The development of novel cell-based therapies requires understanding of distinct human hematopoietic stem and progenitor cell populations. We recently isolated reconstituting hematopoietic stem cells (HSCs) by lineage depletion and purification based on high aldehyde dehydrogenase activity (ALDHhiLin- cells). Here, we further dissected the ALDHhi-Lin- population by selection for CD133, a surface molecule expressed on progenitors from hematopoietic, endothelial, and neural lineages. ALDHhiCD133+Lin- cells were primarily CD34+, but also included CD34-CD38-CD133+ cells, a phenotype previously associated with repopulating function. Both ALDHhiCD133-Lin- and ALDHhiCD133+Lin- cells demonstrated distinct clonogenic progenitor function in vitro, whereas only the ALDHhiCD133+Lin- population seeded the murine bone marrow 48 hours after transplantation. Significant human cell repopulation was observed only in NOD/SCID and NOD/SCID β2M-null mice that received transplants of ALDHhiCD133+Lin- cells. Limiting dilution analysis demonstrated a 10-fold increase in the frequency of NOD/SCID repopulating cells compared with CD133+Lin- cells, suggesting that high ALDH activity further purified cells with repopulating function. Transplanted ALDHhiCD133+Lin- cells also maintained primitive hematopoietic phenotypes (CD34+CD38-) and demonstrated enhanced repopulating function in recipients of serial, secondary transplants. Cell selection based on ALDH activity and CD133 expression provides a novel purification of HSCs with long-term repopulating function and may be considered an alternative to CD34 cell selection for stem cell therapies.
Introduction
Hematopoietic stem cells (HSCs) are required to repopulate all blood cell lineages throughout the life span of an individual. Human HSCs have traditionally been characterized by the expression of cell surface markers such as CD34,1,2 but not all human hematopoietic repopulating cells express CD34,3,4 and cell surface phenotype can be altered by cell cycle progression and ex vivo manipulation.5-10 A purification strategy complementary to the use of surface phenotype involves the assessment of intracellular enzyme activities associated with the protection of primitive cells from oxidative insult during hematopoietic development. One promising purification strategy exploits cytosolic aldehyde dehydrogenase (ALDH), an enzyme implicated in retinoid metabolism and the resistance of HSCs to alkylating agents such as cyclophosphamide.11,12 Murine repopulating cells13,14 and human hematopoietic progenitors have previously been isolated based on increased activity of intracellular ALDH.15,16
We have previously characterized a novel reconstituting HSC population from human umbilical cord blood (UCB) isolated by depletion of cells with mature lineage markers (Lin-) and selection of cells with high ALDH activity.17 ALDHhiLin- cells demonstrated enriched expression of the primitive cell markers CD34 and CD133. Clonogenic progenitor function and in vivo reconstituting ability were restricted to the ALDHhi and not the ALDHlo population.17 Moreover, Storms et al18 were the first to use ALDH activity to delineate distinct CD34+ stem and progenitor cell compartments within human UCB. Thus, ALDH-mediated metabolism of a fluorescent substrate (Aldefluor) and subsequent flow cytometry are valuable tools for the prospective isolation of human hematopoietic stem and progenitor cells with distinct functions.
In this study, we used a similar strategy to purify the ALDHhi-Lin- population based on the expression of CD133 (or AC133; reviewed in Shmelkov et al19 and Fargeas et al20 ), a surface molecule expressed on primitive human progenitor cells of hematopoietic,21-23 endothelial,24-27 and neural epithelial lineages.28-31 CD133 is a unique, 5-membrane-spanning cell surface molecule that does not share homology with previously described HSC surface antigens.23 CD133 is rapidly down-regulated as human HSCs differentiate into phenotypically restricted cells.22,23 Giebel et al32 recently reported that CD34+ cells from UCB redistribute CD133 in lipid rafts during in vitro culture, and polarized CD133 expression is involved in the migration of HSCs in response to stromal-derived factor-1 (SDF-1). Thus, CD133 may be involved in the migration of hematopoietic stem and progenitor cells to the BM microenvironment following irradiation and transplantation.
To dissect the hematopoietic functions of ALDHhiLin- cells from human UCB, ALDHhiCD133-Lin- and ALDHhiCD133+Lin- cells were characterized for primitive cell surface phenotype, in vitro clonogenic progenitor production, and in vivo repopulating capacity. These highly purified populations were primarily CD34+, but the ALDHhiCD133+Lin- population also included CD34-CD38- cells, a primitive phenotype associated with SCID repopulating cell (SRC) function.3,21,22 Both ALDHhiCD133-Lin- and ALDHhiCD133+Lin- cells possessed hematopoietic progenitor function in vitro. However, only the ALDHhiCD133+Lin- population showed efficient homing to the murine BM microenvironment, multilineage hematopoietic repopulation in primary recipients, and the maintenance of primitive cell phenotype (CD34+CD38-) after transplantation into immune-deficient mice. The long-term reconstituting function of ALDHhiCD133+Lin- cells was confirmed by engraftment of recipients of serial, secondary transplants. Collectively, we have identified a novel HSC population with preserved long-term repopulating function that may represent an alternative to CD34 cells for future clinical transplantation applications.
Materials and methods
Human cell purification
UCB was obtained from the cord blood banking facility at Cardinal Glennon Children's Hospital, St Louis, MO, and used in accordance with the ethical authorities at Washington University School of Medicine. UCB mononuclear cells (MNCs) were isolated by Hypaque-Ficoll centrifugation (Pharmacia Biotech, Uppsala, Sweden) and enriched for Lin- cells as previously described.8,33 Lin- cells were purified based on ALDH activity by staining with the Aldefluor reagent (StemCo Biomedical, Durham, NC), according to the manufacturer's specifications. Briefly, Aldefluor substrate (0.625 μg/mL) was added to 1 to 5 × 106 Lin- cells/mL suspended in Aldefluor assay buffer and incubated for 20 to 30 minutes at 37°C to allow the conversion of Aldefluor substrate, a green fluorescent product retained within the cell due to its negative charge.15,34 For each experiment, an aliquot of Aldefluor-stained cells was immediately quenched with 5 μL 1.5-mM diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor, to serve as a negative control. Cells were costained with human CD133-APC (clone-1) antibody (Miltenyi Biotechnology, Gladbach, Germany). Sorted ALDHhiCD133-Lin- or ALDHhiCD133+Lin- cells were checked for purity using CD133-PE (clone-2; Miltenyi Biotechnology). Sorted populations were costained with human CD34-PE-Cy7 and CD38-PE (Becton Dickinson, San Jose, CA) and analyzed on a Coulter FC-500 flow cytometer (Beckman-Coulter, Miami, FL).
Clonogenic progenitor assays
Human clonogenic progenitor assays were performed by culture of purified cell populations in methylcellulose media (Methocult H4434; Stem Cell Technologies, Vancouver, BC), supplemented with 2 U/mL rH erythropoietin 4 days after culture initiation. Colonies were enumerated by microscopy after incubation at 37°C for 14 to 17 days.
Transplantation
Purified cells were transplanted by tail-vein injection into 8- to 10-week-old, sublethally irradiated (300 cGy) NOD/SCID or NOD/SCID β2 microglobulin (β2M)-null mice (Jackson Laboratories, Bar Harbor, ME). Mice that received transplants of low numbers of purified cells were coinjected with 105 irradiated (1500 cGy) Lin+ accessory cells.35
Analysis of human engraftment
At 7 to 8 weeks after transplantation, BM (tibiae and fibiae), spleen, and peripheral blood were harvested, and red cells were lysed with a 0.8% ammonium chloride solution. Cells were incubated for 30 minutes at 4°C with blocking solution and monoclonal antibodies for the human panleukocyte marker CD45, in combination with CD38 or isotype controls (BD). Cells were analyzed on a Coulter FC-500 flow cytometer (Beckman-Coulter). Low-frequency (< 0.2% CD45+) human engraftment was confirmed by human-specific P17H8 sequence polymerase chain reaction (PCR).36 Highly engrafted mouse BM (> 20% CD45+) was further analyzed for the frequency of B-lymphoid cells (CD20, CD19), myeloid cells (CD14, CD33), T-lymphoid cells (CD4, CD8), and primitive cells (CD34, CD38) (BD).
Secondary transplantation
BM cells were isolated from highly engrafted primary NOD/SCID or NOD/SCID β2M-null mice and injected directly into the tail vein of NOD/SCID or NOD/SCID β2M-null secondary recipients. Alternatively, BM cells were cultured for 16 hours in serum-free culture media supplemented with SCF (10 ng/mL) and IL-6 (10 ng/mL) before secondary injection of 2 × 106 surviving cells. BM of mice that received serial, secondary transplants was analyzed as described for primary recipients.
Statistics
Levels of human engraftment were reported as the mean plus or minus standard error of the mean (SEM) for mice grouped according to transplanted cell numbers. Limiting dilution analysis (LDA) was performed using Poisson statistics at 95% confidence intervals. Statistical significance for colony-forming unit (CFU) data and the expression of cell surface markers were assessed by Student t test.
Results
Purification of ALDHhiCD133-Lin- and ALDHhiCD133+Lin- cells
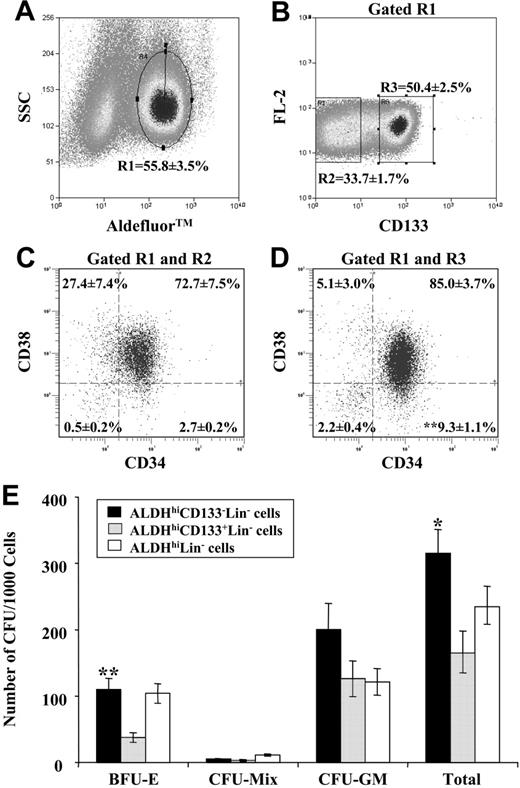
We previously described the purification of ALDHhi cells from Lin- UCB cells.17 A high percentage of ALDHhiLin- cells expressed the cell surface marker CD133. Because CD133 is conserved on stem and progenitor cells from neural, endothelial, and hematopoietic systems, we further purified the ALDHhiLin- UCB population based on CD133 expression (ALDHhiCD133-Lin- or ALDHhiCD133+Lin-). As indicated in Figure 1, the UCB Lin- MNC population was first selected for high ALDH activity (R1, 55.8% ± 3.5%, Figure 1A). Using a stringent gating strategy (Figure 1B), CD133- and CD133+ cells represented 33.7% ± 1.7% (R2) and 50.4% ± 2.5% (R3) of the ALDHhiLin- cells, or 14.7% ± 2.1% and 23.2% ± 4.3% of total Lin-, respectively. Purity for CD133 expression was more than 96% upon reanalysis (data not shown). Using this flow cytometric strategy, purified ALDHhiCD133- and ALDHhiCD133+ cells represented approximately 0.1% and 0.14% of the total UCB MNCs, respectively.
Selection for CD34+CD38- cells purifies HSCs with enhanced repopulating function.2,4,8 However, CD34-CD133+ cells have also shown primitive repopulating ability.21,22 As a consequence, purification of HSCs based solely on CD34 expression may exclude beneficial repopulating cells. Therefore, we analyzed ALDHhiCD133-Lin- and ALDHhiCD133+Lin- cells for CD34 and CD38 expression. ALDHhiLin- cells predominantly express CD34 (> 90%).17 The ALDHhiCD133-Lin- and ALDHhiCD133+Lin- populations highly coexpressed CD34 at 75% and 95%, respectively (Figure 1C). However, only 2.7% ± 0.2% of ALDHhiCD133-Lin- cells were CD34+CD38-, whereas ALDHhiCD133+Lin- cells included an increased (P < .01) proportion of primitive CD34+CD38- cells at 9.3% ± 1.1%. In addition, ALDHhiCD133+Lin- cells included a small cluster of CD34-CD38- cells (2.2% ± 0.4%, Figure 1D). Collectively, the ALDHhiCD133+Lin- population included CD34+ and CD34- cells previously associated with SRC function.1,2,21
Isolation and in vitro progenitor activity of purified ALDHhiCD133-Lin- and ALDHhiCD133+Lin- cell populations. (A) Lin- cells incubated with Aldefluor substrate were used to select ALDHhi cells (R1, 55.8% ± 3.5%). (B) Staining for CD133 expression revealed the ALDHhiCD133-Lin- (R2 = 33.7% ± 1.7%) and ALDHhiCD133+Lin- (R3 = 50.4% ± 2.5%) purified populations. (C-D) Isolated ALDHhiCD133-Lin- and ALDHhiCD133+Lin- sorted cells were analyzed for CD34 and CD38 expression. Purified ALDHhiCD133+Lin- cells were enriched for repopulating CD34+CD38- cells (**P < .01) and included primitive CD34-CD38- cells. Data represent the mean ± SEM for cells isolated from 10 UCB samples. (E) Purified ALDHhiCD133-Lin-, ALDHhiCD133+Lin-, or ALDHhiLin- cells were cultured in methylcellulose media and erythrocyte, mixed, and granulocyte/macrophage colonies (BFU-E, Mix, CFU-GM) were enumerated after 14 to 17 days of in vitro culture. Data represent the number of individual colonies produced per 1000 cells plated from each population. Data are expressed as mean ± SEM for cells isolated from 4 to 6 UCB Lin- samples (*P < .05; **P < .01).
Isolation and in vitro progenitor activity of purified ALDHhiCD133-Lin- and ALDHhiCD133+Lin- cell populations. (A) Lin- cells incubated with Aldefluor substrate were used to select ALDHhi cells (R1, 55.8% ± 3.5%). (B) Staining for CD133 expression revealed the ALDHhiCD133-Lin- (R2 = 33.7% ± 1.7%) and ALDHhiCD133+Lin- (R3 = 50.4% ± 2.5%) purified populations. (C-D) Isolated ALDHhiCD133-Lin- and ALDHhiCD133+Lin- sorted cells were analyzed for CD34 and CD38 expression. Purified ALDHhiCD133+Lin- cells were enriched for repopulating CD34+CD38- cells (**P < .01) and included primitive CD34-CD38- cells. Data represent the mean ± SEM for cells isolated from 10 UCB samples. (E) Purified ALDHhiCD133-Lin-, ALDHhiCD133+Lin-, or ALDHhiLin- cells were cultured in methylcellulose media and erythrocyte, mixed, and granulocyte/macrophage colonies (BFU-E, Mix, CFU-GM) were enumerated after 14 to 17 days of in vitro culture. Data represent the number of individual colonies produced per 1000 cells plated from each population. Data are expressed as mean ± SEM for cells isolated from 4 to 6 UCB Lin- samples (*P < .05; **P < .01).
ALDHhiCD133-Lin- and ALDHhiCD133+Lin- cells show hematopoietic progenitor function
ALDHhiLin- cells not purified on CD133 expression demonstrated in vitro progenitor activity, and produced erythroid (BFU-E), granulocyte/macrophage (CFU-GM), and mixed colonies (CFU-Mix) with a plating efficiency of 1 CFU in 5 cells.17 Figure 1E illustrates the hematopoietic colony formation by purified ALDHhiCD133-Lin- and ALDHhiCD133+Lin- cells. Colony production was enhanced (P < .05) in the ALDHhiCD133-Lin- population, producing 315 colonies per 1000 cells (1 CFU in 3.2 cells), whereas the ALDHhiCD133+Lin- population produced 167 colonies per 1000 cells (1 CFU in 6 cells). The enhanced plating efficiency by ALDHhiCD133-Lin- cells was predominantly due to increased BFU-E production compared with ALDHhiCD133+Lin- cells (P < .01), supporting previous results that BM CD133+CD34+ cells rarely produced BFU-E colonies.22 However, in vitro clonogenic progenitor production does not guarantee repopulating function in immune-deficient mice.37
Human ALDHhiCD133-Lin- cells demonstrate reduced homing to the murine BM microenvironment
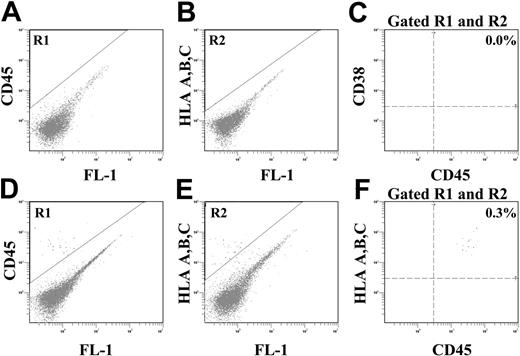
CD133 expression has recently been implicated in the migration of CD34+ hematopoietic progenitor cells in response to a stromal-derived factor-1 (SDF-1) gradient.32 To assay whether ALDHhiCD133-Lin- or ALDHhiCD133+Lin- progenitor cells could efficiently home to the BM microenvironment following transplantation, we injected these purified populations into the tail vein of sublethally irradiated (300 cGy) NOD/SCID β2M-null mice and analyzed for the active homing of human cells to the murine BM. At 48 hours after transplantation, human hematopoietic cells were detected in the murine BM by the stringent coexpression of CD45 and HLA A, B, C by flow cytometry (Figure 2). After the injection of 2 × 105 ALDHhiCD133-Lin- cells (Figure 2A-C), no human cells could be detected in BM or spleen of mice that underwent transplantation. In contrast, transplantation of an equivalent dose (2 × 105) of ALDHhiCD133+Lin- cells (Figure 2D-F) resulted in the detection of BM resident human hematopoietic cells. Mouse BM and spleen were analyzed for a total of 24 mice that underwent transplantation injected with ALDHhiCD133-Lin- (n = 10), ALDHhiCD133+Lin- (n = 11), or unfractioned Lin- (n = 3) cells. In summary, 105 to 4 × 105 ALDHhiCD133+Lin- cells consistently engrafted 9 of 11 mice that underwent transplantation, which confirmed the active homing of these cells to the murine BM (Table 1). Injection of 105 to 4 × 105 ALDHhiCD133-Lin- cells did not result in significant human cell homing to the murine BM after 48 hours. In addition, transplantation of 4 × 105 unfractioned Lin- cells was needed to produce detectable BM engraftment. These short-term engraftment studies indicated that purified ALDHhiCD133+Lin- cells demonstrate enhanced seeding to the murine BM microenvironment when compared with the homing-competent ALDHhiCD133-Lin- cells (Table 1).
Summary of cell homing to the murine BM 48 hours after tail vein injection
Injected cell dose . | Human engraftment, %* . | BM homing at 48 hours, no./no. total (%) . |
|---|---|---|
| 105 ALDHhiCD133-Lin- | 0, 0, 0, 0 | 0/5 (0) |
| 2 × 105 ALDHhiCD133-Lin- | 0, 0, 0 | 0/3 (0) |
| 4 × 105 ALDHhiCD133-Lin- | 0, 0 | 0/2 (0) |
| 105 ALDHhiCD133+Lin- | 0, 0, 0.2, 0.2, 0.3 | 3/5 (60) |
| 2 × 105 ALDHhiCD133+Lin- | 0.2, 0.3, 0.3, 0.4 | 4/4 (100) |
| 4 × 105 ALDHhiCD133+Lin- | 0.3, 0.4 | 2/2 (100) |
| 4 × 105 Lin- | 0, 0, 0.4 | 1/3 (33) |
Injected cell dose . | Human engraftment, %* . | BM homing at 48 hours, no./no. total (%) . |
|---|---|---|
| 105 ALDHhiCD133-Lin- | 0, 0, 0, 0 | 0/5 (0) |
| 2 × 105 ALDHhiCD133-Lin- | 0, 0, 0 | 0/3 (0) |
| 4 × 105 ALDHhiCD133-Lin- | 0, 0 | 0/2 (0) |
| 105 ALDHhiCD133+Lin- | 0, 0, 0.2, 0.2, 0.3 | 3/5 (60) |
| 2 × 105 ALDHhiCD133+Lin- | 0.2, 0.3, 0.3, 0.4 | 4/4 (100) |
| 4 × 105 ALDHhiCD133+Lin- | 0.3, 0.4 | 2/2 (100) |
| 4 × 105 Lin- | 0, 0, 0.4 | 1/3 (33) |
Each value represents one of the mice tested
Human ALDHhiCD133+Lin- cells are enriched for NOD/SCID repopulating function
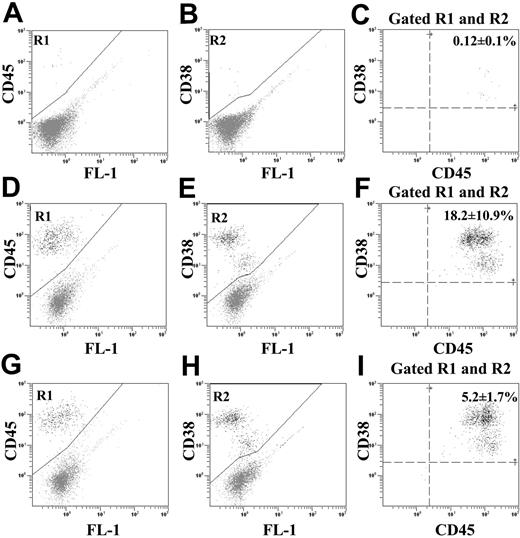
Purified ALDHhiCD133-Lin- or ALDHhiCD133+Lin- cells were transplanted into sublethally irradiated (300 cGy) NOD/SCID β2M-null or NOD/SCID mice. Mice that received transplants of low-dose purified human hematopoietic populations received co-transplants of 105 irradiated (1500 cGy) Lin+ cells to support engraftment.35 Irradiated Lin+ accessory cells possessed no repopulating function when transplanted alone (data not shown). Human hematopoietic cells (CD45+/CD38+) were detected in the BM of NOD/SCID β2M-null recipients 7 to 8 weeks after injection with either ALDHhiCD133-Lin- (Figure 3A-C), ALDHhiCD133+Lin- (Figure 3D-F), or ALDHhiLin- (Figure 3G-I) cells. Flow cytometric analysis after injection of 104 ALDHhiCD133+Lin- cells demonstrated an increased frequency (18.2% ± 10.9%) of human engraftment in the NOD/SCID β2M-null BM (Figure 3F, n = 6) compared with injection of 104 ALDHhiLin- cells (5.2% ± 1.7%) (Figure 3I, n = 5). The engraftment frequency of ALDHhiLin- cells in these experiments was similar to our previously reported analysis.17 Transplantation of 10-fold more (> 105) ALDHhiCD133-Lin- cells was required to observe minimal engraftment (< 0.5% CD45+) in NOD/SCID β2M-null mice (Figure 3C, n = 5). Injection of less than 105 ALDHhiCD133-Lin- cells did not result in human engraftment in either model. Thus, ALDHhiCD133-Lin- cells primarily contained committed myeloerythroid progenitors that did not significantly repopulate NOD/SCID or NOD/SCID β2M-null mice.
BM homing of purified ALDHhiCD133+Lin- or ALDHhiCD133-Lin- cells 48 hours after transplantation. Representative flow cytometric analysis of NOD/SCID β2M-null mice that received transplants of 2 × 105 purified (A-C) ALDHhiCD133-Lin- or (D-F) ALDHhiCD133+Lin- cells. At 48 hours after transplantation, human hematopoietic cells were detected in the murine BM by coexpression of CD45 (R1) and HLA A, B, and C (R2).
BM homing of purified ALDHhiCD133+Lin- or ALDHhiCD133-Lin- cells 48 hours after transplantation. Representative flow cytometric analysis of NOD/SCID β2M-null mice that received transplants of 2 × 105 purified (A-C) ALDHhiCD133-Lin- or (D-F) ALDHhiCD133+Lin- cells. At 48 hours after transplantation, human hematopoietic cells were detected in the murine BM by coexpression of CD45 (R1) and HLA A, B, and C (R2).
Detection of human cell repopulation in mice that received transplants of purified ALDHhiCD133-Lin-, ALDHhiCD133+Lin-, or ALDHhiLin- cells. Representative flow cytometric analysis of NOD/SCID β2M-null mice that received transplants of (A-C) 2 × 105 ALDHhiCD133-Lin-, (D-F) 104 ALDHhiCD133+Lin-, or (G-I) 104 ALDHhiLin- cells. At 7 to 8 weeks after transplantation, human hematopoietic cells in the mouse BM were detected by coexpression of CD45 (R1) with CD38 (R2). Cell suspensions from murine spleen and peripheral blood were analyzed in an identical fashion. Injection of more than 2 × 105 ALDHhiCD133-Lin- cells was required to observe human cell engraftment in the BM of NOD/SCID β2M-null mice (n = 3). Mice that received transplants of 104 ALDHhiCD133+Lin- cells showed enhanced engraftment with human cells (18.2% ± 10.9%, n = 6) in the murine BM, compared with mice that received transplants of 104 ALDHhiLin- cells not selected for CD133 expression (5.2% ± 1.7%, n = 5).
Detection of human cell repopulation in mice that received transplants of purified ALDHhiCD133-Lin-, ALDHhiCD133+Lin-, or ALDHhiLin- cells. Representative flow cytometric analysis of NOD/SCID β2M-null mice that received transplants of (A-C) 2 × 105 ALDHhiCD133-Lin-, (D-F) 104 ALDHhiCD133+Lin-, or (G-I) 104 ALDHhiLin- cells. At 7 to 8 weeks after transplantation, human hematopoietic cells in the mouse BM were detected by coexpression of CD45 (R1) with CD38 (R2). Cell suspensions from murine spleen and peripheral blood were analyzed in an identical fashion. Injection of more than 2 × 105 ALDHhiCD133-Lin- cells was required to observe human cell engraftment in the BM of NOD/SCID β2M-null mice (n = 3). Mice that received transplants of 104 ALDHhiCD133+Lin- cells showed enhanced engraftment with human cells (18.2% ± 10.9%, n = 6) in the murine BM, compared with mice that received transplants of 104 ALDHhiLin- cells not selected for CD133 expression (5.2% ± 1.7%, n = 5).
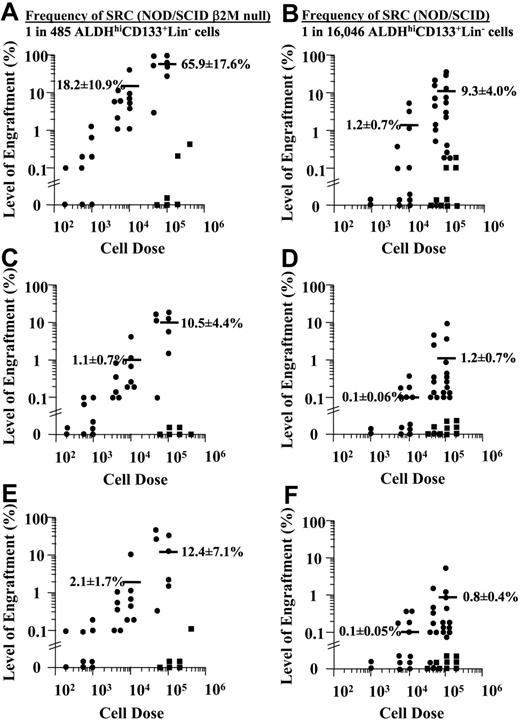
Transplantation of 33 NOD/SCID β2M-null or 41 NOD/SCID mice with 102 to 4 × 105 ALDHhiCD133+Lin- cells produced human engraftment in the BM (Figure 4A-B), spleen (Figure 4C-D), and peripheral blood (Figure 4E-F) 7 to 8 weeks after transplantation. As few as 100 purified ALDHhiCD133+Lin- cells engrafted NOD/SCID β2M-null mice, whereas more than 5 × 103 ALDHhiCD133+Lin- cells were needed to engraft the parental NOD/SCID strain (Figure 4B). Low levels of human engraftment (< 0.2% CD45+) were confirmed independently by human-specific P17H8 PCR (data not shown). Increased doses of ALDHhiCD133+Lin- cells resulted in elevated human chimerism. Overall, engraftment was approximately 10-fold higher in the NOD/SCID β2M-null compared with the less permissive NOD/SCID model, and detection of human cells was enhanced in the periphery of NOD/SCID β2M-null mice. In contrast, 5 × 104 to 4 × 105 ALDHhiCD133-Lin- cells showed only a low level of human engraftment (< 0.5% CD45+) in 2 of 6 NOD/SCID β2M-null mice that underwent transplantation, or 3 of 9 NOD/SCID mice that underwent transplantation. Using LDA and Poisson statistics, SCID repopulating cell (SRC) frequencies for ALDHhiCD133+Lin- cells were 1 SRC in 485 cells in NOD/SCID β2M-null mice and 1 SRC in 16 046 cells in NOD/SCID mice.
Summary of human cell repopulation in mice that received transplants of ALDHhiCD133-Lin- or ALDHhiCD133+Lin- cells. A summary of the level of human engraftment in the BM (A-B), spleen (C-D), and peripheral blood (E-F) of NOD/SCID β2M-null mice (A,C,E; n = 33) or NOD/SCID mice (B,D,F; n = 41) that received transplants of purified 5 × 104 to 4 × 105 ALDHhiCD133-Lin- (▪) or 2 × 102 to 105 purified ALDHhiCD133+Lin- (•) cells. Horizontal bars represent the average level of human engraftment (mean ± SEM) at 104 or 105 injected cells. The frequency of BM repopulating cells by LDA was 1 SRC in 485 ALDHhiCD133+Lin- cells in the NOD/SCID β2M-null mouse or 1 SRC in 16 064 ALDHhiCD133+Lin- in the NOD/SCID mouse. Mice received transplants of the purified cells from 34 cord blood donors.
Summary of human cell repopulation in mice that received transplants of ALDHhiCD133-Lin- or ALDHhiCD133+Lin- cells. A summary of the level of human engraftment in the BM (A-B), spleen (C-D), and peripheral blood (E-F) of NOD/SCID β2M-null mice (A,C,E; n = 33) or NOD/SCID mice (B,D,F; n = 41) that received transplants of purified 5 × 104 to 4 × 105 ALDHhiCD133-Lin- (▪) or 2 × 102 to 105 purified ALDHhiCD133+Lin- (•) cells. Horizontal bars represent the average level of human engraftment (mean ± SEM) at 104 or 105 injected cells. The frequency of BM repopulating cells by LDA was 1 SRC in 485 ALDHhiCD133+Lin- cells in the NOD/SCID β2M-null mouse or 1 SRC in 16 064 ALDHhiCD133+Lin- in the NOD/SCID mouse. Mice received transplants of the purified cells from 34 cord blood donors.
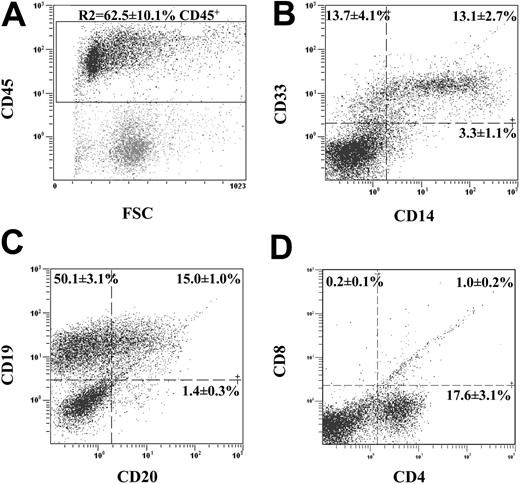
Transplanted human ALDHhiCD133+Lin- cells differentiate into lymphoid and myeloid progeny in vivo. BM from highly engrafted mice that received transplants of 104 to 105 ALDHhiCD133+Lin- cells was stained with human-specific antibodies for mature hematopoietic lineage markers. (A) Human hematopoietic cells were selected by the expression of human CD45 (R2 = 62.5% ± 10.1%, n = 6) and analyzed for myeloid cell markers CD14 and CD33 (B), B-lymphocyte markers CD20 and CD19 (C), and T-lymphocyte markers CD4 and CD8 (D). Lymphoid and myeloid differentiation was observed after the transplantation of purified ALDHhiCD133+Lin- cells. T-lymphocyte production was not supported in the NOD/SCID β2M-null or NOD/SCID mouse.
Transplanted human ALDHhiCD133+Lin- cells differentiate into lymphoid and myeloid progeny in vivo. BM from highly engrafted mice that received transplants of 104 to 105 ALDHhiCD133+Lin- cells was stained with human-specific antibodies for mature hematopoietic lineage markers. (A) Human hematopoietic cells were selected by the expression of human CD45 (R2 = 62.5% ± 10.1%, n = 6) and analyzed for myeloid cell markers CD14 and CD33 (B), B-lymphocyte markers CD20 and CD19 (C), and T-lymphocyte markers CD4 and CD8 (D). Lymphoid and myeloid differentiation was observed after the transplantation of purified ALDHhiCD133+Lin- cells. T-lymphocyte production was not supported in the NOD/SCID β2M-null or NOD/SCID mouse.
CD133-expressing cells demonstrate repopulating function in NOD/SCID mice and in preimmune fetal sheep.21,22 Therefore, we directly compared the repopulating ability of ALDHhiCD133+Lin- and CD133+Lin- cells not purified by ALDH expression, by transplantation at identical doses (2 × 102-105 cells) in NOD/SCID β2M-null siblings. Similar to our previous results,ALDHhiCD133+Lin- cells engrafted mice that received transplants of 2 × 102 to 103 cells, a dose at which CD133+Lin- cells did not engraft (Table 2). Injection of more than 5 × 103 CD133+Lin- cells was needed to observe consistent human engraftment. The repopulating frequency in these mice (n = 42) was 1 SRC in 691 ALDHhiCD133+Lin- cells, confirming our previous frequency in Figure 4, compared with 1 SRC in 6681 CD133+Lin- cells (Table 2). Purification of CD133+Lin- cells with high ALDH activity selects for cells with enhanced hematopoietic repopulating capacity.
Limiting dilution analysis of transplanted ALDHhiCD133+Lin- or CD133+Lin- cells
. | No. of mice engrafted/no. that received transplants (%) . | . | |
|---|---|---|---|
| No. of injected cells . | ALDHhiCD133+Lin- cells . | CD133+Lin- cells . | |
| 200 | 1/2 (50) | 0/2 (0) | |
| 500 | 1/2 (50) | 0/2 (0) | |
| 1 000 | 2/3 (67) | 0/2 (0) | |
| 5 000 | 5/5 (100) | 2/4 (50) | |
| 10 000 | 3/3 (100) | 5/6 (83) | |
| 100 000 | 5/5 (100) | 6/6 (100) | |
. | No. of mice engrafted/no. that received transplants (%) . | . | |
|---|---|---|---|
| No. of injected cells . | ALDHhiCD133+Lin- cells . | CD133+Lin- cells . | |
| 200 | 1/2 (50) | 0/2 (0) | |
| 500 | 1/2 (50) | 0/2 (0) | |
| 1 000 | 2/3 (67) | 0/2 (0) | |
| 5 000 | 5/5 (100) | 2/4 (50) | |
| 10 000 | 3/3 (100) | 5/6 (83) | |
| 100 000 | 5/5 (100) | 6/6 (100) | |
NOD/SCID β2M-null siblings (n = 42) received injections of increasing doses of ALDHhiCD133+Lin- cells or CD133+Lin- cells not purified by ALDH activity and analyzed for human engraftment 7 to 8 weeks after transplantation. SRC frequency and 95% confidence intervals were calculated using Poisson statistics at limiting dilution. ALDHhiCD133+Lin- cells was 1 in 691 cells; for CD133+Lin- cells, it was 1 in 6681 cells. The 95% confidence interval for ALDHhiCD133+Lin- cells was 246-1940 cells; for CD133+Lin- cells, it was 3073-14 525 cells.
Human ALDHhiCD133+Lin- cells demonstrate multilineage engraftment
Chimeric BM was analyzed for the presence of human-specific, lineage-restricted cell surface markers (Figure 5). Transplantation of 5 × 104 to 105 ALDHhiCD133+Lin- cells consistently produced high levels of human engraftment (62.5% ± 10.1% CD45+, n = 6) in the NOD/SCID β2M-null mouse, indicating that ALDHhiCD133+Lin- cells demonstrated extensive proliferation in vivo. Gated human CD45+ cells (R1, Figure 5A) demonstrated surface markers restricted to cells of the myeloid (Figure 5B) and lymphoid (Figure 5C) lineages. Cells with coexpression of CD14 with CD33 (13.1% ± 2.7%) and CD20 with CD19 (15.0% ± 1.0%) confirmed the presence of maturing human monocyte/macrophages and B cells, respectively. Although human cells expressing CD4 were detected (17.6% ± 3.1%, Figure 5D), CD3 and CD8 expression was absent, and demonstrates the lack of mature T-cell development in NOD/SCID mice.2 Lymphoid and myeloid expression patterns were similar in the parental NOD/SCID strain, suggesting thatALDHhiCD133+Lin- cells demonstrate normal hematopoietic differentiation and expansion in vivo.
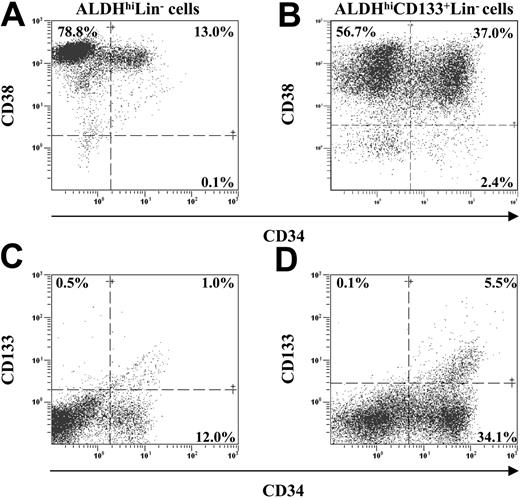
Transplanted human ALDHhiCD133+Lin- cells retain primitive hematopoietic phenotypes. BM from highly engrafted (24.9%-86.6% human CD45+) NOD/SCID β2M-null mice was analyzed for the maintenance of primitive cell surface phenotype 7 to 8 weeks after transplantation. Human cells were analyzed for the coexpression of CD34 with CD38 (A-B) or CD34 with CD133 (C-D).
Transplanted human ALDHhiCD133+Lin- cells retain primitive hematopoietic phenotypes. BM from highly engrafted (24.9%-86.6% human CD45+) NOD/SCID β2M-null mice was analyzed for the maintenance of primitive cell surface phenotype 7 to 8 weeks after transplantation. Human cells were analyzed for the coexpression of CD34 with CD38 (A-B) or CD34 with CD133 (C-D).
ALDHhiCD133+Lin- cells maintain primitive hematopoietic phenotypes in vivo
To assess whether the ALDHhiCD133+Lin- cells maintained primitive cell phenotypes after transplantation, we compared human hematopoietic engraftment after the transplantation of purified ALDHhiCD133+Lin- (n = 6), CD133+Lin- (n = 5), and ALDHhiLin- (n = 5) cells for the coexpression of CD34 with either CD38 or CD133 (Figure 6). Representative analyses of CD45+ human hematopoietic cells expressing these primitive cell surface markers are depicted in Figure 6A-D, and the results for all analyzed mice are summarized in Table 3. We previously reported that a fraction of ALDHhiLin- cells retained the expression of CD34 after transplantation into NOD/SCID β2M-null mice.17 However, transplantation of ALDHhiLin- cells resulted primarily in the production of CD34+CD38+ progenitors (13.8% ± 2.2%), and did not support retention of more primitive repopulating CD34+CD38- (0.2% ± 0.1%) or CD34+CD133+ (1.5% ± 0.6%, Figure 6A,C, and Table 3) cells. A similar loss of primitive repopulating phenotype has also been observed after transplantation of CD34+ populations in NOD/SCID mice.2 Consequently, repopulating ability in secondary recipients, an essential characteristic for long-term reconstitution,22 has been difficult to demonstrate after secondary transplantation of bulk CD34+ cells in the NOD/SCID model.4 Compared with the ALDHhiLin- population, transplanted ALDHhiCD133+Lin- cells showed a 2-fold increase in the overall retention of human CD34 expression (14.0% ± 2.7% versus 30.0% ± 4.7%, P < .05) and significant increases in the frequency of human CD34+CD38- cells (0.2% ± 0.1% versus 3.0% ± 0.3%, P < .001) and CD34+CD133+ cells (1.5% ± 0.6% versus 6.8% ± 1.1%, P < .01) (Figure 6B,D, and Table 3). Transplanted CD133+Lin- cells also maintained primitive marker expression similar to ALDHhiCD133+Lin- cells (Table 3), suggesting that CD133 expression in the original transplanted population may confer the retention of primitive cell phenotype in the expanded progeny. Nevertheless, selection of ALDHhiCD133+Lin- cells isolated a subset of human cells that maintained primitive phenotypes 7 to 8 weeks after transplantation, suggesting that ALDHhiCD133+Lin- cells may also retain repopulating potential after serial transplantation.
Transplanted human ALDHhiCD133+Lin- cells retain primitive hematopoietic surface markers after repopulation in vivo
Transplanted cell population . | CD34+, % . | CD34+CD38+, % . | CD34+CD38-, % . | CD34+CD133+, % . |
|---|---|---|---|---|
| ALDHhiCD133+Lin- | 30.0 ± 4.7* | 27.4 ± 4.7* | 2.6 ± 0.3† | 6.8 ± 1.1‡ |
| CD133+Lin- | 25.3 ± 4.0* | 22.7 ± 3.6* | 2.6 ± 0.6† | 6.1 ± 1.1‡ |
| ALDHhiLin- | 14.0 ± 2.6* | 13.8 ± 2.2 | 0.2 ± 0.1 | 1.5 ± 0.6 |
Transplanted cell population . | CD34+, % . | CD34+CD38+, % . | CD34+CD38-, % . | CD34+CD133+, % . |
|---|---|---|---|---|
| ALDHhiCD133+Lin- | 30.0 ± 4.7* | 27.4 ± 4.7* | 2.6 ± 0.3† | 6.8 ± 1.1‡ |
| CD133+Lin- | 25.3 ± 4.0* | 22.7 ± 3.6* | 2.6 ± 0.6† | 6.1 ± 1.1‡ |
| ALDHhiLin- | 14.0 ± 2.6* | 13.8 ± 2.2 | 0.2 ± 0.1 | 1.5 ± 0.6 |
Mean expression of primitive cell surface markers after the transplantation of purified ALDHhiCD133+Lin- (n = 6) or CD133+Lin- (n = 5) cells was compared with BM repopulation produced by purified ALDHhiLin- cells (n = 5). Populations that received transplants of ALDHhiCD133+Lin- and CD133+Lin- retained cells that expressed CD34 and showed increased frequencies of CD34+CD38+ progenitors and CD34+CD38- repopulating cells.
P < .05
P < .001
P < .01
ALDHhiCD133+Lin- cells repopulate recipients of secondary transplants
Since NOD/SCID mice have a shortened life span due to the premature development of murine lymphomas, long-term HSC function is demonstrated by reconstitution of secondary recipients after serial transplantation.37 Repopulating studies after serial transplantation have clearly demonstrated that purified CD34+CD38- cells from human UCB possess secondary SRC capacity. In contrast, CD34+CD38+ progenitors that rapidly repopulate primary recipients have no secondary repopulating potential.38,39 To test whether ALDHhiLin- populations from human UCB possess repopulating ability after serial transplantation, whole murine BM from highly engrafted primary transplant recipients (22.8%-94.0% human CD45+) of 105 ALDHhiCD133+Lin- or 1 to 2 × 105 ALDHhiLin- cells was harvested and transplanted into secondary recipients. Serial transplantation of BM from primary recipients engrafted with ALDHhiCD133+Lin- cells (secondary dose range, 2.3-5.2 × 106 human CD45+ cells) resulted in human engraftment in 4 of 5 secondary NOD/SCID β2M-null mice (Table 4). As a direct comparison, primary recipients engrafted with ALDHhiLin- cells not subfractioned by CD133 expression (secondary dose range, 3.1-16.2 × 106 human CD45+ cells) resulted in secondary human engraftment in only 1 of 8 NOD/SCID B2M-null recipients of serial transplants (Table 4). As predicted by the retention of primitive cell phenotypes in primary recipients, purified ALDHhiCD133+Lin- cells consistently contain long- term repopulating cells capable of secondary reconstitution after serial transplantation.
ALDHhiCD133+Lin- cells retain long-term repopulating ability after serial transplantation into secondary NOD/SCID β2M-null recipients
Primary transplants . | . | . | Secondary transplants . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mouse . | Cell dose and type of cell . | Human engraftment, % . | Mouse . | Cell dose . | Human cell dose . | Human engraftment, % . | |||||
| P1 | 105 Ahi133+Lin- | 51.9 | S1a | 107 | 5.2 × 106 | 7.8 | |||||
| S1b | 107 | 5.2 × 106 | 6.1 | ||||||||
| P2 | 105 Ahi133+Lin- | 22.8 | S2a | 107 | 2.3 × 106 | 1.9 | |||||
| P3 | 105 Ahi133+Lin- | 55.5 | S3a | 5 × 106 | 2.8 × 106 | 0.5 | |||||
| P4 | 105 Ahi133+Lin- | 52.2 | S4a | 5 × 106 | 2.6 × 106 | 0 | |||||
| P5 | 105 Ahi133+Lin- | 51.9 | S5a (cultured) | 2 × 106 | 1.0 × 106 | 0.3 | |||||
| S5b (cultured) | 2 × 106 | 1.0 × 106 | 0.2 | ||||||||
| P6 | 105 AhiLin- | 31.1 | S6a | 107 | 3.1 × 106 | 0 | |||||
| S6b | 107 | 3.1 × 106 | 0 | ||||||||
| P7 | 2 × 105 AhiLin- | 81.0 | S7a | 2 × 107 | 16.2 × 106 | 0 | |||||
| S7b | 2 × 107 | 16.2 × 106 | 0.2 | ||||||||
| P8 | 2 × 105 AhiLin- | 94.0 | S8a | 107 | 9.4 × 106 | 0 | |||||
| S8b | 107 | 9.4 × 106 | 0 | ||||||||
| P9 | 2 × 105 AhiLin- | 62.2 | S9a | 107 | 6.2 × 106 | 0 | |||||
| S9b | 107 | 6.2 × 106 | 0 | ||||||||
Primary transplants . | . | . | Secondary transplants . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mouse . | Cell dose and type of cell . | Human engraftment, % . | Mouse . | Cell dose . | Human cell dose . | Human engraftment, % . | |||||
| P1 | 105 Ahi133+Lin- | 51.9 | S1a | 107 | 5.2 × 106 | 7.8 | |||||
| S1b | 107 | 5.2 × 106 | 6.1 | ||||||||
| P2 | 105 Ahi133+Lin- | 22.8 | S2a | 107 | 2.3 × 106 | 1.9 | |||||
| P3 | 105 Ahi133+Lin- | 55.5 | S3a | 5 × 106 | 2.8 × 106 | 0.5 | |||||
| P4 | 105 Ahi133+Lin- | 52.2 | S4a | 5 × 106 | 2.6 × 106 | 0 | |||||
| P5 | 105 Ahi133+Lin- | 51.9 | S5a (cultured) | 2 × 106 | 1.0 × 106 | 0.3 | |||||
| S5b (cultured) | 2 × 106 | 1.0 × 106 | 0.2 | ||||||||
| P6 | 105 AhiLin- | 31.1 | S6a | 107 | 3.1 × 106 | 0 | |||||
| S6b | 107 | 3.1 × 106 | 0 | ||||||||
| P7 | 2 × 105 AhiLin- | 81.0 | S7a | 2 × 107 | 16.2 × 106 | 0 | |||||
| S7b | 2 × 107 | 16.2 × 106 | 0.2 | ||||||||
| P8 | 2 × 105 AhiLin- | 94.0 | S8a | 107 | 9.4 × 106 | 0 | |||||
| S8b | 107 | 9.4 × 106 | 0 | ||||||||
| P9 | 2 × 105 AhiLin- | 62.2 | S9a | 107 | 6.2 × 106 | 0 | |||||
| S9b | 107 | 6.2 × 106 | 0 | ||||||||
Murine BM from highly engrafted primary recipients that received transplants of 105 ALDHhiCD133+Lin- (n = 5) or 1 to 2 × 105 ALDHhiLin- (n = 4) cells was harvested, and whole murine BM cells were injected immediately into secondary NOD/SCID β2M-null recipients. Alternatively, secondary recipients S5a and S5b received injections of murine BM cells that were cultured for 16 hours in serum-free media containing SCF and IL-6. BM from primary (n = 9) and secondary (n = 15) recipients was analyzed for human CD45 and human CD38 expression 7 to 8 weeks after transplantation. Transplanted ALDHhiLin- cells contained secondary SRCs in 4 of 5 recipients of primary transplants. In contrast, secondary SRCs were observed in only 1 of 4 primary recipients that received that received transplants of ALDHhiLin- cells not subfractioned by CD133 expression.
Alternatively, BM cells from primary recipients were cultured for 16 hours in serum-free culture media supplemented with SCF and IL-6 before secondary injection of 2 × 106 surviving cells. These conditions have previously shown to promote engraftment of secondary recipients.40,41 Cells derived from a primary recipient that received transplants of the ALDHhiCD133+Lin- population, primed by culture for 16 hours, produced secondary engraftment after the injection of as few as 106 human cells (Table 4). These data confirm that the ALDHhiCD133+Lin- population contains SRCs that maintain primitive phenotype and repopulation capacity in secondary recipients.
Discussion
Methods to safely identify primitive HSCs with enhanced repopulating function are constantly sought for clinical stem cell transplantation. Conventionally, HSCs are purified using a single isolation strategy, such as the selection of cells based on cell surface phenotype (CD34 expression) or efflux of metabolic markers such as Hoechst dye by membrane pumps.1,2,13,21,41-44 However, cell phenotype, such as CD34 surface expression, can vary depending on microenvironmental factors or cellular activation,4,8 and clinical procedures are incompatible with the use of toxic or DNA-intercalating dyes. Nontoxic cell-sorting strategies based on conserved stem cell function, in combination with cell surface phenotype, are necessary for clinical cell purification and may be useful for the study of complex developmental processes such as self-renewal versus the sequential transition from primitive HSCs to restricted progenitors. Our laboratory and others have demonstrated that cells with high intracellular ALDH activity from human UCB comprise a heterogeneous population of clonogenic progenitors and are enriched for NOD/SCID repopulating cells.15,17,34 This isolation strategy uses a nontoxic, fluorescent substrate of ALDH, safely and effectively labeling cells with ALDH activity for selection by flow cytometry. To further delineate the distinct hematopoietic functions of ALDHhiLin- cells from human UCB, we purified ALDHhiCD133-Lin- and ALDHhiCD133+Lin- populations and characterized their hematopoietic engraftment and repopulating ability in immune-deficient mice that received primary and secondary transplants.
Collectively, our analyses demonstrate that the ALDHhiLin- population is enriched for in vivo repopulating ability by the coselection of CD133-expressing cells. TheseALDHhiCD133+Lin- cells actively seeded the murine BM microenvironment within 48 hours after transplantation, expanded efficiently in vivo to produce mature myeloid and lymphoid progeny while maintaining cells with primitive hematopoietic phenotype, and consistently engrafted recipients of serial, secondary transplants. In contrast, ALDHhiCD133-Lin- cells possessed diminished engraftment and repopulating capacity but were enriched for clonogenic progenitor function with particular restriction to the myeloerythroid lineage. Storms et al have recently used ALDH expression to functionally delineate CD34+ progenitors from committed natural killer (NK)-cell progenitors.18 In addition, Pearce et al45 have used ALDH activity to functionally characterize a leukemic stem cell population from human acute myeloid leukemia (AML) samples. Thus, ALDH activity provides an additional tool for the dissection of HSC and progenitor function during normal or malignant hematopoietic development.
Statistical comparison of the repopulating ability of ALDHhiCD133+Lin- cells (1 SRC in 691 cells) by LDA in NOD/SCID β2M-null mice revealed a 10-fold increase in the frequency of SRC compared with CD133+Lin- cells (1 SRC in 6681 cells), and a 3-fold increase in the frequency of SRC compared with ALDHhiLin- cells (1 SRC in 1680 cells). In the NOD/SCID model, LDAproduced a frequency of approximately 1 SRC in 16 000 ALDHhiCD133+Lin- cells. Bhatia et al have previously reported a NOD/SCID repopulating frequency of 1 SRC in 617 cells after the transplantation of highly purified CD34+CD38-Lin- cells at limiting dilution.2 Direct comparison of in vivo repopulating efficiencies of purified subsets of HSCs from different sites can be complicated by UCB isolation procedures affecting sample quality, intercolony variability within NOD/SCID mice, the selected irradiation dose, and the sensitivity of HSC detection after transplantation. Nonetheless, purified human CD34+CD38-Lin- cells remain the gold standard for the isolation of purified human repopulating HSCs, whereas the ALDHhiCD133+Lin- population represents a heterogenous mixture of primarily CD34+ primitive repopulating (CD34+CD38-) cells and committed progenitors (CD34+CD38+) that may not directly contribute to hematopoietic repopulation in the NOD/SCID model. Thus, our purification strategy first selects for UCB stem and progenitor cells primarily conferred by high ALDH activity,17 while CD133 expression may select repopulating cells with the capacity to migrate to and reconstitute hematopoietic tissues after sublethal irradiation.32,44 Thus, primary assessment of ALDH activity, alone or in combination with cell surface molecule expression, can be used to efficiently select cells with heterogeneous hematopoietic repopulating functions, a characteristic corollary to immediate neutrophil and platelet recovery in patients who have undergone transplantation.
The ALDHhiCD133+Lin- population consisted primarily of CD34+ cells and also included rare CD34-CD38-CD133+Lin- cells, a primitive HSC population shown to have NOD/SCID repopulating potential.3,21 This phenotypic heterogeneity resulted in lymphoid and myeloid reconstitution and the maintenance of cells with primitive hematopoietic phenotype. Thus, transplantation of ALDHhiCD133+Lin- donor cells favored the transfer of committed repopulating progenitors and also retained primitive HSCs for prolonged hematopoiesis.
Comprehensive long-term repopulation by human HSCs has been difficult to demonstrate in murine models due to the shortened life span of the NOD/SCID mouse and the inherent differentiation of human HSCs in the murine BM microenvironment after xenotransplantation.37 After serial transplantation, progeny of ALDHhiCD133+Lin- cells consistently engrafted secondary recipients, suggesting that ALDHhiCD133+Lin- cells contain both short-term and long-term repopulating human HSCs. Isolation based on the combination of high ALDH activity and CD133 expression selected a potent stem cell population that produces multilineage reconstitution, maintains primitive cell phenotype in vivo, and possessed long-term repopulating ability in secondary murine recipients. These characteristics are advantageous for clinical cellular transplantation therapies, potentially including stem cell and gene therapy strategies for tissue regeneration. Since ALDH activity, in combination with conserved stem cell surface markers, is a useful tool for the delineation of distinct hematopoietic stem and progenitor cell compartments, similar strategies may be developed to prospectively isolate and functionally characterize nonhematopoietic progenitors from alternate tissues or sources.
Prepublished online as Blood First Edition Paper, November 3, 2005; DOI 10.1182/blood-2005-06-2284.
Supported by R01 DK61848, 2R01 DK53041, and P01 HL54850.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. Hughes from the Siteman Cancer Center flow cytometry core facility for her technical expertise and the staff at Cardinal Glennon Children's Hospital cord blood banking facility for providing human UCB samples. We thank N. Borradaile for editorial review of this article.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal