Abstract
Impaired T-cell reactivity is a feature of C3-deficient mice in several disease models. The mechanism behind the reduced T-cell response is, however, poorly understood. We explored the hypothesis that antigen-presenting cells (APCs) from C3-/- mice have impaired potency to stimulate antigen-specific T cells, in an alloantigen-dependent model. Our results show that C3-/- macrophages have reduced ability to elicit alloreactive T-cell responses in vitro and in vivo, affecting both the primary and secondary responses. The C3 status of donor macrophages had a major impact on the CD4 T-cell response. The impaired CD4 T-cell response was associated with reduced expression of MHC class II on the surface of C3-/- macrophages, without loss of class II gene expression. Furthermore, inhibition of C3 gene expression in C3+/+ macrophages reduced their ability to stimulate alloreactive T cells, suggesting that endogenous production of C3 could in part contribute to the potency of APCs. Our data provide compelling evidence that C3 deficiency modulates the potency of APCs to stimulate the T-cell response, suggesting a critical role for complement in the maintenance of APC function. This could offer a partial explanation as to why the T-cell response is impaired in C3-/- mice. (Blood. 2006;107:2461-2469)
Introduction
Evidence has emphasized the importance of the innate immune system in the establishment of the adaptive immune response.1 The complement system is an important component of innate immunity. In addition to its pivotal role in the regulation of inflammation and host defense, the complement system also participates in regulating the antigen-specific immune response.2,3 The activation of C3 is the converging point of the 3 pathways, from where effector functions of complement are generated. Therefore, C3 is vital for participation of the complement system in both the innate and adaptive immune responses.
C3, the most abundant complement protein in serum (1.3 mg/mL in humans), is an acute-phase protein and rapidly responds to many noxious stimuli, including microbial infection and tissue injury. The liver is the primary source for the synthesis of C34 ; however, many other specialized cells have the capacity to synthesize C3, including myeloid-derived cells and parenchymal cells.5-9 These cells synthesize C3 spontaneously or in response to cytokine stimulation. Extrahepatic synthesis of C3 is thought to serve important physiologic and pathologic functions, in terms of regulation of the inflammatory response, host defense, and allograft rejection.10-13
Human and animal studies have demonstrated the importance of C3 in the adaptive immune response.14,15 C3-/- mice exhibit an impaired B-cell response to both T-cell-independent and -dependent antigens. The mechanism of complement regulation of the B-cell response appears to involve the interaction of complement activation products (C3b, C3d) and complement receptors 1 and 2 (CR1/CD35, CR2/CD21) on cell surfaces, thus increasing the retention of antigen in lymphoid tissue and enhancing the B-cell response.16,17 In addition to the regulation of B-cell responses, participation of complement in T-cell immunity has been described. Impaired T-cell responses in C3-/- mice were reported in several disease models, including viral infection, autoimmune disease, and transplantation.18-21 However, the means by which C3 deficiency leads to impaired antigen-specific T-cell reactivity in such conditions remains unclear.
Although T-cell priming is impaired in C3-/- mice in the influenza virus model, T-cell priming is normal in mice deficient in CD35 and CD21.18 This suggests that the complement receptors CD35 and CD21 are not involved in the development of T-cell reactivity; therefore, the mechanism for C3 regulating T-cell responses may differ from that regulating the B-cell response. Previous studies have found that antigen coupled covalently to C3b is protected from excessive proteolytic degradation in the endosomal/lysosomal compartment of the MHC class II pathway in B cells. This enhances peptide loading onto MHC class II molecules and increases stable MHC class II molecules in this compartment.22-24 These studies suggest that activated C3 fragment functions as a “chaperone” in the MHC class II pathway of APCs, which is essential for maintaining the normal function of APCs. It also raises the possibility that in the absence of C3, APC function may be defective.
In this study, we explored the hypothesis that C3 deficiency reduces the potency of APCs to stimulate T-cell responses. We examined an alloantigen-dependent model of T-cell stimulation using macrophages from C57BL/6 mice as stimulators and splenic T cells from BALB/c mice as responders. We assessed the alloreactive T-cell response in vitro and in vivo following stimulation with C3+/+ and C3-/- macrophages. We assessed the gene and cell-surface protein expression of MHC class II and costimulatory molecules. We also investigated the role of endogenous production of C3 by macrophages in alloreactive T-cell response and the way that this production might increase the capacity of macrophages to stimulate alloreactive T cells.
Materials and methods
Mice
Homozygous C3-/-, C4-/-, and factor B (H2-Bf)-/- mice were derived by homologous recombination in embryonic stem cells25-27 and backcrossed onto the C57BL/6 parental strain for 11 generations. WT (C57BL/6H-2b) mice and BALB/cH-2d mice were purchased from Harlan UK (Oxon, United Kingdom). Male mice (6-7 weeks old) were used in all experiments. All animal procedures were carried out within the Animals (Scientific Procedures) Act, 1986 Untied Kingdom.
Preparation of macrophages
Resident and thioglycollate-elicited peritoneal macrophages and bone marrow (BM) macrophages were prepared using previously described methods28 (see the details in Supplementary Methods, which are available at the Blood website; see the Supplemental Materials link at the top of the online article). Macrophages were further purified with CD11b MicroBeads (Miltenyi Biotec, Surrey, United Kingdom) according to the manufacturer's instruction, unless otherwise specified. Following the purification, 85% to 90% of cells were found to stain positive with anti-mouse F4/80 as determined by flow cytometry (Figure S1). In each experiment, macrophages were prepared from 3 to 5 mice and pooled together for analysis, unless otherwise stated. Thioglycollate-elicited macrophages were used in most of our experiments, unless otherwise specified.
Preparation of T cells
Naive alloreactive T cells were derived from spleens of normal BALB/c mice. Primed alloreactive T cells were derived from BALB/c mice that had received a C57BL/6 skin graft 14 days in advance. CD3+, CD4+, and CD8+ T cells were prepared from splenocytes using Spin-Sep Enrichment Cocktail Kit (StemCell Technologies, London, United Kingdom). Following the isolation, the purity of the T-cell preparation was routinely greater than 90%, as determined by flow cytometry. T cells were prepared from 3 to 6 mice depending on the number of purified T cells needed in each experiment.
Skin grafting
Tail skin from the donor animals was grafted onto the left flank of the recipients under enflurane anesthetic. The graft site was covered with paraffin-embedded gauze and a dressing of dry gauze and clear tape. The dressing was removed on day 7, and the graft was assessed visually on a daily basis for signs of rejection. The time to rejection was determined as the day on which greater than 90% of the graft area was necrotic.
Analysis of alloreactive T-cell response in vitro
Irradiated macrophages (2000 rad [2 Gy]) (2 × 105) and 2 × 105 purified alloreactive T cells (CD3+, CD4+, or CD8+) were cocultured in RPMI-1640 containing 10% heat-inactivated FCS, 50 μM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin for up to 5 days, unless otherwise specified. For measuring the production of IFN-γ and IL-2 by T cells, coculture supernatants were collected and used to perform enzyme-linked immunosorbent assay (ELISA). T-cell proliferation was assessed at 96 hours after culture by measuring the incorporation of 3H-thymidine (see Supplementary Methods) or using the CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay Kit (Promega, Southampton, United Kingdom) in a 3-day coculture, according to the manufacturer's instructions.
Analysis of alloreactive T-cell response in vivo
For priming of alloreactive T cells, BALB/c mice, at day 0, were given either C3+/+ or C3-/- donor macrophages (5 × 106, irradiated at 2000 rad [20 Gy]) by intraperitoneal injection; 14 days after immunization the mice received a C57BL/6 skin graft. On day 21, some of the mice were killed; T-cell responses in those BALB/c mice were then measured ex vivo by mixed lymphocyte reaction (MLR). The remaining mice were kept for monitoring skin graft rejection. For reactivation of alloreactive T cells, BALB/c mice, at day 0, were primed with a C57BL/6 skin graft; 14 days after priming the mice were given either C3+/+ or C3-/- donor macrophages (5 × 106, irradiated at 2000 Rad [20 Gy]) by intraperitoneal injection. After a further 7 days, the mice were killed; T-cell responses in those BALB/c mice were then measured ex vivo by MLR.
MLR
Splenocytes (2 × 105) derived from immunized BALB/c mice (H-2d) and irradiated (2000 rad [20 Gy]) splenocytes (2 × 105) derived from C57BL/6 (H-2b) mice were cocultured in T-cell culture medium for up to 5 days. Supernatants were collected for measuring the production of IFN-γ and IL-2 by ELISA.
ELISA
Sandwich ELISA was performed using OptEIA ELISA Set for mouse IL-2 and IFN-γ (BD Biosciences, Oxford, United Kingdom) according to the manufacturer's instruction. Mouse C3 ELISA was performed using sheep anti-mouse C3 (ICN Biomedicals, London, United Kingdom) and HRP-conjugated goat anti-mouse C3 (Nordic Immunology, Tilburg, The Netherlands). The standard was pooled normal mouse serum, which has a reported concentration of 0.7 mg/mL.29
Conventional reverse transcriptase-polymerase chain reaction (RT-PCR)
cDNA was synthesized as described30 from macrophages. PCR was carried out with 2 μL diluted cDNA (reflecting 0.2 μg total RNA), 12.5 pmol of each 3′ and 5′ primer pair for MHC class II Aβ chain, CD40, ICAM-1, and B7.2 (Cd86) in 25 μL reaction buffer (Promega). The PCR cycle consisted of 1 minute at 94°C, 1 minute at 62°C, and 1 minute at 72°C. Amplified PCR products were visualized after electrophoresis on 1.2% agarose gel containing ethidium bromide. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers, 12.5 pmol each, were also added in every reaction as an internal control. PCR primer sequences and product sizes are shown in Supplementary Methods.
C3 gene silencing
C3+/+ macrophages (5 × 105) were transfected with 100 nM of either C3 siRNA (sense sequence, 5′-GGAAUUCAACUCAGAUAAGtt-3′; anti-sense sequence, 5′-CUUAUCUGAGUUGAAUUCCtt-3′) or silencer-negative control siRNA (Ambion Europe, Cambridgeshire, United Kingdom) using siRNA transfection kit (Qiagen, West Sussex, United Kingdom). After 48 hours of transfection, the reduction of C3 synthesis by macrophages was measured by quantitative real-time RT-PCR and ELISA. After 48 hours of transfection, macrophages were also cocultured with primed alloreactive T cells for up to 2 days. Supernatants were collected and used for measuring the production of cytokine by ELISA.
Flow cytometry
Macrophages (2 × 105 cells) and splenocytes (1 × 106 cells) were stained with either fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibody or the appropriate isotype control antibody. Antibody reagents are described in Supplementary Methods.
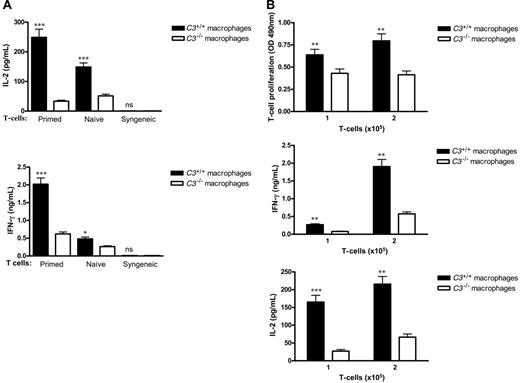
C3-/-macrophages have reduced potency to stimulate alloreactive T cells. Thioglycollate-elicited macrophages were prepared from C3+/+ and C3-/- mice. Alloreactive CD3+ T cells were prepared from either naive BALB/c mice or BALB/c mice that had been primed by grafting with C57BL/6 donor skin. Syngeneic CD3+ T cells were prepared from C57BL/6 mice. (A) Macrophages (2 × 105) and T cells (2 × 105) were cocultured for 3 days; supernatants were collected for measuring the production of IL-2 and IFN-γ by ELISA. (B) Macrophages (2 × 105) and T cells (1 or 2 × 105) were cocultured for 3 days, after which T-cell proliferation and cytokine assays were performed. T-cell proliferation was assessed using Non-Radioactive Cell Proliferation Assay Kit. The absorbance at 490 nm reflecting the amount of formazan in cell culture medium was measured. All data were generated from 3 independent experiments with 3 mice/group in each experiment and analyzed by Student t test. P values are for comparisons between C3+/+ and C3-/- macrophages, *P < .05; **P < .005; ***P < .001; ns, no significant difference. Error bars indicate SEM.
C3-/-macrophages have reduced potency to stimulate alloreactive T cells. Thioglycollate-elicited macrophages were prepared from C3+/+ and C3-/- mice. Alloreactive CD3+ T cells were prepared from either naive BALB/c mice or BALB/c mice that had been primed by grafting with C57BL/6 donor skin. Syngeneic CD3+ T cells were prepared from C57BL/6 mice. (A) Macrophages (2 × 105) and T cells (2 × 105) were cocultured for 3 days; supernatants were collected for measuring the production of IL-2 and IFN-γ by ELISA. (B) Macrophages (2 × 105) and T cells (1 or 2 × 105) were cocultured for 3 days, after which T-cell proliferation and cytokine assays were performed. T-cell proliferation was assessed using Non-Radioactive Cell Proliferation Assay Kit. The absorbance at 490 nm reflecting the amount of formazan in cell culture medium was measured. All data were generated from 3 independent experiments with 3 mice/group in each experiment and analyzed by Student t test. P values are for comparisons between C3+/+ and C3-/- macrophages, *P < .05; **P < .005; ***P < .001; ns, no significant difference. Error bars indicate SEM.
Statistical analysis
ELISA or T-cell proliferation assays were performed in 3 to 6 replicate wells of the cocultures. Results were expressed as a mean plus or minus standard error of measurement (SEM) and subjected to statistical analysis. Student t test or 2-way ANOVA or variance component regression analysis was used when appropriate to determine significant differences between samples. Graft survival was analyzed by log-rank test. All experiments were repeated at least 3 times. Our study protocol was approved by the Home Office, United Kingdom.
Results
C3-/- macrophages have reduced potency to stimulate alloreactive T cells
Initially, we asked whether C3-/- and C3+/+ macrophages have different potencies in stimulating alloreactive T cells. We examined both naive and primed T-cell responses. Naive or primed alloreactive T cells, respectively, were cocultured with either C3+/+ or C3-/- macrophages. After 3-day coculture, the concentrations of secreted IL-2 and IFN-γ were significantly lower with stimulation by C3-/- macrophages compared with C3+/+ macrophages. Both primed and nonprimed T-cell responses were affected by the C3 status of donor macrophages, although the effect was more pronounced with antigen-experienced T cells. Syngeneic controls, where donor macrophages (H-2b) were cocultured with donor T cells (H-2b), exhibited a very low level of IL-2 and IFN-γ production (Figure 1A). On the basis of the result that the primed T-cell response, compared with the naive T-cell response, was more sensitive to the C3 status of donor macrophages, we decided to use primed alloreactive T cells in the remaining in vitro experiments.
In our initial study, we also assessed the proliferative T-cell response after stimulation by allogeneic macrophages. T-cell proliferation following stimulation by C3-/- macrophages was reduced compared with C3+/+ macrophages (Figure 1B).
C3 gene-silenced macrophages have lowered ability to stimulate alloreactive T cells
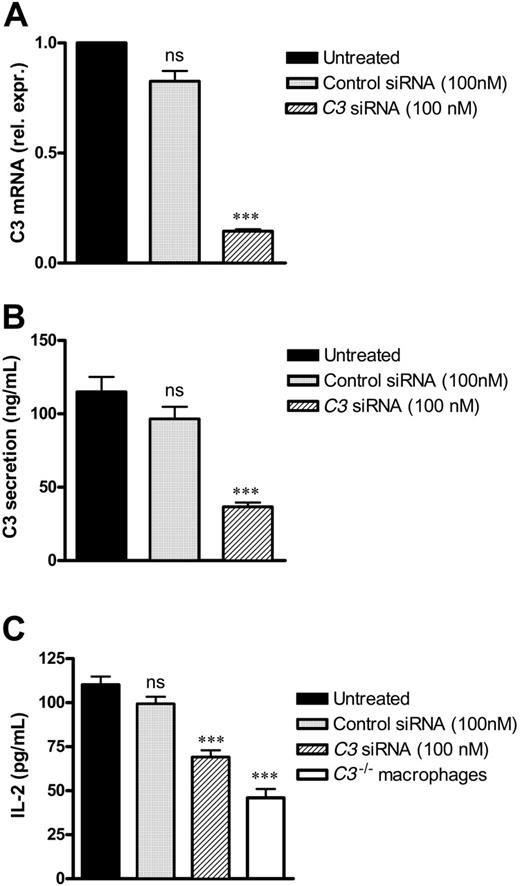
Because macrophages derived from C3-deficient mice exhibited a low level of T-cell stimulation, we wanted to verify whether this effect was attributable to C3 production by the macrophages. For this purpose, we inhibited C3 gene expression in C3+/+ macrophages using small interfering RNA and measured the ability of these macrophages to stimulate alloreactive T cells compared with control-treated macrophages. As shown in Figure 2, treatment with interfering RNA significantly reduced the amount of C3 mRNA and C3 secretion, and coordinately lowered the level of T-cell activation stimulated by these macrophages. However, treatment with irrelevant interfering RNA did not show significant reduction in the amount of C3 produced or in the level of T-cell activation. This is consistent with an effect of endogenous production of C3 on the potency of macrophages to stimulate alloreactive T cells.
C3 gene-silenced macrophages have lowered ability to stimulate alloreactive T cells. Thioglycollate-elicited C3+/+ macrophages were transfected with 100 nM of either C3 siRNA or control siRNA using a siRNA transfection kit (Qiagen). After 48 hours of transfection, C3 expression was measured by quantitative real-time RT-PCR (A) and ELISA (B). After 48-hour transfection, 1 × 105 macrophages were cocultured with 1 × 105 primed alloreactive CD3+ T cells for 2 days. Supernatants were collected and used for measuring the production of IL-2 by ELISA (C). All data were generated from 3 independent experiments, with macrophages pooled from 5 mice in each experiment and analyzed by Student t test. P values are for comparisons between untreated C3+/+ macrophages and other groups of macrophages, ***P < .001; ns, no significant difference. Error bars indicate SEM.
C3 gene-silenced macrophages have lowered ability to stimulate alloreactive T cells. Thioglycollate-elicited C3+/+ macrophages were transfected with 100 nM of either C3 siRNA or control siRNA using a siRNA transfection kit (Qiagen). After 48 hours of transfection, C3 expression was measured by quantitative real-time RT-PCR (A) and ELISA (B). After 48-hour transfection, 1 × 105 macrophages were cocultured with 1 × 105 primed alloreactive CD3+ T cells for 2 days. Supernatants were collected and used for measuring the production of IL-2 by ELISA (C). All data were generated from 3 independent experiments, with macrophages pooled from 5 mice in each experiment and analyzed by Student t test. P values are for comparisons between untreated C3+/+ macrophages and other groups of macrophages, ***P < .001; ns, no significant difference. Error bars indicate SEM.
Dependence of the CD4 T-cell response on the C3 status of donor macrophages
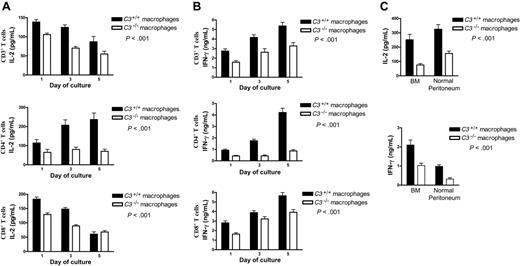
To determine which of the 2 main subpopulations of T cells, CD4 or CD8, was more reactive to the C3 status of the stimulator cells, we performed a 5-day coculture experiment in which the purified CD3+, CD4+, and CD8+ T cells were each stimulated by C3+/+ or C3-/- macrophages. We found that the production of IL-2 (Figure 3A) and IFN-γ (Figure 3B) was lower in all preparations of the T cells stimulated by C3-/- macrophages compared with the T cells stimulated by C3+/+ macrophages. However, this complement-dependent cytokine production was more marked in CD4+ T cells than in CD3+ and CD8+ T cells. These data indicate that both CD4+ and CD8+ T-cell responses were influenced by the C3 status of donor macrophages, but CD4+ T cells were the more sensitive, suggesting that the effect of C3 was primarily on the MHC class II-dependent pathway of donor antigen presentation.
The macrophages used in the experiments (results in Figure 3A-B) were prepared from the inflamed peritoneum of thioglycollate-treated mice. We also prepared macrophages from the normal peritoneal cavity and BM of WT and C3-/- mice without further purification with CD11b beads. Then, we stimulated them with LPS (100 ng/mL) for 24 hours and subsequently cocultured these macrophages with primed alloreactive CD4+ T cells and assessed the T-cell response. We found that the production of IFN-γ and IL-2 was consistently lower in T cells stimulated by C3-/- macrophages compared with the T cells stimulated by C3+/+ macrophages, for macrophages from both of these sources (Figure 3C). C3-dependent T-cell stimulation was thus a feature of macrophages regardless of source or inflammatory status.
Reduced capacity of C3-/- macrophages for immune stimulation in vivo
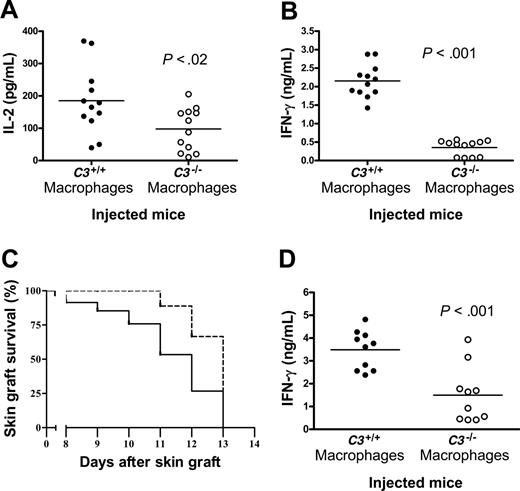
In the next set of experiments, we determined whether C3-/- macrophages have a reduced ability to elicit an alloreactive T-cell response in vivo. We examined 2 protocols. The first tested whether T-cell priming was affected by the C3 status of donor macrophages. At day 0, BALB/c mice were given either C3+/+ or C3-/- C57BL/6 donor macrophages. As a read out of the T-cell response, at day 14 after the injection of macrophages, the mice received a skin graft from a C567BL/6 donor. On day 21, some mice were killed, and their splenocytes were used for analysis of the alloreactive T-cell response ex vivo using MLR. T-cell activation was assessed by measuring the secretion of IL-2 and IFN-γ using ELISA. Other mice were kept for monitoring skin graft rejection. The T-cell response in mice that had received C3-/- macrophages was impaired, with significant lowering of IL-2 (Figure 4A) and IFN-γ (Figure 4B) production and prolonged graft survival (Figure 4C), compared with mice that had received C3+/+ macrophages. Thus immunization with C3-/- macrophages resulted in defective T-cell priming in vivo.
We then tested whether the reactivation of previously primed T cells was affected by the C3 status of donor macrophages. At day 0, BALB/c mice received a C57BL/6 donor skin graft, and after 14 days the mice that received a skin graft were given either C3+/+ or C3-/- macrophages. On day 21, the mice were killed, and their splenocytes were used for analysis of the alloreactive T-cell response ex vivo using MLR. We found that primed alloreactive T cells restimulated by C3-/- macrophages in vivo produced less IFN-γ compared with those restimulated by C3+/+ macrophages in vivo (Figure 4D). However, the production of IL-2 was not significantly different between restimulation with C3-/- and C3-/- macrophages (data not shown). Although the reason for this discrepancy between the IFN-γ and IL-2 responses is unclear, IFN-γ is the hallmark of Th1 lymphocytes, which are thought to be the predominant T-cell population involved in the alloimmune response. On the basis of these results, both T-cell priming and reactivation were impaired when stimulated by C3-/- macrophages.
Reduced cell-surface expression of MHC class II in C3-/- macrophages
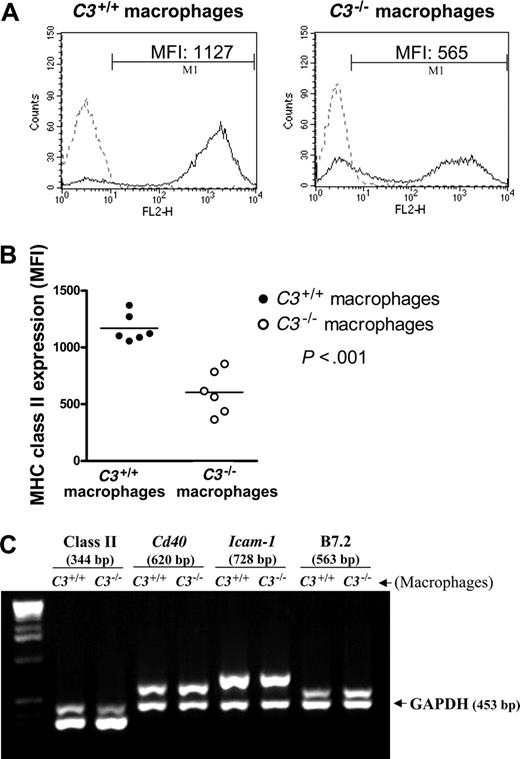
Full activation of T cells requires stimulating signals from both antigen (allo-MHC) and costimulatory molecules present on APCs. To assess the expression of MHC and costimulatory molecules, we first examined thioglycollate-elicited peritoneal macrophages. Using flow cytometry we found that the surface expression of MHC class II was reduced in C3-/- cells compared with C3+/+ cells (Figure 5A-B). However, cell-surface staining for costimulatory molecules showed no reduction of expression of B7.2, ICAM-1, or CD40 on the C3-/- cells (data not shown). To confirm our observations in a second macrophage population, we examined BM-derived macrophages, both before and after activation with LPS. Results showed that activated BM-derived macrophages exhibited higher expression of MHC class II than resting BM-derived macrophages, and the surface expression of MHC class II was lower in C3-/- cells compared with C3+/+ cells by flow cytometry (Figure S4), confirming our observations with peritoneal macrophages. Given the importance of the MHC/peptide complex on the surface of APCs in providing a cognate interaction with T cells, reduced MHC class II surface expression may contribute to the weak property of C3-/- APCs in allostimulation.
Dependence of CD4 T-cell response on the C3 status of donor macrophages. (A-B) Thioglycollate-elicited macrophages were prepared from C3+/+ and C3-/- mice. Primed alloreactive CD3+, CD4+, and CD8+ T cells were prepared from BALB/c mice. Pooled macrophages (2 × 105) from each group were cocultured with 2 × 105 T cells for up to 5 days. Supernatants were collected for measuring the production of IL-2 (A) and IFN-γ (B) by ELISA. (C) Normal peritoneum or BM macrophages were prepared from C3+/+ and C3-/- mice. The pooled macrophages without further purification with CD11b beads were stimulated with LPS (100 ng/mL) for 24 hours and then cocultured with primed alloreactive CD4+ T cells for 3 days. Supernatants were collected for measuring the production of IL-2 and IFN-γ by ELISA. All data in panels A and B were generated from 3 independent experiments, with 5 mice/group in each experiment. Data were analyzed by variance component regression analysis. (C) The data were generated from 3 experiments, with 3 mice/group in each experiment, and analyzed by 2-way ANOVA. P values are for comparisons between C3+/+ and C3-/- macrophages. Six additional T-cell stimulation experiments were performed, in which CD3 or CD4 T cells were incubated for only 3 days with thioglycollate-elicited macrophages. The data are shown in Figure S2. Error bars indicate SEM.
Dependence of CD4 T-cell response on the C3 status of donor macrophages. (A-B) Thioglycollate-elicited macrophages were prepared from C3+/+ and C3-/- mice. Primed alloreactive CD3+, CD4+, and CD8+ T cells were prepared from BALB/c mice. Pooled macrophages (2 × 105) from each group were cocultured with 2 × 105 T cells for up to 5 days. Supernatants were collected for measuring the production of IL-2 (A) and IFN-γ (B) by ELISA. (C) Normal peritoneum or BM macrophages were prepared from C3+/+ and C3-/- mice. The pooled macrophages without further purification with CD11b beads were stimulated with LPS (100 ng/mL) for 24 hours and then cocultured with primed alloreactive CD4+ T cells for 3 days. Supernatants were collected for measuring the production of IL-2 and IFN-γ by ELISA. All data in panels A and B were generated from 3 independent experiments, with 5 mice/group in each experiment. Data were analyzed by variance component regression analysis. (C) The data were generated from 3 experiments, with 3 mice/group in each experiment, and analyzed by 2-way ANOVA. P values are for comparisons between C3+/+ and C3-/- macrophages. Six additional T-cell stimulation experiments were performed, in which CD3 or CD4 T cells were incubated for only 3 days with thioglycollate-elicited macrophages. The data are shown in Figure S2. Error bars indicate SEM.
Reduced capacity of C3-/-macrophages for immune stimulation in vivo. BALB/c mice (n = 12/group) were given either C3+/+ or C3-/- C57BL/6 thioglycollate-elicited macrophages by intraperitoneal injection. The primed mice then received a C57BL/6 donor skin graft. T-cell responses in those BALB/c mice were then measured ex vivo by MLR at day 3 of coculture. The production of IL-2 (A) and IFN-γ (B) was measured by ELISA. Skin graft survival is shown in panel C. Data were analyzed by log-rank test. Solid line indicates C3+/+ macrophages (n = 9); dashed line, C3-/- macrophages (n = 6). P < .001. An additional experiment is shown in panel D, in which BALB/c mice (n = 10/group) were primed by skin grafting from C57BL/6 donors and rechallenged with C3+/+ or C3-/- C57BL/6 macrophages. T-cell responses in those BALB/c mice were then measured ex vivo by MLR. The production of IFN-γ was measured by ELISA (D). (A-B,D) Results from each animal are shown as separate data points, and all data points were analyzed by Student t test. P values are for comparisons between the 2 mouse groups. Additional results for MLR at days 1 to 5 of coculture are presented in Figure S3.
Reduced capacity of C3-/-macrophages for immune stimulation in vivo. BALB/c mice (n = 12/group) were given either C3+/+ or C3-/- C57BL/6 thioglycollate-elicited macrophages by intraperitoneal injection. The primed mice then received a C57BL/6 donor skin graft. T-cell responses in those BALB/c mice were then measured ex vivo by MLR at day 3 of coculture. The production of IL-2 (A) and IFN-γ (B) was measured by ELISA. Skin graft survival is shown in panel C. Data were analyzed by log-rank test. Solid line indicates C3+/+ macrophages (n = 9); dashed line, C3-/- macrophages (n = 6). P < .001. An additional experiment is shown in panel D, in which BALB/c mice (n = 10/group) were primed by skin grafting from C57BL/6 donors and rechallenged with C3+/+ or C3-/- C57BL/6 macrophages. T-cell responses in those BALB/c mice were then measured ex vivo by MLR. The production of IFN-γ was measured by ELISA (D). (A-B,D) Results from each animal are shown as separate data points, and all data points were analyzed by Student t test. P values are for comparisons between the 2 mouse groups. Additional results for MLR at days 1 to 5 of coculture are presented in Figure S3.
In addition we examined mRNA expression for MHC and costimulatory molecules, to ensure that the recombinant DNA procedures used to generate the C3-/- mice had not interfered with the expression of nontargeted genes that were essential for immune stimulation. Using semiquantitative PCR we found that all the genes examined (MHC class II, Cd86, Icam1, and Cd40) were transcribed, and that the level of gene expression was not reduced in the C3-/- macrophages. The RT-PCR data for MHC class II are shown in Figure 5C. Real-time PCR results confirmed that there was no loss of mRNA expression for MHC class II, Cd86, Icam1, and Cd40 (data not shown). Thus, defective immune stimulation by C3-/- macrophages was not due to loss of essential costimulatory molecules. Moreover, the lower frequency of MHC class II-expressing cells in C3-/- macrophages was not caused by a decrease in gene transcription. This raises the possibility that reduced surface expression of MHC class II in the C3-/- mouse cells could be due to a posttranscriptional defect of MHC class II expression.
Reduced cell-surface expression of MHC class II in C3-/-macrophages. (A-B) Thioglycollate-elicited macrophages were prepared from C3+/+ (n = 6) and C3-/- (n = 6) mice. Cells from each mouse were then stained for surface molecule MHC class II using PE-conjugated anti-mouse Ig or isotype control Ig. (A) Representative histogram plots for C3+/+ and C3-/- peritoneal macrophages. The control peak (dashed line) corresponds to staining cells with the isotype control antibody. The detection peak (solid line) shows surface binding of specific antibody. (B) Expression levels (mean fluorescence intensities, MFI) of MHC class II on macrophages. Data were analyzed by Student t test. (C) C3+/+ and C3-/- peritoneal macrophages prepared from 4 mice in each group were pooled and used for RNA extraction and cDNA synthesis and subsequently for semiquantitative PCR. A typical agarose gel shows PCR products for MHC class II, Cd40, Icam1, B7.2, and GAPDH (internal control). Results are representative of 3 independent experiments.
Reduced cell-surface expression of MHC class II in C3-/-macrophages. (A-B) Thioglycollate-elicited macrophages were prepared from C3+/+ (n = 6) and C3-/- (n = 6) mice. Cells from each mouse were then stained for surface molecule MHC class II using PE-conjugated anti-mouse Ig or isotype control Ig. (A) Representative histogram plots for C3+/+ and C3-/- peritoneal macrophages. The control peak (dashed line) corresponds to staining cells with the isotype control antibody. The detection peak (solid line) shows surface binding of specific antibody. (B) Expression levels (mean fluorescence intensities, MFI) of MHC class II on macrophages. Data were analyzed by Student t test. (C) C3+/+ and C3-/- peritoneal macrophages prepared from 4 mice in each group were pooled and used for RNA extraction and cDNA synthesis and subsequently for semiquantitative PCR. A typical agarose gel shows PCR products for MHC class II, Cd40, Icam1, B7.2, and GAPDH (internal control). Results are representative of 3 independent experiments.
Extracellular C3 does not explain the difference in the ability of C3-/- and C3+/+ macrophages to stimulate alloreactive T cells in vitro
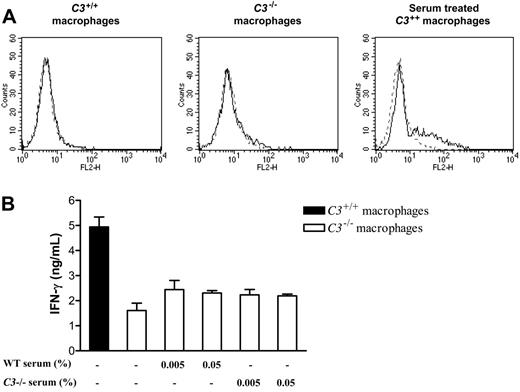
Next, we explored how endogenous production of C3 might increase the capacity of macrophages for stimulation of alloreactive T cells. Previous studies have shown that C3 attached covalently to macrophages can result in stronger T-cell stimulation, presumably via interaction of macrophage-bound C3 with complement receptors on T cells.31,32 To assess the potential for this interaction in our experiments, we prepared thioglycollate-elicited macrophages and performed staining for surface-bound C3 fragment by flow cytometry. There were no detectable differences between C3+/+ and C3-/- macrophages (Figure 6A). Therefore, the differences in T-cell stimulation by C3+/+ and C3-/- macrophages in vitro could not be explained by macrophage-adherent C3.
Extracellular C3 does not account for the different ability of C3-/-and C3+/+macrophages to stimulate alloreactive T cells. Thioglycollate-elicited macrophages were prepared from C3+/+ and C3-/- mice (n = 5/group). (A) C3+/+ or C3-/- macrophages and C3+/+ macrophages that were preincubated with 25% mouse serum at 37°C for 30 minutes and washed 3 times were stained for deposition of C3 using FITC-conjugated goat anti-mouse C3 IgG F(ab′)2 fragment. In all histogram plots, the control peak (dashed line) corresponds to unstained cells. The detection peak (solid line) shows the binding of C3 antibody. (B) C3+/+ or C3-/- macrophages were cocultured with primed alloreactive CD4+ T cells in the absence or presence of either WT (wild-type) normal serum or C3-/- serum for 5 days. Supernatants were collected and used for measuring the production of IFN-γ by ELISA. Data were analyzed by Student t test and showed no significant difference between untreated C3-/- macrophages and serum-treated C3-/- macrophages. Error bars indicate SEM. Results presented in panels A and B are representative of 3 independent experiments.
Extracellular C3 does not account for the different ability of C3-/-and C3+/+macrophages to stimulate alloreactive T cells. Thioglycollate-elicited macrophages were prepared from C3+/+ and C3-/- mice (n = 5/group). (A) C3+/+ or C3-/- macrophages and C3+/+ macrophages that were preincubated with 25% mouse serum at 37°C for 30 minutes and washed 3 times were stained for deposition of C3 using FITC-conjugated goat anti-mouse C3 IgG F(ab′)2 fragment. In all histogram plots, the control peak (dashed line) corresponds to unstained cells. The detection peak (solid line) shows the binding of C3 antibody. (B) C3+/+ or C3-/- macrophages were cocultured with primed alloreactive CD4+ T cells in the absence or presence of either WT (wild-type) normal serum or C3-/- serum for 5 days. Supernatants were collected and used for measuring the production of IFN-γ by ELISA. Data were analyzed by Student t test and showed no significant difference between untreated C3-/- macrophages and serum-treated C3-/- macrophages. Error bars indicate SEM. Results presented in panels A and B are representative of 3 independent experiments.
Another possibility is that soluble fragments released after the cleavage of secreted C3 could interact with C3a and C5a receptors on APCs or T cells.33,34 We measured the amount of C3 secreted during coculture in our experiments with irradiated C3+/+ macrophages and found that only small quantities of C3 were produced (4-14 ng/mL). To determine whether this small amount of secreted C3 could explain the functional difference between C3+/+ and C3-/- macrophages, we performed a reconstitution study. We cocultured C3-/- macrophages with primed T cells in the presence of either 0.005% or 0.05% fresh WT mouse serum (which contains at least 10 × the amount of C3 as secreted by our irradiated macrophages) or C3-/- serum at the same concentration and measured the T-cell response. As shown in Figure 6B, the production of IFN-γ was not significantly increased by the addition of C3 in the coculture medium. The response with C3-/- stimulators remained far below that with C3+/+ stimulators.
Additionally, we performed coculture of WT peritoneal macrophages with primed T cells in the presence of C3 inhibitor (anti-C3 antibody or soluble complement regulator, Crry-Ig35 ). Compared with isotype-matched control Ig, neither of these complement inhibitors led to a reduction in T-cell stimulation, as measured by IL-2 or IFN-γ release at day 3 of incubation (data not shown).
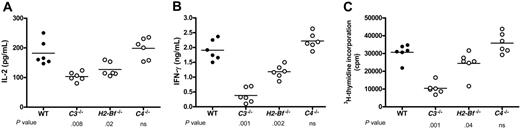
Effect of H2-Bf-/-and C4-/-macrophages on immune stimulation. Primed alloreactive CD4+ T cells were prepared from 4 BALB/c mice. Thioglycollate-elicited macrophages were prepared from WT (C57BL/6), C3-/-, C4-/-, and H2-Bf-/- mice (6 mice in each group). Macrophages (2 × 105) from each mouse were cocultured with 2 × 105 T cells. (A-B) T-cell activation was assessed at 3 days after culture by measuring the production of IL-2 and IFN-γ by ELISA. (C) T-cell proliferation was assessed at 96 hours after culture by measuring the incorporation of 3H-thymidine. Data were analyzed by Student t test. P values are for comparisons between complement-deficient and complement-sufficient macrophages; ns, no significant difference. Two further experiments were undertaken, and the results of these confirm the data shown in panel A (see Figure S5).
Effect of H2-Bf-/-and C4-/-macrophages on immune stimulation. Primed alloreactive CD4+ T cells were prepared from 4 BALB/c mice. Thioglycollate-elicited macrophages were prepared from WT (C57BL/6), C3-/-, C4-/-, and H2-Bf-/- mice (6 mice in each group). Macrophages (2 × 105) from each mouse were cocultured with 2 × 105 T cells. (A-B) T-cell activation was assessed at 3 days after culture by measuring the production of IL-2 and IFN-γ by ELISA. (C) T-cell proliferation was assessed at 96 hours after culture by measuring the incorporation of 3H-thymidine. Data were analyzed by Student t test. P values are for comparisons between complement-deficient and complement-sufficient macrophages; ns, no significant difference. Two further experiments were undertaken, and the results of these confirm the data shown in panel A (see Figure S5).
In all of the aforementioned experiments, the difference between C3-/- and C3+/+ macrophages in their ability to stimulate alloreactive T cells is not explained either by C3 fragment deposited on the macrophage surface or by the small amount of C3 released by irradiated cells in vitro. It is important to note that our data do not exclude the possibility of such effects occurring in vivo, thus promoting the interaction of macrophages and T cells.
H2-Bf-/- macrophages have impaired ability to elicit a T-cell response
Because C3-/- macrophages originate from an environment in which complement activation is lacking, we postulated that other factors required for complement activation might produce a similar effect on macrophage function. We therefore assessed the influence of Factor B (alternative pathway) and C4 (classical and lectin pathways) on macrophage-induced T-cell stimulation. Macrophages derived from C3-/-, H2-Bf-/-, and C4-/- mice were evaluated in T-cell cytokine and proliferation assays. The results are presented in Figure 7A and 7B, respectively. These confirm that the macrophage effect on the stimulation of primed T cells is dependent on C3. Additionally, the data show that macrophages from H2-Bf-/- mice elicit weaker T-cell responses compared with wild-type macrophages, although the effect of factor B is not as pronounced as for C3. In contrast, the T-cell response with C4-/- macrophages was unimpaired. This suggests that the effect of C3 in augmenting the T-cell response may partly depend on activation through the alternative pathway but not through the classical and lectin pathways.
Discussion
Although the effect of complement on B-cell regulation has been comprehensively studied, the way in which C3 stimulates the T-cell response is poorly understood. Our results show that macrophages from C3-deficient mice have reduced ability to stimulate alloreactive T cells. These findings, shown both for T-cell priming and T-cell reactivation, in vitro and in vivo, suggest a critical role for complement in the maintenance of APC function.
The C3-knockout mouse used in our study was an 11th generation backcross that exhibited indefinite skin graft survival with the wild-type strain (C57BL/6). Thus, it is unlikely that the C3-deficient and wild-type macrophages had any significant differences at histocompatibility loci. Moreover, we were unable to demonstrate defects in macrophage gene expression of several vital pathways involved in immune stimulation. Therefore, the differences in T-cell stimulation obtained with wild-type and C3-deficient macrophages were not obviously explained by defects of other genes involved in antigen presentation. Although a mutant strain of mouse with spontaneous deficiency of C3 was not available, an inbred guinea pig strain with partial functional deficiency of C3 exists, and peritoneal macrophages from these guinea pigs were shown to elicit reduced T-cell responses against conventional antigens.32 These observations offer strong support that the differences between deficient and wild-type macrophages detected in our study are due to lack of C3.
The mechanism by which C3 enhances the T-cell stimulatory capacity of macrophages has a number of possible explanations. The simplest of these is that macrophages derived from C3-deficient mice have an immature phenotype and are thus less competent to function as APCs. The reduced cell-surface MHC class II expression and associated defect of CD4 T-cell stimulation found in our study could be an example of this. In vivo, complement activation can occur spontaneously under physiologic conditions and can increase rapidly in response to inflammatory stimuli.36,37 Complement effector products may directly activate macrophages through engagement of C3a or C5a receptors on the macrophage surface or through membrane insertion with a sublethal dose of C5b-9.38,39 In addition, complement-mediated local inflammation could indirectly activate macrophages through cytokine, autocrine, and paracrine regulation.38 Thus, deficiency of C3 or other activating factors required for the cleavage of C3, such as factor B, would result in a less-mature macrophage phenotype with reduced capacity for stimulating alloreactive T cells. It is also apparent from our results that factor B had a weaker effect than C3 on the capacity of APCs for T-cell stimulation, suggesting that the alternative pathway (of which factor B is an essential component) is not the only pathway leading to C3 cleavage in our model. However, our results identified no significant role for the classical and lectin pathway component C4. This apparent discrepancy requires further investigation.
Another possibility to explain the effect of complement is that membrane-bound fragments of C3 behave as adhesion molecules increasing the contact between macrophages and T cells, which are known to express receptors for such fragments.40,41 A similar model has been proposed in the study of conventional antigens.31,32 Thus, C3 receptor-ligand interaction may provide a bridge between these 2 types of cells, facilitating immune adherence and T-cell activation. Our study differed from those with conventional antigen, in that alloantigen is synthesized by the donor APCs, although it may also be taken up by recipient APCs and presented to the immune system. Moreover, we were unable to show that the addition of complement could restore allostimulation by C3-deficient mouse macrophages or show an effect of C3 blockade in vitro. However, our findings do not preclude a role of C3 for opsonization of APCs in vivo.
A third potential mechanism suggested by our data is that local production of C3 by donor APCs could be crucial to their relationship with T cells. Macrophages are able to synthesize C3 and factor B, as well as other components of the complement activation pathways in a cytokine-dependent manner.5,42 Local production of C3 and other activating factors thus has the ability to alter the microenvironment in the region of the APC in vivo and therefore to contribute to their innate and adaptive immune functions. Our findings in C3 gene-silencing studies showing a causal relationship between macrophage expression of C3 and their immunoregulatory capacity support the notion that local production contributes to normal macrophage function.
Finally, it has been proposed that C3 taken up with antigen via a complement-receptor-dependent mechanism can modulate the presentation of antigen to T cells.43 Using B cells as APCs, research has shown that C3-coupled antigen is protected from excessive proteolytic degradation in the endosomal/lysosomal compartment of the MHC class II pathway, suggesting that activated C3 fragment acts as a chaperone in this antigen-presenting pathway.22-24 It is therefore possible that C3, either synthesized or taken up by macrophages, could act as an adjuvant for alloantigen. Thus, it could be speculated that the lowered expression of MHC class II on the surface of C3-deficient APCs, presumably as a result of a posttranscriptional mechanism, arises from defective antigen processing.
In conclusion, our data show that macrophages derived from C3-deficient mice have impaired ability to generate alloreactive T-cell responses in vitro and in vivo. Although it is possible that APCs arising in a complement-deprived environment have defective maturation, the results of C3 gene silencing point to an additional mechanism that is dependent on the local synthesis of C3. This is the subject of ongoing studies. Thus, defective APC function offers an explanation, at least in part, for C3-deficient mice having impaired T-cell responses. Additionally, our data that APC function could depend on the local synthesis of C3 may inform new approaches for vaccine development, cancer therapy, and prevention of organ graft rejection.
Prepublished online as Blood First Edition Paper, November 22, 2005; DOI 10.1182/blood-2005-08-3144.
Supported by grants from the Wellcome Trust and the Medical Research Council of the United Kingdom.
Presented in abstract form at the 20th International Complement Workshop, Honolulu, HI, June 13-18, 2004.44
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Professor M. Carroll for providing the C3 knockout mice. We thank Mrs Min Yang for help with the statistical analysis. We thank Professor M. Peakman, Dr Stipo Jurcevic, and Dr Roseanna Hargreaves for helpful scientific discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal