The extracellular presence of endotoxin-free heat shock protein 70 (HSP70) enhances the rate and capacity of macrophage-mediated phagocytosis at 6 times the basal rate. It is protein-specific, dose- and time-dependent and involves the internalization of inert microspheres, Gram-positive and -negative bacteria and fungi. Structurally, exogenous HSP70 binds the macrophage plasma membrane, specifically on its lipid raft-microdomain. Disruption of lipid rafts, HSP70-LR interaction, or denaturing HSP70 abrogates the HSP-mediated increase in phagocytosis. Further, HSP70-mediated phagocytosis directly enhances the processing and presentation of internalized antigens via the endocytic MHC class-II pathway to CD4+ T lymphocytes. Modulating the HSP70-LR interaction presents an opportunity to intervene at the level of host-pathogen interface: a therapeutic tool for emerging infections, especially when conventional treatment with antibiotics is ineffective (antibiotic resistance) or unavailable (rapidly spreading, endemic). These results identify a new role for HSP70, a highly conserved molecule in stimulating phagocytosis: a primordial macrophage function, thereby influencing both innate and adaptive immune responses.
Introduction
Phagocytosis is a primal protective cellular function that characterizes the innate immune response to microbial invasion.1-4 It is a complex phenomenon implicating several components of the plasma membrane, including pattern recognition receptors (PPRs), cytoskeletal elements, and lipid rafts (LRs).5 Antigen-presenting cells (APCs) act as sentinels of the host that initiate and execute the phagocytic response.2,6-9 These cells are activated in response to certain well-defined substances that provide stimulatory signals that characterize injury or infection. Typically, these APCs, including macrophages, respond either to the presence of pathogens via PRRs or to metabolic, physical, or chemical stress, trauma, or other agents that mediate necrotic-cell lysis and death.
Cell lysis releases a vast number of nonmicrobial, host-derived, intracellular molecules into the environment, some of which are potent activators of the immune system. Of these, heat shock proteins (HSPs) are abundant intracellular molecules, readily released by cell lysis following injury or infection10-12 where they exhibit broad immunoactive properties.13 As shown by Basu et al,10 lysate from 1 g tissue was shown to contain 200 μg HSP70. Injury-causing lysis of as little as 0.5 g tissue could potentially release 100 μg/mL concentration of HSP70 into the extracellular environment where they come into direct contact with cells of the immune system such as macrophages and dendritic cells. HSPs have been shown to play a major role in macrophage activation, preparing the host for defense.10,14,15 Immunologically, HSPs bind several macrophage-surface receptors and up-regulate key antigen-specific and nonspecific functions, including tumor rejection, cytokine release, and up-regulation of costimulatory molecules.16-21 Within the confines of the cell, HSPs are chaperones and facilitate protein synthesis and breakdown.22 They are expressed in all cells in all forms of life and in a variety of intracellular locations: in the cytosol (HSP70 and HSP90), nuclei, endoplasmic reticulum (gp96), and mitochondria. In addition to their ubiquity, the HSPs constitute the single most abundant group of proteins inside cells. They are expressed in vast quantities under normal unstressed conditions, and their expression can be powerfully induced to much higher levels as a result of heat shock or other forms of stress, including exposure to toxins, oxidative stress, glucose deprivation, and so forth, leading up to cell lysis, which releases large quantities of HSPs into the extracellular milieu. Approximately 10 families of HSPs are known, and each family consists of 1 to 5 closely related proteins. Since their discovery, an increasing array of functions such as folding and unfolding of proteins, degradation of proteins, assembly of multisubunit complexes, thermotolerance, buffering of expression of mutations, and others have been attributed to HSPs.22-24
Our study examines whether HSPs provide stimulatory signals to the macrophage and promote phagocytosis of particulate antigen, its processing, and presentation. Here, we show that the ligation of endotoxin-free HSP70 to the LR microdomain on the macrophage-cell surface. Functionally LRs have been implicated in cellular processes by their ability to facilitate protein-protein interaction, raft-mediated endocytosis, signal transduction and to play an important role in the biogenesis of the phagosomes.25-31 The HSP70-LR interaction promotes phagocytosis of particulate antigen and subsequent antigen processing and presentation to CD4+ T lymphocytes in a MHC-II restricted manner.
Taken together, these findings identify HSP70 as a new mammalian agent that can activate a primitive macrophage function: that of phagocytosis. The increase in antigen uptake in response to binding of the HSP70 to the LR microdomain, described here for the first time, defines the new role for HSP70, a highly conserved molecule in influencing a primordial macrophage function. The clinical applications of these findings range from developing new anti-infective therapies, to their value in tissue repair and host defense.
Materials and methods
Cell culture
All cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA). RAW 264.7, RAW 309, and J774A.1 were maintained in DMEM (Gibco, Invitrogen, Grand Island, NY) with 10% heat inactivated FCS and RAW264.7 NO- was maintained in RPMI (ATCC) with 10% heat-inactivated FCS at 37°C, 5% CO2.
Mice
I-Ad-restricted DO11.10 TCR-αβ-transgenic and C57/BL6J mice (6-8 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in the vivarium of the Center of Laboratory Animal Care in University of Connecticut Health Center.
Reagents
All chemicals, unless otherwise specified were purchased from Sigma (St Louis, MO), and all sterile noncharged plastic ware was from Corning (Corning, NY). Alexafluor488-labeled Saccharomyces cerevisiae and Escherichia coli were purchased from Molecular Probes (Eugene, OR); fluorescent and unlabeled polystyrene microspheres (size, 3 μm) were purchased from Sigma. Anti-HSP70 antibodies were obtained from Stressgen (San Diego, CA) respectively.
Heat shock proteins
Heat shock proteins (HSPs) (HSP70, HSP90, and GP96) were purified from murine livers as described earlier.32,33 HSPs were prepared as a complex with endogenous peptide, except when using adenosine triphosphate (ATP)-treated HSP70 that was used to assess the role of peptides in phagocytosis. ATP-treated HSP70 removes all the peptides associated with HSP70 itself, whereas the purification of HSP70 using adenosine diphosphate (ADP) does not.34 Purity was established by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis and immunoblotting, and HSPs were quantified using Bradford analysis. LPS content was measured by the Limulus Amebocyte Lysate (LAL) assay (LAL kit QCL-1000; Biowhittaker, Walkersville, MD).
Phagocytosis assay in vitro
RAW 264.7, RAW264.7 (NO-), and J774A.1 cells were cultured in DMEM or RPMI (RAW NO-) with 10% FCS, harvested, and washed. Cells (3 × 105/well) were incubated in serum-free EMEM in a 24-well plate for 1 hour at 37°C. Alexafluor488-labeled particles (inert polystyrene microspheres, or yeast, S cerevisiae, or Gram-negative bacteria, E coli, 40 particles/macrophage) were added and coincubated in a dark environment for 60 minutes in serum-free EMEM with or without HSPs, LPS, or control proteins. At the end of the 60-minute incubation, the plate was covered in a dark container on ice. Specific measures were taken to exclude the fluorescence that resulted from particles that were outside of the cell or sticking to the surface of the cell. This was done by using trypan blue that quenches all the fluorescence outside of the cell but does not quench the internalized particles. Specifically, trypan blue (0.8 mg/mL) was added for 60 seconds, and plates were analyzed immediately using FluorImager SI (Molecular Dynamics/Amersham, Sunnyvale, CA). Because the emission of Alexafluor488 is approximately 519 nm at excitation approximately, 490 nm falls within the absorbance range of trypan blue (475-675 nm), the fluorescence of the sample only represents the intracellular fluorescence. External or surface-bound fluorescence is quenched. Using Student t test, the results were analyzed, and P values were calculated.
Purification of lipid rafts
Lipid rafts were purified as DRMs (detergent resistant membranes) using nonionic detergents following sucrose gradient centrifugation. The cells used for lipid raft purification were treated with or without 30 mM MCD for 30 minutes at 37°C, after being washed with cold PBS and incubated with HSP70-HRP complexes (HRP conjugation kit; Alpha Diagnostic, San Antonio, TX) for 30 minutes on ice. After incubation, cells were washed 3 times with PBS and lysed with 2 mL MBS (150 mM NaCl, 20 mM MES, pH 6.5, 500 mM PMSF, and 5 mM iodoacetamide) containing 0.5% Ttriton X-100 or 1% Brij98 for 30 minutes on ice or 7 minutes at 37°C, respectively. The cells were mixed with an equal volume of 90% sucrose in MEB and placed at the bottom of the centrifuge tube. The sample was overlaid with 5.5 mL 30% sucrose and 4.5 mL 5% sucrose in MBS and centrifuged at 100 000g for 16 hours (SW28; Beckman Instruments, Palo Alto, CA). Fractions of 1 mL each were collected from the bottom of the tube, and each fraction was analyzed by SDS-PAGE and immunoblotting.
Proliferation of CD4+ T cells and IFN-γ release
Unlabeled S cerevisiae (80 μg) was incubated with excess chicken ovary albumin (OVA) at 37°C for 1 hour and washed repeatedly to eliminate free OVA protein. S cerevisiae-OVA complexes (Ova-coated yeast) were administered to macrophages treated with or without HSP70. Macrophages were then washed and irradiated at 120 Gy (12 000 rad) and assessed for viability by trypan blue exclusion. Subsequently, they were cocultured with CD4+ T lymphocytes that were purified from spleens of DO11.10 mice and labeled with CSFE. Purification of CD4+ T lymphocytes was performed using the magnetic-activated cell sorting (MACS) purification columns and αCD4 microbeads (Miltenyi Biotech, Auburn, CA). Following the 48-hour incubation, proliferation of CD4+ cells was determined with fluorescence-activated cell sorting (FACS), and IFN-γ release in the supernatant was tested by enzyme-linked immunosorbent assay (ELISA; Pierce, Rockford, IL).
Results
HSPs enhance macrophage-mediated antigen uptake
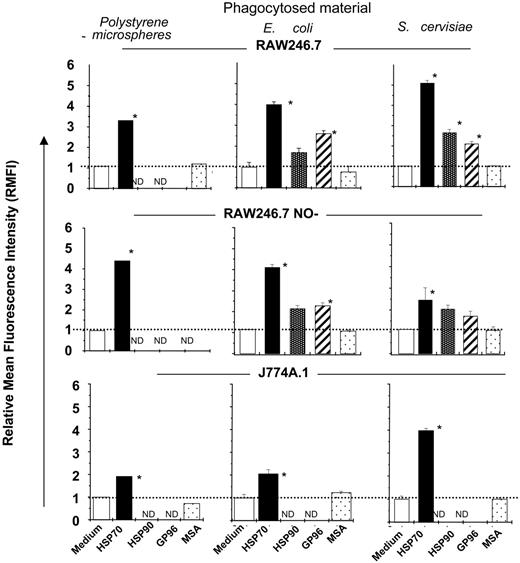
Murine macrophage lines (RAW264.7, J774.A1, or RAW264 NO-) were treated with either one of the HSPs (HSP70, HSP90, or gp96) (100 μg/mL) or with non-HSP control proteins and coincubated with either Alexafluor488-labeled inert polystyrene microspheres, yeast (S cerevisiae [Sc]), or Gram-negative bacteria (E coli [Ec]) (40 particles/macrophage). A phagocytosis assay was performed using specific techniques to exclude external cell-surface binding of particles and measuring the actual fluorescence from internalized material alone (see “Materials and methods”). Macrophages treated with any of the 3 HSPs consistently showed an increase in uptake of microbial or nonmicrobial materials as compared with those treated with control proteins or with buffer (Figure 1A). The increase in uptake ranged from 2 to 6 times the basal rate and included the internalization of a variety of materials tested.
We focused on HSP70, because of our knowledge of its molecular functions and its influence on various aspects of tissue protection.35-45 Specificity of HSP70-mediated antigen uptake was tested by treating macrophages with either HSP70 (100 μg/mL) or with equimolar amounts of HSP controls, including β galactosidase; phosphorylase B; and mouse serum albumin (molecular weights of these proteins correspond to those of the HSPs tested); bovine serum albumin (BSA; 100 μg/mL); concavalin A (6 μg/mL); and complete and incomplete Freund adjuvant (10 μL/well) (potent stimulators of APC-function). None of the control proteins used enhanced uptake as compared with treatment with HSP70. Further, reagents commonly used in HSP purification, including buffers, ADP (3 mM); and ATP (3 mM); were ineffective (Figure 2A).
Macrophage-mediated phagocytosis is modulated by HSPs. The ability of macrophage cell lines (as indicated) to phagocytose either Alexafluor-labeled inert microspheres or S cerevisiae or E coli in the absence or presence of each of the HSPs indicated (HSP70, HSP90, or gp96) or serum albumin (MSA) was tested in vitro. HSP-mediated phagocytosis was quantified by measuring the internalized fluorescence measured by FluorImager SI, and relative mean fluorescence intensity (RMFI) was compared between the treatment groups (as indicated). All the treatment groups were compared with medium alone using the Student t test, and significance was denoted by P < .01. The error bar represents 1 SD. The results shown are a cumulative analysis of 3 experiments, 3 wells/group. *P < .05 when compared to medium.
Macrophage-mediated phagocytosis is modulated by HSPs. The ability of macrophage cell lines (as indicated) to phagocytose either Alexafluor-labeled inert microspheres or S cerevisiae or E coli in the absence or presence of each of the HSPs indicated (HSP70, HSP90, or gp96) or serum albumin (MSA) was tested in vitro. HSP-mediated phagocytosis was quantified by measuring the internalized fluorescence measured by FluorImager SI, and relative mean fluorescence intensity (RMFI) was compared between the treatment groups (as indicated). All the treatment groups were compared with medium alone using the Student t test, and significance was denoted by P < .01. The error bar represents 1 SD. The results shown are a cumulative analysis of 3 experiments, 3 wells/group. *P < .05 when compared to medium.
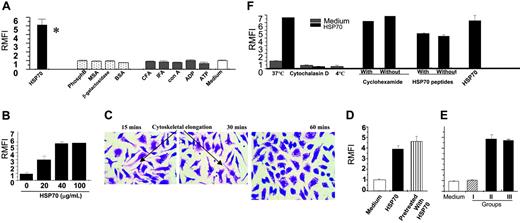
HSP70-mediated phagocytosis is specific, titratable, actin dependent, and independent of protein synthesis and HSP-peptides. (A) Macrophages were treated with HSP70 or non-HSP controls (as indicated) to examine their specific effects on phagocytosis of Alexafluor-labeled yeast (S cerevisiae) at the concentration of 40 particles/macrophage. *P < .05 when compared to medium. (B) Macrophages were treated with HSP70 (doses indicated) and subsequently tested for their ability to phagocytose Alexafluor-labeled yeast (S cerevisiae) at the concentration of 40 particles/macrophage. (C) Representative microphotographs (× 10 magnification) of macrophages (treated for minutes as indicated). At 15 and 30 minutes the macrophages show ongoing phagocytic activity (elongated cells with cytoskeletal alterations), whereas at 60 minutes the cells are round and appear quiescent. Images were visualized under a Nikon Optiphot microscope (Nikon, Melville, NY) equipped with objective lenses ranging from 10 ×/2.5 to 40 ×/16.0. Images were captured with a Kodak DC 120-zoom camera (Kodak, Rochester, NY) and processed with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA). (D) Macrophages were pretreated with HSP70 (100 μg/mL), as indicated, and washed free of residual HSP70. Macrophages were then administered yeast and tested in a phagocytosis assay. For comparison, macrophages were with HSP70 and administered the yeast at the same time. (E) HSP70-coated yeast was prepared by coincubating Alexafluor-labeled S cerevisiae (Sc) with HSP70 and washed until free of unbound HSP70. Experimental groups (as indicated) were tested for their ability to enhance phagocytosis. (F) Macrophages were treated with HSP70 (100 μg/mL) under (1) conditions that prevent actin-mediated cytoskeletal changes (low temperature and cytochalasin D as indicated) or (2) in the presence or absence of cycloheximide (as indicated) and subjected to a phagocytosis assay or (3) phagocytosis-enhancing effects of ATP-treated HSP70 (peptide free) was compared with that of ADP-purified HSP70 (with peptides). The error bar represents 1 SD. The results shown are a cumulative analysis of 3 experiments, 3 wells/group.
HSP70-mediated phagocytosis is specific, titratable, actin dependent, and independent of protein synthesis and HSP-peptides. (A) Macrophages were treated with HSP70 or non-HSP controls (as indicated) to examine their specific effects on phagocytosis of Alexafluor-labeled yeast (S cerevisiae) at the concentration of 40 particles/macrophage. *P < .05 when compared to medium. (B) Macrophages were treated with HSP70 (doses indicated) and subsequently tested for their ability to phagocytose Alexafluor-labeled yeast (S cerevisiae) at the concentration of 40 particles/macrophage. (C) Representative microphotographs (× 10 magnification) of macrophages (treated for minutes as indicated). At 15 and 30 minutes the macrophages show ongoing phagocytic activity (elongated cells with cytoskeletal alterations), whereas at 60 minutes the cells are round and appear quiescent. Images were visualized under a Nikon Optiphot microscope (Nikon, Melville, NY) equipped with objective lenses ranging from 10 ×/2.5 to 40 ×/16.0. Images were captured with a Kodak DC 120-zoom camera (Kodak, Rochester, NY) and processed with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA). (D) Macrophages were pretreated with HSP70 (100 μg/mL), as indicated, and washed free of residual HSP70. Macrophages were then administered yeast and tested in a phagocytosis assay. For comparison, macrophages were with HSP70 and administered the yeast at the same time. (E) HSP70-coated yeast was prepared by coincubating Alexafluor-labeled S cerevisiae (Sc) with HSP70 and washed until free of unbound HSP70. Experimental groups (as indicated) were tested for their ability to enhance phagocytosis. (F) Macrophages were treated with HSP70 (100 μg/mL) under (1) conditions that prevent actin-mediated cytoskeletal changes (low temperature and cytochalasin D as indicated) or (2) in the presence or absence of cycloheximide (as indicated) and subjected to a phagocytosis assay or (3) phagocytosis-enhancing effects of ATP-treated HSP70 (peptide free) was compared with that of ADP-purified HSP70 (with peptides). The error bar represents 1 SD. The results shown are a cumulative analysis of 3 experiments, 3 wells/group.
Increasing the doses of HSP70 (range, 10-100 μg/mL) revealed that doses less than 20 μg/mL were unable to stimulate uptake, and the peak effect of HSPs occurred at 40 μg/mL (Figure 2B) and reached a plateau through 100 μg/mL. Doses up to 200 μg/mL did not result in any further increase in the quantity of phagocytosis. Physiologically, this dose is well within the range of the concentration of HSP70 that is observed from lysis of 1 g tissue lysis. As shown by Basu et al,10 lysate from 1 g tissue was shown to contain 200 μg HSP70. In our experiments we used HSP70 in the dose of 100 μg/mL that would correspond to 0.5 g lysed tissue. Injury-causing lysis of as little as 0.5 g tissue could potentially mimic the conditions we use in vitro wherein macrophages are treated with 100 μg/mL concentration of HSP70. Viability of cells treated was confirmed by using trypan blue exclusion to ensure that the HSP treatment was not cytotoxic. Time titration was performed by treating macrophages with HSPs or controls for increasing time periods, ranging from 10 to 90 minutes. In the HSP-treated group, antigen uptake reached a plateau within 45 minutes. Time points beyond 90 minutes were not tested. Representative microphotographs of macrophages visualized at × 10 magnification using H&E stain at 15, 30, and 60 minutes showed morphologic changes indicative of ongoing uptake, which ceased at 60 minutes (Figure 2C).
To address whether HSP70 somehow coated the yeast particles, thereby acting like an “opsonizing agent,” the following experiment were performed. First, macrophages were pretreated with HSP70 and washed until no free HSPs remained in the wash. Then they were administered Alexafluor-labeled yeast (S cerevisiae). Pretreating the macrophages with HSP prior to administering the yeast enhanced the uptake of yeast to the same extent as when both (HSP and yeast) were administered simultaneously (Figure 2D).
Second, yeast particles were coincubated with HSP70 (100 μg/80 μg yeast, 37°C, 60 minutes) to create a HSP-coated yeast complex. Unbound HSP70 was removed by washing with PBS. Presence of the HSP-coated yeast complex was confirmed by SDS-PAGE and immunoblotting with HSP70 antibodies. These complexes were then coincubated with macrophages with the following experimental groups: group I, HSP70-coated yeast (HSP-Sc*); group II, yeast (Sc*) + HSP70; group III, HSP70-treated macrophages + HSP70-coated yeast (HSP-Sc*). As shown in Figure 2E, macrophages that were treated with HSP70-coated yeast (group I) did not demonstrate increased uptake of yeast as compared with group II in which both the HSP70 and yeast were administered simultaneously, indicating that HSPs do not act as opsonizing agents.
Macrophages treated with HSP70 at conditions that inhibit actin polymerization,46 that is, at either 4°C instead of 37°C or in the presence of cytochalasin-D (4 μM), showed inhibition of the effects of HSP70 (Figure 2F), indicating that HSP70-mediated internalization was via actin-dependent, cytoskeletal rearrangement: essential characteristics of phagocytosis.
Macrophages treated with HSP70 either in the presence or absence of cycloheximide (1 mM; ribosomal protein synthesis inhibitor)47 showed similar increase in HSP-mediated phagocytic uptake as did macrophages treated with HSP70 alone (Figure 2F), indicating that synthesis of new intracellular proteins is not essential for HSP-mediated phagocytosis. Trypan blue exclusion was undertaken to confirm that the cells were viable through the cycloheximide treatment.
Finally, macrophages treated with either ATP-purified HSP70 (ATP removes peptide from the HSP70)34 or with ADP-purified HSP70 (peptides remain bound to the HSP70) demonstrated the same degree of HSP-mediated phagocytosis (Figure 2F), indicating that the HSP70-mediated effect was independent of the presence of chaperoned peptides.
HSP70-mediated phagocytosis is not due to contamination by endotoxin
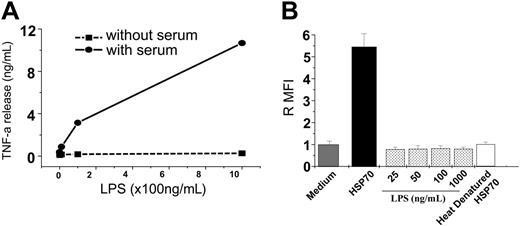
HSP70 used in all assays was purified using LPS-free sterile-packaged plastic ware, baked glass ware (420°F for 4 hours) and using endotoxin-free culture grade reagents to minimize inadvertent LPS contamination. Further, LPS levels and activity in the purified HSPs were quantified and confirmed to be less than 1 EU/mg protein. Next, HSPs and LPS were compared for their influence on phagocytosis in the presence or absence of serum. These conditions were specifically chosen because LPS-binding protein (LBP), an essential mediator for LPS function, is not present in serum-free conditions.48 As a control, macrophages were treated with LPS in the presence or absence of serum, and their ability to elicit TNF-α release was compared (Figure 3A). Having established that serum-free conditions rendered LPS ineffective at stimulating macrophages, the same conditions (serum-free) was used to test the ability of HSP70 to stimulate macrophages. Briefly, macrophages were treated with HSP70 (100 μg /mL) or LPS (different doses ranging from 25 ng to 1 μg/mL) in serum-free conditions for 1 hour, and a phagocytosis assay was performed. HSP70 was able to enhance phagocytosis equally whether in the presence or absence of serum, whereas LPS was unable to enhance phagocytosis in serum-free conditions (Figure 3B). Because HSPs do not act via the LBP, their activity is not affected by the absence of serum.
HSP70-mediated phagocytosis is independent of LPS. (A) Macrophages were treated with LPS in serum-free conditions and resultant TNF-α production was measured. (B) Macrophages maintained in serum-free conditions were treated with either HSP70 or of LPS (as indicated), or heat-denatured HSP70 and a phagocytosis assay was performed. The error bar is 1 SD. The results shown are a cumulative analysis of 3 experiments, 3 wells/group.
HSP70-mediated phagocytosis is independent of LPS. (A) Macrophages were treated with LPS in serum-free conditions and resultant TNF-α production was measured. (B) Macrophages maintained in serum-free conditions were treated with either HSP70 or of LPS (as indicated), or heat-denatured HSP70 and a phagocytosis assay was performed. The error bar is 1 SD. The results shown are a cumulative analysis of 3 experiments, 3 wells/group.
Last, because heat denatures HSPs but not LPS, HSP70 preparations were heated at 100°C for 20 minutes and tested for phagocytosis. Heat-denatured HSP70 samples lost the ability to enhance phagocytosis as compared with intact HSPs (Figure 3B), indicating that the HSP70-mediated phagocytosis was due to intact HSP70 alone and not due to any contaminating LPS. Taken together, these measures indicate that contaminating LPS (if any) is not responsible for HSP-mediated phagocytosis.
Exogenous HSP70 binds the lipid raft microdomain of the macrophage plasma membranex
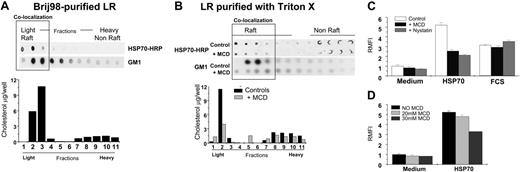
Briefly, RAW264.7 macrophages were administered exogenous HRP-labeled HSP70 (100 μg/mL) or were left untreated for 60 minutes and washed with PBS 3 times to remove free HSPs. Subsequently, they were lysed in MES buffer and processed for purification of lipid rafts (see “Materials and methods”). One of 2 different types of detergents was used: Brij98 or Triton X-100 to fractionate the material to isolate the fraction of DRMs. This fraction was further purified into light and heavy components using a sucrose gradient. These fractions obtained (light, LR; heavy, non-LR) were then analyzed using immunoblotting to detect HRP activity of HSP70-HRP complexes or tested for the presence of an LR-associated ganglioside GM1 by using HRP-cholera toxin B. Similarly, these fractions were tested for the amount of cholesterol using (Biovision Research Products, Palo Alto, CA). LR fractions were purified from HSP70-treated macrophages using Brij98 (Figure 4A) or Triton X (Figure 4B) methods showed that both, exogenously administered HSP70 and GM1, colocalized on the LR-microdomain of the macrophage plasma-membrane binding (Figure 4A). Similarly, cholesterol levels of the fractions that bound exogenous HSP70 were higher. Collectively, these results indicated that HSP70 bound to the same fractions that were enriched in LR-associated molecules, including GM1 and cholesterol and copurified with the LR-microdomains as purified by using 2 separate detergents.
Further, macrophages were treated with exogenous HRP-labeled HSP70 (100 μg/mL) in the presence of MCD (30 μM), which is known to disrupt the LR-integrity.26 Subsequently, macrophages were processed for purification of LRs by using Triton X-100. As shown in Figure 4B, there was a significant reduction in the binding of exogenously administered HSP70 when macrophages were treated with MCD. Similar reduction in the presence of GM1 and cholesterol further supported the evidence that this reduction in HSP70 binding was due to the disruption of the LR. To ensure that the viability of cells was not affected by treatment with MCD, we performed a trypan blue exclusion that revealed a 95% viability in both MCD-treated or untreated cells.
HSP70-macrophage interaction occurs on the lipid raft microdomain of macrophage plasma membranes. (A) Macrophages treated with exogenous HRP-labeled HSP70 (100 μg/mL, at 4°C) were washed, lysed with MBS buffer with 1% Brij98. Further fractionation using a sucrose gradient into lipid rafts (LR) (light fractions) or non-LR (heavy fractions) microdomains was undertaken. These fractions were tested by using detecting HRP activity of HRP-HSP70 complexes (as indicated); the presence of GM1 by using HRP-cholera toxin-B (as indicated); and assayed for the amount of cholesterol. (B) Similar to the conditions in panel A macrophages were treated with HSP70-HRP, and the LR-fractions were purified using Triton X-100 (as indicated). These fractions were tested for the presence of HSP70 and GM1 and were assayed for the amount of cholesterol. Further, the ability of macrophages to bind HSP70 was tested in the presence of LR-disrupting drug MCD. Macrophages were treated methyl-β-cyclodextrin (MCD; 30 mM), washed with cold PBS, and then incubated with exogenous HRP-labeled HSP70 (100 μg/mL) for 30 minutes on ice. Subsequently, macrophages were processed for purification of LRs by using Triton X-100 and tested for the presence of HSP70 and GM1 and were assayed quantitatively for the amount of cholesterol. (C) The influence of the LR-integrity on HSP70-mediated phagocytosis was tested by treating macrophages with nystatin or MCD (both drugs disrupt LRs), and a phagocytosis assay was performed. Macrophages treated with FCS served as controls to assess the effects of LR-disrupting drugs on opsonic phagocytosis. The results shown are a cumulative analysis of 3 experiments, 3 wells/group. (D) RAW264.7 macrophages were treated with HSP70 (100 μg/mL) in the presence of varying doses of MCD (as indicated). Error bars indicate one standard deviation.
HSP70-macrophage interaction occurs on the lipid raft microdomain of macrophage plasma membranes. (A) Macrophages treated with exogenous HRP-labeled HSP70 (100 μg/mL, at 4°C) were washed, lysed with MBS buffer with 1% Brij98. Further fractionation using a sucrose gradient into lipid rafts (LR) (light fractions) or non-LR (heavy fractions) microdomains was undertaken. These fractions were tested by using detecting HRP activity of HRP-HSP70 complexes (as indicated); the presence of GM1 by using HRP-cholera toxin-B (as indicated); and assayed for the amount of cholesterol. (B) Similar to the conditions in panel A macrophages were treated with HSP70-HRP, and the LR-fractions were purified using Triton X-100 (as indicated). These fractions were tested for the presence of HSP70 and GM1 and were assayed for the amount of cholesterol. Further, the ability of macrophages to bind HSP70 was tested in the presence of LR-disrupting drug MCD. Macrophages were treated methyl-β-cyclodextrin (MCD; 30 mM), washed with cold PBS, and then incubated with exogenous HRP-labeled HSP70 (100 μg/mL) for 30 minutes on ice. Subsequently, macrophages were processed for purification of LRs by using Triton X-100 and tested for the presence of HSP70 and GM1 and were assayed quantitatively for the amount of cholesterol. (C) The influence of the LR-integrity on HSP70-mediated phagocytosis was tested by treating macrophages with nystatin or MCD (both drugs disrupt LRs), and a phagocytosis assay was performed. Macrophages treated with FCS served as controls to assess the effects of LR-disrupting drugs on opsonic phagocytosis. The results shown are a cumulative analysis of 3 experiments, 3 wells/group. (D) RAW264.7 macrophages were treated with HSP70 (100 μg/mL) in the presence of varying doses of MCD (as indicated). Error bars indicate one standard deviation.
Next, macrophages were treated with medium alone, HSP70, or fetal calf serum (FCS) in the presence of either nystatin or MCD (30μM) (both agents are known to disrupt the LR integrity26 ; see Figure 4B). Subsequently, the cells were administered Alexafluor-labeled yeast (40 particles/macrophage), and the amount of yeast internalized was quantified as described in “Materials and methods.” As a control, macrophages treated with serum were also included in the assay to test the effects the LR-disrupting drugs on FcRγ-mediated antigen uptake. As shown in Figure 4C, HSP70-mediated phagocytosis was inhibited in the presence of LR-disrupting drugs, whereas opsonic phagocytosis via the FcRγ was not.
Further, macrophages were treated with HSP70 (100 μg/mL) in the presence of varying doses of MCD (ranging from 0 to 30 mM). As seen in Figure 4D, the inhibitory effect that MCD has on the macrophage response to HSP70 is titratable depending on the dose of MCD used.
Increased HSP70-mediated phagocytosis enhances MHC-II antigen processing and presentation
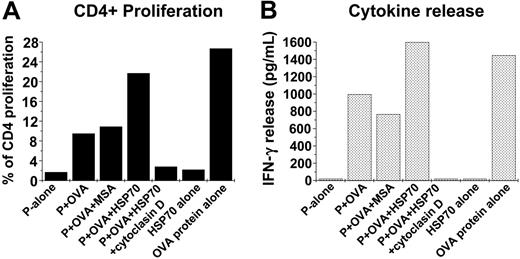
Alexafluor-labeled S cerevisiae were coated with whole ovalbumin protein 1 mg/mL by coincubation at 37°C for 20 minutes and washed until free ovalbumin protein was removed (washes were tested by gel electrophoresis using SDS-PAGE and immunoblotting). The association itself was confirmed by SDS-PAGE analysis and immunoblotting using antibody to ovalbumin (Sigma). The ovalbumin protein-coated-yeast complexes (Ova-coated yeast) were administered to 4 × 105 macrophages (40 yeast/macrophage) that were treated with either HSP70 (100 μg/mL) or control proteins in the presence or absence of cytochalasin-D to confirm that the process is phagocytosis dependent (Figure 5A). After 2 hours, the macrophages were washed, irradiated to prevent macrophage-proliferation, and cocultured with CSFE-labeled 2 × 105 CD4+ T cells purified from spleens of DO11.10 mice (I-Ad-restricted DO11.10 TCR-αβ-transgenic mice). The DO11.10 strain is transgenic against a MHC-II epitope of ovalbumin protein, and CD4+ T cells from these animals proliferate on being exposed to the antigenic epitope but not to the whole ovalbumin protein. Resultant proliferation of the CSFE-labeled CD4+ was measured by FACS as an indirect measure of the amount of ovalbumin protein processed and its peptide epitope presented in context of MCH-II. To quantify the effector function of the CD4+ cells, the production of IFN-γ by the CD4+ T cells was measured using ELISA. As shown in Figure 5B HSP70-treated macrophages cause an increase in CD4+ proliferation to 20% as compared with those treated with medium alone (1.7%). Further, cytochalasin-D treatment abrogated the proliferation caused by HSP70 treatment, indicating the net result was phagocytosis dependent. The net increase in CD4+ cells in response to HSP70 treatment was concordant with the increase in their ability to produce IFN-γ as compared with treatment with ova-coated yeast alone or HSP70 given alone (Figure 5C). Taken together, these results indicated that HSP70 mediated a sharp rise in the uptake of the yeast coated with ovalbumin protein antigen, enhanced its processing to generate the MHC-II-restricted antigenic epitope, and presented it effectively to generate a CD4+ T-cell response.
Increased HSP70-mediated phagocytosis enhances antigen presentation. (A) Yeast (S cerevisiae) was coated with ovalbumin (the whole protein) by coincubation at 37°C for 20 minutes. The complexes (Ova-coated yeast) were administered to macrophages treated with HSP70 (100 μg/mL) or control proteins in the presence or absence of cytochalasin-D. After 2 hours, the macrophages were washed, irradiated (to prevent macrophage-proliferation), and cocultured with CSFE-labeled CD4+ T cells purified from spleens of DO11.10 mice (I-Ad-restricted DO11.10 TCR-αβ-transgenic). (The DO11.10 strain is transgenic against a specific MHC-II epitope of ovalbumin protein.) Resultant CD4+ proliferation was measured using FACS as an indicator of the amount of the ovalbumin peptide presented in context of MCH-II antigen presentation. (B) Concurrent production of interferon γ (IFNγ) by the CD4+ T cells was quantified using ELISA as a measure of their effector function. The data shown represents 1 of 3 independent experiments.
Increased HSP70-mediated phagocytosis enhances antigen presentation. (A) Yeast (S cerevisiae) was coated with ovalbumin (the whole protein) by coincubation at 37°C for 20 minutes. The complexes (Ova-coated yeast) were administered to macrophages treated with HSP70 (100 μg/mL) or control proteins in the presence or absence of cytochalasin-D. After 2 hours, the macrophages were washed, irradiated (to prevent macrophage-proliferation), and cocultured with CSFE-labeled CD4+ T cells purified from spleens of DO11.10 mice (I-Ad-restricted DO11.10 TCR-αβ-transgenic). (The DO11.10 strain is transgenic against a specific MHC-II epitope of ovalbumin protein.) Resultant CD4+ proliferation was measured using FACS as an indicator of the amount of the ovalbumin peptide presented in context of MCH-II antigen presentation. (B) Concurrent production of interferon γ (IFNγ) by the CD4+ T cells was quantified using ELISA as a measure of their effector function. The data shown represents 1 of 3 independent experiments.
Discussion
The interaction of extracellular HSP70, a highly conserved intracellular molecule with the macrophage, stimulates them to phagocytose at 6 times the basal rate. Soon after HSP70 treatment, macrophages rapidly internalize a variety of particulate materials, including Gram-positive and -negative bacteria (Staphylococcus aureus, E coli), fungi (S cerevisiae, Candida albicans), and inert particles (polystyrene microspheres).
Further, HSP70-macrophage interaction occurs on the LR microdomain of the macrophage-cell surface. Our study identifies an important role of LRs in the HSP70-macrophage interaction and its downstream effects. Structurally, it appears that the exogenous HSP70 binds the macrophage on the lipid raft microdomain of the plasma membrane. Whether this binding is on a specific LR-bound receptor or whether it binds certain structural components of the LR is not currently known and is being investigated. By the same token, it is possible that the HSP70 is taken up by a different macrophage receptor and binds the inner leaflet of the LR. This possibility was not tested. Our study defines a functional role of lipid rafts. Although their physical existence has been questioned by some,49 our results define the influence of LRs on HSP phagocytosis. Disruption of the LRs partially abrogates the HSP-mediated effects on phagocytosis. Lipid rafts could provide a platform for the interaction between the HSP70 and the phagocytic receptors. Although our results indicate that the interaction with HSP70 occurs on the lipid raft, the nature of further signaling to involve the phagocytic receptors is not clear. Our preliminary work in defining the signaling pathway indicates that it is dependent on endocytosis (data not shown). Whether the entire HSP70-LR complex is endocytosed and if so, how this complex actually activates the phagocytic receptors needs further study. The fact that cycloheximide is unable to block HSP-mediated phagocytosis indicates that new protein synthesis is not required, partly explaining the quick onset of HSP-mediated phagocytosis. Disruption of the LRs abrogates the HSP-mediated phagocytosis, suggesting that LRs provide a platform for HSP70 and subsequent activation of the phagocytic receptors. Although several studies have shown that the endogenous HSP70 binds the LR,26,50 this provides further evidence of exogenous HSP70 binding the LR. Whether LRs play any other role in the HSP70-mediated phagocytic uptake (other than providing a structural platform for the HSP70 binding and facilitating further activation) needs further scrutiny. Functionally, LRs have been implicated in several cellular processes, including their ability to facilitate protein-protein interaction, raft-mediated endocytosis, signal transduction, and the biogenesis of the phagosome.27-29,31 Our current work did not study the signaling pathway involved; however, some of our findings provide vital clues to its characteristics. The quick onset of phagocytosis, within minutes of HSP70 treatment, and the nondependence on synthesis of new proteins (cycloheximide independent) suggest that HSP70-mediated enhancement of phagocytosis occurs via a short signaling pathway, possibly membrane bound and does not involve a gene up-regulation.
These results provide evidence that HSP70, a phylogenetically conserved molecule, plays an important role in the innate host response to pathogens. Physiologically, necrotic-cell lysis from injury or infection is known to release HSPs into the extracellular compartment where they activate APC-mediated defenses.10 It has been shown that the local concentration of HSP70 is in the range of 200 μg/g tissue lysed.10 In the context of earlier data, as little as 100 μg/mL HSP70 (which would correspond to 0.5 g tissue lysate) released extracellularly either from infection or injury could potentially elicit macrophage phagocytosis. It is important to note that the HSP70 is able to stimulate phagocytosis whether it carries peptides on it or not. Both ADP- and ATP-purified HSP70 are equally capable of stimulating phagocytosis. Because the nonspecific responses to HSPs are independent of peptides chaperoned by HSPs, they act similarly to bacterial lipopolysaccharides (LPSs). As seen in our results, our study has taken special precautions to exclude the effect of LPS.51,52
Our results reveal that extracellular HSPs are recognized by LR microdomain of the macrophage-triggering phagocytosis, a primal mechanism of self-defense. The significance of these findings is far reaching. This report indicates that the presence of extracellular HSP70, basically an intracellular molecule whose presence outside of the cell is unnatural, a result of tissue breakdown from infection or injury.10 It appears from our findings that a component of the LR microdomain recognizes the extracellularly released HSP70 to represent cell lysis and responds to it by up-regulating phagocytic antigen uptake. Modulating the HSP70-LR interaction presents an opportunity to intervene at the level of host-pathogen interface, a therapeutic tool for emerging infections, especially where conventional treatment with antibiotics is ineffective (antibiotic resistance) or unavailable (rapidly spreading, endemic). Sequentially, HSP70-mediated phagocytosis leads to increased antigenic processing and presentation to CD4+ T lymphocytes, which proliferate and release IFN-γ. In this context, HSP70-LR interaction plays an important role, not only clearing invading agents but also processing and presenting their antigens to host immune system. Although prokaryotic HSPs have been shown to be a potential source of microbial peptide antigens during phagocytic processing of bacteria during infection,53 our results indicate that antigen presentation is increased when macrophages are treated or pretreated with HSP70 and not otherwise. The wide range of microbial and nonmicrobial agents phagocytosed in response to HSP70-LR interaction as well as its remarkably short time of onset presents the opportunity to develop a rapidly deployable therapeutic intervention. Potentially, it could not only facilitate early elimination of the invading agent but also activate the immune system into a state of heightened preparedness.
Prepublished online as Blood First Edition Paper, November 1, 2005; DOI 10.1182/blood-2005-06-2559.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal