Comment on Zhang et al, page 1989
In this issue, Zhang and colleagues describe a subversive molecular pathway through which the HIV envelope glycoprotein (Env) may limit the immune response by suppressing the expression of CD40 ligand (CD40L, also called CD154) on CD4+ T cells, thereby preventing helper T-cell activation of dendritic cells.
Following T-cell receptor (TCR) engagement with peptide/MHC-II complexes on a dendritic cell (DC), CD4+ T cells express CD40L on their surface. CD40L then stimulates the DC to express CD80 and CD86, both ligands for CD28 on T cells. When a CD8+ T cell recognizes peptide/MHC-I on that DC, it receives both TCR and CD28 stimuli and departs as a fully activated CD8+ T cell. In the context of HIV infection, such CD8+ T cells may be especially important for holding down viral replication. A new realization, however, is that CD4+CD25+ regulatory T cells (“Tregs”) act on DCs to down-regulate CD80 and CD86 expression, leading to suppressed CD4+ and CD8+ T-cell responses1 and immune tolerance. However, strongly activated CD4+CD25- T cells express enough CD40L to overcome Treg effects on DCs and thereby promote effective CD8+ T-cell responses.2
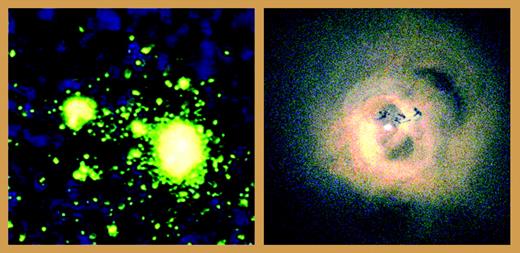
Areas of intense virion production in a lymph node were visualized by in situ hybridization autoradiography (left panel, from Zhang et al6; copyright 2004 National Academy of Sciences, USA). Radiation from the black hole in Perseus was visualized by x-ray detection (right panel, Chandra X-Ray Observatory, NASA, by permission).
Areas of intense virion production in a lymph node were visualized by in situ hybridization autoradiography (left panel, from Zhang et al6; copyright 2004 National Academy of Sciences, USA). Radiation from the black hole in Perseus was visualized by x-ray detection (right panel, Chandra X-Ray Observatory, NASA, by permission).
Enter now HIV. As suggested by Zhang and colleagues, HIV Env binding to the CD4 molecule suppresses CD40L expression by stimulated CD4+ T cells. This deprives DCs of strong CD40 stimulation, giving Tregs the upper hand over DCs and leading to the suppression of both CD4+ and CD8+ T cells. This concept is partly supported by the findings of Pitcher et al,3 who used intracytoplasmic staining for IFN-γ to study anti-HIV CD4+ T-cell responses in long-term nonprogressors. Even though these subjects had significant CD4+ T-cell responses to Gag, they had little or no detectable responses to Env.3 The results of Zhang et al suggest one explanation for this finding: Env suppresses CD40L expression leading to reduced DC activation and the induction of Tregs that suppress CD4+ T-cell responses. As Kinter et al4 found, removing Tregs can significantly augment the in vitro responses of T cells from HIV-infected subjects.
If the defective CD4+ T-cell response were limited to Env, it might play only a minor role in the response to a multiantigenic pathogen such as HIV. However, DCs lacking a strong CD40 stimulus can subsequently engage additional antigen-specific CD4+ T cells and drive them to become new Tregs. Using unactivated DCs as an intermediary, Tregs give rise to more Tregs, leading to what transplant immunologists call “infectious tolerance,”5 a kind of immunologic black hole. And because Treg suppression is antigen nonspecific, the resulting Tregs threaten to suppress effective T cell responses to other HIV antigens, as might occur in subjects progressing to AIDS.
Zhang et al found that a considerable quantity of HIV virions is needed to suppress CD40L expression. This makes it likely that the Env-centered immunologic black holes are superimposed in time and space over hot spots of viral replication and accumulation. By in situ hybridization, such sites gleam brightly under the microscope. From the immune system's perspective, however, they may be dark regions spouting out Tregs that suppress effective anti-HIV immunity. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal