 8 | 17/16 | Small/spleen, kidneys | 119/0.6 | <5 | 1040 | 23.8 | 43.7 | 30 | 0 | – | |||
All patients were male and had NYHA (New York Heart Association) class IV heart failure. ECHO indicates echocardiography; IVS, intraventricular septum; LVPW, left ventricular posterior wall; PCs, plasma cells in bone marrow; LOS, length of stay in the hospital; ISHLT, International Society of Heart and Lung Transplantation; and –, not applicable.
Normal ranges are as follows: FLC(K), 3.3-19.4 mg/L; FLC(λ), 5.7-26.3 mg/L; and k/λ ratio, 0.26-1.65
Patient 2 received cyclical intravenous melphalan and oral dexamethasone before heart transplantation (see “Patients”)
One patient (patient 2) received chemotherapy before heart transplantation consisting of three 28-day cycles of 25 mg/m2 intravenous melphalan on day 1 and oral dexamethasone 20 mg daily on days 1 to 4.
Heart transplantation
Heart transplantation, performed in 4 different centers, was uncomplicated in 3 patients; one patient had a tonic-clonic convulsion, and another had a hemorrhage that precipitated transient acute renal failure. Initial immunosuppression therapy consisted of cyclosporin A, azathioprine, and prednisolone in 4 patients and included antithymocyte globulin in 5 patients. All acute rejection episodes responded to intravenous methylprednisolone (Table 1).
Chemotherapy and stem cell transplantation
The median time from heart transplantation to SCT was 13 months (range, 10-24 months), during which SAP scintigraphy demonstrated accumulation of extracardiac amyloid in each patient, but there was no evidence of amyloid in the cardiac allografts. Patient 1 received 6 cycles of oral melphalan (total dose, 504 mg) and prednisolone before SCT, which, on retrospective FLC testing, was ineffective.
Azathioprine was substituted by an increase in prednisolone dosage for stem cell harvesting and the duration of SCT. Stem cells were mobilized for apheresis collection with G-CSF alone in 4 patients and with cyclophosphamide and G-CSF in the remaining patient. Conditioning consisted of melphalan at a median dose of 140 mg/m2 (range, 140-200 mg/m2), and engraftment occurred successfully in all 5 patients. SCT was uncomplicated in 3 patients, but fungal pneumonia and reversible dialysis-dependent acute renal failure, respectively, developed in 2 patients; however, TRM did not occur.
Response to chemotherapy and stem cell transplantation
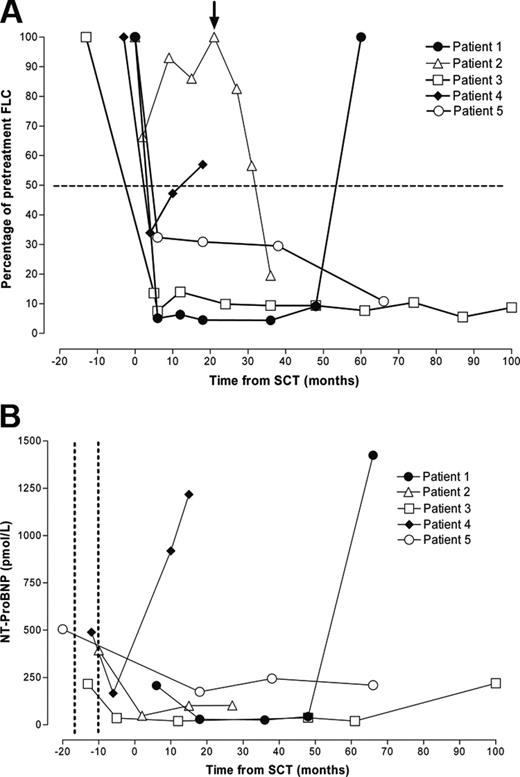
SCT resulted in complete normalization of FLCs in patient 3 and an approximately 90% response in patient 5 (Figure 1A), each of which was sustained at the time of censor. Today, more than 9 years after heart transplantation, neither patient has clinical, biochemical, or echocardiographic evidence of cardiac graft amyloid or extracardiac amyloid deposition. Furthermore, amyloid has not been detected histologically during routine annual posttransplantation cardiac biopsy of either patient.
Patient 2 had only a minor FLC response and experienced relapse within 10 months of SCT but had a substantial and sustained FLC response to subsequent high-dose dexamethasone (40 mg daily, days 1-4) (Figure 1A). At the time of censor, he was clinically well and had no evidence of amyloid in the cardiac allograft.
In 2 patients, the plasma cell dyscrasia relapsed after an initial response to SCT. Patient 1 was clinically well until relapse, 56 months after SCT, whereupon his general condition deteriorated rapidly. In patient 4, a transient partial response to SCT was followed by a progressive increase in the aberrant FLC and ultimately by fatal amyloid accumulation in the liver, spleen, and cardiac allograft. In each patient, relapse of the plasma cell dyscrasia was accompanied by development of characteristic amyloid cardiomyopathy on echocardiography and a substantial increase in serum NT-ProBNP (Figure 1B).
Serial serum FLC and NT-ProBNP concentration. Each line represents a single patient. Open symbols represent patients alive at censor; filled symbols, patients who had died. (A) Response of the FLC concentration to chemotherapy. FLC concentration decreased after SCT to less than 50% of pretreatment levels in 4 patients. Patient 3 experienced a decrease in FLC concentration after heart transplantation and before SCT that was further consolidated by SCT. Patient 2 had a poor initial FLC response to SCT and then had a relapse but responded to high-dose dexamethasone therapy (arrow). Patients 1 and 4 experienced FLC relapse associated with progressive intracardiac and extracardiac amyloid accumulation and died. (B) Serial serum NT-proBNP concentration decreased after heart transplantation (timing indicated between the dotted vertical lines) in the 4 patients in whom it was measured. Relapse of the plasma cell dyscrasia and accumulation of amyloid in the cardiac allograft and major viscera in 2 patients were associated with a marked increase in NT-ProBNP concentration and patient death.
Serial serum FLC and NT-ProBNP concentration. Each line represents a single patient. Open symbols represent patients alive at censor; filled symbols, patients who had died. (A) Response of the FLC concentration to chemotherapy. FLC concentration decreased after SCT to less than 50% of pretreatment levels in 4 patients. Patient 3 experienced a decrease in FLC concentration after heart transplantation and before SCT that was further consolidated by SCT. Patient 2 had a poor initial FLC response to SCT and then had a relapse but responded to high-dose dexamethasone therapy (arrow). Patients 1 and 4 experienced FLC relapse associated with progressive intracardiac and extracardiac amyloid accumulation and died. (B) Serial serum NT-proBNP concentration decreased after heart transplantation (timing indicated between the dotted vertical lines) in the 4 patients in whom it was measured. Relapse of the plasma cell dyscrasia and accumulation of amyloid in the cardiac allograft and major viscera in 2 patients were associated with a marked increase in NT-ProBNP concentration and patient death.
Survival
Despite a median 95-month (range, 37-118 months) follow-up from diagnosis, median overall survival by Kaplan-Meier estimate was not reached at censor; the projected mean confidence interval patient survival times from diagnosis, heart transplantation, and SCT were 95.5 months (range, 68-123 months), 91.1 months (range, 64-118 months), and 76.4 months (range, 47-105 months), respectively. Patients 1 and 4 died of progressive amyloidosis after relapse of the underlying plasma cell dyscrasia 95 and 37 months after diagnosis of amyloid and 90 and 33 months after heart transplantation, respectively.
This is the first reported series of heart transplantation followed by autologous peripheral blood SCT for systemic AL amyloidosis, and it illustrates the feasibility and potential for prolonged survival in selected patients with predominant cardiomyopathy.
Heart transplantation in AL amyloidosis is controversial because of the systemic and progressive nature of the disease.13,18 Five-year survival rates after heart transplantation were recently reported as 38% in amyloidosis and 67% in all other conditions.18 A previous series reported a 4-year patient survival rate of 39% and the development of allograft amyloid in 4 of 9 recipients.19 However, 3 of 5 (60%) patients in the present series, who had sustained suppression of clonal disease after chemotherapy, had normal performance status and no evidence of cardiac or extracardiac amyloid accumulation at the time of censor (64, 116, and 118 months after diagnosis of amyloidosis).
All 5 patients reported here were carefully selected for the absence of clinically significant extracardiac amyloid, and heart transplantation was therefore expected to restore normal functional status, thereby permitting SCT to be performed afterward with relatively little risk for TRM.11
The degree to which monoclonal light chain production must be suppressed to prevent amyloid deposition in transplanted hearts is unknown, though the lack of amyloid in the allografts of the 3 surviving patients in this series suggests that an 80% reduction in the aberrant serum FLC concentration is sufficient. The 2 patients who died, 90 and 33 months after heart transplantation, did so after relapse of the clonal disease accompanied by an accumulation of amyloid in the cardiac allograft and elsewhere.
Although our experience demonstrates the feasibility and substantial survival benefit of sequential heart transplantation and SCT in AL amyloidosis, only a small minority of patients with severe cardiac involvement are likely to benefit from this tandem approach. To date, these 5 patients are the only ones among more than 2500 patients with AL amyloidosis attending the NAC in the past 10 years who have been considered eligible, by all their attending medical and surgical specialists, for this tandem approach.
Prepublished online as Blood First Edition Paper, October 6, 2005; DOI 10.1182/blood-2005-08-3253.
Supported by Medical Research Council (MRC) Programme Grant G97900510 (M.B.P., P.N.H.), Wolfson Foundation, University College London (UCL) Amyloidosis Research Fund, and NHS Research and Development Funds.
J.D.G. performed research, analyzed the data, and wrote the paper. H.J.G. performed research and analyzed the data. H.J.L. performed research. M.O. performed research. A.D.W. performed research and wrote the paper. J.J. performed research. M.B.P. designed the research and wrote the paper. P.N.H. designed and performed the research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the following physicians and surgeons who contributed to the care of these patients: Dr J. Parameshwar, Papworth Hospital, Cambridge, United Kingdom; Professor J.F. Apperley, Hammersmith Hospital, London, United Kingdom; Dr F. Serfontein, Sinoville, South Africa; Professor P. Jacobs, Cape Town, South Africa; Mr P.C. Braidley, Northern General Hospital, Sheffield, United Kingdom; Dr E.A. Vandenberghe, Royal Hallamshire Hospital, Sheffield, United Kingdom; Dr L.J. Olsen, Mayo Clinic, Rochester, MN; and Dr M.A. Gertz, Mayo Clinic, Rochester, MN.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal