Abstract
Many B-lineage-specific genes are down-regulated in Hodgkin and Reed-Sternberg (HRS) cells of classical Hodgkin lymphoma (cHL). We investigated the involvement of epigenetic modifications in gene silencing in cHL cell lines and in microdissected primary HRS cells. We assessed the expression and methylation status of CD19, CD20, CD79B, SYK, PU.1, BOB.1/OBF.1, BCMA, and LCK, all of which are typically down-regulated in cHL. We could reactivate gene expression in cHL cell lines with the DNA demethylating agent 5-aza-deoxycytidine (5-aza-dC). Using methylation-specific polymerase chain reaction (MSP), bisulfite genomic sequencing, and digestion with methylation-sensitive endonuclease followed by polymerase chain reaction (PCR), we determined the methylation status of promoter regions of PU.1, BOB.1/OBF.1, CD19, SYK, and CD79B. Down-regulation of transcription typically correlated with hypermethylation. Using bisulfite genomic sequencing we found that in microdissected HRS cells of primary cHL SYK, BOB.1/OBF.1, and CD79B promoters were also hypermethylated. Ectopic expression of both Oct2 and PU.1 in a cHL cell line potentiated endogenous PU.1 and SYK expression after 5-aza-dC treatment. These observations indicate that silencing of the B-cell-specific genes in cHL may be the consequence of a compromised regulatory network where down-regulation of a few master transcription factors results in silencing of numerous genes. (Blood. 2006;107:2493-2500)
Introduction
B-cell-type Hodgkin and Reed-Sternberg (HRS) cells of classical Hodgkin lymphoma (cHL) are characterized by either the complete absence or only low-level expression of many B-cell-specific genes. Whereas initially this was seen for immunoglobulin (Ig) genes and specific cell-surface markers, later analyses also identified the lack of a variety of B-cell-specific transcription factors like Oct2 (octamer binding factor 2), BOB.1/OBF.1 (B-cell Oct binding protein/Oct-binding factor), and PU.1 (purine-reach GGAA binding site) (for review, see Kuppers et al1 ). Recent gene expression array studies on cHL cell lines added to the list of down-regulated genes or confirmed the down-regulation of CD79A and CD79B, CD19, CD20, SYK (spleen tyrosine kinase), BCMA (B-cell maturation antigen), LCK (lymphocyte-specific protein tyrosine kinase), and some others.2,3 Extensive immunohistochemical studies in primary tumors by and large were in agreement with the gene expression array data obtained from cHL cell lines.4,5 These findings gave rise to the concept of “loss of the B-cell identity” as part of the neoplastic transformation process leading to cHL.1
Many of the affected genes are involved in regulation of the death/survival choice in B-cells upon B-cell-receptor (BCR) activation. The BCR is made up from immunoglobulin heavy and light chains as well as CD79a and CD79a. CD19 is a B-cell coreceptor that augments the signals delivered through the BCR.6 CD20 is a nonglycosylated phosphoprotein expressed on the surface of almost all normal and malignant B cells. CD20 probably functions as a store-operated calcium channel, activation of which can lead to apoptosis.7 LCK is involved in signal transduction and regulation of apoptosis induced by anti-CD20 antibody stimulation.8 SYK is a protein kinase proximal to BCR involved in activating phospholipase Cγ2 (PLCγ2).9 Activation of PLCγ2 then results in Ca2+ release. The stimulation of SYK, PLCγ2, and Ca2+ release is necessary both for BCR signaling and BCR-induced apoptosis.9,10
The mechanisms of simultaneous down-regulation of many B-cell-specific genes in cHL are still not understood. In theory, gene silencing could be achieved by specific mutations, absence of transcription factors, or by epigenetic silencing. These processes may operate alone or in combination. Down-regulation of a large set of genes can be explained by the inhibition of few critical transcription factors. Indeed, several of the down-regulated genes listed above are regulated by BOB.1/OBF.1/Oct2 and/or PU.1.11,12 Genetic mutations as the cause of simultaneous silencing of numerous genes seem unlikely considering their relative scarcity and their mainly stochastic nature. Apparently, mutations and translocations of the genes, playing a crucial role in the pathogenesis of non-Hodgkin lymphomas, are rare or absent in cHL.13 Genomic imbalances or rearrangements are not the causes of PU.1, BOB.1/OBF.1, and OCT2 silencing in cHL.14 The only known consistent oncogenic feature of cHL, the constitutive nuclear factor (NF)-κB activation, can be explained in only a few cases by mutations of a regulatory gene.15 Even the recently described mutations of the tumor suppressor gene SOCS1 in about 40% of primary cHL cases16 is most likely not the only tumorigenic mechanism.
In malignant neoplasias, epigenetic events often cause the down-regulation of tumor suppressor genes (for reviews, see Egger et al,17 Di Croce et al,18 Esteller and Herman,19 and Teodoridis et al20 ). Two main processes are involved in epigenetic gene silencing: DNA methylation and histone modifications. DNA methylation, the addition of a methyl group to position 5 of the cytosine pyrimidine ring, occurs in eukaryotes specifically in cytosinephosphate-guanosine (CpG) dinucleotides. The DNA regions with high CpG density are called CpG islands. The CpG islands are often located in the promoter region and spread into the first exon of the respective gene. They are important for the regulation of gene transcription.21 It was shown in numerous studies that CpG islands of transcriptionally active genes are hypomethylated. In contrast, silencing of a gene is often associated with CpG island hypermethylation.22 In cHL, DNA methylation is involved in silencing of the tumor suppressor genes p16INK4a, p15INK4b,23 RASSF1A (RAS-associated domain family 1),24 and p18INK4c.25 We found that inhibition of immunoglobulin transcription in cHL cells may at least in part be explained by epigenetic silencing.26 The fact that epigenetic silencing often is nonrandom, but can occur in a pathway-specific manner and affect numerous genes,27,28 led us to ask whether this mechanism might be involved in down-regulation of B-lineage genes in HRS cells of cHL. Probing cHL cell lines, we show that this is indeed the case. In addition, by analyzing microdissected cells we found that promoter methylation is not a cell culture artifact but is detectable also in primary cHL HRS cells.
Materials and methods
Cell lines and treatments
The human Burkitt lymphoma cell lines Namalwa and BJAB, as well as the cHL-derived cell lines L1236, KM-H2, and L428 were cultured in RPMI 1640 medium (Life Technologies, Karlsruhe, Germany) supplemented with 10% fetal bovine serum (PAN; Biotech, Aidenbach, Germany), antibiotics, l-glutamine, and 50 μM 2-mercaptoethanol at 37°C and 5% CO2. 5-aza-2′-deoxycytidine (5-aza-dC) was purchased from Calbiochem (Darmstadt, Germany). L428-Oct2-BOB.1 cell lines were created by transfection of L428-Oct2 cell lines with pCFG5-BOB.1 vector, expressing human BOB.1/OBF.1 as described earlier.26 The clones stably expressing BOB.1/OBF.1 were selected by limiting dilutions. Transfected cells were incubated with zeozin (InvivoGen, San Diego, CA) at a concentration of 100 μg/mL. As a control we used the L428-Oct2-zeo cell lines, which were generated by transfection of the L428-Oct2 cell lines with pCFG5 IEGZ (empty vector) and selection with zeozin. Mouse PU.129 was transiently overexpressed in L428 cells using the Nucleofector device (Nucleofector Kit T, program T-13; Amaxa, Cologne, Germany) as described earlier.26
RT-PCR
Total RNA was isolated from 1 × 106 cells using High Pure RNA Isolation Kit (Roche, Mannheim, Germany). RNA (2 μg) and 0.5 μg dT15 primer (MWG-Biotech, Ebersberg, Germany) were heated for 5 minutes at 70°C. After cooling on ice, the first-strand cDNA was synthesized by Moloney murine leukemia virus (M-MLV) reverse transcriptase (RT; Promega, Madison, WI). The following pairs of primers were used to amplify the resulting cDNA (5′ to 3′, sense and antisense, annealing temperature): BOB.1/OBF.1: TCAGATGTGCAAGATGAATCC and CACAGCTCCGGAGCAAGCC, 60°C30 ; PU.1: CGACCATTACTGGGACTTCC and TTCTTCTTCACCTTCTTGACC, 48°C31 ; mouse Pu.1: GATGGAGAAGCTGATGGCTTGG and TTCTTCACCTCGCCTGTCTTGC, 56°C32 ; CD79B: GGAGCCTCGGACGTTGTCA and CGACCTGGCTCTCACTCCT, 61°C33 ; CD19: TCACCGTGGCAACCTGACCATG and GAGACAGCACGTTCCCGTTACT, 55°C34 ; CD20: GAAAAACTCCCCATCTACCCAATAC and AAAAAGGAAACAGAAAACAGAAGAAATC, 54°C; BCMA: GGCAGTGCTCCCAAAATGAATA and CTGGGAGTGGAAAGCAATGGTC, 56°C; SYK: TGTCAAGGATAAGAACATCATAG and CACCACGTCATAGTAGTAATTG, 62°C35 ; PBGD: AGCTGCAGAGAAAGTTCCC and GTTACGAGCAGTGATGCC, 60°C; OCT2: GAGGAGCTGGAGCAATTCG and CTCTTCTCTAAGGCGAAGCG, 51°C31 ; and LCK: TGGTGGGAGGACGAGTGGGAGGTTC and GGATGCGGCCGTGGGTGACAAT, 66°C. Polymerase chain reaction (PCR) products were resolved on 1.5% agarose gel and stained with ethidium bromide (EtBr).
PCR amplification of genomic DNA digested with methylation-sensitive enzymes
CpG islands in promoter regions of the genes were located using the MethPrimer program (Urology Research Center, Veterans Affairs Medical Center and University of California, San Francisco).36 Genomic DNA was purified using DNease tissue kit, no. 69504 (Qiagen, Hilden, Germany). DNA (1 μg) was digested with 20 U MspI or HpaII (both from New England Biolabs, Beverly, MA) in 20 μL total reaction volume for 2 hours at 37°C. The reaction (1 μL) was amplified with the primers flanking the 5′-CCGG-3′ recognition sites in promoter regions of the genes. SYK: GGCAGCCCCACCTTCTCT and CGCGGCTCTTCCTCATTT, 51°C; CD19: AGCGTGGCAGGGAGGAGGCAAGTGTT and GCGAGGAGGTGGCATGGTGGTCAGA, 62°C; and PU.1: TTAGCCCCCAAAGTCATCCCTCTCA and ACCCTTCCATTTTCGACTCCTGTAAC, 62°C. The PCR products were resolved on 1.5% agarose gel and stained with EtBr.
Methylation-specific PCR
The primers inside of the BOB.1/OBF.1 CpG island were located using the MethPrimer program.36 Genomic DNA was isolated as described in “PCR amplification of genomic DNA digested with methylation-sensitive enzymes.” DNA (1 μg) was used for bisulfite modification using the CpGenome DNA modification kit, no. S7820 (Chemicon, Temecula, CA) according to the manufacturer's instructions. The bisulfite-converted DNA (100 ng) was amplified 35 times using primers specific for methylated CpG cytosines: TGGTTGTTCGCGTTGC and CCTCAAACACCGATACAACGT, 49°C; and primers specific for unmethylated CpGs: TTATATATAGTAGGTTTTTGCGGGGTTG and TAAATTCCCACTACATAAACCACAT, 49°C. The PCR products were separated on 6% polyacrylamide gels and stained with EtBr.
Bisulfite sequencing
To analyze DNA methylation in macrosamples (cell cultures or tissue samples), genomic DNA was isolated and nonmethylated cytosines were converted to uridines by bisulfite treatment as described in “Methylation-specific PCR.” The templates (100 ng) were amplified with specific primers: SYK promoter CpG island: GTTTGTGGGTTTTGGGTAGTTATAG and ACTCTTCCTCATTTTAAACAACTTCC, 57°C; and BOB.1/OBF.1 promoter CpG island: GTTTTTGGGTTTATAATTGGTTTG and AAACTTTTTAAAAACCTAAATTCCC, 57°C.
The HotStarTaq polymerase master kit (Qiagen) was used followed by 20 amplification cycles with Pwo DNA polymerase (Roche) to blunt the ends. PCR products were then cloned into the pCAPs vector using the PCR cloning kit (blunt end; Roche). XL-1-competent cells (Stratagene, Amsterdam, The Netherlands) were transformed with the vector. The cloned plasmids were purified with Quiaprep Spin Miniprep Kit (Qiagen) and sequenced using the BigDye Terminator v1.1 cycle sequencing kit and the ABI Prism 310 Genetic Analyzer (both from Applied Biosystems, Warrington, United Kingdom).
Human material, laser capture microdissection, and bisulfite sequencing of the HRS DNA samples
Tumor tissues were drawn from our bank of fresh tissues. The tumor material was pseudonymized to comply with the German law for correct usage of archival tissue for clinical research (Deutsches Ärzteblatt 2003; 100 A1632). Approval for this procedure was obtained from the ethics committee of the University of Ulm. According to the morphologic criteria, cHL cases HL10B, HL61, HL10, HL77, HL41, and HL26 were characterized as nodular sclerosis and HL59 and HL83 as mixed cellularity forms. In addition, 2 DLCL cases, DLCL-1 and DLCL-2 (both centroblastic subtype), and 2 FL cases, FL-A and FL-B (both grade 1), were analyzed.
Human primary B cells were isolated from buffy coats of 3 healthy donors using B-cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Frozen tonsil tissue was cut to 25-μm slices on a cryostat microtome using a clean blade for every new tissue sample. Genomic DNA from B lymphocytes and from tonsil tissue samples was extracted with the DNeasy kit (Qiagen) following the manufacturer's instructions. HRS cells were isolated from tumor specimens by laser capture microdissection. Membrane-covered glass slides were mounted with 7-μm lymphoma frozen sections. Sections were fixed in acetone at room temperature for 5 minutes and stained with CD30 (Ber-H2; Dako, Hamburg, Germany) by immunohistochemistry using 3-amino-9 ethyl-carbazole (AEC) as a color substrate. Nuclei were counterstained with hematoxylin. HRS cells were identified by CD30 expression and by their characteristic cytomorphology. Microdissection and laser pressure catapulting was performed using a Robot-Microbeam system (P.A.L.M. Microlaser Technologies, Bernried, Germany) equipped with an IX50 microscope (Olympus, Hamburg, Germany). From each lymphoma specimen, 50 to 100 HRS cells were catapulted into the cap of 1 PCR tube. DNA was extracted using PicoPure DNA extraction kit no. KIT0103 (Arcturus, Mountain View, CA) and purified with phenol/chloroform. Cytosines were deaminated by the bisulfite conversion protocol as described.37 Deaminated DNA was desalted by QiaexII gel extraction kit (Qiagen) and desulfonated as described.37 The DNA was precipitated with ethanol. The pellet was resuspended in 10 μL water. The whole sample was PCR amplified using HotStarTaq polymerase master kit (Qiagen; 35 cycles) followed by 20 cycles with Pwo DNA polymerase (Roche) to blunt the ends. For CD79b amplification we used primers GGTTTTAATTTGTATGGTAGGAAGG and CCAATAACTAAACACAAAAAACAAC, 49°C. For SYK and BOB.1/OBF.1 CpG island amplification we used seminested primers. First, the converted DNA was amplified with 35 cycles with respective outer primers (described in “Bisulfite sequencing”). The reactions (2 μL) were then amplified with a new nested forward primer and the same reverse primer. The PCR products were cloned and sequenced as described for macrosamples. Nested forward primer for SYK GGGTAGTTTTATTTTTTTTGTTTG, 52°C and for BOB.1/OBF.1 GGTTTTTATAGTTTGTTTTATATTATTAAAA, 56°C, were used.
Results
Reactivation of silenced genes by 5-aza-dC
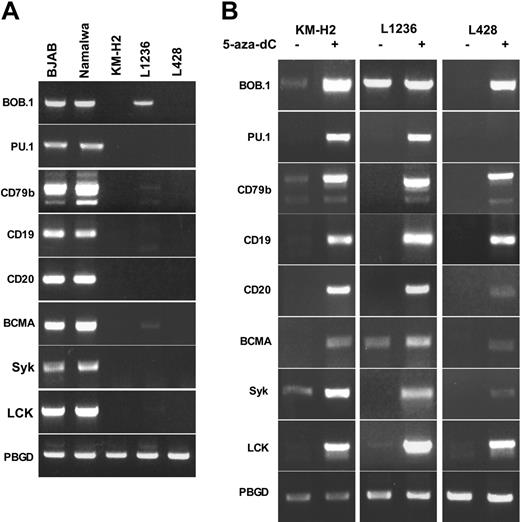
As outlined in “Introduction,” expression of multiple B-cell-specific genes had been shown to be down-regulated in primary HRS and cHL cell lines. Before we went on to check the potential involvement of epigenetic processes in this down-regulation we first confirmed the lack of expression of these genes in several cHL-derived cell lines. We chose the transcription factors BOB.1/OBF.1 and PU.1 as well as several genes encoding proteins involved in signaling (CD79B, CD19, CD20, BCMA, SYK, and LCK). Expression of the selected genes was assessed in the cHL cell lines KM-H2, L1236, and L428. These cell lines share many properties of primary HRS cells.38,39 The Burkitt lymphoma cell lines Namalwa and BJAB were used as positive controls (Figure 1A). Whereas expression of all the genes tested was readily detectable in the Burkitt lymphoma cell lines, no significant expression for most of the B-lineage-specific genes was detected in cHL-derived cell lines. However, consistent with our earlier observations L1236 cells showed expression of BOB.1/OBF.1 mRNA.
We then asked whether epigenetic silencing might contribute to the lack of expression of these genes in the cHL-derived cell lines. Cells were treated with 1 μM 5-aza-dC for 24 hours and subsequently were washed and incubated in complete medium for an additional 72 hours (Figure 1B). This schedule of treatment was found superior to continuous 5-aza-dC treatment (data not shown). When expression of the various silenced genes was analyzed in 5-aza-dC-treated cells, we found that all genes were reexpressed in KM-H2 cells and in L1236 cells. Although we also observed gene activation in L428 cells, we were unable to detect PU.1 expression, and genes like CD20, BCMA, and SYK were expressed at very low levels.
Reactivation of the B-lineage genes in cHL-derived cell lines by treatment with 5-aza-dC. (A) Silencing of B-cell-specific genes in cHL cell lines. Transcriptional activity of B-lineage-specific genes was assayed by RT-PCR in Burkitt lymphoma (Namalwa and BJAB) and cHL (KM-H2, L1236 and L428) cell lines. (B) Reactivation of genes by 5-aza-dC treatment. 5 × 106 cells were seeded in complete medium at a density of 0.2 × 106 cells/mL. The next day, cells were treated with 1 μM 5-aza-dC for 24 hours. Then, cells were washed and resuspended in the fresh medium. After 72 hours cells were harvested and gene expression was assessed by RT-PCR. Whereas 35 PCR cycles were performed for most genes, signals for CD20, BCMA, and SYK could be detected only after 40 cycles in L428 cells. Cycles of amplification (25) were used for PBGD. PCR products were separated on the 1.5% agarose gel and visualized by EtBr staining. All experiments were done at least in triplicate.
Reactivation of the B-lineage genes in cHL-derived cell lines by treatment with 5-aza-dC. (A) Silencing of B-cell-specific genes in cHL cell lines. Transcriptional activity of B-lineage-specific genes was assayed by RT-PCR in Burkitt lymphoma (Namalwa and BJAB) and cHL (KM-H2, L1236 and L428) cell lines. (B) Reactivation of genes by 5-aza-dC treatment. 5 × 106 cells were seeded in complete medium at a density of 0.2 × 106 cells/mL. The next day, cells were treated with 1 μM 5-aza-dC for 24 hours. Then, cells were washed and resuspended in the fresh medium. After 72 hours cells were harvested and gene expression was assessed by RT-PCR. Whereas 35 PCR cycles were performed for most genes, signals for CD20, BCMA, and SYK could be detected only after 40 cycles in L428 cells. Cycles of amplification (25) were used for PBGD. PCR products were separated on the 1.5% agarose gel and visualized by EtBr staining. All experiments were done at least in triplicate.
The ability of 5-aza-dC to reactivate expression of the silenced genes depends on the presence of specific transcription factors
We went on to investigate why PU.1 could not be reactivated by 5-aza-dC treatment in L428 cells. An obvious difference between L428 and the other 2 cell lines KM-H2 and L1236 is a different expression level of Oct transcription factors/cofactors. KM-H2 expresses low-levels of Oct2,26,40 and L1236 expresses low levels of BOB.1/OBF.1.26,41 We therefore hypothesized that reactivation of PU.1 might require not only the “opening” of the promoter region by inhibition of the DNA methyltransferase, but in addition the presence of at least low concentrations of relevant transcription factors. To test this hypothesis, we investigated the ability of Oct2 alone or in combination with the coactivator BOB.1/OBF.1 to reactivate expression of PU.1 in L428 cells. Our choice was based on the known role of Oct2 in the regulation of PU.1 transcription.29 We found that overexpression of Oct2 alone did not reactivate PU.1 expression, whereas treatment of L428-Oct2 cells with 5-aza-dC resulted in the appearance of the PU.1 signal (Figure 2A). However, Oct2 did not increase expression of CD20 and BCMA, which had shown only partial reactivation by 5-aza-dC in L428 cells (Figure 1B and data not shown).
When L428-Oct2-BOB.1 cells expressing both Oct2 and BOB.1/OBF.1 were treated with 5-aza-dC, PU.1 expression was slightly higher then that observed in L428 cells ectopically expressing Oct2 only. However, the sole expression of BOB.1/OBF.1 alone in the absence of Oct2 did not result in a measurable effect on PU.1 expression (data not shown). Interestingly, we found that PU.1 reactivation was always accompanied by reactivation of SYK. This might be explained by the fact that SYK has a PU.1 binding site at position -45 relative to the start of transcription. PU.1 has also been identified previously as a positive regulator of its own promoter, suggesting a positive autoregulatory loop.42 We therefore asked whether the endogenous SYK and PU.1 genes in L428 cells could be reactivated by ectopic expression of PU.1. We used mouse PU.1, which shares substantial homology with the human PU.1 and is able to activate target promoters of human PU.1.43 By RT-PCR we found that in combination with 5-aza-dC treatment, mouse PU.1 reactivated expression of the endogenous PU.1 and SYK genes (Figure 2B). Of note, ectopic PU.1 was able to reactivate SYK expression even without 5-aza-dC treatment. However, PU.1 alone or in combination with 5-aza-dC again was unable to further activate CD20 and BCMA (data not shown). Thus, re-expression of silenced genes induced by 5-aza-dC is dependent on the presence of specific transcription factors.
Expression of PU.1 and Syk requires the combination of 5-aza-dC treatment and Oct2 or PU.1 expression. (A) L428 cells stably overexpressing Oct2 or Oct2 plus BOB.1/OBF.1 transcription factors as well as L428 cells stably transfected with the corresponding empty vectors were treated with 5-aza-dC as described in the legend to Figure 1. The gene expression was assessed by RT-PCR using 35 amplification cycles. (B) 5 × 106 L428 cells were either left untreated or treated with 1 μM 5-aza-dC. After 48 hours the cells were transiently transfected with mouse PU.1 expression vector or the empty vector as control. Another 48 hours later, cells were harvested and used for RT-PCR analysis. PU.1m corresponds to the ectopic murine PU.1, and PU.1h represents the endogenous human PU.1 gene. The experiments were done at least in triplicate.
Expression of PU.1 and Syk requires the combination of 5-aza-dC treatment and Oct2 or PU.1 expression. (A) L428 cells stably overexpressing Oct2 or Oct2 plus BOB.1/OBF.1 transcription factors as well as L428 cells stably transfected with the corresponding empty vectors were treated with 5-aza-dC as described in the legend to Figure 1. The gene expression was assessed by RT-PCR using 35 amplification cycles. (B) 5 × 106 L428 cells were either left untreated or treated with 1 μM 5-aza-dC. After 48 hours the cells were transiently transfected with mouse PU.1 expression vector or the empty vector as control. Another 48 hours later, cells were harvested and used for RT-PCR analysis. PU.1m corresponds to the ectopic murine PU.1, and PU.1h represents the endogenous human PU.1 gene. The experiments were done at least in triplicate.
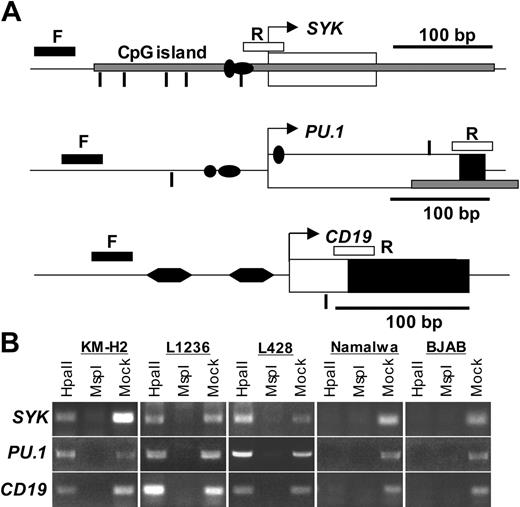
Promoter regions of SYK, PU.1, and CD19 are methylated in cHL cell lines. (A) Analysis of promoter methylation by amplification of genomic DNA digested with methylation-sensitive endonucleases. The positions of the forward and reverse primers are marked with F and R, respectively. Genomic DNA was digested with the methylation-sensitive enzyme HpaII or with its methylation-insensitive isoschizomer MspI followed by amplification with primers, located on both sides of the recognized sequence 5′-CCGG-3′ (shown as vertical lines). Amplification of HpaII-digested DNA is possible only when the cytosine of the CpG dinucleotide is methylated and the DNA is therefore not digested. The SYK amplicon is 264 bp in length and contains five 5′-CCGG-3′ motifs at positions -30, -107, -136, -186, and -214 bp counting from the start site of transcription (arrow) as predicted by the National Center for Biotechnology Information (NCBI) Entrez Gene program.68 The SYK CpG island (shown as a gray horizontal bar) spreads over exon 1 (thick filled bar). Sp1 binding sites are shown as horizontal filled ovals. For PU.1 promoter methylation analysis, we used NCBI sequence (accession no. U34046). The amplified region contains two 5′-CCGG-3′ sites at positions -66 and +187, an octamer motif (•), and Sp1 and PU.1 (vertical filled oval) binding sites. The coding region of exon 1 is shown in black. The 211-bp-long amplicon of CD19 5′ untranslated region (NCBI accession no. M84371) included 2 BSAP (B-cell-specific activator protein)/Pax5 binding sites (filled hexagons) and one 5′-CCGG-3 HpaII/MspI recognition site at position +26. (B) Methylation of the SYK, PU.1, and CD19 promoter regions. The PCR amplification products were separated on 1.5% agarose gels and visualized by EtBr staining. All experiments were performed at least in triplicate.
Promoter regions of SYK, PU.1, and CD19 are methylated in cHL cell lines. (A) Analysis of promoter methylation by amplification of genomic DNA digested with methylation-sensitive endonucleases. The positions of the forward and reverse primers are marked with F and R, respectively. Genomic DNA was digested with the methylation-sensitive enzyme HpaII or with its methylation-insensitive isoschizomer MspI followed by amplification with primers, located on both sides of the recognized sequence 5′-CCGG-3′ (shown as vertical lines). Amplification of HpaII-digested DNA is possible only when the cytosine of the CpG dinucleotide is methylated and the DNA is therefore not digested. The SYK amplicon is 264 bp in length and contains five 5′-CCGG-3′ motifs at positions -30, -107, -136, -186, and -214 bp counting from the start site of transcription (arrow) as predicted by the National Center for Biotechnology Information (NCBI) Entrez Gene program.68 The SYK CpG island (shown as a gray horizontal bar) spreads over exon 1 (thick filled bar). Sp1 binding sites are shown as horizontal filled ovals. For PU.1 promoter methylation analysis, we used NCBI sequence (accession no. U34046). The amplified region contains two 5′-CCGG-3′ sites at positions -66 and +187, an octamer motif (•), and Sp1 and PU.1 (vertical filled oval) binding sites. The coding region of exon 1 is shown in black. The 211-bp-long amplicon of CD19 5′ untranslated region (NCBI accession no. M84371) included 2 BSAP (B-cell-specific activator protein)/Pax5 binding sites (filled hexagons) and one 5′-CCGG-3 HpaII/MspI recognition site at position +26. (B) Methylation of the SYK, PU.1, and CD19 promoter regions. The PCR amplification products were separated on 1.5% agarose gels and visualized by EtBr staining. All experiments were performed at least in triplicate.
Hypermethylation of SYK, PU.1, and CD19 promoter regions in cHL cell lines
Reactivation of B-cell-specific genes by treatment with 5-aza-dC led us to conclude that promoter methylation is involved in gene silencing in cHL cell lines. To directly investigate the methylation status of the promoter regions of the selected genes (PU.1, SYK, and CD19) we used PCR amplification of genomic DNA digested with methylation-sensitive enzymes.44 This assay is based on the inability of the methylation sensitive restriction enzyme HpaII to digest a methylated 5′-CCmGG-3′ site. The primers were positioned in the promoter region from both sites of the selected 5′-CCGG-3′ recognition site(s) as indicated in Figure 3A. We found that in the PU.1, CD19, and SYK promoters the 5′-CCGG-3′ motifs were always methylated in cHL cell lines but not in Burkitt lymphomas (Figure 3B). Absence of the PCR product in the samples digested with the methylation-insensitive enzyme MspI indicates that the 5′-CCGG-3′ sequence is preserved in the amplified regions.
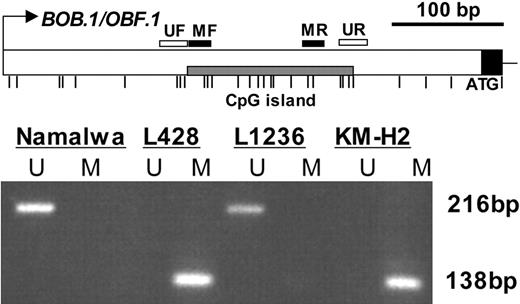
For analysis of BOB.1/OBF.1 CpG island methylation, we used methylation-sensitive PCR (MSP).45 We generated primers for amplification of methylated (MF and MR) and unmethylated CpG islands (UF and UR) and used these primers to amplify bisulfitetreated DNA (Figure 4). Methylation was detected only in KM-H2 and L428 cell lines, which lack BOB.1/OBF.1 expression. In contrast, only primers specific for unmethylated BOB.1/OBF.1 amplified the bisulfite-modified DNA of L1236 cells and the Burkitt lymphoma control cell line Namalwa, both of which express BOB.1/OBF.1 mRNA. These results are absolutely consistent with the mRNA expression data shown in Figure 1A and 1B.
SYK, BOB.1/OBF.1, and CD79B promoters are hypermethylated in cHL cell lines and in HRS of primary cHL
The restriction enzyme-based method gives information only about the methylation status of the HpaII restriction site. Similarly, MSP provides information only about the methylation status of a few CpG dinucleotides within the primer binding sites. To obtain a more detailed assessment, we examined CpG island methylation of the SYK, BOB.1/OBF.1, and CD79B genes using sequencing of clones obtained from bisulfite-treated DNA. Importantly, bisulfite sequencing is suitable for limited amounts of DNA37 and was successfully used for DNA methylation analysis in samples containing as few as 50 microdissected HRS cells.24,25
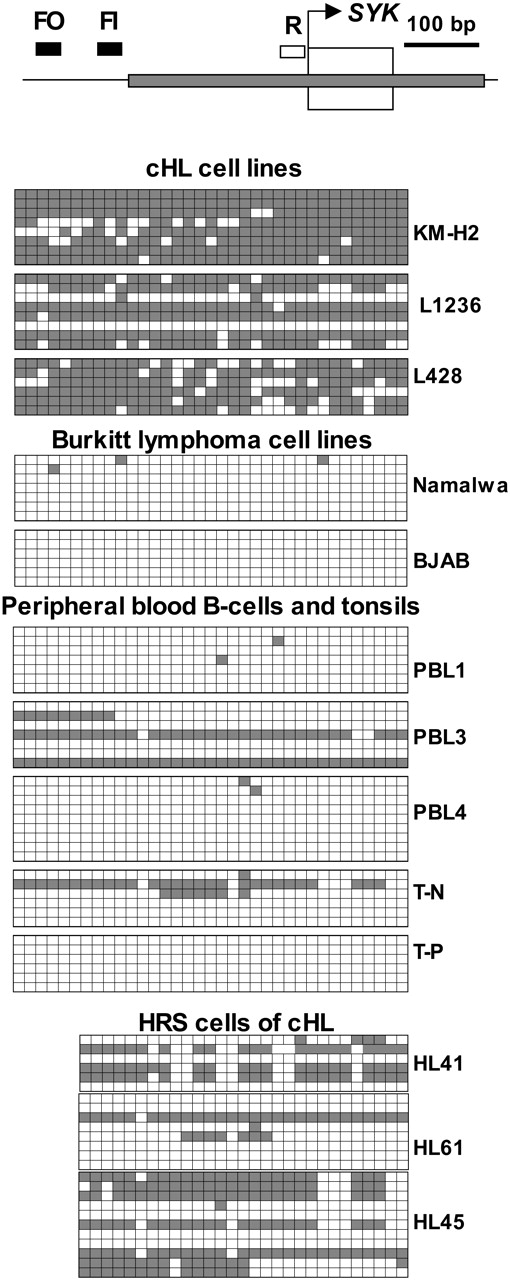
SYK was chosen based on (1) the presence of the well studied CpG island spanning the 5′ regulatory region and the first exon, which was found to be hypermethylated in various types of tumors35,46,47 ; (2) its known tumor-suppressor activity; and (3) participation in oxidative stress-mediated apoptosis in B cells.48,49
In the cHL cell lines, a very high level of methylation was observed. In only L1236 cells did we find some unmethylated alleles. In contrast to the situation in cHL lines, methylated CpG dinucleotides were seen infrequently in the Burkitt lymphoma cell lines (Figure 5). Therefore, these results on CpG island methylation were in agreement with the results described (Figure 3). Given the fact that hypermethylation can arise as a consequence of cell culture,50,51 we wanted to move this analysis to primary HRS cells. We first analyzed SYK methylation in normal peripheral B lymphocytes (PBLs) and in normal tonsil tissue (Figure 5). In these samples, the promoter region was predominantly hypomethylated, whereas methylated alleles were rare (see PBL3 and T-N, Figure 5). We then analyzed the methylation pattern of the SYK CpG island in HRS cells from 3 cases of the primary cHL. Because HRS cells comprise only 1% to 2% of the tumor mass, we used laser capture microdissection to isolate about 100 HRS cells from each sample. We found that in 2 of 3 cHL the SYK CpG island was hypermethylated in more then 50% of the clones. Only in 1 cHL tumor specimen hypomethylated alleles prevailed (Figure 5). Thus, in contrast to the preferentially hypomethylated status of SYK CpG island in normal tissue, the HRS cells of cHL were predominantly hypermethylated.
The BOB.1/OBF.1 CpG island is methylated in cHL cell lines. Analysis of the BOB.1/OBF.1 CpG island by methylation-specific PCR (MSP). Bisulfite-converted DNA samples were used for amplification of the BOB.1/OBF.1 CpG island. The sequence of the 5′-untranslated region of BOB.1/OBF.1 was found by alignment of the longest mRNA sequence Z49194 to genomic DNA. The primers, specific for methylated (MF and MR) and unmethylated (UF and UR) CpGs were positioned in the CpG-rich area inside exon 1. Thin vertical lines represent the positions of CpG dinucleotides. The PCR amplification products specific for methylated (M) and unmethylated (U) DNA (138 bp and 216 bp, respectively) were separated on 6% polyacrylamide gels. MSP data shown are representative for 3 independent experiments.
The BOB.1/OBF.1 CpG island is methylated in cHL cell lines. Analysis of the BOB.1/OBF.1 CpG island by methylation-specific PCR (MSP). Bisulfite-converted DNA samples were used for amplification of the BOB.1/OBF.1 CpG island. The sequence of the 5′-untranslated region of BOB.1/OBF.1 was found by alignment of the longest mRNA sequence Z49194 to genomic DNA. The primers, specific for methylated (MF and MR) and unmethylated (UF and UR) CpGs were positioned in the CpG-rich area inside exon 1. Thin vertical lines represent the positions of CpG dinucleotides. The PCR amplification products specific for methylated (M) and unmethylated (U) DNA (138 bp and 216 bp, respectively) were separated on 6% polyacrylamide gels. MSP data shown are representative for 3 independent experiments.
SYK is methylated in cHL cell lines and in HRS cells of primary cHL. The methylation status of the SYK promoter was analyzed by genomic sequencing. The bisulfite-treated genomic DNA was amplified with seminested primers as indicated: FO indicates forward outer primer; FI, forward inner primer; and R, reverse primer. For amplification of DNA from cell lines and normal tissues only FO and R primers were needed (since there was enough material). The obtained amplicon contained 35 CpG dinucleotides. The first and last CpGs were located at positions -294 and -23, respectively, counting from the start site of transcription (bent arrow, predicted by Entrez Gene, NCBI). Samples of 50 to 100 microdissected HRS cells were reamplified with seminested primers FI and R. This amplicon included 29 CpGs; the first CpG was at the position -228. The gray bar represents the CpG island. The first exon is depicted as an open box. The PCR products were cloned into the pCAPsvector and sequenced. Peripheral B lymphocytes (PBLs) PBL1, PBL3, and PBL4 were isolated from the blood of healthy donors. HRS cells were isolated from primary cHL biopsies (HL41, HL61, and HL45) by laser capture microdissection. Clones (5 to 11) were sequenced from each sample. Every row represents a single PCR clone. □ indicates unmethylated CpG dinucleotides; ▪, methylated CpGs.
SYK is methylated in cHL cell lines and in HRS cells of primary cHL. The methylation status of the SYK promoter was analyzed by genomic sequencing. The bisulfite-treated genomic DNA was amplified with seminested primers as indicated: FO indicates forward outer primer; FI, forward inner primer; and R, reverse primer. For amplification of DNA from cell lines and normal tissues only FO and R primers were needed (since there was enough material). The obtained amplicon contained 35 CpG dinucleotides. The first and last CpGs were located at positions -294 and -23, respectively, counting from the start site of transcription (bent arrow, predicted by Entrez Gene, NCBI). Samples of 50 to 100 microdissected HRS cells were reamplified with seminested primers FI and R. This amplicon included 29 CpGs; the first CpG was at the position -228. The gray bar represents the CpG island. The first exon is depicted as an open box. The PCR products were cloned into the pCAPsvector and sequenced. Peripheral B lymphocytes (PBLs) PBL1, PBL3, and PBL4 were isolated from the blood of healthy donors. HRS cells were isolated from primary cHL biopsies (HL41, HL61, and HL45) by laser capture microdissection. Clones (5 to 11) were sequenced from each sample. Every row represents a single PCR clone. □ indicates unmethylated CpG dinucleotides; ▪, methylated CpGs.
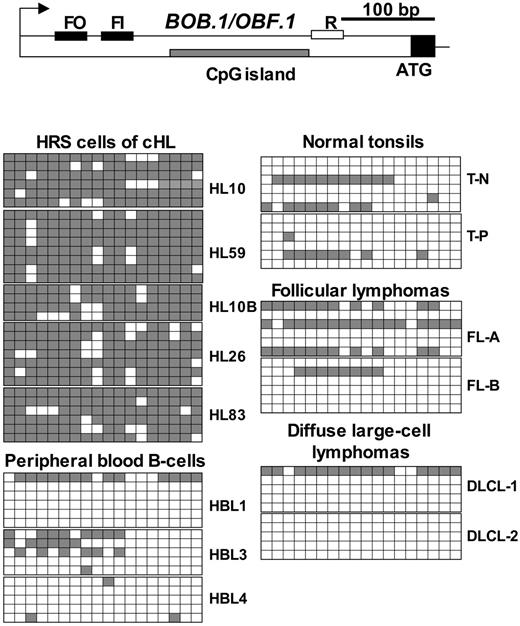
Next, we investigated the BOB.1/OBF.1 and CD79B methylation status using genomic bisulfite sequencing in isolated HRS cells. CD79B is interesting because it does not have a bona fide CpG island in the vicinity of the promoter, but its silencing correlated with promoter methylation.33 In addition, both Oct1/Oct2 and BOB.1/OBF.1 regulate the CD79b promoter. For analysis of methylation of the BOB.1/OBF.1 CpG island we used seminested primers to amplify the CpG island located in the first exon (Figure 6). We analyzed the methylation status of the BOB.1/OBF.1 CpG island in normal tissues, including PBLs and tonsils, and compared it with that of primary HRS cells, derived from 5 different cases of cHL. In addition, we analyzed the BOB.1/OBF.1 methylation status in follicular lymphomas (FLs) and in diffuse large-cell lymphomas (DLCLs), which are known to express BOB.1/OBF.1.41 All 5 cases of cHL demonstrated extensive methylation of the BOB.1/OBF.1 CpG island (Figure 6). In contrast, most of the sequenced clones from normal tissues did not contain methylated cytosines or showed low levels of methylation in a mosaic pattern. The methylation intensity in FL and DLCL tumor samples was slightly higher than in normal tissues, but significantly lower compared with cHL samples.
The BOB.1/OBF.1 CpG island is methylated in HRS cells of primary cHL. Bisulfite-treated genomic DNA was amplified with seminested primers (FO, FI, and R). The primers were located to amplify the CpG island within exon 1. The amplicon obtained using FI and R primers contained 18 CpG dinucleotides. The first and last CpGs are at positions +183 and +373, respectively, counting from the start of transcription. The filled part of exon 1 indicates the coding sequence. All other symbols are the same as for Figure 5.
The BOB.1/OBF.1 CpG island is methylated in HRS cells of primary cHL. Bisulfite-treated genomic DNA was amplified with seminested primers (FO, FI, and R). The primers were located to amplify the CpG island within exon 1. The amplicon obtained using FI and R primers contained 18 CpG dinucleotides. The first and last CpGs are at positions +183 and +373, respectively, counting from the start of transcription. The filled part of exon 1 indicates the coding sequence. All other symbols are the same as for Figure 5.
Finally, the methylation pattern of the CD79B promoter region was investigated in 5 cases of cHL and in normal-tissue DNA samples (Figure 7). Similar to the situation observed for SYK, all cHL samples contained hypermethylated clones, albeit with an overall reduced prevalence compared with the BOB.1/OBF.1 CpG island (Figure 6). In one case, all the sequenced clones were hypermethylated. In the other 4 cases, the frequency of the hypermethylated clones was about 50%. In normal tissues, methylation was sporadic. Thus, promoter hypermethylation of B-lineage genes is a frequent event in cHL cell lines and in primary cHL clinical cases.
Discussion
In this study, we investigated the contribution of epigenetic modifications to repression of B-cell-specific genes in cHL. We have shown that silencing of the B-cell-specific genes correlates with promoter methylation in cHL cell lines and in primary cases of cHL. Although the silenced genes could be reactivated by treatment with DNA methyltransferase inhibitor 5-aza-dC, the reactivation effect varied among different cHL-derived cell lines and for different genes. Combining the 5-aza-dC treatment with overexpression of the transcription factors Oct2 and PU.1 reactivated expression of the endogenous PU.1 and SYK genes, which were resistant to 5-aza-dC alone in the L428 cell line. In our previous work,26 we reported the involvement of chromatin modification in Ig silencing in cHL cell lines. Recently, the promoter regions of BOB.1/OBF.1, CD79B, and TCL1 were shown to be methylated cHL cell lines.52 We not only substantially extended the list of the B-cell-specific genes silenced by DNA methylation in cHL adding PU.1, BCMA, LCK, CD19, SYK, and CD20 genes, but we also investigated the methylation status of selected genes in HRS cells isolated from primary cases of cHL.
CD79B promoter region is methylated in HRS cells of primary cHL. Methylation status of CD79B promoter was analyzed by bisulfite genomic sequencing. The sequenced region contained 9 CpGs. The first and last CpGs are at positions -5 and +106, respectively, counting from the start of transcription. The binding sites for Oct2, Sp1, and PU.1 are marked as filled circles, horizontal ovals, and vertical ovals, respectively. All other symbols and designations are the same as for Figures 5 and 6. The polymorphism/mutation in expected CpG dinucleotides is marked by X.
CD79B promoter region is methylated in HRS cells of primary cHL. Methylation status of CD79B promoter was analyzed by bisulfite genomic sequencing. The sequenced region contained 9 CpGs. The first and last CpGs are at positions -5 and +106, respectively, counting from the start of transcription. The binding sites for Oct2, Sp1, and PU.1 are marked as filled circles, horizontal ovals, and vertical ovals, respectively. All other symbols and designations are the same as for Figures 5 and 6. The polymorphism/mutation in expected CpG dinucleotides is marked by X.
DNA methylation is a frequent mechanism of tumor suppressor gene inactivation. There are 2 models to explain the gene silencing by DNA methylation. The first model considers the promoter methylation as a stochastic event. Such “methylation errors”53 would then became inherited due to some positive effects on cell growth, similar to the selection processes known for genetic mutations. Typically, such a stochastic event would occur in only one allele. To silence the second allele, other events, such as loss of heterozygosity (LOH), are likely to be involved. Examples for stochastic methylation in cHL might be the tumor suppressor genes p18INK4c25 and RASSF1A (RAS effector).24 Their silencing is observed in only some of the cases, and it is not specific for cHL. The second model considers methylation as a determined process. This second model predicts that functionally or structurally related genes are targeted to methylation by transcriptional silencing due to the initial down-regulation of specific transcription factor(s).28,54,55 The silencing of B-lineage-specific genes might be an example of such a systemic gene silencing, assuming the existence of common factor(s) causing the cooperative down-regulation of all these B-cell-specific target genes. Down-regulation of transcription factors such as Oct2, BOB.1/OBF.1, and PU.1 in cHL56,57 might be an initial step in silencing other B-lineage-specific genes. Indeed, Oct2 and BOB.1/OBF.1 regulate the expression of CD79B,58 CD20,59 BCL-2,60 CD19,61 BCMA,62 LCK,11 and PU.1.63 PU.1, in turn, regulates the transcription of CD79A, CD79B, CD19,64 and CD2065 as well as its own expression. PU.1 is expressed earlier in the hematopoetic lineage compared with Oct1 and BOB.1/OBF.1; nevertheless, Oct2 and BOB.1/OBF.1 might be higher in the hierarchy of B-lineage-specific transcription factors than PU.1, because overexpression of Oct2 and/or BOB.1/OBF.1, but not PU.1, prevented extinguishing of B-cell-specific genes in plasmacytoma cells, fused to T lymphoma (immunoglobulin heavy chain [IgH], Oct2, and PU.1).66 Our data on PU.1 and SYK reactivation in Oct2-overexpressing L428 cells treated with 5-aza-dC and the ability of PU.1 to reactivate SYK indicate their important role in the reactivation process. Both SYK and PU.1 were reactivated in KM-H2 and L1236 cells, and both were absent or very low in L428 cHL cells. The simultaneous reactivation of both genes in Oct2- and PU.1-overexpressing cells treated with 5-aza-dC is a strong hint that regulatory networks and hierarchies are involved in the silencing process.
The comparison of the methylation patterns of B-cell-specific genes in cHL-derived cell lines and in primary HRS cells suggests 2 different scenarios. For genes such as SYK and CD79B, both hypo- and hypermethylated clones were observed in primary cHL cases. Roughly equal frequency of hyper- and hypomethylated clones of SYK and CD79B promoter regions indicates monoallelic methylation. Alternatively, a mixture of cells with either fully methylated or completely nonmethylated alleles might coexist. Hypermethylation of all clones of the CD79B promoter was observed only in 1 of 5 cases, indicating the ongoing character of the methylation. At the same time, hypermethylation was observed in virtually all sequenced SYK clones in cHL-derived cell lines (excluding L1236, where 2 of 8 clones were hypomethylated). This might mean that in the SYK and CD79B genes the hypermethylation is not clonal but arises asynchronously in different alleles and/or cells. The homogenous methylation of all SYK alleles in cHL cell lines might be a result of the positive selection or ongoing methylation during cell culture, as it was described for the calcitonin gene in colon carcinoma.50 In contrast to SYK and CD79B, all clones of BOB.1/OBF.1 in primary HRS cells of cHL were hypermethylated. Given the coactivator function of BOB.1/OBF.1, this might mean that BOB.1/OBF.1 silencing precedes inactivation of the target genes and probably occurs simultaneously with malignant transformation. Interestingly, BOB.1/OBF.1 hypermethylated alleles were obviously more frequent in normal tissues than hypermethylated alleles of SYK and CD79B, suggesting an involvement of methylation in physiologic regulation of BOB.1/OBF.1 expression. HRS cells might be derived from B cells with methylated BOB.1/OBF.1 promoters. Thus, we hypothesize that the methylation not only is involved in the silencing of the target genes after down-regulation of the relevant transcription factors, but also causes silencing of “master” transcription factors.
The silencing of several components of the BCR signaling cascade in HRS cells at first glance seems to be at odds with the essential role of BCR signaling for B-cell survival.67 However, earlier work15 has established that activation of the antiapoptotic NF-κB pathway represents a common scenario in cHL. Given the described roles of several BCR-signaling components in regulation not only of B-cell proliferation but also of B-cell apoptosis, silencing the expression of these genes might actually be a selective advantage for HRS. Our data clearly demonstrate the involvement of the epigenetic regulation in B-lineage-specific gene silencing not only in cHL cell lines but also in HRS cells of primary cHL tumor tissues. The systemic character of the gene silencing argues for the existence of a common factor or factors, down-regulation of which might result in promoter methylation of target genes. To date, oncogenic mutations and translocations known to play a pivotal role in formation of other B-cell malignancies are rare or absent in cHL. Although this might be due to technical problems with respect to analyzing the rare HRS cells, our results demonstrating the extensive systemic methylation of B-lineage-specific genes suggest that epigenetic control mechanisms play a critical role in cHL pathogenesis.
Prepublished online as Blood First Edition Paper, November 22, 2005; DOI 10.1182/blood-2005-09-3765.
Supported by grants from the Deutsche Forschungsgemeinschaft (DFG SFB497, C5) and the Fonds der Chemischen Industrie to T.W.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Anita Kick and Beate Wotschke for excellent technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal