Abstract
Recent studies have demonstrated that the cellular contribution of the bone marrow to tumor neovascularization is highly complex. In this context, the extent to which bone marrow–derived cells incorporate as bona fide endothelial (nonhematopoietic) cells into perfused tumor vessels, or any new vessels formed postnatally (vasculogenesis), is unclear. To this end, we developed models to characterize local vessel–derived and bone marrow–derived endothelial cells (BMD-ECs). Then, we characterized the BMD-ECs based on a set of endothelial markers and morphology. Finally, we quantified their contribution to perfused blood vessels in tumors using transplanted as well as spontaneous primary and metastatic tumor models. We demonstrate that BMD-ECs incorporate in perfused tumor vessels, and that this contribution varies with organ site and mouse strain.
Introduction
The contribution of bone marrow–derived endothelial cells (BMD-ECs) to blood vessels in postnatal life (adult vasculogenesis) remains controversial. For example, BMD-EC involvement in tumor neovascularization has been reported to be significant1-5 to undetectable6-8 in some of the same tumor models.9 Other studies have identified hematopoietic cells incorporated into the endothelial lining of tumor vessels.10-12 We hypothesized that the reasons for these discordant findings may be the inability of some of the reported models to detect and distinguish both bone marrow– and local vessel–derived endothelial cells and the fact that most studies relied on ambigous markers to identify the endothelial-cell phenotype.9 Other potential confounding factors may result from the genetic deficiency or transgenic models used, tumor type and organ site.2,13 To address these issues, we quantified BMD-ECs in perfused blood vessels in tumors using bone marrow transplantation (BMT) in 2 strains of mice and 2 tumor models: isograft and spontaneous metastasis.
Study design
We quantified vasculogenesis with a set of markers for bone marrow–derived cells incorporated in perfused tumor blood vessels. To detect and analyze donor-derived cells, we used flow cytometry and fluorescence microscopy. In one transgenic mouse line, green fluorescence protein (GFP) was constitutively expressed in all cells (under β-actin promoter activation, Actb-Gfp/C57BL614 ); in another, GFP was expressed in cells upon Tie2 promoter activation (Tie2-Gfp/FVB15 ). We employed restorative BMT from these mice to wild-type (WT) nontransgenic syngeneic counterparts after lethal irradiation. Using the resulting chimeric mice (WT/Tie2-Gfp-BMT and WT/Actb-Gfp-BMT), we analyzed isografted and spontaneous primary tumors, as well as distant metastases. Then, using fluorescence confocal microscopy, we directly compared the incorporation of BMD-ECs and locally derived endothelial cells into lectin-perfused vessels (see supplemental methods, available on the Blood website; see the Supplemental Materials link at the top of the online article). We identified BMD-ECs by simultaneous cytoplasmic GFP expression and luminal-side lectin perfusion staining, and further confirmed BMD-EC identity by morphologic analysis (large, elongated nuclei; see detailed supplemental methods).
Results and discussion
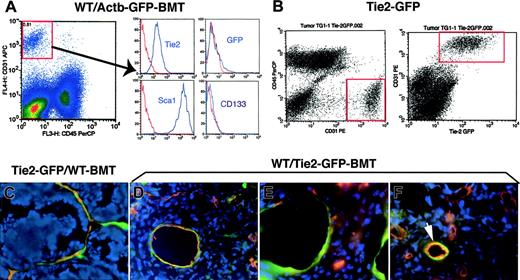
The contribution of BMD-ECs to blood vessels has been previously studied by immunohistochemistry in tumors grown in C57BL6 mice3,7,8 including in the WT/Actb-Gfp-BMT model.16 Therefore, we first analyzed BMDC phenotype by flow cytometry using single-cell suspensions obtained by collagenase digestion of subcutaneously grown Lewis lung carcinoma in WT/Actb-Gfp-BMT mice (see supplemental methods). The massive GFP+-cell infiltration consisted mostly of myelomonocytic (CD11b+CD45+) cells and of a small (∼5%) Sca1+-CD31–CD133–CD45– putative mesenchymal population (Figure S1A and not shown). The vast majority of tumor endothelial cells (CD31+Tie2+CD45–) were Sca1+ but CD133– and GFP–, indicating that they were mature, local tissue–derived endothelial cells (Figure 1A). A similar phenotype and minimal BMD-EC incorporation was detected by flow cytometry in cell suspensions obtained from spontaneous lung metastases arising after primary Lewis lung carcinoma resection (not shown). In addition, analyses of perfused vessels in 2 types of subcutaneously implanted syngeneic tumors (Lewis lung carcinoma or B16 melanoma), and Lewis lung carcinoma lung metastases in WT/Actb-Gfp-BMT mice showed minimal involvement of BMD-ECs in perfused tumor vessels (Table 1 and Figure S1B-C). Thus, our phenotypic and functional data indicate that vasculogenesis in these primary and metastatic tumors in genetically unmodified C57BL6 mice is marginal.
Analysis of GFP expression in tumor endothelial cells and BMD-ECs by fluorescence flow cytometry and microscopy in 2 GFP-transgenic mouse models: Tie2-2Gfp and Actb-Gfp. (A) Identification of tumor endothelial cells as CD31brightCD45– in single-cell suspensions of lung carcinoma tissue from WT/Actb-Gfp-BMT mice (left plot). The surface phenotype of these lung tumor endothelial cells was Tie2+Sca1+CD133–, and the vast majority of them were GFP– (ie, local vessel–derived). In red, isotype-matched IgG staining; for GFP, the control represents cell suspensions of tumors grown in wild-type mice. (B) Identification of tumor endothelial cells (CD31brightCD45–) in single-cell suspensions of mammary carcinoma tissue from Tie2-Gfp mice (left plot). All the Tie2+ cells (identified by detecting the Gfp reporter gene using flow cytometry) were also positive for CD31 (right plot). (C-F) Representative images of histochemical analysis of GFP+ cells in functional tumor vessels after perfusion staining using tomato lectin (shown in red; blue represents DAPI nuclear staining). (C) Mammary carcinoma vessels in Tie2-Gfp mice after BMT from nontransgenic FVB donors; most of the blood-perfused endothelial lining was composed of locally derived GFP+ cells. (D) Spontaneous metastasis of lung carcinoma after primary tumor resection in a WT/Tie2-Gfp-BMT mouse; BMD-ECs were detected in larger diameter-size blood vessels. (E) The new vessels formed shortly after mammary tumor-cell implantation under the pial surface of the brain in a WT/Tie2-Gfp-BMT mouse contained a high proportion of BMD-ECs. (F) Spontaneous squamous cell carcinoma vessels in a WT/Tie2-Gfp-BMT mouse; BMD-ECs were occasionally detected in perfused blood vessels (arrow). Functional endothelial cells in C-F were identified by Texas-Red–labeled streptavidin (Molecular Probes, Eugene, OR) staining of biotinylated-lypopersicon lectin (Vector Labs, Burlingame, CA) infused in the mice prior to sacrifice, and were imaged using a BX61WI confocal microscope (Olympus, Tokyo, Japan). The images were captured using Olympus FluoView software and were analyzed using Adobe Photoshop CS2 software (Adobe Systems, San Jose, CA). Images are 423 μm across in panels C, E, and F and 310 μm across in panel D, using Olympus 40×/1.00 NA (panels C, E, and F) and 60×/1.35 NA (panel D) oil objectives.
Analysis of GFP expression in tumor endothelial cells and BMD-ECs by fluorescence flow cytometry and microscopy in 2 GFP-transgenic mouse models: Tie2-2Gfp and Actb-Gfp. (A) Identification of tumor endothelial cells as CD31brightCD45– in single-cell suspensions of lung carcinoma tissue from WT/Actb-Gfp-BMT mice (left plot). The surface phenotype of these lung tumor endothelial cells was Tie2+Sca1+CD133–, and the vast majority of them were GFP– (ie, local vessel–derived). In red, isotype-matched IgG staining; for GFP, the control represents cell suspensions of tumors grown in wild-type mice. (B) Identification of tumor endothelial cells (CD31brightCD45–) in single-cell suspensions of mammary carcinoma tissue from Tie2-Gfp mice (left plot). All the Tie2+ cells (identified by detecting the Gfp reporter gene using flow cytometry) were also positive for CD31 (right plot). (C-F) Representative images of histochemical analysis of GFP+ cells in functional tumor vessels after perfusion staining using tomato lectin (shown in red; blue represents DAPI nuclear staining). (C) Mammary carcinoma vessels in Tie2-Gfp mice after BMT from nontransgenic FVB donors; most of the blood-perfused endothelial lining was composed of locally derived GFP+ cells. (D) Spontaneous metastasis of lung carcinoma after primary tumor resection in a WT/Tie2-Gfp-BMT mouse; BMD-ECs were detected in larger diameter-size blood vessels. (E) The new vessels formed shortly after mammary tumor-cell implantation under the pial surface of the brain in a WT/Tie2-Gfp-BMT mouse contained a high proportion of BMD-ECs. (F) Spontaneous squamous cell carcinoma vessels in a WT/Tie2-Gfp-BMT mouse; BMD-ECs were occasionally detected in perfused blood vessels (arrow). Functional endothelial cells in C-F were identified by Texas-Red–labeled streptavidin (Molecular Probes, Eugene, OR) staining of biotinylated-lypopersicon lectin (Vector Labs, Burlingame, CA) infused in the mice prior to sacrifice, and were imaged using a BX61WI confocal microscope (Olympus, Tokyo, Japan). The images were captured using Olympus FluoView software and were analyzed using Adobe Photoshop CS2 software (Adobe Systems, San Jose, CA). Images are 423 μm across in panels C, E, and F and 310 μm across in panel D, using Olympus 40×/1.00 NA (panels C, E, and F) and 60×/1.35 NA (panel D) oil objectives.
Quantification of BMD-EC-positive perfused tumor vessels using GFP-reporter mice
. | . | Percent GFP+ functional vessels . | . | |
|---|---|---|---|---|
| Tumor type . | Implantation site . | WT/Actb-Gfp-BMT (C57BL6)* . | WT/Tie2-Gfp-BMT (FVB)† . | |
| Lung adenocarcinoma | Subcutaneous | < 1%‡ (Lewis lung carcinoma) | 4.5 ± 1.6‡ (LA-P0297) | |
| Lung adenocarcinoma metastasis | Lung§ | < 1%∥ (Lewis lung carcinoma) | 10.6 ± 3.6∥¶ (LA-P0297) | |
| Melanoma | Subcutaneous | < 1%‡ (B16) | N/A | |
| Mammary carcinoma | Mammary fat pad/subcutaneous | N/A | 1.5 ± 0.3# (TG1-1)/1.3 ± 0.1# (MCa8) | |
| Mammary carcinoma metastasis | Brain | N/A | 58.4 ± 8.4#** (TG1-1) | |
. | . | Percent GFP+ functional vessels . | . | |
|---|---|---|---|---|
| Tumor type . | Implantation site . | WT/Actb-Gfp-BMT (C57BL6)* . | WT/Tie2-Gfp-BMT (FVB)† . | |
| Lung adenocarcinoma | Subcutaneous | < 1%‡ (Lewis lung carcinoma) | 4.5 ± 1.6‡ (LA-P0297) | |
| Lung adenocarcinoma metastasis | Lung§ | < 1%∥ (Lewis lung carcinoma) | 10.6 ± 3.6∥¶ (LA-P0297) | |
| Melanoma | Subcutaneous | < 1%‡ (B16) | N/A | |
| Mammary carcinoma | Mammary fat pad/subcutaneous | N/A | 1.5 ± 0.3# (TG1-1)/1.3 ± 0.1# (MCa8) | |
| Mammary carcinoma metastasis | Brain | N/A | 58.4 ± 8.4#** (TG1-1) | |
Percent values are shown ± SD obtained after counting GFP+ vessels for different mice (in at least 5 random regions of interest in 5 nonconsecutive sections) in frozen tumor tissue.
N/A indicates not available.
C57BL6 mice that received a bone marrow transplant from Actb-Gfp/C57BL6 mice following lethal irradiation.
FVB mice that received a bone marrow transplant from Tie2-Gfp/FVB mice following lethal irradiation.
Experimental group size was n = 3 mice.
Spontaneous metastases analyzed after primary tumor resection.
Experimental group size was n = 6 mice.
The percentage of BMD-EC-positive vessels was significantly increased in lung metastases in FVB mice compared with primary subcutaneous tumors in FVB mice (P < .05).
Experimental group size was n = 4 mice.
The percentage of BMD-EC-positive vessels was highly significantly increased in mammary carcinoma isografted in brain compared with mammary pad isografts in FVB mice (P < .01).
To distinguish BMD-ECs from local vessel–derived endothelial cells in FVB mice and independently quantify their contribution to blood vessels, we used Tie2-Gfp/FVB mice. Tie2 is a marker expressed on all endothelial cells, including in tumors,15 but also on hematopoietic cells.8,17 Moreover, the level of Tie2 expression in endothelial cells depends on vessel type.18 Therefore, we first confirmed that intrinsic Tie2-GFP expression is detectable in tumor endothelial cells by flow cytometric analysis of single-cell suspensions of mammary carcinoma tissue from orthotopically implanted tumors in Tie2-Gfp mice. We detected the colocalization of Tie2-Gfp expression on tumor CD31+CD45– endothelial cells (Figure 1B). Consistent with the flow cytometry data in control experiments using Tie2-Gfp mice, GFP was uniformly detectable on endothelial cells in perfused tumor vessels evaluated by confocal microscopy (Figures S2 and S3).
To measure the contribution of BMD-ECs, we quantified the number of GFP-positive vessels (ie, Tie2+lectin+ BMDCs) in tumors using 2 BMT models. All recipient mice were lethally irradiated and restorative BMTs were performed using bone marrow cells from Tie2-GfP to WT-FVB mouse recipients (WT/Tie2-Gfp-BMT mice) and, conversely, from WT-FVB to Tie2-Gfp mice (Tie2-Gfp/WT-BMT mice). Syngeneic mammary tumors were implanted in all mice after reconstitution of hematopoiesis (∼3 months after BMT). We quantified lectin-perfused mammary tumor (MCa8) vessels in Tie2-Gfp/WT-BMT mice and found that the vast majority (94% ± 1%, n = 4 mice) of vessels in subcutaneous tumors were locally derived Tie2-Gfp+CD11b– endothelial cells (Figure 1C and not shown). Conversely, in WT/Tie2-Gfp-BMT mice we detected a very small percentage of GFP+ endothelial cells vessels of tumors implanted subcutaneously or in the mammary fat pad (Table 1 and Figure S3B). A similar degree of BMD-EC incorporation was detected when we used a lung adenocarcinoma cell line LA-P0297, as well as a different mammary carcinoma line (TG1-1, Table 1).
Next, we measured BMD-EC contribution in spontaneous metastases in WT/Tie2-Gfp-BMT mice. BMD-ECs contributed significantly more to functional vessels in lung metastases than in the primary subcutaneous LA-P0297 tumors (P < .05, Table 1). BMD-ECs were more prevalent in larger tumor vessels in the lung (with an average diameter of GFP+ vessels of approximately 45 μm, compared with an average tumor vessel diameter of approximately 14 μm) unlike those in the subcutaneous space (with an average diameter of GFP+ vessels of approximately 16 μm) (Figure 1D).
To further explore the issue of site-dependent vasculogenesis, we injected TG1-1 mammary carcinoma cells superficially under the pial surface of the brain in WT/Tie2-Gfp-BMT mice. In this experimental model of breast cancer metastasis, blood-perfused GFP+CD31+ vessels comprised the majority (∼60%) of the tumor vessels (Figure 1E, Table 1, and Figure S3C-D). Thus, the extent of vasculogenesis in FVB mice did not seem to depend on the availability of local endothelial cells—as brain and lung tissues are highly vascularized compared with the skin—but rather on the specific tumor-stroma/microenvironment interactions.
Four aging WT/Tie2-Gfp-BMT mice developed spontaneous tumors. In a spontaneous mammary carcinoma, the contribution of BMD-ECs was detectable in approximately 20% of the total number of perfused blood vessels. Three other spontaneous tumors had detectable but lower BMD-EC contribution (5% or less) to endothelium (one hemangiosarcoma, one fibrosarcoma, and one squamous cell carcinoma; Figure 1F and not shown). It is unclear whether the BMD-EC contribution to tumor vessels in these aging mice was the result of an active recruitment for vasculogenesis or due to a long-term replenishment by bone marrow–derived cells of the host tissues.19
In summary, we employed models that allowed detection of both local endothelial cells and BMD-ECs in perfused blood vessels. By defining the endothelial cells based on their morphology, phenotype (ie, cell-surface markers), and function (ie, blood perfusion), we demostrate that the process of tumor neovascularization20 directly involves BMD-ECs. BMD-ECs incorporated into blood vessels with a high degree of heterogeneity between tumor types, in line with emerging human data.21 BMD-EC incorporation in vessels also varied with tumor site and with mouse strain, consistent with a recent report that documented the heterogeneity in concentration of blood circulating endothelial precursors among mouse strains.13 The BMD-ECs may also derive from hematopoietic stem cells with “hemangioblast” activity14 or from multipotent progenitor cells.22 Collectively, these findings offer direct evidence for vasculogenesis during isografted tumor growth and metastasis in genetically unaltered mice. Many tumors are associated with extensive bone marrow–derived cell infiltration8,10,12,23,24 and thus, characterization of the roles of different subsets of bone marrow–derived cells in tumor development, progression, metastasis, and response to treatment may identify new therapeutic targets.
Prepublished online as Blood First Edition Paper, December 8, 2005; DOI 10.1182/blood-2005-08-3210.
Supported by a National Cancer Institute grants (PO1-CA80124 and RO1-CA115767 to R.K.J., and RO1-CA96915 to D.F.), an American Association for Cancer Research (AACR)–Genentech BioOncology Career Development Award grant (D.G.D.), and the Swiss National Science Foundation (J.Y.P.). D.G.D. is a Cancer Research Institute fellow.
D.G.D. designed research, performed research, analyzed data, and wrote the paper; K.S.C., S.V.K., and J.Y.P. performed research and analyzed data; D.F. designed research and analyzed data; D.T.S. contributed vital reagents and analytic tools; and R.K.J. designed research, contributed vital reagents and analytic tools, and edited the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to acknowledge the outstanding technical assistance of J. Kahn, M. Riley, and S. Roberge with animal models; N. Hosseini, M. Kim, and C. Smith for imunohistochemical analyses; and R. Klein for flow cytometry studies. Many thanks to Drs G. Adams, A. Kadambi, K. Kozak, B. Stoll, and H. Suit for helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal