Abstract
CD47 on red blood cells (RBCs) reportedly signals “self” by binding SIRPα on phagocytes, at least in mice. Such interactions across and within species, from mouse to human, are not yet clear and neither is the relation to cell adhesion. Using human SIRPα1 as a probe, antibody-inhibitable binding to CD47 was found only with human and pig RBCs (not mouse, rat, or cow). In addition, CD47-mediated adhesion of human and pig RBCs to SIRPα1 surfaces resists sustained forces in centrifugation (as confirmed by atomic force microscopy) but only at SIRPα-coating densities far above those measurable on human neutrophils, monocytes, and THP-1 macrophages. While interactions strengthen with deglycosylation of SIRPα1, low copy numbers explain the absence of RBC adhesion to phagocytes under physiologic conditions and imply that the interaction being studied is not responsible for red cell clearance in humans. Evidence of clustering nonetheless suggests mechanisms of avidity enhancement. Finally, using the same CD47 antibodies and soluble SIRPα1, bone marrow-derived mesenchymal stem cells were assayed and found to display CD47 but not bind SIRPα1 significantly. The results thus demonstrate that SIRPα-CD47 interactions, which reportedly define self, exhibit cell type specificity and limited cross-species reactivity. (Blood. 2006;107:2548-2556)
Introduction
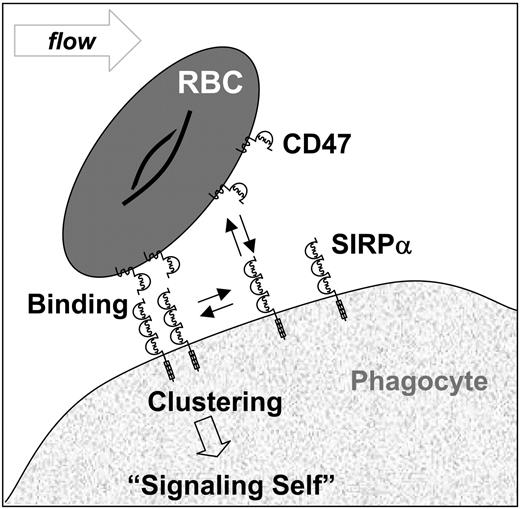
Heterotypic interactions between ubiquitously expressed CD47 (or IAP) and signal regulatory protein (SIRPα) are believed to regulate a wide range of cellular processes that include neutrophil transmigration,1 T-cell and dendritic cell activation,2,3 and even phagocytosis-based clearance (Figure 1).4-6 On mouse red blood cells (RBCs), CD47 is reportedly a “marker of self” that “signals” against phagocytosis by macrophages expressing SIRPα: CD47-/- RBCs are cleared rapidly from the bloodstream of normal mice,5 and anti-CD47 bound to normal mouse RBCs leads to enhanced phagocytosis in vitro.4 Signaling cascades initiated through SIRPα have also been demonstrated in vitro through cell aggregation or through use of excess soluble protein, although neither approach is physiologic.5,8 Similar results to those with RBCs were also reported with mouse platelets.9 In human as opposed to mouse, however, severe reduction of CD47 levels on RBCs (∼10%-25% of normal), as occurs in Rhnull individuals as well as several other human deficiencies,10,11 shows little to no initial evidence of enhanced RBC interactions with peripheral blood monocytes.12 Such conditions in humans could certainly introduce compensating perturbations as seem to occur in CD47-/- mice13 and so here we begin to characterize distinctive interactions of CD47 from human through mouse.
If CD47-mediated binding of RBCs to phagocytes leads to strong adhesion because of large attractive forces or high protein numbers, detachment from phagocytes will be difficult. Indeed, high expression of CD47 on apoptotic mouse lymphocytes leads to their engulfment by mouse phagocytes.14 If CD47-mediated adhesion is too weak or immeasurable, signaling would seem tenuous. Although protein binding assays with mouse and human systems have demonstrated interactions,1,15-18 a physiologic context for CD47-mediated adhesion is lacking, especially with human RBCs. A CD47-SIRPα interaction that has broad interactions across species should just be classified as antagonistic to phagocyte activity rather than a marker of self. Likewise, human cells (eg, stem cells) that express SIRPα (or CD47), which do not interact with human CD47 (or SIRPα), severely restrict the meaning of a marker of self.
Conserved function from mice to humans is questionable based on molecular-scale differences. The human sequence of SIRPα's N-terminal Ig domain, which binds CD47, differs from mouse by 34%,16 whereas the human sequence of CD47's lone Ig domain differs from mouse by almost 50%. Additionally, mouse CD47 contains a unique 21-amino acid insert. Within the RBC membrane, interactions of CD47 are also very distinct. In human RBCs but not in mouse RBCs, a majority of CD47 is part of an immobile macro-complex with Rh proteins (2 genes in human, but 1 in mouse) plus band 3 and other proteins, forming a “metabolon” that coordinates transport.10,19 In human RBCs but not in mouse RBCs, a majority of CD47 is linked to the membrane skeleton by protein 4.2.11,20
CD47-mediated interactions between intact RBCs and SIRPα have not been studied in any detail. We demonstrate that CD47 on intact human RBCs binds the recombinant extracellular domain of human SIRPα1 (SIRPα1ex) in flow cytometric assays and this binding is verified by centrifugation-based and atomic force microscopy (AFM)-based adhesion measurements. Species specificity of human SIRPα1ex binding is characterized and shows, as 2 notable examples, that mouse CD47 does not bind human SIRPα1ex although pig CD47 does bind. Additionally, CD47 is found on human bone marrow-derived mesenchymal stem cells (MSCs) but does not appear to significantly bind human SIRPα1ex.
Adhesive interactions of SIRPα-CD47 on cell surfaces. Based on mouse studies, binding between CD47's Ig domain on RBCs and the N-terminal Ig domain of SIRPα (or SHPS-1, P84) on phagocytes is thought to trigger clustering of SIRPα. Phosphorylation events at SIRPα's cytoplasmic tail are then believed to ultimately signal “self” and inhibit phagocytosis of RBCs. This occurs through tyrosine phosphorylation of SIRPα5 and subsequent recruitment of phosphatases (SHP-1 predominantly).7
Adhesive interactions of SIRPα-CD47 on cell surfaces. Based on mouse studies, binding between CD47's Ig domain on RBCs and the N-terminal Ig domain of SIRPα (or SHPS-1, P84) on phagocytes is thought to trigger clustering of SIRPα. Phosphorylation events at SIRPα's cytoplasmic tail are then believed to ultimately signal “self” and inhibit phagocytosis of RBCs. This occurs through tyrosine phosphorylation of SIRPα5 and subsequent recruitment of phosphatases (SHP-1 predominantly).7
Patients, materials, and methods
Chemicals
DPBS without Ca2+ or Mg2+ (Invitrogen, Carlsbad, CA) was supplemented with either 1% BSA (Roche, Indianapolis, IN) or 1% BSA and 0.05% Tween 20 (Sigma-Aldrich, St Louis, MO). TBS (Tris-buffered saline; BIORAD, Hercules, CA) and TTBS (TBS with Tween 20) were used in Western blotting. All other reagents were from Sigma-Aldrich unless noted.
Antibodies
Primary antibodies are listed in Table 1. Several secondary reagents were used, including R-phycoerythrin-conjugated goat antimouse, AlexaFluor-488 rabbit anti-GST (Invitrogen), and extravidin conjugated with R-phycoerythrin or Quantum Red. Alkaline phosphatase (AP)-conjugated goat antimouse and horseradish peroxidase (HRP)-conjugated goat anti-GST (Rockland Immunochemicals, Gilbertsville, PA) were used for enzyme-linked immunosorbent assay (ELISA). Rabbit anti-GST (Invitrogen) and AP-conjugated goat antirabbit were used in immunoblotting.
List of primary antibodies used in this study
Antibody . | Antigen . | Source . | Species . | Application . |
|---|---|---|---|---|
| B6H12 | Human CD47 | BD Pharmingen (San Diego, CA) | Mouse | Flow cytometry |
| BRIC126 | Human CD47 | IBGRL (Bristol, United Kingdom) | Mouse | Flow cytometry |
| 2D3 | Human CD47 | EMD Biosciences (La Jolla, CA) | Mouse | Flow cytometry |
| 6H9 | Human CD47 | Dr M. Telen (Duke) | Mouse | Flow cytometry |
| CIKm1 | Human CD47 | Chemicon (Temecula, CA) | Mouse | Flow cytometry |
| mIAP301 | Mouse CD47 | BD Pharmingen (San Diego, CA) | Rat | Flow cytometry |
| P3C4 | Human SIRPα | MBL International (Woburn, MA) | Mouse | Flow cytometry |
| Anti-SIRPα | Human SIRPα (aa's 487-503) | Affinity BioReagents (Golden, CO) | Rabbit | Western blotting |
Antibody . | Antigen . | Source . | Species . | Application . |
|---|---|---|---|---|
| B6H12 | Human CD47 | BD Pharmingen (San Diego, CA) | Mouse | Flow cytometry |
| BRIC126 | Human CD47 | IBGRL (Bristol, United Kingdom) | Mouse | Flow cytometry |
| 2D3 | Human CD47 | EMD Biosciences (La Jolla, CA) | Mouse | Flow cytometry |
| 6H9 | Human CD47 | Dr M. Telen (Duke) | Mouse | Flow cytometry |
| CIKm1 | Human CD47 | Chemicon (Temecula, CA) | Mouse | Flow cytometry |
| mIAP301 | Mouse CD47 | BD Pharmingen (San Diego, CA) | Rat | Flow cytometry |
| P3C4 | Human SIRPα | MBL International (Woburn, MA) | Mouse | Flow cytometry |
| Anti-SIRPα | Human SIRPα (aa's 487-503) | Affinity BioReagents (Golden, CO) | Rabbit | Western blotting |
aa's indicates amino acids.
Cells
COS-1, Lec-1, and THP-1 cells (ATCC, Manassas, VA) were respectively maintained in DMEM, αMEM, and RPMI 1640 media (Invitrogen) supplemented with 10% FBS (Sigma-Aldrich). Differentiation of THP-1 cells was achieved in 100 ng/mL phorbol myristate acetate (PMA) for 2 days21 and confirmed by attachment of these cells to tissue-culture plastic. Bone marrow-derived human MSCs (Osiris Therapeutics; Baltimore, MD)22 were maintained in low-glucose DMEM (Invitrogen) with 10% FBS at low passage. Cells were detached using PBS/1 mM EDTA to assay for CD47/SIRPα. Human blood was obtained from finger pricks of healthy donors. Blood from other species was obtained from Covance (Denver, PA) and washed 3 × in PBS plus 1% BSA. Mononuclear cells and neutrophils were isolated from anticoagulated blood, which was layered over polymorphonuclear leukocyte (PMN) isolation medium (Robbins Scientific, Sunnyvale, CA), and spun at 700g for 30 minutes followed by 2 × PBS washes of fractions.
SIRPα expression and production
Plasmid encoding the extracellular domain of human SIRPα1 fused to GST (SIRPα1ex) was transfected into COS-1 cells using Lipofectamine-2000 (Invitrogen) and purified as described.17 Aglycosylated SIRPα1ex was prepared by transfection in 10 μg/mL tunicamycin. Core-glycosylated SIRPα1ex (high mannose) was prepared by transfecting Lec-1 cells. The extracellular domain of mouse SIRPα16 was also prepared as a GST fusion. A pcDNA3.1-based (Invitrogen) vector encoding human SIRPα1 was used to express full-length protein in COS-1.
Centrifugation adhesion assay
Immulon 2HB 96-well plates (ThermoElectron, Waltham, MA) were incubated with 100 μL protein solution in PBS for at least 1 hour at room temperature (RT) followed by blocking with 200 μL blocking solution (PBS, 1% BSA, 0.05% Tween 20) under similar conditions. An ELISA was used to assay function and site density of immobilized protein (for methods, see Supplemental Document S1, available on the Blood website by clicking the Supplemental Materials link at the top of the online article). Five microliters of packed RBCs was added to 20 mL of PBS containing 1% BSA. Four hundred microliters of RBC suspension was added to each well and incubated for 10 minutes at RT. The plate was then sealed, inverted, and spun at 100g for 10 minutes using a centrifuge equipped with a swinging bucket rotor (Beckman Coulter, Fullerton, CA). After centrifugation, the plate was inverted and immersed in PBS, where the sealer was removed and the plate was turned upright under liquid. Two to 4 fields were imaged per well using a 4 × objective. The image was binarized and the area fraction occupied by RBCs was calculated using ImageJ 1.31v (http://rsb.info.nih.gov/ij/).43 Initial RBCs added were optimized to obtain a sparse monolayer.
Fluorescent labeling of RBCs and Ig-CD47 beads with SIRPα1ex and CD47 antibodies
Five microliters of 0.5 mg/mL SIRPα1ex, 5 μL of 2 mg/mL AlexaFluor 488 rabbit anti-GST, 40 μL of PBS/1% BSA, and 0.5 μL packed RBCs were mixed and incubated at RT for 30 minutes. Cells were pelleted and resuspended in 1 mL PBS and analyzed immediately. For blocking, saturating antibody was added prior to SIRPα1ex. For antibody labeling, saturating anti-CD47 antibody in 50 μL PBS/1% BSA and 0.5 μL packed RBCs were mixed together and incubated as described for SIRPα1ex labeling. Cells were washed in 0.5 mL PBS/1% BSA, then resuspended in 50 μL of PBS/1% BSA containing 5 μL secondary antibody. After an incubation of at least 20 minutes at RT, cells were washed once in 0.5 mL PBS/1% BSA and resuspended in 1 mL PBS and kept on ice. Ig-CD47 beads were made by incubating streptavidin-coated microspheres (Spherotech, Libertyville, IL) with biotinylated human CD47 Ig domain (fused to domains 3 and 4 of rat CD4; Ig-CD47).18 To microscopically analyze CD47 clustering, saturating levels of 2D3 complexed with anti-mouse IgG1 fluorescent Fab (Zenon; Invitrogen) were incubated with human RBCs and Ig-CD47 beads at RT for at least 30 minutes.
Measurement of SIRPα density on phagocytes
Neutrophils, monocytes, and THP-1 cells were labeled with 60 μg/mL P3C4 (MBL International, Woburn, MA) against human SIRPα for 1 hour on ice. Cells were washed twice with PBS/1% BSA and incubated with the secondary antibody for at least 30 minutes on ice. Cells were washed and resuspended in isotonic PBS and stored on ice until flow cytometric analysis. Mean fluorescence intensities were calibrated with human RBCs labeled with saturating B6H12 levels and the same secondary antibody. The upper-bound estimate of 50 000 CD47 molecules on the surface of the human RBCs was used.11,23,24 Surface areas (SAs) were determined microscopically for human neutrophils, monocytes, and undifferentiated THP-1 cells, approximating these cells as spherical (SA = 4 × projected area); for PMA-differentiated THP-1 cells, a “pancake” shape was used for a simplified analysis (SA = 2 × projected area). These approximate shapes probably underestimate SAs and therefore lead to overestimates of SIRPα surface densities. For clustering SIRPα on phagocytes, incubations were done at RT. In some experiments, cells were labeled on ice with recombinant human Ig-CD47 complexed with Quantum Red-conjugated extravidin (Sigma-Aldrich) after preclustering SIRPα using P3C4 for at least 30 minutes at 37°C.
Flow cytometry, microscopy, ELISA, AFM, and Western blotting
For more details regarding the methods for each of these procedures, see Document S1.
Results
Human SIRPα binds CD47 on human RBCs
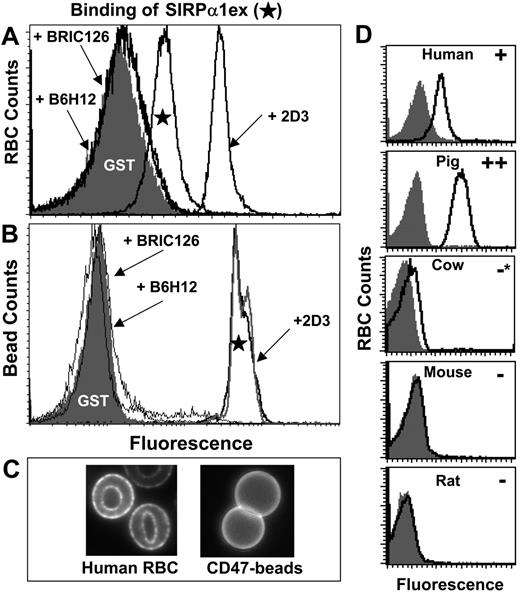
To demonstrate SIRPα interactions with CD47 on intact human RBCs, cells were incubated with purified recombinant human SIRPα1 extracellular domain fused to GST (SIRPα1ex). SIRPα1ex was fluorescently labeled using polyclonal anti-GST. Binding clearly exceeded that of similarly labeled GST (lacking SIRPα1ex; Figure 2A) and specificity was confirmed with blocking antibodies (BRIC126 and B6H12).
Surprisingly, the nonblocking antibody 2D3 enhances SIRPα1ex binding to human RBCs. Increased binding might occur through a preclustering of CD47 that increases the avidity of the interaction. To demonstrate this, biotinylated human Ig-CD47 was immobilized on streptavidin-coated beads and stained with SIRPα1ex (Figure 2B). While blocking antibodies inhibited SIRPα1ex binding as with RBCs, 2D3 did not enhance binding to beads. To visualize clustering or a lack thereof, Ig-CD47 beads and human RBCs were labeled with fluorescent 2D3: Figure 2C shows that 2D3 clusters CD47 on RBCs but not Ig-CD47 on beads. This suggests that clustered CD47 on RBCs enhances soluble ligand binding.
Although SIRPα binding of RBCs is clear, it is weak compared with anti-CD47 labeling (Table 2; Figure S1). This holds even at high concentrations (50 μg/mL) of SIRPα1ex (which is a dimer as a result of GST dimerization) and maximal levels of anti-GST. A low signal is consistent with the reportedly weak CD47-SIRPα binding affinity (low μM range).18
Binding of human-SIRPα and anti-CD47 to RBCs from multiple species
. | SIRPα1ex . | BRIC126 . | B6H12 . | 2D3 . | 6H9 . | CIKm1 . | mIAP301 . |
|---|---|---|---|---|---|---|---|
| Human | + | ++ | ++ | ++ | ++ | ++ | − |
| Pig | ++ | + | − | − | ++ | ++ | ND |
| Cow | −* | − | − | − | + | ++ | ND |
| Rat | − | − | − | − | − | − | ND |
| Mouse | − | ND | ND | ND | ND | ND | ++ |
. | SIRPα1ex . | BRIC126 . | B6H12 . | 2D3 . | 6H9 . | CIKm1 . | mIAP301 . |
|---|---|---|---|---|---|---|---|
| Human | + | ++ | ++ | ++ | ++ | ++ | − |
| Pig | ++ | + | − | − | ++ | ++ | ND |
| Cow | −* | − | − | − | + | ++ | ND |
| Rat | − | − | − | − | − | − | ND |
| Mouse | − | ND | ND | ND | ND | ND | ++ |
+ indicates weak, >2×BKGD (background) and <10×BKGD; ++, strong, >10×BKGD; −, very weak, <2×BKGD; and ND, not determined.
Visibly distinct but very weak.
Human SIRPα 1ex binding to RBCs is CD47 specific and species specific. Human RBC (A) or Ig-CD47-coated beads (B) were incubated with soluble SIRPα1ex or GST, and bound protein was detected in flow cytometry using fluorescent anti-GST. SIRPα1ex specifically binds to human RBCs whereas GST does not. Blocking antibodies (B6H12 and BRIC126) inhibit the CD47-SIRPα interaction. 2D3, a nonblocking antibody, enhances SIRPα1ex binding to RBCs but not Ig-CD47 beads, probably by ability to cluster CD47 (C). Using the same methodology, RBCs from 5 mammalian species were labeled with SIRPα1ex or GST (D) under standardized conditions of cell number and reagent concentration. Human SIRPα1ex binds to human RBCs as expected and does not bind to RBCs from cow, mouse, or rat. Human SIRPα1ex also binds to pig RBCs and results in higher intensity in comparison to human RBCs. Note that * indicates a slight signal above background.
Human SIRPα 1ex binding to RBCs is CD47 specific and species specific. Human RBC (A) or Ig-CD47-coated beads (B) were incubated with soluble SIRPα1ex or GST, and bound protein was detected in flow cytometry using fluorescent anti-GST. SIRPα1ex specifically binds to human RBCs whereas GST does not. Blocking antibodies (B6H12 and BRIC126) inhibit the CD47-SIRPα interaction. 2D3, a nonblocking antibody, enhances SIRPα1ex binding to RBCs but not Ig-CD47 beads, probably by ability to cluster CD47 (C). Using the same methodology, RBCs from 5 mammalian species were labeled with SIRPα1ex or GST (D) under standardized conditions of cell number and reagent concentration. Human SIRPα1ex binds to human RBCs as expected and does not bind to RBCs from cow, mouse, or rat. Human SIRPα1ex also binds to pig RBCs and results in higher intensity in comparison to human RBCs. Note that * indicates a slight signal above background.
Human SIRPα binding to RBCs is species specific
To elucidate any species-specific definition of “self” by CD47, the extent of human SIRPα1ex binding to RBCs from 5 mammalian species was assessed by flow cytometry (Figure 2D). While human SIRPα1ex does not bind to rat or mouse RBCs (Figure 2D; Table 2) and binds only very weakly to cow RBCs, it binds strongly to pig RBCs. Human SIRPα1ex binds pig RBCs even more strongly than human RBCs under the same conditions. The SIRPα1ex interaction with pig RBCs is clearly mediated by CD47 since it was inhibited by BRIC126 (data not shown), which is an anti-human CD47 monoclonal antibody (mAb) known to bind pig CD4725 and also known to block SIRPα interaction.18 Human SIRPα1ex also does not bind to sheep RBCs (data not shown), which are commonly used to study opsonization-dependent phagocytosis.
To determine whether immunogenic epitopes were conserved across species, antibodies raised against human CD47 were tested broadly for cross-reactivity to RBCs from the species tested (Table 2; Figure S1). Antibodies that block SIRPα binding (BRIC126, B6H12) as well as those that do not (2D3) were included in the analysis. All antibodies tested reacted with human RBCs as expected. BRIC126, a blocking antibody, reacted with pig RBCs in addition to human RBCs, matching the binding profile observed with SIRPα1ex. B6H12, another blocking antibody, however, does not show reactivity to RBCs from any species other than human, indicating a distinct epitope from BRIC126. Surprisingly, 2D3, a nonblocking antibody, shows no cross-reactivity, indicating that its cluster-allowing epitope is also not conserved.
All of the negative results with rat RBCs are consistent with CD47's high homology (84% identity) between rat and mouse; all of the antibodies were made in mice. Likewise, one rat-derived antibody specific for mouse CD47 (mIAP301)16 that blocks P84 binding does not react with human RBCs (Table 2), confirming the divergence in the SIRPα binding region. Consistent with this, the extracellular domain of mouse SIRPα16 binds mouse RBCs but does not bind human RBCs significantly (Figure S2).
CD47-mediated adhesion of RBCs to SIRPα-coated surfaces
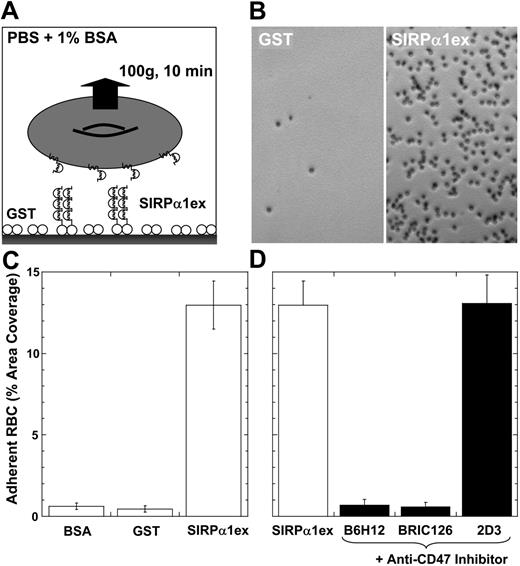
Low signals in flow cytometry might reflect dilution, prompting development of a centrifugation-based adhesion method (Figure 3A). Human RBCs adhering to SIRPα1ex-coated surfaces did not detach under sustained centrifugation (10 minutes at 100g), whereas RBCs adhering to control GST- or BSA-coated surfaces did detach (Figure 3B-C). The adhesion of RBCs to immobilized SIRPα1ex also proved CD47 specific, as blocking antibodies (BRIC126, B6H12) inhibited adhesion efficiently, reducing adhesion to background levels. The nonblocking antibody 2D3, which enhanced binding in the flow cytometry assay (Figure 2A), did not enhance or inhibit adhesion, suggesting that clustering of both CD47 and SIRPα is needed for avidity enhancement.
RBC adhesion to human SIRPα confirms species specificity of interaction
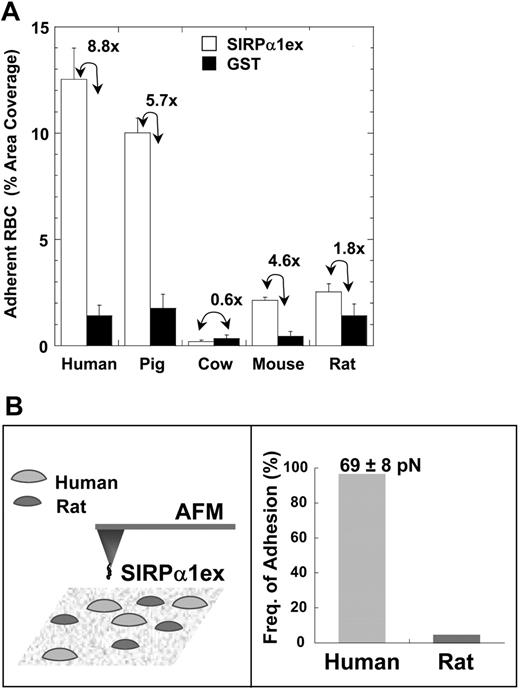
RBCs from the 5 species were also tested for adhesion to a surface coated with saturating levels of SIRPα1ex, approximately 10 000 sites/μm2. This density will be shown to be high relative to SIRPα expression levels on phagocytes later in “Results” (Figure 6). Following centrifugation, the adherent cells were counted and reported with a specificity ratio, defined as the ratio of coverage on the SIRPα1ex surface to that on the GST surface (Figure 4A). Cell attachment was largest for human and pig RBCs and proved specific, with the highest specificity ratios. Prelabeling pig RBCs with BRIC126 also blocked adhesion (data not shown).
Mouse RBCs showed little attachment to the SIRPα surface with a modest specificity ratio, much lower than that of pig or human RBCs. While rat RBCs showed SIRPα binding similar to that of mouse RBCs, the low specificity ratio (∼1) clearly indicated nonspecific adhesion. Cow RBCs did not bind to either surface, confirming the minimal binding of SIRPα seen in flow cytometry.
Finally, to rule out variation in receptor density between RBCs from the different species as a contributing factor to observed species-specific adhesion, CD47 levels were measured for mouse and human RBCs using labeled, primary antibodies. CD47 densities on mouse and human RBCs (obtained by normalizing CD47 levels to cell surface area) proved similar (data not shown), demonstrating that variation in density is not the reason for variation in binding.
The force required to disrupt the SIRPα-CD47 interaction was directly determined with a SIRPα1ex-coated AFM tip (Figure 4B). First, at a high density of SIRPα1ex that would tend to amplify avid interactions, the frequency of adhesion was nearly 100% with human RBCs but less than 5% with rat RBCs (Figure 4B). At a 10-fold lower density of SIRPα, the force needed to break a single CD47-SIRPα bond was approximately 70 pico-Newtons (pN), which is typical for adhesion bonds such as ICAM and VCAM on intact cells.26 The species specificity and finite force of the CD47-SIRPα interaction were thus consistent between AFM and centrifugation.
CD47-mediated adhesion of human RBCs to human SIRPα1ex-coated surfaces. (A) Human RBCs were allowed to adhere at modest density to SIRPα1excoated wells in a 96-well plate for 10 minutes and centrifuged inverted for 10 minutes at 100g (cells remained wet). (B) Microscopy showed significant, intact adhesion of human RBCs to SIRPα1ex-coated wells but minimal adhesion to GST. (C) The fraction of well area covered by adherent RBCs after centrifugation was very small for bovine serum albumin (BSA)- and glutathione S-transferase (GST)-compared with SIRPα1ex-coated surfaces. (D) Prelabeling of human RBCs with blocking antibodies (B6H12 and BRIC126) prevented cell adhesion, whereas 2D3 did not inhibit or significantly enhance adhesion. The G-forces used here exert a sustained peeling force of approximately 100 pN on a human red cell (volume = 95 fL; density = 1.09 g/mL). Error bars indicate plus or minus 1 SD from multiple experiments.
CD47-mediated adhesion of human RBCs to human SIRPα1ex-coated surfaces. (A) Human RBCs were allowed to adhere at modest density to SIRPα1excoated wells in a 96-well plate for 10 minutes and centrifuged inverted for 10 minutes at 100g (cells remained wet). (B) Microscopy showed significant, intact adhesion of human RBCs to SIRPα1ex-coated wells but minimal adhesion to GST. (C) The fraction of well area covered by adherent RBCs after centrifugation was very small for bovine serum albumin (BSA)- and glutathione S-transferase (GST)-compared with SIRPα1ex-coated surfaces. (D) Prelabeling of human RBCs with blocking antibodies (B6H12 and BRIC126) prevented cell adhesion, whereas 2D3 did not inhibit or significantly enhance adhesion. The G-forces used here exert a sustained peeling force of approximately 100 pN on a human red cell (volume = 95 fL; density = 1.09 g/mL). Error bars indicate plus or minus 1 SD from multiple experiments.
Glycosylation of SIRPα is not required for interaction but modulates binding
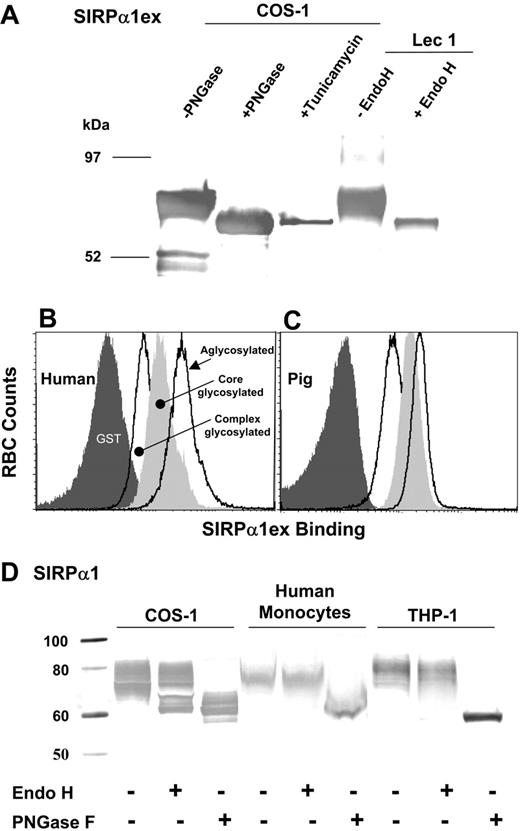
Galactosylation level of rat SIRPα has been shown to alter its affinity for CD47 expressed on neuronal cells and lymphocytes.27 Aglycosylated human SIRPα1ex, with no N-linked sugars, was therefore prepared using tunicamycin. The molecular weight of the SIRPα1ex product corresponded to what would be expected if the amino acid sequence had no posttranslational modifications (64.7 kDa; Figure 5A) and it appeared identical to PNGase-treated SIRPα1ex produced in COS-1 cells without any inhibitors (Figure 5A). O-linked sugars in human SIRPα have not been reported; indeed, SIRPα from rat brain or spleen lacked GalNAc, suggesting the absence of O-linked glycans.27 Human SIRPα1ex with a more defined sugar composition (Man5GlcNac2) was also produced using Lec-1 cells that block the formation of complex or hybrid glycans at occupied N-linked sites.28 This “core-glycosylated” protein was Endo H sensitive as expected (Figure 5A), whereas the COS-1-secreted protein required PNGase treatment for removal of hybrid/complex sugars (Figure 5A; data not shown).
Species-restricted adhesion of RBCs to human SIRPα1ex-coated surfaces. (A) RBCs from 5 mammalian species were allowed to adhere to either human SIRPα1ex- or GST-coated wells (∼10 000 molecules/μm2) and the fraction of well area covered by adherent RBCs after centrifugation was determined. Adhesion results closely matched the results from the flow cytometry-based SIRPα1ex binding assay. Only RBCs from human and pig showed significant adhesion to SIRPα1excoated wells among the species tested. The ratio of RBC area coverage on SIRPα1ex to that on GST is indicated. (B) Human and rat RBCs are probed using atomic force microscopy using human SIRPα1ex-coated tips, leading to almost 100% adhesion with human cells. Almost no adhesion is seen with rat cells. The force required to disrupt single bonds of CD47-SIRPα is approximately 70 pN. See Document S1 and Figures S1-S3 for details on AFM experiments. Error bars represent 1 SD from multiple experiments.
Species-restricted adhesion of RBCs to human SIRPα1ex-coated surfaces. (A) RBCs from 5 mammalian species were allowed to adhere to either human SIRPα1ex- or GST-coated wells (∼10 000 molecules/μm2) and the fraction of well area covered by adherent RBCs after centrifugation was determined. Adhesion results closely matched the results from the flow cytometry-based SIRPα1ex binding assay. Only RBCs from human and pig showed significant adhesion to SIRPα1excoated wells among the species tested. The ratio of RBC area coverage on SIRPα1ex to that on GST is indicated. (B) Human and rat RBCs are probed using atomic force microscopy using human SIRPα1ex-coated tips, leading to almost 100% adhesion with human cells. Almost no adhesion is seen with rat cells. The force required to disrupt single bonds of CD47-SIRPα is approximately 70 pN. See Document S1 and Figures S1-S3 for details on AFM experiments. Error bars represent 1 SD from multiple experiments.
Binding of aglycosylated human SIRPα1ex to RBCs showed the same species specificity as glycosylated protein (Figure S3). For the same amounts of protein, the quantity of bound SIRPα was higher when it was aglycosylated (Figure 5B-C). The core-glycosylated form from Lec-1 cells showed intermediate levels of binding (Figure 5B-C) on both human and pig RBCs. This indicates that both the presence and type of sugars on human SIRPα influence binding to CD47 on RBCs. Previous studies on SIRPα expressed in mouse melanocytes/melanoma demonstrated that CD47 binding does not require any sugars to be present on SIRPα.29 Similarly, reduced glycosylation on SIRPα expressed in human astrocytomas, when compared with recombinant SIRPα expressed on Chinese hamster ovary (CHO) cells, does not compromise CD47 binding.30
The extent of glycosylation and the predominance of complex/hybrid glycans (limited Endo H sensitivity; Figure 5D) suggest that COS-1 glycosylation is similar, but not identical, to native glycosylation in human monocytes and THP-1 cells. Changes in glycosylation of SIRPα on phagocytes, perhaps under cancerous conditions,29 could thus affect interactions with RBCs in vivo.
Characterization of glycosylation in recombinant and native SIRPα and effects on binding to RBCs. (A) Purified soluble human SIRPα1ex produced in COS-1 cells (in the presence or absence of tunicamycin) or from Lec-1 cells detected using anti-GST is shown. The Lec-1 product is Endo H sensitive, whereas PNGase is required to remove all glycans from the COS-1 product (without inhibitors). Human RBCs (B) or pig RBCs (C) were labeled with complex glycosylated, core glycosylated, and aglycosylated human SIRPα1ex complexed with fluorescent anti-GST. Alteration in the type of N-linked glycans (complex/hybrid to mannose) or removal of N-linked glycans enhances binding to both human and pig RBCs. (D) Recombinant human SIRPα1 from COS-1 cells and native SIRPα from human monocytes and THP-1 cells is detected using a C-terminus peptide-specific anti-SIRPα. Recombinant and native SIRPα show limited Endo H sensitivity, requiring PNGase for complete deglycosylation, indicating complex/hybrid glycans. Extent of glycosylation is also similar between these forms of SIRPα. See Document S1 and Figures S1-S3 for Western blotting details.
Characterization of glycosylation in recombinant and native SIRPα and effects on binding to RBCs. (A) Purified soluble human SIRPα1ex produced in COS-1 cells (in the presence or absence of tunicamycin) or from Lec-1 cells detected using anti-GST is shown. The Lec-1 product is Endo H sensitive, whereas PNGase is required to remove all glycans from the COS-1 product (without inhibitors). Human RBCs (B) or pig RBCs (C) were labeled with complex glycosylated, core glycosylated, and aglycosylated human SIRPα1ex complexed with fluorescent anti-GST. Alteration in the type of N-linked glycans (complex/hybrid to mannose) or removal of N-linked glycans enhances binding to both human and pig RBCs. (D) Recombinant human SIRPα1 from COS-1 cells and native SIRPα from human monocytes and THP-1 cells is detected using a C-terminus peptide-specific anti-SIRPα. Recombinant and native SIRPα show limited Endo H sensitivity, requiring PNGase for complete deglycosylation, indicating complex/hybrid glycans. Extent of glycosylation is also similar between these forms of SIRPα. See Document S1 and Figures S1-S3 for Western blotting details.
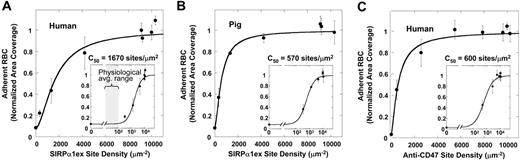
RBC adhesion is dependent on ligand density
To better understand the dependence of adhesion on SIRPα1ex density, surfaces were coated with varying levels of SIRPα1ex up to saturation. Adherent RBCs increased almost linearly up to 2000 sites/μm2 and then leveled off as is typical of saturable interactions (Figure 6A). The relatively noncooperative nature of detachment was confirmed using Hill equation fits: a fitted Hill coefficient of less than 1.5 for all the data is consistent with minimal cooperativity in adhesion and is perhaps indicative of no more than small ligand-receptor clusters for this system where SIRPα is certainly immobilized as is most of CD47 on human RBCs.19,20 The ligand density at half-maximal adhesion (C50) for human RBC binding to human SIRPα1ex exceeded that for pig RBC adhesion by about 3-fold (Figure 6B). This is consistent with the 4-fold stronger interaction between pig RBCs and human SIRPα1ex observed in flow cytometry measurements (Figure 2D).
RBC adhesion is dependent on immobilized ligand density. Immobilized ligand density was varied to measure the effect on RBC adhesion in centrifugation. (A) Human RBCs adhere to human SIRPα1ex-coated wells with half-max adhesion at C50 = 1670 sites/μm2. Inset shows log scale with the average physiologic range of SIRPα1ex on various phagocytes, per Table 3. (B) Pig RBCs adhere to human SIRPα1ex-coated wells more tightly than human RBCs. (C) Human RBCs adhere more strongly to B6H12 antibody-coated wells than to human SIRPα1ex. Averages (± SEM) of % area covered by RBCs after centrifugation from multiple experiments are shown and the ligand density at half-maximal adhesion (C50) was determined by data fit. Note that SIRPα1 immobilized here prevents clustering and limits avidity effects, consistent with the low Hill coefficients. Error bars represent plus or minus 1 SEM from multiple experiments.
RBC adhesion is dependent on immobilized ligand density. Immobilized ligand density was varied to measure the effect on RBC adhesion in centrifugation. (A) Human RBCs adhere to human SIRPα1ex-coated wells with half-max adhesion at C50 = 1670 sites/μm2. Inset shows log scale with the average physiologic range of SIRPα1ex on various phagocytes, per Table 3. (B) Pig RBCs adhere to human SIRPα1ex-coated wells more tightly than human RBCs. (C) Human RBCs adhere more strongly to B6H12 antibody-coated wells than to human SIRPα1ex. Averages (± SEM) of % area covered by RBCs after centrifugation from multiple experiments are shown and the ligand density at half-maximal adhesion (C50) was determined by data fit. Note that SIRPα1 immobilized here prevents clustering and limits avidity effects, consistent with the low Hill coefficients. Error bars represent plus or minus 1 SEM from multiple experiments.
In addition, the C50 for RBC adhesion to B6H12 as a coated ligand is more like that of the pig RBC result (Figure 6C). This agrees well with the competition results in flow cytometry (Figure 2) and thus with higher affinity of B6H12 for CD47 (∼50 nM; data not shown) versus SIRPα's affinity for CD47 (8 μM).18
SIRPα density and clustering induced by ligands
The physiologic context of ligand densities used in our in vitro assays was clarified by determining SIRPα densities on human neutrophils, monocytes, and the macrophage-like THP-1 cells. Average SIRPα levels were determined using saturating, calibrated levels of SIRPα-specific antibody (P3C4) on these cells and then dividing by cell surface area estimates to obtain average densities (Table 3).
SIRPα density on phagocytes and clustering analysis
Human cell type . | SIRPα, per cell . | Cell area, μm2 . | SIRPα density, per μm2 . | % Area clustered with at least 2 × unclustered intensity . | % Area clustered with at least 5 × unclustered intensity . |
|---|---|---|---|---|---|
| Neutrophils | 6 432 | 370 | 17 | 18.1 | 2.1 |
| Monocytes | 13 883 | 575 | 24 | 34.4 | 8.5 |
| THP-1, undifferentiated | 56 202 | 620 | 91 | 19.1 | 1.7 |
| THP-1, differentiated | 53 249 | 530 | 100 | 17.8 | 1.6 |
Human cell type . | SIRPα, per cell . | Cell area, μm2 . | SIRPα density, per μm2 . | % Area clustered with at least 2 × unclustered intensity . | % Area clustered with at least 5 × unclustered intensity . |
|---|---|---|---|---|---|
| Neutrophils | 6 432 | 370 | 17 | 18.1 | 2.1 |
| Monocytes | 13 883 | 575 | 24 | 34.4 | 8.5 |
| THP-1, undifferentiated | 56 202 | 620 | 91 | 19.1 | 1.7 |
| THP-1, differentiated | 53 249 | 530 | 100 | 17.8 | 1.6 |
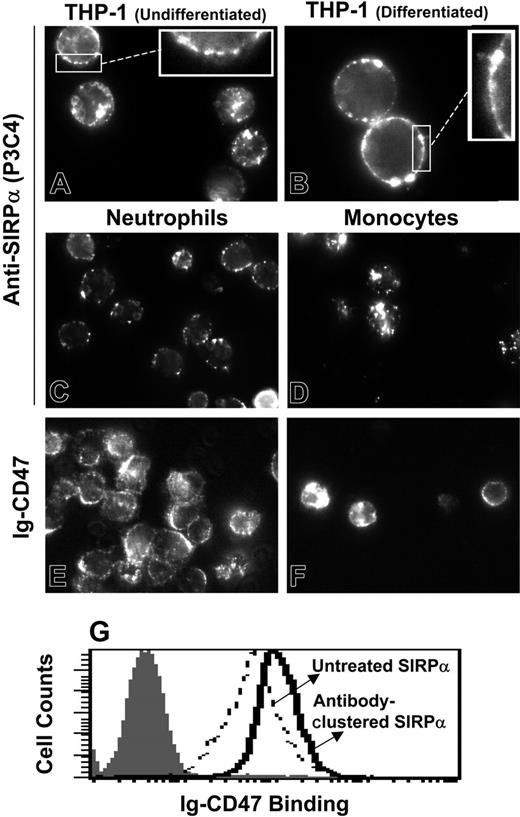
Estimated SIRPα densities on all of these phagocytes are probably overestimates (see “Measurement of SIRPα density on phagocytes”) and clearly fall on the low side of levels required to mediate significant RBC adhesion to immobilized SIRPα (Figure 6A inset). Receptor clustering could potentially increase the local density of SIRPα and thereby the avidity significantly. To explore this further, SIRPα clustering was induced and monitored on live phagocytes by labeling these cells using saturating levels of either anti-SIRPα IgG or else recombinant human Ig-CD47 complexed by extravidin at room temperature (Figure 7). Specific labeling and clustering of SIRPα is clearly observed for all cell types (Figure 7). The clusters are clearly located at the cell surface for THP-1 cells, as seen from the edge brightness (Figure 7A-B insets). For other cell types such as monocytes this is not clear since the cells spread on the glass coverslips needed for microscopy. Similar clustering was however observed when monocytes were labeled at 4°C (data not shown), where endocytosis is not expected to occur.31 RBCs in the neutrophil preparation and lymphocytes in the monocyte preparation were negative for SIRPα labeling as expected (data not shown). Simple analyses of image intensity histograms reveal, however, that the area occupied by clusters is small compared with the cell area (Table 3). Labeling of THP-1 cells (undifferentiated) with Ig-CD47 after clustering SIRPα with P3C4 does show higher binding than with cells where SIRPα was not clustered. The enhancement is relatively weak, but whether this is sufficient to result in adhesion between RBCs and phagocytes needs to be investigated.
While avidity effects with SIRPα clustering need to be investigated further (using surfaces with mobile SIRPα, for example), it is clear on the CD47 side of the interaction that the majority of CD47 on human RBCs is kept from clustering by attachment to the RBC's spectrin skeleton.19,20 Such restraints are absent in mouse RBCs, where CD47 appears fully extractable11 and mobile (data not shown), highlighting again the species differences.
MSCs express CD47 but do not bind SIRPα
In order to understand whether the CD47-SIRPα interactions are conserved within the same species, bone marrow-derived human MSCs were tested for functional expression of CD47 and SIRPα. SIRPα expression was confirmed using the P3C4 mAb consistent with results obtained earlier32 (data not shown). Using B6H12, we show here that CD47 is also expressed in human MSCs and at levels 3-fold lower than in human RBCs (Table 4). However, no significant binding of SIRPα1ex is seen with MSCs, whereas significant binding is seen with RBCs when labeled under identical conditions (Table 4). SIRPα1ex binding can be detected on beads with about 3-fold lower levels of CD47 in comparison to MSCs (9-fold lower with respect to RBCs; data not shown). Therefore, the lack of binding is not simply a result of the lower levels of CD47 present in MSCs.
CD47 expression and SIRPα1ex binding to human RBCs versus human MSCs
Cell type . | CD47 expression . | SIRPα1ex binding . |
|---|---|---|
| RBC | 3775 (2.1) | 59.6 (21.6) |
| MSC | 924 (5.5) | 12.8 (10.2) |
Cell type . | CD47 expression . | SIRPα1ex binding . |
|---|---|---|
| RBC | 3775 (2.1) | 59.6 (21.6) |
| MSC | 924 (5.5) | 12.8 (10.2) |
Mean fluorescence intensity from flow cytometric data are shown with background values within parentheses.
Ligand-induced clustering of SIRPα on phagocytes. Freshly isolated human neutrophils and monocytes as well as undifferentiated THP-1 cells and PMA-differentiated THP-1 cells were labeled (at RT, without fixation) with antibody P3C4 against human SIRPα (A-D). Bound antibody was detected with R-phycoerythrin-tagged secondary antibody. Inset images magnify the membrane association. (E-F) Neutrophils and monocytes were also stained with biotinylated Ig-CD4718 and fluorescent extravidin. Ligand-induced SIRPα clusters are clearly visible for both ligands and for all phagocytes. Average pixel intensity in unclustered regions was calculated to determine the fraction of cell area with at least 2- or 5-fold the mean unclustered pixel intensity. Antibody-mediated preclustering of SIRPα with P3C4 enhances Ig-CD47 binding (G).
Ligand-induced clustering of SIRPα on phagocytes. Freshly isolated human neutrophils and monocytes as well as undifferentiated THP-1 cells and PMA-differentiated THP-1 cells were labeled (at RT, without fixation) with antibody P3C4 against human SIRPα (A-D). Bound antibody was detected with R-phycoerythrin-tagged secondary antibody. Inset images magnify the membrane association. (E-F) Neutrophils and monocytes were also stained with biotinylated Ig-CD4718 and fluorescent extravidin. Ligand-induced SIRPα clusters are clearly visible for both ligands and for all phagocytes. Average pixel intensity in unclustered regions was calculated to determine the fraction of cell area with at least 2- or 5-fold the mean unclustered pixel intensity. Antibody-mediated preclustering of SIRPα with P3C4 enhances Ig-CD47 binding (G).
Discussion
While CD47-SIRPα interactions have been reported in mouse studies to inhibit phagocytosis of RBCs4,5 and platelets,9 there is at least one recent brief report12 that concludes that severely reduced levels of CD47 on human RBCs does not lead to enhanced phagocytosis by human monocytes. Because of the many known differences in CD47 and SIRPα between mouse and human, 3 assays were used here to examine species-specific interactions of red cell CD47 with human SIRPα1ex. Direct binding of soluble human SIRPα1ex to RBCs assayed by flow cytometry showed that SIRPα interacts specifically with human and porcine RBCs (Figures 2, 4, 6, S3), whereas little or no binding is found with cow, rat, or mouse RBCs. Cell adhesion measurements confirm species-specific binding and indicate that CD47-SIRPα interactions can be strong enough to resist significant forces in centrifugation and AFM; furthermore, these interactions prove highly specific and saturable (Figures 3, 6). In order to have physiologic meaning, such interactions must be renormalized by the calibrated assays of SIRPα receptor numbers and clustering on various human phagocytes (Figure 7; Table 3). These numbers indicate that SIRPα-CD47 is not likely to mediate adhesion and imply that this interaction being studied is not responsible for red cell clearance. Given well-established binding and adhesion results with mice, the results here certainly reveal divergence in the molecular interactions and lend insight into any species-specific meaning of self. Furthermore, based on the interaction data here, it is clearly possible that the original conclusions of Oldenborg et al4,5 based on mouse knock-outs are not appropriate to clearance processes in humans.
Species-restricted binding and adhesion
Human SIRPα1ex binding shows a species-restricted pattern: notably, human SIRPα1ex interacts with CD47 on pig but not mouse, cow, or rat RBCs. While species-specific posttranslational modifications of CD47 such as glycosylation might have a role in mechanism, the results here for RBCs reveal native state interactions. Paradoxically, for a putative marker of self, the pig CD47 responds more strongly to human SIRPα1ex (Figures 2D and 6). It remains to be seen if these results mean that CD47 on pig RBCs can signal self through human SIRPα, or perhaps adhesion of pig RBCs to human phagocytes would prove so strong that the phagocytes cannot detach from the pig RBCs before committing to engulfment. Moreover, the compatibility implications of such findings for pig-human xenotransplantation could prove significant.
Whether increased binding of human SIRPα1ex to pig RBCs is due to affinity or avidity effects is not clear. Higher avidity, as a result of increased clustering, could be due to increased receptor or ligand mobility. In the case of CD47, mobility (lack of cytoskeletal connectivity) varies across species. For instance, CD47 is only 40% mobile in human RBCs,19 whereas mobility is 100% in mouse RBCs.11 Enhanced mobility may strengthen pre-existing ligand-receptor binding but it cannot, by itself, mediate binding. Thus, the fully mobile CD47 in murine RBCs is nevertheless unable to bind human SIRPα; the mobility of CD47 and the extent to which it augments CD47-human SIRPα binding in pig RBCs is presently unknown. However, by using recombinant human SIRPα1ex that lacks N-linked glycans (Figures S3 and 5B), it is clear that the interaction is amino acid mediated on the SIRPα1ex side and interacts in a species-specific manner with red cell CD47. Differences in antibody binding (Table 2; Figure S3) further highlight divergence in accessible surface sites.
Binding, avidity, and adhesion
The SIRPα-CD47 interaction is surmised to be weak, in the low μM range.18 Such interactions are difficult to measure in typical binding assays, requiring long incubations at 37°C to promote receptor clustering and internalization.33 Indeed, all past reports appear to have used multimers of CD47 or SIRPα (multimerized via fusions to multimeric partners) in order to detect binding.14,17,27,30,34 Here, using polyclonal antibodies against the GST moiety, we were able to prepare SIRPα1ex dimers into a multimeric complex (≥ 1000-kDa sized by gel filtration; data not shown). Binding of this complex is specific, since CD47-specific blocking antibodies reduce labeling to control levels. Interestingly, one nonblocking antibody (2D3) enhanced the binding greater than 10-fold (Figure 2A). A similar observation was made while staining astrocytomas expressing SIRPα using a CD47-Fc fusion protein.30 Antibody clustering of CD47 likely increases the avidity. Indeed, 2D3 is unable to enhance SIRPα1ex binding when CD47 is immobilized on Ig-CD47 beads (Figure 2B) but 2D3 does visibly cluster CD47 on RBCs (Figure 2C).
Cluster analysis of SIRPα indicates that only a small fraction of a phagocyte's area is occupied with clusters of higher SIRPα density (Figure 7; Table 3). However, we show enhancement of Ig-CD47 binding when SIRPα on THP-1 cells was preclustered using a nonblocking antibody (P3C4).17 These enhancements in avidity may be sufficient for transient and/or local adhesion between RBCs and phagocytes, enabling signaling of self.
Consistent with flow cytometry, human RBCs resist sustained centrifugal forces (∼100 pN for 10 minutes) after adhering to surfaces coated with human SIRPα1ex (Figure 3). Blocking antibodies again show that adhesion is specifically mediated by CD47. The ligand density at half-maximal adhesion is about 3-fold lower for SIRPα1ex than for a well-tested blocking antibody, B6H12 (Figure 6). Orders of magnitude differences are seen, in comparison, between solution affinities of SIRPα1 (8 μM)18 and B6H12 (∼50 nM; data not shown). Strikingly, however, the average density of SIRPα measured on several phagocyte types is well below the range that effectively mediates RBC adhesion (Table 3; Figure 6A inset).
Unbinding force measurements using AFM (Figure 4B) suggest that the forces are not low in comparison to well-established interactions among cell adhesion molecules.35 The single-molecule disruption force, which is seen on time scales of 2 to 20 ms (based on ∼10-100 nm membrane extensions at the 5 μm/s detachment rates), appears reasonably consistent with centrifugation results at 100 pN load per cell when the number of bonds per cell, given by C50 (1670 bonds/μm2) multiplied by contact area (60 μm2),36 is divided by 10-minute centrifugation times to give 6 ms per bond. However, the low density of SIRPα observed in phagocytes again means that adhesions will be rare, limited by density and mobility.
Binding of CD47 is further modulated by SIRPα glycosylation, with reduced or no glycosylation leading to increased binding (Figures 5B and S3). This is consistent with findings on mouse B16 melanoma, where reduced or no glycosylation of SIRPα is actually required for CD47 binding and hyperglycosylation prevents detectable interaction.29 However, the extent of glycosylation is very different between these systems, where COS-1 cells produced human SIRPα1 migrating at approximately 80 kDa and the hyperglycosylated form of mouse SIRPα in melanoma cells migrating at greater than 121 kDa. Divergence in the number of potential N-linked glycosylation sites makes the result with human SIRPα novel as well as consistent.
Even within species, CD47-SIRPα interactions do not seem conserved. The example here is the lack of SIRPα1ex binding to human MSCs (Table 4), even in a highly sensitive assay. An interaction between SIRPα1ex and CD47 (Figures 2-3) thus cannot be assumed from past work on mouse16 RBCs or human cell types.3,17
The state of human stem cells in mice37 could depend on whether the interaction between human stem cell CD47 and mouse macrophages is functional. In fact, CD47 has already been implicated in developmental processes such as erythropoiesis38 and neuronal development39-41 and more recently synaptogenesis,42 making efforts to understand the requirements of CD47-SIRPα signaling all the more important.
Prepublished online as Blood First Edition Paper, November 15, 2005; DOI 10.1182/blood-2005-04-1463.
Supported by funding from National Institutes of Health (NIH)-R21 and NIHR01 grants (D.E.D.), a National Science Foundation (NSF) Career grant (E.T.B.), and a predoctoral fellowship from Merck (S. Subramanian).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr Axel Ullrich of Max-Planck-Institute fur Biochemie (Martinsreid, Germany) for the human SIRPα1 expression vector, Dr Marion Brown of Sir William Dunn School of Pathology (Oxford, United Kingdom) for the human CD47 expression vector and recombinant CD47 protein, and Dr Mary Telen of Duke University (Raleigh, NC) for a generous gift of the CD47 antibody 6H9.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal