Therapy for patients with chronic lymphocytic leukemia (CLL) has greatly changed over the past few years. After years of stagnation, with treatment revolving around the use of rather ineffective drugs such as alkylators, many patients are now being treated with more effective agents such as purine analogs either alone or combined with other drugs and/or monoclonal antibodies. Treatment of patients refractory to these treatments is particularly challenging and should be decided only upon a careful evaluation of the disease, patient characteristics, and prognostic factors. Refractory disease should be clearly separated from relapsing disease. The only curative therapy for patients with CLL, including those with refractory disease, is allogeneic stem cell transplantation. However, the use of allogeneic transplantation is limited because of the advanced age of most patients and the high transplant-related mortality (TRM). Transplants with nonmyeloablative regimens may reduce TRM and allow more patients to receive transplants more safely. For patients in whom an allogeneic transplantation is not feasible or in whom it is deemed inappropriate, participation in phase 2 trials should be encouraged. Finally, to investigate mechanisms to overcome resistance to therapy in CLL and to identify patients that might gain benefit from early, intensive therapies (eg, based on biologic markers) constitute a challenge that needs active investigation.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by the progressive accumulation of monoclonal peripheral (mature) CD5+ B cells in lymphoid tissues, bone marrow, and peripheral blood.1-3 The basic physiopathologic defect in CLL is the resistance of neoplastic cells to programmed cell death or apoptosis. CLL is the most frequent form of leukemia in Western countries and predominates in older people, the median age of patients at diagnosis being around 65 years.4 Median survival is about 10 years but it largely varies from one patient to another, from less than 3 years to a normal life expectancy. Treatment should therefore be individualized on the basis of the risk to each patient.5,6
For many decades, treatment of patients with CLL was based on the use of chlorambucil and other alkylating agents, which resulted in a complete response (CR) rate of less than 10% along with palliation of symptoms and only a modest, if any, impact on survival. Over the last few years, the management of CLL has greatly improved. The introduction of purine analogs and monoclonal antibodies has made possible combined treatments that result in CR rates of up to 60% to 70%; this translates into a longer progression-free interval.5-7
It is important to emphasize that no matter how exciting the results with new, experimental combination chemotherapy and other innovative treatment approaches are, the advantages of these strategies in terms of survival have not yet been demonstrated. In this regard, it is important to keep in mind that the ultimate goal of therapy is to prolong survival and improve quality of life. Consequently, the use of intensive regimens is only justified within clinical trials. However, after so many years of stagnation in treatment possibilities, it is understandable that physicians encourage their patients to participate in trials and also that well-informed patients are willing to participate in them. The downside of this approach is the important difficulties posed by the treatment of patients who do not respond to, or who relapse after, these treatments.
What is refractory CLL?
There is no widely accepted definition for refractory CLL. Refractory CLL is not defined in either the International Workshop on CLL (IWCLL) or in the National Cancer Institute (NCI)-CLL Working Group proposals for evaluating response to therapy.8,9 This is understandable since those guidelines were published when treatment of CLL was quite ineffective and significant and durable responses were not being obtained. But even in more recent guidelines, refractory CLL has not been defined.10-12 A reasonable definition of refractory CLL is either the lack of response to purine analogs or disease progression within 6 months of a partial response (PR) or CR to purine analog therapy.13-15 Likewise, patients progressing or relapsing shortly (eg, within 12 months) after stem-cell transplantation should be considered as having refractory disease. The prognosis of patients with refractory CLL is very poor, their median survival being measured in months.16,17
Refractory disease should be separated from progressing disease, the prognosis of the latter situation being much better. Unfortunately, in some studies these 2 situations are not separated, making it difficult to interpret the results obtained with salvage regimens.
Mechanisms of resistance
The great majority of cytotoxic drugs, like purine analogs and alkylators, act inducing DNA double-strand breaks, this leading to the activation of ATM and ATM-effector proteins like p53. Thus, the functional integrity of the p53 pathway is required to induce cell death.
Two types of p53 dysfunction have been demonstrated in CLL: one associated with TP53 mutations/deletions, and the other due to the inactivation of the p53-regulation machinery, mainly the ATM gene. Low levels of ATM protein and/or TP53 mutations are found in approximately 30% of patients with CLL at diagnosis. The presence of these molecular abnormalities has been associated with other adverse prognostic parameters, such as unmutated IgVH genes or high CD38 expression, and with a poor clinical outcome.18,19 More interestingly, deletions and/or mutations of TP53 are more commonly found in relapsed or refractory CLL patients, this being associated with resistance to alkylating agents and purine analogs alone or in combination with rituximab.20-23 Interestingly, the higher the percentage of cells with deletions of 17p, the greater the proportion of nonresponders.24 In contrast, alemtuzumab seems to be active in TP53-mutated cases, probably as a consequence that this drug induces cell death in a p53-independent manner.15,25
As per ATM, given the role of this gene as an activator of p53, it would seem that CLL patients with inactivation of ATM might also respond poorly to therapy with purine analogs. However, this hypothesis has not been fully supported.26 Recently, it has been suggested that ATM induces prosurvival responses in a p53-independent pathway. Thus, the more aggressive clinical course of CLL with mutated TP53 has been associated with both defective apoptosis and increased survival response related to the ATM protein integrity.27
The response to purine analogs and alkylators also correlates with a high ratio of Bcl-2 to Bax proteins and the expression of certain Bcl-2 family proteins (eg, Mcl-1).28 Recently, the overexpression of BCL2A1 an apoptosis-regulating gene, has been associated with resistance to therapy29 and, of greater concern, it has also been shown that treatment with fludarabine induces a TP53-dependent gene expression response, this raising the important question as to whether fludarabine may select TP53-mutant CLL cells.30
Diagnostic workup of patients with refractory CLL
A necessary first step when confronting a patient with refractory CLL is to make sure that the patient actually has CLL and not another chronic lymphoproliferative disorder that mimics CLL. In fact, some patients deemed to have refractory CLL turn out to have another disease, mantle-cell lymphoma in leukemic phase being among the most frequent. A careful examination of peripheral-blood smears and cytofluorometry is essential for an accurate diagnosis. The typical CLL immunophenotype is SmIgweak, CD5+, CD20weak, FMC7-, and CD23+. In some difficult cases, lymph node biopsy, cytogenetics, and molecular studies may be necessary to establish the correct diagnosis. The differential diagnosis of chronic lymphoproliferative disorders is beyond the scope of this paper, but we would like to emphasize that the diagnosis of atypical CLL should not be accepted without discarding other chronic lymphoproliferative disorders.
Another important point is to rule out disease transformation. In about 10% of patients, CLL undergoes transformation into a more aggressive tumor, most commonly diffuse large B-cell lymphoma (DLBCL), also known as Richter syndrome.31,32
Although fever, weight loss, night sweats, enlarged lymphadenopathy, increased lactate dehydrogenase (LDH) serum levels, anemia, hypercalcemia, thrombocytopenia, and monoclonal gammopathy are considered the most frequent and typical features of the disease, in many instances the clinical features are less obvious: for example increasing levels of serum LDH in a patient whose general clinical situation is deteriorating. The DLBCL arising in this context may be clonally related or unrelated to the preceding CLL. CLL transformation is associated with different genetic alterations including increased number of chromosomal alterations; losses of 8p, 9p, and 17p; homozygous deletions of p16; and mutations of TP53 gene.33 DLBCL associated with the presence of Epstein-Barr virus (EBV) in the transformed tumor cells has been observed in some patients. Usually, these tumors are not clonally related to the previous CLL. CLL can also transform into Hodgkin lymphoma. This transformation is usually also associated with EBV and may be clonally related or unrelated to the preceding CLL. Of note, EBV+ B cells and disease transformation have been associated with immunosuppression related to fludarabine and other therapies.34
The ultimate diagnosis of disease transformation relies on the biopsy of a lymph node or tissue showing DLBCL. However, disease transformation can be limited to a given territory and as a result the diagnosis may be missed if the lymph node biopsied is not involved. It has been suggested that Ga-67 scintingraphy might be useful to identify lymph nodes whose biopsy may lead to the diagnosis35 but this could not be corroborated in a study performed by our group (Cobo et al36 ).
Disease transformation has an extremely poor prognosis, the median survival being 6 months. We treat these patients with regimens active in aggressive lymphoma and if a response is obtained, the possibility of stem-cell transplantation is envisaged. In one study, the superiority of allogeneic stem cell transplantation over autologous transplantation and conventional chemotherapy in such a setting has been suggested.37
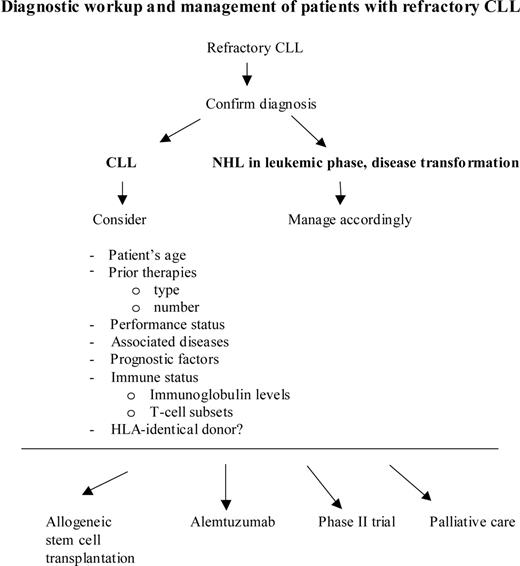
Once the diagnosis of refractory CLL has been established, the patient has to undergo a complete clinical and laboratory evaluation, as is done in newly diagnosed patients. Key parameters to consider are patient's age and performance status, associated diseases, prognostic factors, past treatments, bone marrow function, and, of great importance, the patient's own expectations (Figure 1).
Median age of patients at diagnosis is about 65 years; roughly one third of the patients are 60 to 70 years old, another third are older than 70 years, and the remaining third are younger than 60 years.4 Treatment strategies that are feasible in younger individuals can be more difficult (or impossible) to apply in older patients. Treatment of elderly patients with CLL is a real challenge and is clearly an area where specific investigation is required. However, when making treatment recommendations the biologic age rather than the chronologic age should be considered, and fit elderly patients should not be denied potentially effective treatments just because of their age.
Performance status and comorbidity also need careful consideration. In addition, the type and number of lines of therapies that the patient has received before becoming refractory to treatment should be considered. For instance, bone marrow function may be impaired as a result of prior therapy, this precluding or making further treatment with myelotoxic agents extremely hazardous.
Diagnostic workup and management of patients with refractory CLL. NHL indicates non-Hodgkin lymphoma.
Diagnostic workup and management of patients with refractory CLL. NHL indicates non-Hodgkin lymphoma.
On the other hand, a unique feature of CLL is the immune disturbances (eg, hypogammaglobulinemia, autoimmune cytopenias) that frequently form part of the disease; these abnormalities are more profound in patients with a long natural history who were extensively treated. Moreover, currently employed therapies may cause immunodepression, this also favoring the appearance of infections.
Finally, to ascertain whether an HLA (human leukocyte antigen)-identical bone marrow donor, either related or unrelated, is available is important to advise therapy. In this regard, it has recently been demonstrated that up to 13.5% of first-degree relatives of patients with CLL may harbor in their peripheral blood a monoclonal population with an immunophenotype that is identical to that found in CLL and that this may also occur in 3.5% of healthy individuals.38-40 Therefore, potential HLA-identical donors should be studied by cytofluorometry to discard such a possibility.
Treatment of patients with refractory CLL
It is important to separate refractory from relapsing disease. Whereas treatment of patients with relapsing disease, particularly when the progression-free interval has been long (eg, > 12 months), can be envisaged as in newly diagnosed patients, patients with truly refractory disease have an extremely poor prognosis and require immediate intervention. Unfortunately, however, in a significant proportion of patients (eg, those heavily pretreated, with important comorbidity and poor performance status) palliation of symptoms is the more reasonable approach.
The treatment to which a given patient is deemed to be “refractory” is very important. For example, resistance to chlorambucil or other alkylating agents at low/standard doses can sometimes be overcome by purine analogs. In a study comparing chlorambucil with fludarabine, 46% of 79 patients not responding to chlorambucil did respond to fludarabine, whereas only 7% of the 29 patients who were crossed over from fludarabine to chlorambucil had a response.41 In other studies, the response rate to fludarabine or fludarabine-based treatment in patients refractory to alkylating agents has been between 20% and 40%.6,13,16,42-45 As previously discussed, this emphasizes that treatment refractoriness is a concept that should only be applied to patients not responding to purine analogs.
When purine analogs were first introduced in the treatment of patients with CLL, the issue of whether or not these agents showed cross-resistance was a matter of extensive debate. The current notion is that cross-resistance does exist. However, pentostatin combined with cyclophosphamide has been reported to produce responses (77% including one CR) in a small series of 13 patients who did not respond to fludarabine-based therapy.14 It should be noted, however, that the median response duration in that study was inferior to 10 months and that the toxicity was high.
High-dose methylprednisolone given alone or combined with other drugs has been reported to produce responses in 77% of 25 heavily pretreated patients; responders included 5 of 10 patients with abnormal TP53, of whom 2 achieved nodular PR. Infections were frequent and responses short lived.46
An effective agent to treat patients refractory to purine analogs is the humanized anti-CD52 monoclonal antibody alemtuzumab (Table 1).47-58 Notably, alemtuzumab is effective in patients with TP53 abnormalities.15
Alemtuzumab in previously treated CLL
Study . | Treatment and regimen . | No. patients . | Patients refractory to fludarabine, % . | OR, % . | CR, % . | OR, % refractory . |
|---|---|---|---|---|---|---|
| Osterborg et al (1997)47 | Alemtuzumab 30 mg, 3× weekly intravenously, up to 12 w | 29 | — | 42 | 4 | — |
| Kennedy et al (2001)48 | Alemtuzumab 30 mg, 3× weekly intravenously until maximum response | 77 | 99 | 44 | 25 | 44 |
| Rai et al (2002)49 | Alemtuzumab 30 mg, 3× weekly intravenously, up to 16 w | 24 | 71* | 33 | 0 | 29 |
| Keating et al (2002)50 | Alemtuzumab 30 mg, 3× weekly intravenously, up to 12 w | 93 | 48* | 33 | 2 | 29 |
| Stilgenbauer et al (2004)51 | Alemtuzumab 30 mg, 3× weekly subcutaneously, up to 12 w | 50 | 100 | 36 | 2 | 36 |
| Cortelezzi et al (2005)52 | Alemtuzumab 10 mg, 3× weekly subcutaneously, up to 18 w | 16 | 88 | 50 | 25 | 50 |
| Moreton et al (2005)53 | Alemtuzumab 30 mg, 3× weekly intravenously until maximum response | 91 | 48* | 55 | 36 | 50 |
| Kennedy et al (2002)54 | Alemtuzumab 30 mg, 3× weekly intravenously; fludarabine 25 mg/m2, 3× monthly until maximum response | 6 | 100 | 83 | 16 | 83 |
| Nabhan et al (2004)55 | Alemtuzumab 3-30 mg, 3× weekly intravenously, up to 4 w; rituximab 375 mg/m2 weekly intravenously, up to 4 w | 12 | 100 | 8 | 0 | 8 |
| Faderl et al (2003)56 | Alemtuzumab 30 mg, 2× weekly intravenously, up to 4 w; rituximab 375 mg/m2 weekly intravenously, up to 4 w | 48† | 54 | 52 | 8 | — |
| Wierda et al (2004)57 | Cyclophosphamide 250 mg/m2 days 3-5 intravenously; fludarabine 25 mg/m2 days 3-5 intravenously; alemtuzumab 30 mg/m2 days 1, 3, 5 intravenously; rituximab 375-500 mg/m2 day 2, (monthly, 6 cycles) | 17 | 59 | 52 | 14 | — |
| Osuji et al (2005)58 | Alemtuzumab 30 mg, 3× weekly intravenously until maximum response | 24 | 62 | 54 | 18 | — |
Study . | Treatment and regimen . | No. patients . | Patients refractory to fludarabine, % . | OR, % . | CR, % . | OR, % refractory . |
|---|---|---|---|---|---|---|
| Osterborg et al (1997)47 | Alemtuzumab 30 mg, 3× weekly intravenously, up to 12 w | 29 | — | 42 | 4 | — |
| Kennedy et al (2001)48 | Alemtuzumab 30 mg, 3× weekly intravenously until maximum response | 77 | 99 | 44 | 25 | 44 |
| Rai et al (2002)49 | Alemtuzumab 30 mg, 3× weekly intravenously, up to 16 w | 24 | 71* | 33 | 0 | 29 |
| Keating et al (2002)50 | Alemtuzumab 30 mg, 3× weekly intravenously, up to 12 w | 93 | 48* | 33 | 2 | 29 |
| Stilgenbauer et al (2004)51 | Alemtuzumab 30 mg, 3× weekly subcutaneously, up to 12 w | 50 | 100 | 36 | 2 | 36 |
| Cortelezzi et al (2005)52 | Alemtuzumab 10 mg, 3× weekly subcutaneously, up to 18 w | 16 | 88 | 50 | 25 | 50 |
| Moreton et al (2005)53 | Alemtuzumab 30 mg, 3× weekly intravenously until maximum response | 91 | 48* | 55 | 36 | 50 |
| Kennedy et al (2002)54 | Alemtuzumab 30 mg, 3× weekly intravenously; fludarabine 25 mg/m2, 3× monthly until maximum response | 6 | 100 | 83 | 16 | 83 |
| Nabhan et al (2004)55 | Alemtuzumab 3-30 mg, 3× weekly intravenously, up to 4 w; rituximab 375 mg/m2 weekly intravenously, up to 4 w | 12 | 100 | 8 | 0 | 8 |
| Faderl et al (2003)56 | Alemtuzumab 30 mg, 2× weekly intravenously, up to 4 w; rituximab 375 mg/m2 weekly intravenously, up to 4 w | 48† | 54 | 52 | 8 | — |
| Wierda et al (2004)57 | Cyclophosphamide 250 mg/m2 days 3-5 intravenously; fludarabine 25 mg/m2 days 3-5 intravenously; alemtuzumab 30 mg/m2 days 1, 3, 5 intravenously; rituximab 375-500 mg/m2 day 2, (monthly, 6 cycles) | 17 | 59 | 52 | 14 | — |
| Osuji et al (2005)58 | Alemtuzumab 30 mg, 3× weekly intravenously until maximum response | 24 | 62 | 54 | 18 | — |
OR indicates overall response; —, not reported.
Definition of refractoriness to fludarabine: no CR or PR with such a treatment or progression within the 6 months from last course. These two series (Rai et al49 and Keating et al50 ) share some patients
Includes patients with CLL/prolymphocytic leukemia (PLL), PLL, and mantle-cell lymphoma (MCL)
An important limitation of alemtuzumab is that it is more effective in peripheral blood than in other sites. Lymphadenopathy is particularly resistant to alemtuzumab, the presence of lymph nodes larger than 5 cm being correlated with poor response,53 hence computed tomography (CT) scans may be useful to predict response. Toxicity is not negligible and includes fever, rigors, immunosuppression, and infections; cytomegalovirus (CMV) reactivation and clinically overt CMV infection are of particular concern, particularly in heavily pretreated patients, this making the monitoring of the CMV status mandatory during and after treatment. To improve its efficacy, alemtuzumab is being actively investigated in combination with other drugs and/or monoclonal antibodies (Table 1). A reasonable strategy is to use alemtuzumab to improve responses obtained with chemotherapy, the best dose and treatment schedule for alemtuzumab having not yet been determined; the administration of alemtuzumab subcutaneously appears to be safer than the intravenous route.59,60
Rituximab alone is not effective in treating refractory CLL but it may be useful by enhancing the effect of other drugs such as fludarabine or cyclophosphamide. A number of trials combining rituximab with other agents such as fludarabine, mitoxantrone, cyclophosphamide, and alemtuzumab have shown that these regimens are active in CLL, including refractory cases (Table 2).61-66
Fludarabine-based regimens in relapsed or refractory CLL
Study . | Treatment and regimen . | No. patients* . | Refractory, %† . | OR, % . | CR, % . | OR, % refractory . |
|---|---|---|---|---|---|---|
| O'Brien et al (2001)61 | Fludarabine 30 mg/m2 intravenously d 1-3, for 4-6 w, up to 6 courses; cyclophosphamide 300-500 mg/m2 intravenously d 1-3 | 94 | 69 | 80 | 12 | 39 |
| Hallek et al (2001)62 | Fludarabine 30 mg/m2 intravenously d 1-3, for 28 d, up to 6 courses; cyclophosphamide 250 mg/m2 intravenously d 1-3 | 18 | — | 94 | 11 | — |
| Bosch et al (2002)63 | Fludarabine 25 mg/m2 intravenously d 1-3 for 28 d, up to 6 courses; cyclophosphamide 200-600 mg/m2 intravenously d 1-3; mitoxantrone 6-8 mg/m2 d 1 | 60 | 58 | 78 | 50 | 34 |
| Mauro et al (2002)64 | Fludarabine 25 mg/m2 intravenously d 1-3, for 28 d, up to 6 courses; Ara-C 1 g/m2 d 1-2; novantrone 10 mg/m2 d 1; dexamethasone 20 mg intravenously d 1-3 | 23 | 60 | 70 | 48 | 50 |
| Schulz et al (2002)65 | Fludarabine 25 mg/m2 intravenously d 1-5 for 28 d, courses 1 to 4; rituximab 375 mg/m2 intravenously d 1 for 28 d, courses 3 to 7 | 11 | — | 90 | 27 | — |
| Wierda et al (2005)66 | Fludarabine 25 mg/m2 intravenously d 1-3, for 28 d, up to 6 courses; cyclophosphamide 250 mg/m2 intravenously d 1-3; rituximab 375-500 mg/m2 d 1 intravenously | 177 | 18‡ | 73 | 25 | 58 |
Study . | Treatment and regimen . | No. patients* . | Refractory, %† . | OR, % . | CR, % . | OR, % refractory . |
|---|---|---|---|---|---|---|
| O'Brien et al (2001)61 | Fludarabine 30 mg/m2 intravenously d 1-3, for 4-6 w, up to 6 courses; cyclophosphamide 300-500 mg/m2 intravenously d 1-3 | 94 | 69 | 80 | 12 | 39 |
| Hallek et al (2001)62 | Fludarabine 30 mg/m2 intravenously d 1-3, for 28 d, up to 6 courses; cyclophosphamide 250 mg/m2 intravenously d 1-3 | 18 | — | 94 | 11 | — |
| Bosch et al (2002)63 | Fludarabine 25 mg/m2 intravenously d 1-3 for 28 d, up to 6 courses; cyclophosphamide 200-600 mg/m2 intravenously d 1-3; mitoxantrone 6-8 mg/m2 d 1 | 60 | 58 | 78 | 50 | 34 |
| Mauro et al (2002)64 | Fludarabine 25 mg/m2 intravenously d 1-3, for 28 d, up to 6 courses; Ara-C 1 g/m2 d 1-2; novantrone 10 mg/m2 d 1; dexamethasone 20 mg intravenously d 1-3 | 23 | 60 | 70 | 48 | 50 |
| Schulz et al (2002)65 | Fludarabine 25 mg/m2 intravenously d 1-5 for 28 d, courses 1 to 4; rituximab 375 mg/m2 intravenously d 1 for 28 d, courses 3 to 7 | 11 | — | 90 | 27 | — |
| Wierda et al (2005)66 | Fludarabine 25 mg/m2 intravenously d 1-3, for 28 d, up to 6 courses; cyclophosphamide 250 mg/m2 intravenously d 1-3; rituximab 375-500 mg/m2 d 1 intravenously | 177 | 18‡ | 73 | 25 | 58 |
— indicates not reported.
Only refers to relapsed or refractory patients included in these series
Definition of refractoriness: resistance to the previous treatment including alkylating agents or fludarabine
Fludarabine refractory patients defined as failure to achieve at least a PR with the last fludarabine-based treatment or progression within 6 months of treatment
Wierda et al66 have recently reported treatment results in patients with either relapsing or refractory CLL using a combination of fludarabine, cyclophosphamide, and rituximab. Response rate among 33 patients refractory to fludarabine was 58% (6% CR, 9% nodular PR [nPR], and 42% PR) compared with 77% (33%CR, 19% nPR, 24% PR) in 78 fludarabine-sensitive patients; time to progression, however, was quite short (median, 18 months; range, 3 to 49 months).66
Another approach to try to overcome resistance to therapy is to use agents that target antiapoptotic proteins. Oblimersen is an antisense oligonucleotide that targets Bcl-2 and that in vitro enhances the activity of fludarabine, dexamethasone, and rituximab. The combination of oblimersen, fludarabine, and cyclophosphamide is being compared with fludarabine and cyclophosphamide. Preliminary results are encouraging, with a higher rate of major responses (CR/nPR) in the arm including the antisense oligonucleotide (16% vs 7%; P = .039) and also a shorter time to response with the experimental treatment.67 Clearly, more patients and a longer follow-up are needed to draw definitive conclusions from this study.
Flavopiridol has demonstrated in vitro activity against CLL cells that acts as a potent inhibitor of cyclin-dependent kinases inducing p53-independent apoptosis in CLL cells. In a phase 1 trial, 41% of patients (9/22) achieved PR including 8 patients refractory to fludarabine and 8 with high-risk cytogenetic features (6 del 11q and 3 del 17p); toxicity (eg, tumor lysis), however, has been high.68
With all the therapies discussed above and many others not covered in this article,69-76 the response duration is unfortunately short, ranging from 10 to 20 months, and all patients eventually relapse and die because of the disease.
In this context, it is not surprising that the number of patients with CLL in whom a hematopoietic stem cell transplantation is performed is increasing. The necessary condition for the success of autologous transplantation is that the patient receives the transplant while in CR. Therefore such a procedure has no role in the treatment of patients with refractory CLL. Allogeneic transplantation is the only treatment with curative potential in CLL. In most series a plateau is observed, with 40% to 60% of the patients remaining alive and free of disease 3 to 6 years after transplantation, this being thanks to a well-demonstrated graft-versus-CLL effect. Of note, the graft-versus-CLL effect seems to revert poor prognostic biologic variables.77,78 Although nonrelapse mortality (NRM) in registry studies is extremely high, in single-center series it may be much lower (Table 3).79-89
Summary of results according to larger studies analyzing the outcome after myeloablative allogeneic stem cell transplantation in CLL
Study . | No. patients . | Refractory patients, % . | Characteristics of the study . | NRM, % . | Relapse risk, % (year of projection) . | PFS, % (year of projection) . | OS, % (year of projection) . |
|---|---|---|---|---|---|---|---|
| Pavletic et al (Omaha, 2000)80 | 23 | 61 | Single-center series | 30 | 2 (5) | 62 (5) | 65 (5) |
| Michallet et al (EBMT, 2000)81 | 209 | 44 | Registry study | 40 | 25 (3) | NR | 55 (3) |
| Horowitz et al (IBMTR, 2000)82 | 242 | 50 | Registry study | 30 | 25 (3) | 44 (3) | 45 (3) |
| Khouri et al (MDACC, 2002)83 | 28 | 68 | Single-center series | NA | NA | 26 (5)* | 31 (5)* |
| Doney et al (Seattle, 2002)84 | 25 | 64 | Single-center series | 40 | < 10 | NR | 32 (5) |
| Esteve et al (International series, 2001)85 | 46 | 55 | Multicenter study | 41 | 18 (5) | 51 (5) | 58 (5) |
| Michallet et al (EBMT/IBMTR, 2003)86 | 54 | NR | Registry study | 46 | NR | 37 (10) | 41 (10) |
| Esteve et al (Hospital Clinic, 2005)87 | 17 | 35 | Single-center series | 24 | 9 (6) | 66 (6) | 76 (6) |
| Pavletic et al (NMDP, 2005)88 | 38 | 55 | Unrelated donor registry study | 38 | 32 (5) | 30 (5) | 33 (5) |
| Gribben et al (Dana-Farber, 2005)89 | 27 | 0 | Single-center series | 27 | 68 (6) | 24 (6) | 58 (6) |
Study . | No. patients . | Refractory patients, % . | Characteristics of the study . | NRM, % . | Relapse risk, % (year of projection) . | PFS, % (year of projection) . | OS, % (year of projection) . |
|---|---|---|---|---|---|---|---|
| Pavletic et al (Omaha, 2000)80 | 23 | 61 | Single-center series | 30 | 2 (5) | 62 (5) | 65 (5) |
| Michallet et al (EBMT, 2000)81 | 209 | 44 | Registry study | 40 | 25 (3) | NR | 55 (3) |
| Horowitz et al (IBMTR, 2000)82 | 242 | 50 | Registry study | 30 | 25 (3) | 44 (3) | 45 (3) |
| Khouri et al (MDACC, 2002)83 | 28 | 68 | Single-center series | NA | NA | 26 (5)* | 31 (5)* |
| Doney et al (Seattle, 2002)84 | 25 | 64 | Single-center series | 40 | < 10 | NR | 32 (5) |
| Esteve et al (International series, 2001)85 | 46 | 55 | Multicenter study | 41 | 18 (5) | 51 (5) | 58 (5) |
| Michallet et al (EBMT/IBMTR, 2003)86 | 54 | NR | Registry study | 46 | NR | 37 (10) | 41 (10) |
| Esteve et al (Hospital Clinic, 2005)87 | 17 | 35 | Single-center series | 24 | 9 (6) | 66 (6) | 76 (6) |
| Pavletic et al (NMDP, 2005)88 | 38 | 55 | Unrelated donor registry study | 38 | 32 (5) | 30 (5) | 33 (5) |
| Gribben et al (Dana-Farber, 2005)89 | 27 | 0 | Single-center series | 27 | 68 (6) | 24 (6) | 58 (6) |
NRM indicates nonrelapse mortality; PFS, progression-free survival; OS, overall survival; NR, not reported; IBMTR, International Bone Marrow Transplant Registry; MDACC, MD Anderson Cancer Center at Houston; NA, not available; and NMDP, National Marrow Donor Program.
Referred to the subset of refractory patients
The use of allogeneic transplants in CLL is, unfortunately, limited due to the advanced age of most patients and the high NRM rate. Nonmyeloablative regimens may reduce the NRM rate and permit an increase in the age limit up to which patients can receive transplants (Table 4). Results are better and NRM rate lower in younger patients with chemosensitive disease.90-93
Results of allogeneic stem cell transplantation with nonmyeloablative regimens for CLL
Study . | No. patients . | NRM, % (follow-up time, y) . | Conditioning regimen . | Patients with related donor, % . | PFS, % (year of projection) . | OS, % (year of projection) . |
|---|---|---|---|---|---|---|
| Dreger et al (EBMT, 2003)90 | 77 | 18 (1) | Several | 82 | 56 (2) | 72 (2) |
| Schetelig et al (2003)91 | 30 | 15 (2) | Fluda + Bu + ATG | 50 | 67 (2) | 72 (2) |
| Khouri et al (2004)92 | 17 | 22 (2) | Fluda + Cy ± Rituximab | 100 | 60 (2) | 80 (2) |
| Sorror et al (2005)93 | 64 | 22 (2) | Fluda ± TBI (2 Gy) | 69 | 52 (2) | 60 (2) |
Study . | No. patients . | NRM, % (follow-up time, y) . | Conditioning regimen . | Patients with related donor, % . | PFS, % (year of projection) . | OS, % (year of projection) . |
|---|---|---|---|---|---|---|
| Dreger et al (EBMT, 2003)90 | 77 | 18 (1) | Several | 82 | 56 (2) | 72 (2) |
| Schetelig et al (2003)91 | 30 | 15 (2) | Fluda + Bu + ATG | 50 | 67 (2) | 72 (2) |
| Khouri et al (2004)92 | 17 | 22 (2) | Fluda + Cy ± Rituximab | 100 | 60 (2) | 80 (2) |
| Sorror et al (2005)93 | 64 | 22 (2) | Fluda ± TBI (2 Gy) | 69 | 52 (2) | 60 (2) |
NRM indicates nonrelapse mortality; PFS, progression-free survival; OS, overall survival; Fluda, fludarabine; Bu, busulfan; ATG, antithymocyte globulin; and Cy, cyclophosphamide.
In a retrospective study, Dreger et al90 analyzed 77 patients (median age, 54 years), most of them having received a transplant from an HLA-identical sibling donor. Patients received a variety of conditioning regimens, mostly low-dose total body irradiation (TBI) or fludarabine-cyclophosphamide with or without alemtuzumab. With a median follow-up of 18 months, the cumulative transplant-related mortality (TRM) was 18% after 12 months. The 2-year probability of relapse was 31%. Event-free survival (EFS) and overall survival at 2 years were 56% and 72%, respectively.90
In another study, Schetelig et al91 reported on 30 patients (median age, 50 years) with advanced CLL conditioned with fludarabine, melphalan, and ATG who received a transplant from related (n = 15) or unrelated (n = 15) donors. Twelve patients achieved CR, this being molecular in some cases.91
Khouri et al92 treated 17 patients (median age, 54 years) who were refractory or had relapsed after a prior response to fludarabine. All patients received fludarabine and cyclophosphamide and 10 patients were also given rituximab as preparation regimen. Ten patients required donor lymphocyte infusion (DLI) because of persistence of the disease after transplantation. The overall procedure was well tolerated and, although the series is small, it is worth noting that the overall survival of patients who received rituximab was 100%, with 6 patients remaining in CR from 11 to 43 months after the procedure.92
The Seattle Group (Sorror et al93 ) has recently reported on 64 patients diagnosed with advanced CLL treated with nonmyeloablative conditioning with (n = 53) or without (n = 11) fludarabine who also received a transplant from related (n = 44) or unrelated (n = 20) donors. The overall response rate in 61 evaluable patients was 67% (50% CR). The 2-year incidence of relapse/progression was 26%, whereas the 2-year relapse rate and nonrelapse mortality rate were 18% and 22%, respectively. Although the number is small, unrelated transplants resulted in higher CR and lower relapse rates than related transplants, suggesting more effective graft-versus-leukemia activity.93
A recent published case-matched study from the European Group for Blood and Marrow Transplantation (EBMT) database has shown that as expected from the reduction of the intensity conditioning regimen, reduced intensity conditioning (RIC) transplants, compared with conventional allogeneic transplants, are associated not only with lower TRM rates, between 10% and 20%, but also with higher incidence of relapse (hazard ratio, 2.5).94 Comparative studies (myeloablative vs nonmyeloablative allogeneic transplants) are necessary to determine the relative merits and most appropriate candidates for each procedure.
Taking into consideration all of the above, we propose allogeneic stem cell transplantation for patients who have failed fludarabine-based chemotherapy or autologous transplantation and have a good performance status, no associated medical conditions precluding the procedure, an HLA-identical sibling or unrelated donor, and, obviously, full understanding of the procedure and its risks. If a transplant is not feasible or it is rejected by the patient, participation in phase 2 trials or palliative care are offered.
In short, our transplantation strategy is as follows. Before transplantation, patients receive salvage therapy (regimens currently investigated include R-CHOP [rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone] and R-FCM [rituximab, fludarabine, cyclophosphamide, mitoxantrone]). We indicate allogeneic transplantation with myeloablative conditioning for patients younger than 50 years. In patients between 50 and 65 years of age and in all those relapsing after an autologous transplantation, an allogeneic stem cell transplantation with a reduced-intensity conditioning regimen is offered. In our experience and that of others, the combination of melphalan and fludarabine is an effective regimen that is associated with low toxicity and mortality.95,96 Recently, alemtuzumab has been added to this regimen, which seems to result in a lower incidence of graft-versus-host disease (GVHD) but also, and of concern, in a higher incidence of graft rejection, relapse, and infections.97-99 DLIs are used in case of disease progression, relapse, or lack of complete donor chimerism.
Regarding transplantation procedures, a number of important points need to be stressed. First, the age limit for patients to be offered standard or reduced-intensity conditioning regimens has not been well established; the general condition and comorbidity of the patient are somehow more important than the age itself. Second, given the limited activity of RIC transplants in patients with chemorefractory disease,93,94 in well-fitted individuals whose disease does not respond at all to therapy, myeloablative transplants should not be discarded just because of the patient's age. Third, the best salvage and conditioning regimens have not been determined. All this emphasizes the need for including patients in trials aimed at ascertaining these important issues.
Summary and conclusions
Treatment of patients with refractory CLL is extremely challenging. Although there are a number of agents that may be useful to induce response in patients in such a situation, these are usually short lived and, unless some other procedure is undertaken, all patients will eventually progress or die. The only treatment with curative potential for CLL, including refractory cases, is allogeneic stem cell transplantation. Allogeneic stem cell transplantation has been limited by the high TRM rate and the advanced age of many patients with CLL. With the use of nonmyeloablative transplants, TRM rates seem to be decreasing, this opening the door to a wider use of this therapy and to increasing the upper age limit up to which transplants are indicated. Allotransplants, however, should be reserved for patients refractory to purine analogs and in whom transplantation constitutes the only possibility for a long-term control of their disease, not to say cure. Future research should be aimed at identifying mechanisms of resistance to therapy and modalities to either prevent or circumvent them, as well as to the design of specific therapies based on the biologic diversity of the disease. In this regard, ongoing studies in which treatment is based on biologic features such as IgVH mutations, ZAP-70 expression, or cytogenetic abnormalities are worthy of being pursued.
Prepublished online as Blood First Edition Paper, October 4, 2005; DOI 10.1182/blood-2005-02-0819.
Supported in part by grants from the José Carreras International Foundation (EM/04 and CR/04), Lilly Spain, and Redes Temáticas de Cáncer G03/008.
Thanks are due to Carmen Torres and Montse Soriano for their excellent secretarial support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal