Abstract
The two genes mutated in the bone marrow failure syndrome dyskeratosis congenita (DC) both encode components of the telomerase complex responsible for maintaining the ends of chromosomes in stem cells and in the germ line. In reviewing the mutation profile that is found in DC, we describe 9 novel mutations in the DKC1 gene and 3 novel TERC mutations responsible for the X-linked and autosomal dominant forms of the disease, respectively, but find that two thirds of the families do not have mutations in either of these genes. In a significant subset of these uncharacterized families, the index case presents with severe disease previously defined as the Hoyeraal Hreidarsson (HH) syndrome. The diverse clinical phenotype seen in patients with X-linked DC is not explained by the different amino acid substitutions: Presentation of the recurrent A353V substitution ranges from classic DC to the severe HH variant. However, we do see that patients with HH have significantly shorter telomeres than those with a relatively mild presentation. In the new families described with TERC mutations, there is further evidence of disease anticipation associated with shorter telomeres in the younger generations. This study highlights the considerable genetic and phenotypic diversity of DC.
Introduction
Dyskeratosis congenita (DC) is a bone marrow failure syndrome classically associated with a triad of mucocutaneous features: nail dystrophy, oral leukoplakia, and abnormal reticulate skin pigmentation.1 In contrast to Fanconi anemia, in which a diagnosis can be made on the basis of increased sensitivity of cells to agents that promote chromosomal breakage,2 no definitive laboratory test is available for DC. The absence of such a test makes the diagnosis of DC difficult in some cases, particularly because there is a great range of clinical presentation.3 At its most severe, both male and female children can present within the first year of life with immunodeficiency, developmental delay, cerebellar hypoplasia, and gastrointestinal problems. This had previously been recognized as the Hoyeraal Hreidarsson (HH) syndrome4,5 but is now known in some cases to be allelic to the X-linked form of DC.6 At the other extreme, asymptomatic affected individuals of middle age with only mild hematologic abnormalities have been observed in families with the autosomal dominant form of DC.7
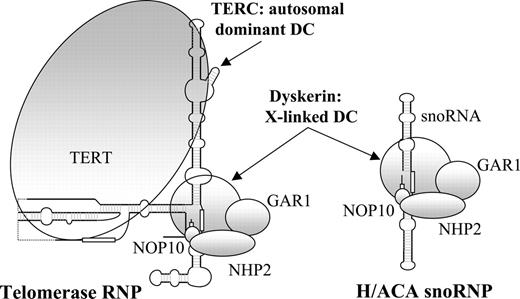
The clear definition of the range of severity of DC and the ability to offer a definitive diagnosis has become possible over recent years through the cloning of 2 of the disease genes. The X-linked form is the most commonly recognized and is caused almost entirely by missense mutations in the DKC1 gene.8 This gene encodes a nucleolar protein called dyskerin that is involved in both ribososme biogenesis9-11 and telomere maintenance12 (Figure 1). The autosomal dominant form of DC is caused by mutations in the RNA component of telomerase (TERC),17 and it has been suggested that DC is therefore a disorder of human telomerase.18,19 This ribonucleoprotein complex is responsible for maintaining the repeat structure that caps the end of each chromosome. During each round of cell division this repeat is incompletely replicated, resulting in telomere loss that is compensated for by the action of telomerase in the germ line and in some stem cells.13,20,21
In mice, Terc knockouts are initially viable but do begin to display symptoms overlapping DC in later generations, which may be explained by the effects of the gradual telomere loss.22 In contrast, mice that lack Dkc1 are embryonic lethal, indicating that dyskerin has an additional essential function distinct from telomere maintenance.23 Mice in which Dkc1 expression is reduced are viable and have DC-like symptoms attributed mainly to inadequate processing of ribosomal RNA.24 However, mouse embryonic stem (ES) cells that harbor missense mutations in Dkc1 show defects in both telomerase and pseudouridylation.25
Several interesting questions therefore remain. We do not know to what extent the defect in ribosome biogenesis is important in the development of X-linked DC. The precise mechanism whereby missense mutations in a TERC-associated protein and heterozygous mutations in TERC itself lead to remarkably similar phenotypes remains to be determined. It has been shown that disease anticipation in families with autosomal dominant DC is associated with telomere shortening,7 but we do not know whether telomere length has anything to do with the severity of the disease in X-linked families. We are also interested to determine whether any of the other members of these ribonucleoprotein complexes are involved in DC, including the reverse transcriptase component of telomerase (TERT), which has recently been shown to be mutated in some patients with aplastic anemia (AA).26,27
To try to answer some of these questions we can refer to the DC Registry (DCR) at the Hammersmith Hospital in London that now represents more than 228 families. In this paper, we review the mutations that have been identified in these families, assess genotype-phenotype correlations, and investigate whether there is any relationship between telomere length and severity of DC.
A sketch of the telomerase and H/ACA small nucleolar ribonucleoprotein complexes. sno indicates small nucleolar; RNP, ribonucleoprotein. Based on information from Chen and Greider,13 Wang and Meier,14 Narayanan et al,15 and Dragon et al.16
Patients, materials, and methods
The DC Registry
Families are recruited to the DCR from all over the world. We currently have clinical and genetic information for 228 families registered from 40 different countries, comprising 354 affected individuals. The criteria for admission to the DCR have changed over the years. Initially, families were included only when the index case presented with the triad of diagnostic mucocutaneous features (nail dystrophy, leukoplakia, and abnormal skin pigmentation). As it became clear that not all DC patients have all of these features, we have extended recruitment to include families in which the index case has 1 or more of these mucocutaneous features, combined with a hypoplastic bone marrow and at least 2 of the other somatic features known to occur in DC.3 Patients are also entered into the DCR if they have at least 4 of 6 of the most commonly recognized features of HH: interuterine growth retardation, developmental delay, microcephaly, cerebellar hypoplasia, immunodeficiency, or bone marrow failure. Finally, families are included in the DCR when patients, presenting initially with AA, have been shown to have mutations in TERC. Approval was obtained for these studies from the Research Ethics Committee of the Hammersmith Hospitals NHS Trust, the institutional review board. Informed consent was provided in accordance with the Declaration of Helsinki.
Mutation screening
In families with only affected males, the entire coding sequence and 5′ flanking sequence of the DKC1 gene is screened for point mutation by denaturing high-performance liquid chromatography (HPLC) analysis on a WAVE DNA fragment analysis system (Transgenomic, Paris, France). The gene is amplified in 15 different fragments using the polymerase chain reaction (PCR) conditions and primer pairs previously described.28 After mixing with amplified wild-type DNA, these fragments are denatured and slowly cooled to allow for heteroduplex formation prior to screening at 2 different temperatures selected using Wavemaker software (Transgenomic). When abnormal elution patterns are detected, the appropriate fragments are reamplified and subjected to direct sequence analysis by BigDye chain termination cycle sequencing and fragment analysis on the 3700 DNA Analyzer (Applied Biosystems, Foster City, CA). Any mutations identified are confirmed either by sequencing of the reverse strand or by reamplification and restriction enzyme digestion using sites that are created or destroyed by the mutation. All families are also screened for mutation in the TERC gene by both Southern blot and denaturing HPLC analysis using the probe, primer pair, and PCR conditions previously described.17 Screening of the gene encoding TERT was performed by denaturing HPLC and direct DNA sequence analysis as previously described.27
Telomere length measurement
Telomere length was measured by Southern blot analysis using a subtelomeric probe from chromosome 7 (pTelBam8) as described elsewhere.29 The size of the peak of the signal intensity was determined using Gel Blot Pro software (UVP, Upland, CA). Unaffected siblings and spouses in families in which DKC1 or TERC mutations have been identified were used as age-matched healthy controls. To adjust telomere length measurement for age, a linear regression line was calculated for telomere length against age in these 100 healthy individuals. The telomere length of affected individuals was then expressed as the difference between the observed length and the age-adjusted normal telomere length predicted from the linear regression line (deltaTEL).30
Results
Inheritance patterns in DC
Although DC is thought of primarily as an X-linked recessive disorder, of 228 families entered into the DCR, only 22 of them show true X-linked inheritance, with affected maternal uncles or male cousins of the index case. In 19 families there are at least 2 affected brothers, but in most of the families (123) the affected male is a sporadic case. In the remaining 64 families there is 1 or more affected female. Again, in most of these (35) there is only 1 sporadic female case while 18 families show autosomal recessive inheritance and 11 show autosomal dominant inheritance (Table 1).
Patterns of inheritance and the mutation profile in DC
Pattern of inheritance . | DKC1 mutated* . | TERC mutated† . | No mutation identified . | Total . |
|---|---|---|---|---|
| X-linked | 21 (4) | 0 (0) | 1 (1) | 22 (5) |
| Two affected male siblings | 11 (3) | 1 (0) | 7 (1) | 19 (4) |
| Sporadic affected male | 40 (8) | 3 (0) | 80 (15) | 123 (23) |
| Autosomal dominant | 0 (0) | 7‡ (0) | 4 (0) | 11‡ (0) |
| Autosomal recessive | 0 (0) | 0 (0) | 18 (9) | 18 (9) |
| Sporadic affected female | 0 (0) | 0 (0) | 35 (7) | 35 (7) |
| All | 72 (15) | 11 (0) | 144 (33) | 228 (48) |
Pattern of inheritance . | DKC1 mutated* . | TERC mutated† . | No mutation identified . | Total . |
|---|---|---|---|---|
| X-linked | 21 (4) | 0 (0) | 1 (1) | 22 (5) |
| Two affected male siblings | 11 (3) | 1 (0) | 7 (1) | 19 (4) |
| Sporadic affected male | 40 (8) | 3 (0) | 80 (15) | 123 (23) |
| Autosomal dominant | 0 (0) | 7‡ (0) | 4 (0) | 11‡ (0) |
| Autosomal recessive | 0 (0) | 0 (0) | 18 (9) | 18 (9) |
| Sporadic affected female | 0 (0) | 0 (0) | 35 (7) | 35 (7) |
| All | 72 (15) | 11 (0) | 144 (33) | 228 (48) |
Numbers in parentheses refer to the number of families in each category with HH.
All families with only affected males have been screened for DKC1 mutation; of the families with affected females, 27 have been screened for DKC1 mutation, and all of those are negative.
All families have been screened for TERC mutation.
Includes 2 families in which both parents are asymptomatic and that were initially thought to show autosomal recessive inheritance but are now known to have autosomal dominant DC due to TERC mutations.
Mutations in X-linked DC
In 21 of 22 families that show X-linked inheritance of DC, we have found mutations in the DKC1 gene. In one family showing clear X-linked inheritance, and in which an obligate carrier shows the characteristically skewed pattern of X-chromosome inactivation, we have not found a mutation despite sequencing all coding exons and flanking intron sequences of the DKC1 gene. Two thirds (11 of 19) of the families with affected brothers but only approximately one third (40 of 123) of the sporadic male cases have DKC1 mutations (Table 1). Notably, among the HH families with affected males, the proportion of the different types of families (X-linked, 2 affected male siblings, and sporadic males) that have DKC1 mutations is broadly similar to that seen in DC as a whole (Table 1).
As previously noted, these mutations almost always cause amino acid substitutions, which appear to cluster in 2 distinct regions of the dyskerin protein (Figure 2). Most of these mutations are unique although 4 have been seen twice, 1 has been seen 4 times, and 1 (A353V) has been seen 30 times. Those that have not been described previously are listed in Table 2. One mutation presented here that is an exception to the rule is the A>G substitution occurring at the AG acceptor splice site of the last intron of the gene. RNA is not available from this case, and so we cannot establish what this mutation does to the 3′ end of the DKC1 transcript. However, it may be relevant that another DC case has a 2-kb deletion that removes the 3′ end of this intron as well as the final exon of DKC1.31
Novel DKC1 mutations
Mutation . | AA substitution . | Exon . | Family . | Presentation . |
|---|---|---|---|---|
| 29C>T | P10L | 2 | DCR160 | S-DC/HH |
| 200C>T | T67I | 4 | DCR108 | X-DC/HH |
| 204C>A | H68Q | 4 | DCR187 | S-DC/HH |
| 941A>G | K314R | 10 | DCR163, DCR204 | X-DC/HH, S-DC |
| 1075G>A | D359N | 11 | DCR190 | S-DC |
| 1156G>A | A386T | 12 | DCR216 | S-DC/HH |
| 1223C>T | T408I | 12 | DCR125 | X-DC |
| 1258, 1259AG>TA | S420Y | 12 | DCR217 | S-DC |
| IVS14 nt 473A>G | N/A | IVS14 | DCR192 | S-DC |
Mutation . | AA substitution . | Exon . | Family . | Presentation . |
|---|---|---|---|---|
| 29C>T | P10L | 2 | DCR160 | S-DC/HH |
| 200C>T | T67I | 4 | DCR108 | X-DC/HH |
| 204C>A | H68Q | 4 | DCR187 | S-DC/HH |
| 941A>G | K314R | 10 | DCR163, DCR204 | X-DC/HH, S-DC |
| 1075G>A | D359N | 11 | DCR190 | S-DC |
| 1156G>A | A386T | 12 | DCR216 | S-DC/HH |
| 1223C>T | T408I | 12 | DCR125 | X-DC |
| 1258, 1259AG>TA | S420Y | 12 | DCR217 | S-DC |
| IVS14 nt 473A>G | N/A | IVS14 | DCR192 | S-DC |
S indicates sporadic case; DC/HH, patients with features of both classic DC and HH syndrome; X, X-linked inheritance; and nt, intronic mutation.
Amino acid substitutions in dyskerin. A linear representation of the protein shows the location of the nuclear localization signals (NL) and the TruB and PUA domains. Vertical bars show the positions of the amino acid substitutions. Those in bold are presented in this paper; those underlined are seen in patients with HH; those with asterisks are seen repetitively.
Amino acid substitutions in dyskerin. A linear representation of the protein shows the location of the nuclear localization signals (NL) and the TruB and PUA domains. Vertical bars show the positions of the amino acid substitutions. Those in bold are presented in this paper; those underlined are seen in patients with HH; those with asterisks are seen repetitively.
There does not seem to be any clear correlation between the location of the dyskerin mutation and the severity of the disease: Mutations that cause the most severe form of DC, previously recognized as the HH syndrome, are located in both mutation clusters, although 2 of these amino acid substitutions (S121G and R158W) do not fall into either cluster. Of the 48 families in which the index case presents with or has features of HH, 32 are male and 15 of them have 8 different DKC1 mutations (underlined in Figure 2). One of these (T49M) has been seen in 4 different families and is only seen in HH patients, indicating that there is a consistent phenotype for this amino acid substitution. However, 4 other families with HH have the commonly recurring A353V mutation. In the 16 HH families in which there is an affected female, none have DKC1 mutations, 7 are sporadic, 9 appear to have an autosomal recessive form, and none appear to have an autosomal dominant form of the disease. These observations highlight the existence of a significant fraction of autosomal (non DKC1) HH, the genetic basis of which presently remains unknown.
Phenotypic diversity in patients with A353V
Remarkably, 30 of the 72 DC families in the DCR that have DKC1 mutations have the same mutation (1058C>T) causing an A353V substitution, representing about 42% of X-linked DC. These families are unrelated, and in 12 of them the mutation has occurred de novo. To investigate the relationship between genotype and phenotype in this group of patients, we have stratified the severity of the disease into 4 categories: (1) those that presented at the age of 15 years or more and have DC but do not have AA, defined as a cytopenia of 2 or more lineages (hemoglobin [Hb], less than 80 g/L [8 g/dL]; neutrophils, less than 0.5 × 109/L; and/or platelets, less than 100 × 109/L); (2) those that presented at less than 15 years of age and have DC but do not have AA; (3) those that have DC and AA; and (4) those that have features of HH.
It becomes clear from this analysis that patients with the same mutation can present with an extremely variable phenotype. Of 28 patients from 25 families with the A353V mutation for whom we have good clinical records, 8 fall into category 1, 7 into category 2, 9 into category 3, and 4 into category 4 (Table 3). The presentation ranges from a 34-year-old man with the triad of mucocutaneous features of DC, sparse hair, bilateral lacrimal duct obstruction, and a reduced Hb, to a 1-year-old boy with many of the features of both DC and HH: leukoplakia, thrombocytopenia, anemia, interuterine growth retardation, developmental delay, cerebellar hypoplasia, immunodeficiency (with low B and natural killer [NK] cells), and gastrointestinal tract problems.
Phenotypic diversity in 28 patients with the A353V dyskerin mutation
Disease category . | Phenotype . | No. of patients . |
|---|---|---|
| 1 | DC ≥ 15 y | 8 |
| 2 | DC < 15 y | 7 |
| 3 | DC + AA | 9 |
| 4 | HH | 4 |
Disease category . | Phenotype . | No. of patients . |
|---|---|---|
| 1 | DC ≥ 15 y | 8 |
| 2 | DC < 15 y | 7 |
| 3 | DC + AA | 9 |
| 4 | HH | 4 |
The disease categories are explained in the text, under “Phenotypic diversity in patients with A353V.”
Telomere length and disease severity in patients with DKC1 mutations
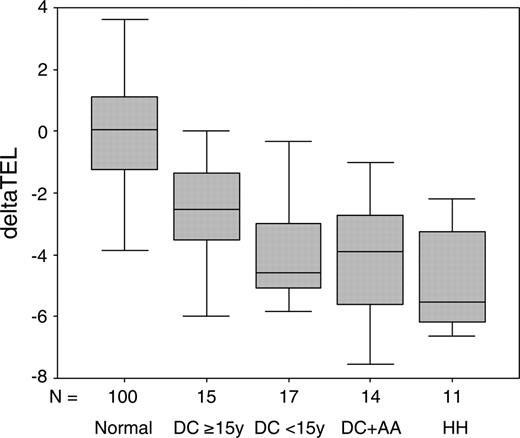
It has been reported previously that patients with DKC1 mutations have significantly shorter telomeres compared with age-matched controls.18,32 We have now investigated the relationship between clinical severity and telomere length in 57 patients with DKC1 mutations for whom we have information on both of these aspects. The patients are divided into 4 clinical categories based on age of presentation (categories 1 and 2), the presence of AA (category 3), and the presence of HH (category 4). Telomere lengths are corrected for age by subtracting the predicted telomere length (obtained from a line of best fit drawn through a plot of telomere length against age for 100 healthy subjects) from the observed telomere length to obtain a deltaTEL measurement, as described elsewhere.30
The results of this analysis are shown in Figure 3 and Table 4. The deltaTEL values for patients with DKC1 mutations are significantly reduced compared with the healthy individuals (Mann-Whitney test P < .001), in confirmation of previous observations. We also find that patients with the most severe phenotype (HH, category 4) have significantly shorter telomeres than those with the mildest phenotype (DC, no AA, 15 years or more, category 1, Mann-Whitney test P = .012). The young patients (less than 15 years, category 2) and those with AA (category 3) also appear to have shorter telomeres than the milder group (category 1), but this is marginally significant (Mann-Whitney test P = .044 and .045, respectively). There is no difference in the telomere lengths between the young DC patients (less than 15 years), those with DC and AA, and those with HH.
Clinical severity and telomere lengths in patients with DKC1 mutations
Disease category . | Phenotype . | No. of subjects . | Median deltaTEL . | Range of deltaTEL . |
|---|---|---|---|---|
| 0 | Normal | 100 | 0.058 | -3.86 to 3.63 |
| 1 | DC ≥ 15 y | 15 | -2.54 | -6.0 to 0 |
| 2 | DC < 15 y | 17 | -4.57 | -5.84 to -0.32 |
| 3 | DC + AA | 14 | -3.90 | -7.54 to -1.03 |
| 4 | HH | 11 | -5.55 | -6.64 to -2.18 |
Disease category . | Phenotype . | No. of subjects . | Median deltaTEL . | Range of deltaTEL . |
|---|---|---|---|---|
| 0 | Normal | 100 | 0.058 | -3.86 to 3.63 |
| 1 | DC ≥ 15 y | 15 | -2.54 | -6.0 to 0 |
| 2 | DC < 15 y | 17 | -4.57 | -5.84 to -0.32 |
| 3 | DC + AA | 14 | -3.90 | -7.54 to -1.03 |
| 4 | HH | 11 | -5.55 | -6.64 to -2.18 |
Mann-Whitney test: category 0 deltaTEL versus categories 1 through 4 deltaTEL, P < .001; category 1 versus 2, P = .044; category 1 versus 3, P = .045; category 1 versus 4, P = .012.
Box-whisker plots of telomere lengths. DeltaTEL values are shown in a box-whisker plot for 100 healthy subjects, 15 DC patients age 15 years or more (DC ≥ 15y), 17 DC patients age less than 15 years (DC < 15y), 14 DC patients with AA (DC+AA), and 11 patients with HH.
Box-whisker plots of telomere lengths. DeltaTEL values are shown in a box-whisker plot for 100 healthy subjects, 15 DC patients age 15 years or more (DC ≥ 15y), 17 DC patients age less than 15 years (DC < 15y), 14 DC patients with AA (DC+AA), and 11 patients with HH.
Autosomal dominant DC and TERC mutations
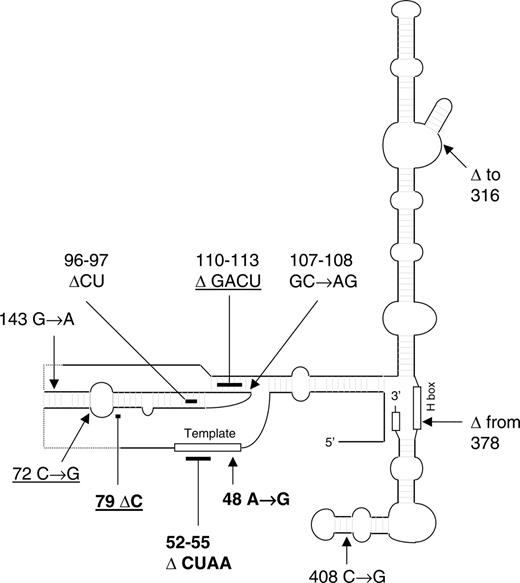
Nine families referred to the DCR showed apparent autosomal dominant inheritance of the disease. Of these, 5 have TERC mutations, but in 4 we have been unable to find mutations in TERC. Six other families are now known to have autosomal dominant DC due to TERC mutations, but their presentation did not initially suggest this pattern of inheritance. These include 1 family with apparent autosomal recessive inheritance of DC, 1 with apparent autosomal recessive inheritance of AA, and 1 sporadic case of AA that we have reported previously as well as 3 new families detailed in the next paragraph. Of the 11 different TERC mutations that we have identified in the 228 DC families, 3 have not been reported previously and are shown in Table 5. Unlike other natural mutations that have been described in TERC, 2 of these are located in the template region of the molecule (Figure 4).
Novel TERC mutations
Mutation . | Location . | Family . | Presentation . |
|---|---|---|---|
| 79delC | Pseudoknot domain | DCR207 | FAA |
| 52-55delCTAA | Template region | DCR209 | S-MDS/DC |
| 48A>G | Template region | DCR230 | S-DC |
Mutation . | Location . | Family . | Presentation . |
|---|---|---|---|
| 79delC | Pseudoknot domain | DCR207 | FAA |
| 52-55delCTAA | Template region | DCR209 | S-MDS/DC |
| 48A>G | Template region | DCR230 | S-DC |
FAA indicates familial aplastic anemia; S, sporadic case; MDS, myelodysplastic syndrome.
Two of these new mutations were identified in males without a family history, one of whom had bone marrow failure and nail dystrophy at the age of 16, while the other was a 13-year-old with hypoplastic myelodysplasia and nail dystrophy. The third new mutation was identified in a family in which 2 men presented with AA/pancytopenia in their 40s, one of whom had leukoplakia developing into squamous cell carcinoma of the tongue. In none of these 3 families did the parents of the affected children present with features related to DC, supporting the observation that there is disease anticipation in families with TERC mutations.7 In 2 of the families, an asymptomatic parent has been shown to carry the TERC mutation, confirming that these are constitutional. In both cases, the age-adjusted telomere length (deltaTEL) of the affected children is shorter than that seen in the asymptomatic affected parent (child deltaTEL–parent deltaTEL =–2.9 and –2.16, respectively).
TERT mutations in the DCR
We have previously reported on the screening of the TERT gene in 24 families from the DCR.27 Among these we identified one heterozygous missense mutation in a girl with HH in a family with apparent autosomal recessive inheritance of the disease. Her asymptomatic mother was also heterozygous for the mutation. It has been shown elsewhere that heterozygous TERT mutations can impair telomerase activity by haploinsufficiency and that they may be risk factors for marrow failure.26
We have now screened an additional 50 patients from the DCR from families with autosomal dominant and recessive inheritance as well as sporadic affected males and females (Table 6). In this group of patients we have identified 8 novel sequence changes, including one that causes a missense mutation (Table 6), in addition to 5 other previously described synonymous and intronic mutations. The P721R substitution, which is predicted to result from the novel 2162C>G missense mutation, lies in motif A of the RT domain of TERT.33 It was identified in a 2-year-old girl with DC in a family of consanguineous marriage and apparent autosomal recessive inheritance of the disease. She is heterozygous for this mutation, as are her older asymptomatic sister and her asymptomatic mother. Two maternal aunts and one first cousin who have nail dystrophy are also heterozygous for this mutation. It was not detected among 95 ethnically matched healthy individuals.
TERT mutations in DCR families
Pattern of inheritance . | No. of subjects screened* . | Amino acid substitutions†(n) . | Novel synonymous and intronic sequence changes‡(n) . |
|---|---|---|---|
| Sporadic affected male | 26 | A279T (5) | 1849C>T (2) |
| IVS8 nt-88 G>A (1) | |||
| IVS10 nt-10 T>C (1) | |||
| IVS12 nt-10 G>T (1) | |||
| Autosomal dominant | 4 | None | None |
| Autosomal recessive | 17 | F1127L (1) | None |
| P721R (1) | |||
| Sporadic affected female | 27 | A279T (2) | IVS10 nt-63C>T (1) |
| IVS12 nt 74 C>T (1) | |||
| IVS12 nt 79insCG (1) |
Pattern of inheritance . | No. of subjects screened* . | Amino acid substitutions†(n) . | Novel synonymous and intronic sequence changes‡(n) . |
|---|---|---|---|
| Sporadic affected male | 26 | A279T (5) | 1849C>T (2) |
| IVS8 nt-88 G>A (1) | |||
| IVS10 nt-10 T>C (1) | |||
| IVS12 nt-10 G>T (1) | |||
| Autosomal dominant | 4 | None | None |
| Autosomal recessive | 17 | F1127L (1) | None |
| P721R (1) | |||
| Sporadic affected female | 27 | A279T (2) | IVS10 nt-63C>T (1) |
| IVS12 nt 74 C>T (1) | |||
| IVS12 nt 79insCG (1) |
“n” indicates is the number of times each mutation has been observed in this series of 74 patients; and ins, insertion.
TERT screening of 24 of these subjects has been reported previously.
The A279T substitution has previously been shown to be polymorphic; the F1127L substitution has also been described previously; the P721R substitution is described in this paper.
Previously published synonymous and intronic sequence changes are not included.
Discussion
Recent interest in the bone marrow failure syndrome DC has largely focused on the fact that some families with this disease have mutations in the gene encoding the RNA component of telomerase.17 However, the most widely recognized form of this disease is caused by mutations in dyskerin, a protein that is involved both in the telomerase complex12 as well as the H/ACA small nucleolar ribonucleoprotein complex that is responsible for the site-specific modification of uracil residues during ribosomal RNA processing.9-11 In this paper we have reviewed the mutation profile among 228 DC families that have been referred to the DCR at the Hammersmith Hospital in London and describe 8 novel missense mutations and 1 splicing mutation in the DKC1 gene as well as 3 novel mutations in the TERC gene. However, one of the most striking findings of this survey is the large proportion of DC families (144 of 228, 63%) in which we have not found a mutation in either of these genes. Although some mutations might have been be missed by the denaturing HPLC screen, we can infer that one or a few DC genes are still to be discovered. In a significant subset of these uncharacterized families (33 of 144) the index case has features of the severe variant HH.
TERC mutations in the DCR. The 11 TERC mutations that we have identified in patients in the DCR are shown on a sketch of the TERC molecule (based on information from Chen and Greider13 ). Mutations identified in patients presenting with AA are underlined. The 3 novel mutations are in bold. Arrows indicate point mutations or the location of breakpoints of large deletions; bars, small deletions.
TERC mutations in the DCR. The 11 TERC mutations that we have identified in patients in the DCR are shown on a sketch of the TERC molecule (based on information from Chen and Greider13 ). Mutations identified in patients presenting with AA are underlined. The 3 novel mutations are in bold. Arrows indicate point mutations or the location of breakpoints of large deletions; bars, small deletions.
Obvious candidates for mutation in these uncharacterized families are the other protein components that are found in both telomerase and the small nucleolar ribonucleoprotein complex, namely GAR1, NHP2, and NOP10. Previous work has shown that these are not commonly mutated in autosomal recessive DC, and the gene(s) responsible for this subtype of DC remain unknown.34 Of the 18 families that show autosomal recessive inheritance of DC, 10 involve a consanguineous marriage where it is likely that one pathogenic mutation is present in the homozygous state.
Recently it has been shown that some patients with familial AA and DC have heterozygous mutations in the gene that encodes TERT.26,27 Although some of these mutations have been shown to result in reduced telomerase activity in vitro, they do not appear to segregate with the disease in the few families described. The novel missense mutation described here seems to fit this pattern. So while it appears that they may act as risk factors for bone marrow failure, their direct pathogenic role remains to be established. What is clear is that TERT mutations do not account for many of the uncharacterized DC families.
Relatively little is known about the functional consequences of the many missense mutations that have been described in dyskerin. This is partly due to the fact that any subtle effects that these may have are difficult to assay, although an artificial mutation in the catalytic center has been shown in other species to abolish pseudouridylation activity and significantly reduce H/ACA RNA association.14,35 On the other hand, in one patient with a Q31E substitution, a reduction in the level of telomerase RNA in peripheral blood has been demonstrated.36 However, it is clear that there is quite a substantial variation in the levels of telomerase activity seen in normal blood samples, and this does not seem to be significantly different in patients with DKC1 mutations.32 The 3D structure of dyskerin has not been elucidated, and functional domains of the protein can only be inferred through reference to orthologs in other species (Figure 2). It can be seen that several mutations fall in the PUA domain, conserved in pseudouridine synthase and archaeosine transglycosylases. Only 2 amino acid substitutions fall in the TruB domain, in which the catalytic 125Asp residue lies, but there is a significant cluster near the N-terminal of the protein, the function of which is unknown. It is possible that some of these simply cause the protein to be unstable.
There does not seem to be any clear relationship between the location of the amino acid substitution and the severity of the disease, although 2 missense mutations (I38T and T49M) have now been seen to be repeatedly associated with a severe clinical phenotype, previously characterized as HH syndrome. The one amino acid substitution (A353V) that accounts for more than 40% of the families with DC due to dyskerin mutations can cause a full spectrum of disease severity, indicating that other genetic or environmental factors are clearly important in the disease phenotype.
One thing that is clear is that patients with DC have significantly shorter telomeres than age-matched controls.18,32 We have also shown here that among the patients with DKC1 mutations, those with the most severe form of DC have significantly shorter telomeres than those with a milder form of the disease. This is consistent with the idea that a defective dyskerin results in impaired telomerase activity, leading to shorter telomeres and premature aging of the tissues that require constant turnover. These data, together with the disease anticipation (increasing disease severity with succeeding generations) associated with progressive telomere shortening observed in autosomal dominant DC families7 and Terc–/– mice,22 also suggest that the shortest telomeres cause the most severe disease. Further evidence in support of this observation comes from the 2 families presented here with novel TERC mutations for which we have the appropriate clinical and genetic information: In both cases, the affected children have shorter age-adjusted telomere lengths than their asymptomatic affected parents. The clinical features observed in these families provide further support for the overlap of autosomal dominant DC with aplastic anemia observed previously.37-39
Of the 3 TERC mutations described here, 2 are particularly interesting in that they are the first natural mutants to be described that disrupt the template region of the molecule that is directly responsible for the synthesis of the telomeric repeat sequences. It will be interesting to investigate whether these mutations cause DC through haploinsufficiency, like those that have been described previously,40-42 or whether they may have some dominant negative effect on the telomerase complex.
Prepublished online as Blood First Edition Paper, December 6, 2005; DOI 10.1182/blood-2005-07-2622.
Supported by the Wellcome Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to the DC families and clinicians (particularly Drs Arn, Benke, Bonfim, Donato, Gungor, Kratz, Leblanc, Neven, Oliva, Paller, Porea, Shashi, Sullivan, and Warren) who have sent samples and to Richard Szydlo for assistance with the statistical analysis.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal