Abstract
The outcomes of 293 patients with leukemia undergoing HLA-identical sibling (n = 158) or related HLA-mismatched (n = 135) hematopoietic cell transplantation (HCT) performed during the same time period were compared. Patients received BUCY2 in HLA-identical sibling HCT or BUCY2 + ATG in mismatched HCT as conditioning regimens, followed by unmanipulated marrow and/or peripheral blood (PB) transplantation. All patients achieved full engraftment. The cumulative incidences of grades II to IV acute graft-versus-host disease (aGVHD) in the matched and mismatched cohorts were 32% (CI, 25%-39%) versus 40% (CI, 32%-48%, P = .13), respectively, with the relative risk (RR) = 0.64 (95% CI, 0.43-0.94), P = .02. The incidence of chronic GVHD did not differ significantly between the cohorts (P = .97). Two-year incidences of treatment-related mortality and relapse for matched versus mismatched were 14% (range, 9%-20%) versus 22% (range, 15%-29%) with P = .10 and 13% (range, 8%-19%) versus 18% (range, 10%-27%) with P = .40, respectively. Two-year adjusted leukemia-free survival (LFS) and overall survival were 71% (range, 63%-78%) versus 64% (range, 54%-73%) with P = .27 and 72% (range, 64%-79%) versus 71% (range, 62%-77%) with P = .72, respectively. Multivariate analyses showed that only advanced disease stage and a diagnosis of acute leukemia had increased risk of relapse, treatment failure, and overall mortality. In summary, HCT performed with related HLA-mismatched donors is a feasible approach with acceptable outcomes.
Introduction
Currently, allogeneic hematopoietic cell transplantation (HCT) is the only curative therapy for a majority of malignant hematologic diseases. But lack of HLA-matched sibling or unrelated donors has restricted its application, particularly as family sizes shrink. Related HLA-mismatched HCT is a viable alternative since almost every patient has at least a haplotype-sharing parent, child, or sibling available to donate stem cells, but it has been limited by high risk of severe graft-versus-host disease (GVHD), graft rejection, life-threatening infections, and relapse—all of which are partially caused by intensive immunosuppressive therapy and T-cell depletion.1-4 T-cell add-back after purified HCT decreases the risk of complications after transplantation but cannot fully solve these problems.5-7 Since 1997, availability of CD34+ selection and high doses of mobilized peripheral blood stem cells (PBSCs) have stimulated a “megadose” HCT approach to improve engraftment and results, particularly in HLA-mismatched haploidentical transplantations.8-13 But only about 80% of all grafts can reach the level of cell doses required.14,15 Additionally, the relatively high cost of ex vivo CD34+ cell selection makes it difficult to use this approach widely.
Family mismatched donors at least share complete haplotypes, including examined and unexamined HLA and minor histocompatibility loci. The potential donor pool for related HLA-mismatched/haploidentical donors may be quite large. The parents, siblings, children, and even cousins of the patient may be acceptable donors. Particularly in those patients with advanced or resistant disease in need of urgent HCT, the ready availability of a family haploidentical donor can be beneficial compared with an extended search for a matched unrelated donor. Our goal in this study was to investigate the feasibility and clinical value of this approach in the treatment of otherwise incurable hematologic malignancies. We included children and adult recipients with leukemia who received identical sibling donor transplants if a matched sibling donor was available, or mismatched/haploidentical transplants from a family donor; all transplantations were performed during the same time period.
Patients, materials, and methods
Patients
Consecutive patients (N = 293) younger than 50 years who received an allogeneic hematopoietic cell transplant for leukemia from either an HLA-identical sibling if available (n = 158) or a mismatched family donor (n = 135) between January 25, 2002, and July 31, 2004, at Peking University Institute of Hematology, People's Hospital, or at Beijing Dao-Pei Hospital, were analyzed. All patients were treated by the same attending physician staff. The protocols were approved by the institutional review board (IRB) at Peking University Institute of Hematology, and all patients or their guardians signed consent forms approved by the IRB. Patients were not eligible for HCT if they had severe liver or renal disease, corrected pulmonary diffusion capacity less than 35%, cardiac ejection fraction lower than 40%, Karnofsky performance status less than 80, or any active infections. Patient characteristics are summarized in Table 1. Patients were classified as having early, intermediate, or advanced disease stages based on the status of their leukemia at the time of HCT. Early-stage patients included (1) chronic myeloid leukemia (CML) patients in chronic phase (CP) and (2) acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) in first remission (CR1), while intermediate stage included (1) CML in accelerated phase (AP), (2) AML or ALL in second remission (CR2), and (3) myelodysplastic syndrome (MDS) with refractory anemia. Advanced disease stage included (1) advanced or resistant AML or ALL, (2) MDS–refractory anemia with excess of blasts (RAEB) or MDS-AML, and (3) CML in blast phase (BP). Pretransplantation comorbidities were noted using the Charlson Comorbidity Index (CCI)16,17 and showed no significant difference between the 2 cohorts. Pretransplantation cytomegalovirus (CMV) serologic analysis showed that low risk (recipient [R]–, donor [D]–), intermediate risk (R–, D+), and high risk (R+) patients for CMV reactivity after HCT were 3.9%, 3.9%, and 92.2%, respectively, in the matched HCT group and 11.4%, 6.8%, and 81.8%, respectively, in the mismatched HCT group, with no significant difference between the 2 groups (P = .25). Follow-up for all patients went through February 28, 2005.
Patient, donor, and graft characteristics
Variable . | HLA-identical sibling HCT . | Related mismatched HCT . | P . |
|---|---|---|---|
| Median age, y (range) | 37 (5-50) | 24 (3-50) | < .001 |
| Age 0 to 20 y, no. (%) | 8 (5) | 51 (38) | < .001 |
| Age 21 to 35 y, no. (%) | 65 (41) | 51 (38) | |
| Age older than 35 y, no. (%) | 85 (54) | 33 (24) | |
| Disease, no. (%) | .05 | ||
| CML | 68 (43) | 42 (31) | |
| AML | 39 (25) | 30 (22) | |
| ALL | 39 (25) | 53 (39) | |
| MDS | 12 (8) | 10 (7) | |
| Disease status, no. (%) | .07 | ||
| Early | 100 (63) | 68 (50) | |
| Intermediate | 33 (21) | 35 (26) | |
| Advanced | 25 (16) | 32 (24) | |
| Conditioning regimens, no. (%) | — | ||
| BUCY | 147 (93) | 0 (0) | |
| BUCY + ATG | 11 (7) | 135 (100) | |
| Donor-patient sex match, no. (%) | .38 | ||
| MM | 54 (34) | 37 (27) | |
| MF | 30 (19) | 21 (16) | |
| FM | 54 (34) | 55 (41) | |
| FF | 20 (13) | 22 (16) | |
| ABO match, no. (%) | .12 | ||
| Matched | 85 (54) | 68 (50) | |
| Minor mismatched | 23 (15) | 32 (24) | |
| Major mismatched | 50 (32) | 35 (26) | |
| Donor-patient relationship, no. (%) | — | ||
| Mother to child | 0 (0) | 60 (44) | |
| Father to child | 0 (0) | 21 (16) | |
| Child to parent | 0 (0) | 13 (10) | |
| Sibling | 158 (100) | 37 (27) | |
| Cousin | 0 (0) | 4 (3) | |
| Graft type, no. (%) | < .001 | ||
| BM + PB | 103 (65) | 130 (96) | |
| BM alone | 31 (20) | 4 (3) | |
| PB alone | 24 (15) | 1 (1) | |
| G-CSF use after HCT, no. (%) | 85 (54) | 132 (98) | < .001 |
| Median MNCs, × 108/kg (range) | 6.2 (2.3-13.6) | 7.5 (3.1-16.3) | < .001 |
| MNCs less than 7 × 108/kg, no. (%) | 106 (67) | 44 (33) | < .001 |
| MNCs 7 × 108/kg or more, no. (%) | 52 (33) | 91 (67) | |
| Median CD34+ count, × 106/kg (range)* | 2.4 (0.3-6.7) | 2.3 (0.2-9.7) | .79 |
| CD34+ count less than 2 × 106/kg, no. (%) | 46 (29) | 51 (38) | .14 |
| CD34+ count 2 × 106/kg or more, no. (%) | 90 (57) | 73 (54) | |
| CD34+ count NA, no. (%) | 22 (14) | 11 (8) | |
| Median CD3+ count, × 106/kg (range)† | 187 (9-676) | 177 (15-991) | .55 |
| CD3+ count less than 100 × 106/kg, no. (%) | 31 (20) | 12 (9) | .001 |
| CD3+ count 100 to 200 × 106/kg, no. (%) | 41 (26) | 63 (47) | |
| CD3+ count 200 × 106/kg or more, no. (%) | 63 (40) | 45 (33) | |
| CD3+ count NA, no. (%) | 23 (15) | 15 (11) | |
| Median CD4+ count, × 106/kg (range)† | 109 (5-489) | 98 (4-527) | .34 |
| CD4+ count less than 100 × 106/kg, no. (%) | 57 (36) | 62 (46) | .14 |
| CD4+ count 100 to 200 × 106/kg, no. (%) | 57 (36) | 49 (36) | |
| CD4+ count 200 × 106/kg or more, no. (%) | 21 (13) | 9 (7) | |
| CD4+ count NA, no. (%) | 23 (15) | 15 (11) | |
| Median CD8+, × 106/kg (range)† | 81 (4-382) | 76 (8-497) | .65 |
| CD8+ count less than 50 × 106/kg, no. (%) | 40 (25) | 33 (24) | .60 |
| CD8+ count 50 to 100 × 106/kg, no. (%) | 40 (25) | 43 (32) | |
| CD8+ count 100 × 106/kg or more, no. (%) | 55 (35) | 44 (33) | |
| CD8+ count NA, no. (%) | 23 (15) | 15 (11) | |
| Median follow-up time among living patients, mo (range)‡ | 17 (7-35) | 14 (6-35) | .01 |
Variable . | HLA-identical sibling HCT . | Related mismatched HCT . | P . |
|---|---|---|---|
| Median age, y (range) | 37 (5-50) | 24 (3-50) | < .001 |
| Age 0 to 20 y, no. (%) | 8 (5) | 51 (38) | < .001 |
| Age 21 to 35 y, no. (%) | 65 (41) | 51 (38) | |
| Age older than 35 y, no. (%) | 85 (54) | 33 (24) | |
| Disease, no. (%) | .05 | ||
| CML | 68 (43) | 42 (31) | |
| AML | 39 (25) | 30 (22) | |
| ALL | 39 (25) | 53 (39) | |
| MDS | 12 (8) | 10 (7) | |
| Disease status, no. (%) | .07 | ||
| Early | 100 (63) | 68 (50) | |
| Intermediate | 33 (21) | 35 (26) | |
| Advanced | 25 (16) | 32 (24) | |
| Conditioning regimens, no. (%) | — | ||
| BUCY | 147 (93) | 0 (0) | |
| BUCY + ATG | 11 (7) | 135 (100) | |
| Donor-patient sex match, no. (%) | .38 | ||
| MM | 54 (34) | 37 (27) | |
| MF | 30 (19) | 21 (16) | |
| FM | 54 (34) | 55 (41) | |
| FF | 20 (13) | 22 (16) | |
| ABO match, no. (%) | .12 | ||
| Matched | 85 (54) | 68 (50) | |
| Minor mismatched | 23 (15) | 32 (24) | |
| Major mismatched | 50 (32) | 35 (26) | |
| Donor-patient relationship, no. (%) | — | ||
| Mother to child | 0 (0) | 60 (44) | |
| Father to child | 0 (0) | 21 (16) | |
| Child to parent | 0 (0) | 13 (10) | |
| Sibling | 158 (100) | 37 (27) | |
| Cousin | 0 (0) | 4 (3) | |
| Graft type, no. (%) | < .001 | ||
| BM + PB | 103 (65) | 130 (96) | |
| BM alone | 31 (20) | 4 (3) | |
| PB alone | 24 (15) | 1 (1) | |
| G-CSF use after HCT, no. (%) | 85 (54) | 132 (98) | < .001 |
| Median MNCs, × 108/kg (range) | 6.2 (2.3-13.6) | 7.5 (3.1-16.3) | < .001 |
| MNCs less than 7 × 108/kg, no. (%) | 106 (67) | 44 (33) | < .001 |
| MNCs 7 × 108/kg or more, no. (%) | 52 (33) | 91 (67) | |
| Median CD34+ count, × 106/kg (range)* | 2.4 (0.3-6.7) | 2.3 (0.2-9.7) | .79 |
| CD34+ count less than 2 × 106/kg, no. (%) | 46 (29) | 51 (38) | .14 |
| CD34+ count 2 × 106/kg or more, no. (%) | 90 (57) | 73 (54) | |
| CD34+ count NA, no. (%) | 22 (14) | 11 (8) | |
| Median CD3+ count, × 106/kg (range)† | 187 (9-676) | 177 (15-991) | .55 |
| CD3+ count less than 100 × 106/kg, no. (%) | 31 (20) | 12 (9) | .001 |
| CD3+ count 100 to 200 × 106/kg, no. (%) | 41 (26) | 63 (47) | |
| CD3+ count 200 × 106/kg or more, no. (%) | 63 (40) | 45 (33) | |
| CD3+ count NA, no. (%) | 23 (15) | 15 (11) | |
| Median CD4+ count, × 106/kg (range)† | 109 (5-489) | 98 (4-527) | .34 |
| CD4+ count less than 100 × 106/kg, no. (%) | 57 (36) | 62 (46) | .14 |
| CD4+ count 100 to 200 × 106/kg, no. (%) | 57 (36) | 49 (36) | |
| CD4+ count 200 × 106/kg or more, no. (%) | 21 (13) | 9 (7) | |
| CD4+ count NA, no. (%) | 23 (15) | 15 (11) | |
| Median CD8+, × 106/kg (range)† | 81 (4-382) | 76 (8-497) | .65 |
| CD8+ count less than 50 × 106/kg, no. (%) | 40 (25) | 33 (24) | .60 |
| CD8+ count 50 to 100 × 106/kg, no. (%) | 40 (25) | 43 (32) | |
| CD8+ count 100 × 106/kg or more, no. (%) | 55 (35) | 44 (33) | |
| CD8+ count NA, no. (%) | 23 (15) | 15 (11) | |
| Median follow-up time among living patients, mo (range)‡ | 17 (7-35) | 14 (6-35) | .01 |
Except where otherwise noted, n = 158 for HLA-identical sibling donors, and n = 135 for related mismatched donors.
NA indicates that data were not available; —, data not comparable.
n = 136 for HLA-identical sibling donors, and n = 124 for related mismatched donors.
n = 135 for HLA-identical sibling donors, and n = 120 for related mismatched donors.
n = 119 for HLA-identical sibling donors, and n = 94 for related mismatched donors.
Donor source and HLA disparity
Initially, HLA-A, HLA-B, and HLA-C typing was performed by serology, while intermediate resolution DNA typing and HLA-DRB1, HLA-DQB1, and HLA-DPB1 typing were done at the allele level by high-resolution techniques. However, since 2003, all typing has been performed using high-resolution DNA techniques (56 donor-recipient pairs in 135 HLA-mismatched cohorts were included). The reagents (Special Monoclonal Tray-Asian HLA Class I and Micro SSP HLA Class I and II ABDR DNA Typing Tray; One Lambda, Canoga Park, CA) were commercially imported and approved by the FDA. One hundred fifty-eight sibling donor-recipient pairs were fully HLA matched. The HLA disparities in the mismatched cohort are shown in Table 2.
Donor-recipient relationships and histocompatibility in the HLA-mismatched group
. | . | Donor, no. (%) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | Total, no. (%) . | Mother . | Father . | Sibling . | Offspring . | Cousin . | ||||
| Number | 135 (100) | 60 (44) | 21 (16) | 37 (27) | 13 (10) | 4 (3) | ||||
| HLA-antigen class mismatched | ||||||||||
| Class I | 32 (24) | 10 (17) | 10 (48) | 9 (24) | 1 (8) | 2 (50) | ||||
| Class II | 9 (7) | 4 (6) | 1 (4.8) | 4 (11) | 0 (0) | 0 (0) | ||||
| Classes I and II | 94 (70) | 46 (77) | 10 (48) | 24 (65) | 12 (92) | 2 (50) | ||||
| No. of HLA-antigen mismatched | ||||||||||
| 1 Ag | 21 (16) | 7 (12) | 4 (19) | 8 (22) | 1 (8) | 1 (25) | ||||
| 2 Ag | 62 (46) | 24 (40) | 15 (71) | 17 (46) | 3 (23) | 3 (75) | ||||
| 3 Ag | 52 (39) | 29 (48) | 2 (10) | 12 (32) | 9 (69) | 0 (0) | ||||
| No. and location of mismatched loci | ||||||||||
| Only at HLA-A | 6 (4) | 1 (2) | 3 (14) | 1 (3) | 0 (0) | 1 (25) | ||||
| Only at HLA-B | 6 (4) | 2 (3) | 0 (0) | 3 (8) | 1 (8) | 0 (0) | ||||
| Only at HLA-DRB1 | 9 (7) | 4 (7) | 1 (4) | 4 (11) | 0 (0) | 0 (0) | ||||
| Mismatches at HLA-A, -B | 20 (15) | 7 (12) | 7 (33) | 5 (14) | 0 (0) | 1 (25) | ||||
| Mismatches at HLA-A, -DRB1 | 11 (8) | 2 (3) | 2 (10) | 7 (19) | 0 (0) | 0 (0) | ||||
| Mismatches at HLA-B, -DRB1 | 31 (23) | 15 (25) | 6 (29) | 5 (13) | 3 (23) | 2 (50) | ||||
| Mismatches at HLA-A, -B, and -DRB1 | 52 (39) | 29 (48) | 2 (10) | 12 (32) | 9 (69) | 0 (0) | ||||
. | . | Donor, no. (%) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | Total, no. (%) . | Mother . | Father . | Sibling . | Offspring . | Cousin . | ||||
| Number | 135 (100) | 60 (44) | 21 (16) | 37 (27) | 13 (10) | 4 (3) | ||||
| HLA-antigen class mismatched | ||||||||||
| Class I | 32 (24) | 10 (17) | 10 (48) | 9 (24) | 1 (8) | 2 (50) | ||||
| Class II | 9 (7) | 4 (6) | 1 (4.8) | 4 (11) | 0 (0) | 0 (0) | ||||
| Classes I and II | 94 (70) | 46 (77) | 10 (48) | 24 (65) | 12 (92) | 2 (50) | ||||
| No. of HLA-antigen mismatched | ||||||||||
| 1 Ag | 21 (16) | 7 (12) | 4 (19) | 8 (22) | 1 (8) | 1 (25) | ||||
| 2 Ag | 62 (46) | 24 (40) | 15 (71) | 17 (46) | 3 (23) | 3 (75) | ||||
| 3 Ag | 52 (39) | 29 (48) | 2 (10) | 12 (32) | 9 (69) | 0 (0) | ||||
| No. and location of mismatched loci | ||||||||||
| Only at HLA-A | 6 (4) | 1 (2) | 3 (14) | 1 (3) | 0 (0) | 1 (25) | ||||
| Only at HLA-B | 6 (4) | 2 (3) | 0 (0) | 3 (8) | 1 (8) | 0 (0) | ||||
| Only at HLA-DRB1 | 9 (7) | 4 (7) | 1 (4) | 4 (11) | 0 (0) | 0 (0) | ||||
| Mismatches at HLA-A, -B | 20 (15) | 7 (12) | 7 (33) | 5 (14) | 0 (0) | 1 (25) | ||||
| Mismatches at HLA-A, -DRB1 | 11 (8) | 2 (3) | 2 (10) | 7 (19) | 0 (0) | 0 (0) | ||||
| Mismatches at HLA-B, -DRB1 | 31 (23) | 15 (25) | 6 (29) | 5 (13) | 3 (23) | 2 (50) | ||||
| Mismatches at HLA-A, -B, and -DRB1 | 52 (39) | 29 (48) | 2 (10) | 12 (32) | 9 (69) | 0 (0) | ||||
Conditioning regimen
Conditioning therapy was modified BUCY2 in matched sibling transplantations consisting of cytarabine (2 g/m2 per day) intravenously on days –10 to –9; busulfan (4 mg/kg per day) orally on days –8 to –6; cyclophosphamide (1.8 g/m2 per day) intravenously on days –5 to –4; and Me-CCNU (250 mg/m2) orally once on day –3. In HLA-mismatched HCT, patients received the BUCY2 regimen consisting of a higher dose of cytarabine (4 g/m2 per day) intravenously on days –10 to –9, but otherwise an identical regimen to the HLA-matched patients, along with ATG (thymoglobuline, 2.5 mg/kg per day; Sang Stat, Lyon, France, now marketed by Genzyme, Cambridge, MA) intravenously for 4 consecutive days, on days –5 to –2.
Collection of hematopoietic cells
Donor BM and/or PB cells were collected using standard mobilization protocols. G-CSF (5 μg/kg per day; filgrastim) was used to mobilize bone marrow (G-BM) and peripheral blood (G-PB). Both G-BM (harvested on day 0, after 4 days of G-CSF) and G-PB (harvested on day 1, after 5 days of G-CSF) cells were harvested. In general, one leukapheresis was performed per donor to yield sufficient cells along with the bone marrow graft. Patients who received only G-PB received 2 days of leukapheresis collections from their donors. Six patients in the G-BMPB group also received a second leukapheresis PB graft on day 2 to increase the cell dose. Data on the composition of the G-BM (n = 35), G-PB (n = 25), and G-BMPB (n = 233) grafts are shown in Table 1 and Table 3.
Comparison of graft composition
Cells . | G-BMPB . | G-BM . | G-PB . | P . |
|---|---|---|---|---|
| MNCs, × 108/kg* | 7.54 (3.1-16.3) | 5 (2.31-11.44) | 6.2 (4.4-8) | < .001 |
| CD34+, × 106/kg† | 2.8 (0.2-9.7) | 2.3 (0.28-5.95) | 2.4 (0.7-5.65) | .002 |
| CD3+, × 106/kg | 217 (15-991) | 84 (9.13-676) | 306 (39.9-638) | < .001 |
| CD4+, × 106/kg | 124 (4.19-527) | 62 (4.59-489) | 166 (23.3-370) | < .001 |
| CD8+, × 106/kg | 104 (6.8-497)‡ | 50 (3.9-210)‡ | 120 (12.6-266) | < .001 |
Cells . | G-BMPB . | G-BM . | G-PB . | P . |
|---|---|---|---|---|
| MNCs, × 108/kg* | 7.54 (3.1-16.3) | 5 (2.31-11.44) | 6.2 (4.4-8) | < .001 |
| CD34+, × 106/kg† | 2.8 (0.2-9.7) | 2.3 (0.28-5.95) | 2.4 (0.7-5.65) | .002 |
| CD3+, × 106/kg | 217 (15-991) | 84 (9.13-676) | 306 (39.9-638) | < .001 |
| CD4+, × 106/kg | 124 (4.19-527) | 62 (4.59-489) | 166 (23.3-370) | < .001 |
| CD8+, × 106/kg | 104 (6.8-497)‡ | 50 (3.9-210)‡ | 120 (12.6-266) | < .001 |
Values represent median, with range in parentheses. Unless otherwise indicated, n = 205 for G-BMPB, n = 31 for G-BM, and n = 19 for G-PB.
n = 233 for G-BMPB, n = 35 for G-BM, and n = 25 for G-PB.
n = 209 for G-BMPB, n = 31 for G-BM, and n = 20 for G-PB.
G-BMPB versus G-BM in CD8+ group (P = .371).
Evaluation of engraftment and transplantation-related toxicity
Neutrophil engraftment was defined as absolute neutrophil count (ANC) of 0.5 × 109/L or more for 3 consecutive days and platelet engraftment, as 20 × 109/L or more for 5 consecutive days without transfusion. Transplantation-related toxicity (TRT) was evaluated by common toxicity criteria set by the National Cancer Institute (NCIC, www.ecog.org/general/ctc.pdf). Time of onset of grades III to IV toxicities was defined as occurring within 40 days after HCT. Organ damage due to GVHD and/or infectious complications was excluded.
GVHD prophylaxis and management
All patients were given a combination of cyclosporine (CSP), a short course of methotrexate (MTX), and MMF. On day +1, MTX (15 mg/m2) was administered intravenously and then 10 mg/m2 was given on days +3, +6, and +11 after transplantation. CSP (2.5 mg/kg twice a day) intravenously was started on day –10 and continued until patients were able to tolerate oral medication. Then CSP (3.25 mg/kg) was given orally twice a day with trough levels targeted at 150 to 250 ng/mL during the first 40 days and then tapered, taking about 60 days to be fully discontinued in the high-risk patients. MMF (7.5 mg/kg twice a day) was begun on day –10 and tapered on day 14 in the HLA-matched patients. But in the mismatched patients, tapering of MMF was delayed until beginning on days 30 to 80, based on the presence or absence of severe GVHD, infectious diseases, and relapse risk. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were defined according to Fred Hutchinson Cancer Research Center (FHCRC) criteria.18-20 GVHD was treated with 1 to 2 mg/kg per day of prednisolone equivalents and resumption of full-dose CSP administration. Second-line immunosuppressive therapy such as tacrolimus (FK506), MMF, and CD25 monoclonal antibody (daclizumab; Roche, Basel, Switzerland) or MTX was given for steroid refractory aGVHD.
Infection prevention and surveillance
All patients received prophylactic antibiotics when the ANC was less than 1 × 109/L. Fluconazole was given to all patients from day –5 to engraftment. Trimethoprim sulfamethoxazole was administered for prophylaxis of Pneumocystis carinii infection. Acyclovir was given orally from days –10 to +30 and ganciclovir (5 mg/kg twice daily) was routinely administrated intravenously from days –10 to –2. Patients were monitored weekly by CMV pp65 antigenemia test, and CMV-positive patients were treated with either ganciclovir or foscarnet. CMV-related interstitial pneumonia (IPn) was defined according to reported criteria.21 Human herpes virus 6 (HHV6), HHV7, and adenovirus were tested by polymerase chain reaction (PCR) and herpes simplex virus (HSV) and Epstein-Barr virus (EBV), by enzyme-linked immunosorbent assay (ELISA). Surveillance for bacterial, fungal, Pneumocystis carinii, and other viral infections was based on clinical requirements. Blood products were irradiated to 2500 cGy. CMV-seronegative recipients received leucodepleted and irradiated blood products. When fever, severe mucositis, or new infections occurred while on prophylaxis, additional agents were added based on clinical status and results of pathogen reports.
Chimerism analyses and minimal residual disease (MRD) monitor
Chimerism was evaluated on recipient BM cells usually on days +30, +180, and +365 after HCT by cytogenetic G-banding or fluorescence in situ hybridization (FISH). Sex-matched donor-recipient chimerism was assessed by using PCR-based analyses of polymorphic minisatellite or microsatellite regions (variable number tandem repeat [VNTR]). HLA typing was performed for patients after mismatched HCT. As a rule, PCR assay of Bcr-Abl, IgH, WT-1, and ETO according to the type of leukemia was used for minimal residual disease monitoring after HCT.
Donor lymphocyte infusion (DLI)
DLI was given when patients without aGVHD had evidence of recipient chimerism or MRD detected by molecular, cytogenetic, or hematologic methods, following a trial of immunosuppressant withdrawal. G-CSF–mobilized PB was used for DLI. CD3+ cell dose was initially administered at 1 × 105 cells/kg in the mismatched transplantation cohort and 1 × 107 cells/kg in the matched sibling transplantation cohort. Ten high-risk patients who underwent matched and 8 who underwent mismatched HCT received DLI for prophylactic purposes, while therapeutic DLI was performed in 11 patients receiving matched and 14 receiving mismatched transplants. Four patients in the matched and 4 in the mismatched groups also received G-CSF–mobilized DLI due to persistent thrombocytopenia.
Statistical analyses
Patient-, disease-, and transplant-related variables for patients receiving HLA-identical sibling and mismatched related donor transplants were compared using chi-square statistics for categoric variables and the Kruskal-Wallis test for continuous variables. Univariate probabilities of ANC and platelet engraftment, CMV infection, aGVHD and cGVHD, treatment-related mortality (TRM), and relapse were calculated using cumulative incidence curves to accommodate competing risks.22 Variables considered in multivariate analysis are shown in Table 1. The Cox proportional hazards model was used in multivariate analyses for outcomes given in Table 4. First, the test indicated that the proportionality assumptions hold. The final multivariate models were built using a forward stepwise model selection approach. Each model contained the main effect for mismatched related versus sibling-matched groups since it is the main interest of this study. The potential interaction between main effect and all significant covariates was tested. No interactions were detected. Finally, adjusted probabilities of LFS and survival were calculated using the multivariate models, stratified on type of transplantation and weighted by the pooled sample proportion value for each prognostic factor.23 These adjusted probabilities estimate likelihood of outcomes in populations with similar prognostic factors.
Multivariate analysis for aGVHD, cGVHD, TRM, relapse, treatment failure, and overall survival
Outcome . | RR (95% CI) . | P . |
|---|---|---|
| aGVHD | ||
| Mismatch related vs HLA-identical sibling | 1.57 (1.06-2.33) | .024 |
| Other significant risk factors | ||
| Disease stage before transplantation | ||
| Advanced vs early/intermediate | 1.68 (1.09-2.60) | .020 |
| ABO blood group | ||
| Matched | 1.00 | .033† |
| Minor mismatched | 0.42 (0.22-0.81) | .009 |
| Major mismatched | 0.93 (0.61-1.42) | .733 |
| cGVHD | ||
| Mismatch related vs HLA-identical sibling | 0.99 (0.72-1.38) | .969 |
| TRM* | ||
| Mismatch related vs HLA-identical sibling | 1.75 (0.99-3.09) | .054 |
| Relapse | ||
| Mismatch related vs HLA-identical sibling | 0.86 (0.45-1.65) | .653 |
| Other significant risk factors | ||
| Disease stage before transplantation | ||
| Advanced vs early/intermediate | 5.69 (2.92-11.09) | < .001 |
| Disease type | ||
| ALL | 1.00 | .002† |
| AML | 0.75 (0.36-1.56) | .436 |
| CML | 0.24 (0.09-0.59) | .002 |
| MDS | 0.12 (0.03-0.53) | .005 |
| Treatment failure | ||
| Mismatch related vs HLA-identical sibling | 1.23 (0.80-1.89) | .351 |
| Other significant risk factors | ||
| Disease stage before transplantation | ||
| Advanced vs early/intermediate | 3.23 (1.99-5.24) | < .001 |
| Disease type | ||
| ALL | 1.00 | .015† |
| AML | 0.92 (0.55-1.57) | .771 |
| CML | 0.55 (0.33-0.94) | .027 |
| MDS | 0.29 (0.12-0.74) | .010 |
| Overall survival | ||
| Mismatch related vs HLA-identical sibling | 1.12 (0.71-1.76) | .620 |
| Other significant risk factors | ||
| Disease stage before transplantation | ||
| Advanced vs early/intermediate | 3.30 (2.00-5.45) | < .001 |
| Disease type | ||
| ALL | 1.00 | .028† |
| AML | 0.85 (0.49-1.50) | .584 |
| CML | 0.57 (0.33-0.98) | .043 |
| MDS | 0.27 (0.10-0.74) | .011 |
Outcome . | RR (95% CI) . | P . |
|---|---|---|
| aGVHD | ||
| Mismatch related vs HLA-identical sibling | 1.57 (1.06-2.33) | .024 |
| Other significant risk factors | ||
| Disease stage before transplantation | ||
| Advanced vs early/intermediate | 1.68 (1.09-2.60) | .020 |
| ABO blood group | ||
| Matched | 1.00 | .033† |
| Minor mismatched | 0.42 (0.22-0.81) | .009 |
| Major mismatched | 0.93 (0.61-1.42) | .733 |
| cGVHD | ||
| Mismatch related vs HLA-identical sibling | 0.99 (0.72-1.38) | .969 |
| TRM* | ||
| Mismatch related vs HLA-identical sibling | 1.75 (0.99-3.09) | .054 |
| Relapse | ||
| Mismatch related vs HLA-identical sibling | 0.86 (0.45-1.65) | .653 |
| Other significant risk factors | ||
| Disease stage before transplantation | ||
| Advanced vs early/intermediate | 5.69 (2.92-11.09) | < .001 |
| Disease type | ||
| ALL | 1.00 | .002† |
| AML | 0.75 (0.36-1.56) | .436 |
| CML | 0.24 (0.09-0.59) | .002 |
| MDS | 0.12 (0.03-0.53) | .005 |
| Treatment failure | ||
| Mismatch related vs HLA-identical sibling | 1.23 (0.80-1.89) | .351 |
| Other significant risk factors | ||
| Disease stage before transplantation | ||
| Advanced vs early/intermediate | 3.23 (1.99-5.24) | < .001 |
| Disease type | ||
| ALL | 1.00 | .015† |
| AML | 0.92 (0.55-1.57) | .771 |
| CML | 0.55 (0.33-0.94) | .027 |
| MDS | 0.29 (0.12-0.74) | .010 |
| Overall survival | ||
| Mismatch related vs HLA-identical sibling | 1.12 (0.71-1.76) | .620 |
| Other significant risk factors | ||
| Disease stage before transplantation | ||
| Advanced vs early/intermediate | 3.30 (2.00-5.45) | < .001 |
| Disease type | ||
| ALL | 1.00 | .028† |
| AML | 0.85 (0.49-1.50) | .584 |
| CML | 0.57 (0.33-0.98) | .043 |
| MDS | 0.27 (0.10-0.74) | .011 |
Based on final model, a GVHD and cGVHD had significant effect on TRM: RR (95% CI) for aGVHD of grades II to IV versus 0 to I = 1.90 (1.08-3.34; P = .026), for cGVHD of yes versus no = 2.77 (1.26-6.07; P = .011).
Three degrees of freedom test.
Results
Patient characteristics prior to transplantation
Characteristics of patients prior to transplantation are shown in Tables 1, 2. Mismatched patients were younger than patients in the matched group (median age of 24 years [range, 3-50 years] versus median age of 37 years [range, 5-50 years], respectively, P < .001). Compared with matched patients, more patients in the mismatched group received a combination of BM and PB for their graft (96% versus 65%, respectively, P < .001), and this resulted in higher cell doses in the mismatched group (mononuclear cells [MNCs]: 7.5 [range, 3.1-16.3] × 108/kg versus 6.2 [range, 2.3-13.6] × 108/kg, respectively; P < .001). The median CD34+, CD3+, CD4+, and CD8+ cell doses of the grafts are shown in Table 3. Data analyses showed that G-BMPB contained higher cell doses in MNCs, CD34+ cells, CD3+ cells, and CD4+ cells than either G-BM or G-PB graft (P < .001, P < .002, P < .001, and P < .001, respectively; Table 3).
Hematopoietic reconstitution
Analyses of chimerism indicated that all patients achieved full donor chimerism by day 30 after HCT. All patients engrafted to ANC exceeding 0.5 × 109/L, with a median time to neutrophil engraftment of 15 days (range, 10-25 days) in matched HCT versus 12 days (range, 10-25 days) in mismatched HCT (P < .001). Two patients had secondary graft failure at days 63 and 345 after transplantation. They received a second allo–hematopoietic cell transplant from the original donor. As a result, one patient achieved full hematologic reconstitution and the other failed to engraft and died of severe infection at day 98 after transplantation. One hundred fifty-six and 128 patients achieved platelet engraftments in both matched and mismatched groups, respectively, at 15 days (range, 2-108 days) versus 15 days (range, 7-151 days), P = .57. Primary platelet engraftment failure or secondarythrombocytopenia occurred in 19 patients, with 8 patients in the matched HCT group and 11 in the mismatched HCT group. Among them, 8 patients finally received additional mobilized donor PBSCs, and 6 of them are alive and well.
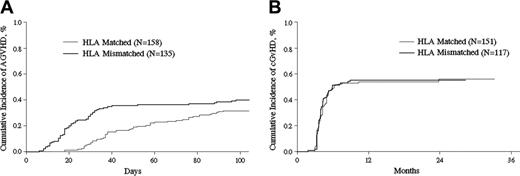
GVHD after transplantation. Cumulative incidence of aGVHD (A) and cGVHD (B) after HLA-identical sibling or HLA-mismatched related transplantations (P = .13 and P = .97, respectively).
GVHD after transplantation. Cumulative incidence of aGVHD (A) and cGVHD (B) after HLA-identical sibling or HLA-mismatched related transplantations (P = .13 and P = .97, respectively).
GVHD incidence and severity
Mismatched patients had a higher risk for and faster rate of developing grades II to IV aGVHD than matched sibling HCT patients (relative risk [RR] = 0.64 [95% CI, 0.43-0.94], P = .02). The cumulative incidences of grades II to IV aGVHD on day 100 in the matched and mismatched cohorts were 32% (CI, 25%-39%) versus 40% (CI, 32%-48%, P = .13), respectively (Figure 1A). Clinical manifestation of grades III to IV GVHD included severe diarrhea, 17 (44%); bloody diarrhea, 9 (23%); hepatic dysfunction, 16 (41%); skin rash, 12 (31%); and noncardiac edema, 7 (18%). Corticosteroids (1 mg/kg per day) were given intravenously and then tapered as scheduled or based on therapeutic response. Thirty-nine patients (17 in matched and 22 in mismatched transplantation groups) resistant to corticosteroids received a second line of immunosuppressive drugs (tacrolimus [FK506], MMF, or MTX). One hundred fifty-one patients in matched and 117 patients in mismatched cohorts with survival longer than 100 days after HCT were eligible to be evaluated for the incidence of cGVHD. Matched and mismatched patients had the same risk of developing cGVHD (RR = .99 [CI, 0.72-1.38]; P = .97). The 2-year cumulative incidence of cGVHD in matched and mismatched transplantation was 56% (CI, 47%-64%) versus 55% (CI, 46%-64%), respectively (P = .90; Figure 1B).
Infectious complications and transplantation-related toxicities
Although the patients undergoing matched HCT had a lower 100-day cumulative incidence of CMV antigenemia (39% [CI, 32%-47%] in the matched versus 65% [CI, 57%-72%] in the mismatched group, P < .001; Figure 2A), the incidence of CMV-associated IPn was the same: 17% (CI, 12%-23%) in the matched versus 17% (CI, 11%-24%) in the mismatched HCT group (P = .98, Figure 2B). Mismatched patients had a higher incidence of hemorrhagic cystitis (HC) within 100 days after HCT, 35% (CI, 27%-43%) compared with 13% (CI, 9%-19%) in the matched patients (P < .001, Figure 2C). Grade 3 HC was noted in 5 matched patients and 15 mismatched patients. Hepatitis viral infections (including hepatitis A, B, and C) were present in 12 matched and 9 mismatched patients.
Grades II to IV organ toxicities during the 40 days after HCT were evaluated as follows (matched vs mismatched cohort, respectively): cardiovascular, 1% versus 6%; neurologic, 0% versus 4%; hepatic, 7% versus 10%; renal, 1% versus 0%; metabolism, 3% versus 5%; and hepatic veno-occlusive disease, 1% versus 4%. No deaths resulted from lethal organ toxicities during the 40 days after HCT.
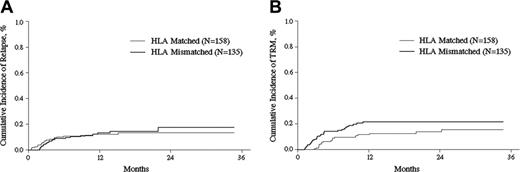
Relapse and treatment-related mortality (TRM)
Patients who underwent matched and mismatched transplantation had similar risks of relapse and TRM. In multivariate analyses, the relative risk of relapse and TRM for patients who underwent mismatched versus matched transplantation were 0.91 (95% CI, 0.48-1.72; P = .774) and 1.75 (CI, 0.99-3.09; P = .054), respectively (Table 4). The 2-year relapse and TRM rates were 13% (8%-19%) versus 18% (10%-27%) (P = .40) and 14% (9%-20%) versus 22% (15%-29%) (P = .10) for patients who underwent matched versus mismatched transplantation, respectively (Figure 3A-B). Thirty-nine patients had relapsed by the time of the last follow-up, which included 20 (13%) patients in the matched and 19 (14%) patients in the mismatched cohort. To treat relapse, 25 patients received immunosuppressive agent taper and DLI. They included 11 in the matched and 14 in the mismatched groups. Among 39 relapsed patients, 32 patients died after relapse, 18 in the matched and 14 in the mismatched groups, with a median time to death of 127 days (range, 35 to 465 days) and 204 days (range, 72 to 395 days), respectively. Analyses of non–relapse-related mortality (NRM) showed that aGVHD and pulmonary infection were the major causes of death in both the matched and mismatched transplantation cohorts.
Infectious complications after transplantation. Cumulative incidence of CMV antigenemia (A), CMV-related IPn infection (B), and HC (C) after HLA-identical sibling or HLA-mismatched related transplantations (P < .001, P = .98, and P < .001, respectively).
Infectious complications after transplantation. Cumulative incidence of CMV antigenemia (A), CMV-related IPn infection (B), and HC (C) after HLA-identical sibling or HLA-mismatched related transplantations (P < .001, P = .98, and P < .001, respectively).
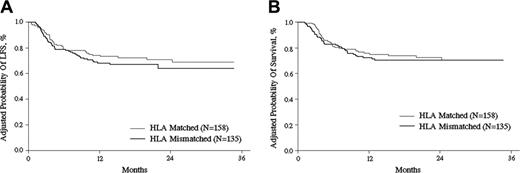
Leukemia-free survival (LFS) and survival
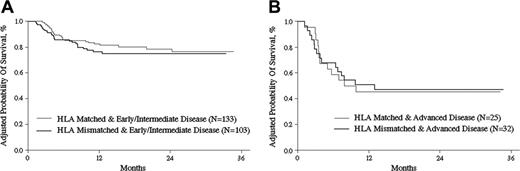
Adjusted for other prognostic variables identified in the multivariate analyses, the 2-year probabilities of LFS were 71% (CI, 63%-78%) and 64% (CI, 54%-73%) after matched and mismatched transplantations, respectively, with P = .27 (Figure 4A). Adjusted 2-year overall survival probabilities were 72% (CI, 64%-79%) and 71% (CI, 62%-77%) after matched and mismatched transplantations, respectively, with P = .72 (Figure 4B). There were no differences in adjusted survival probabilities according to disease status before transplantation between the 2 cohorts. The 2-year overall survival probabilities for patients with early and intermediate disease were 79% (CI, 70%-85%) and 75% (CI, 65%-82%) with P = .55, while patients with advanced disease were 45% (CI, 24%-64%) and 47% (CI, 28%-64%) with P = .90, for the matched and mismatched cohorts, respectively (Figure 5A-B). No statistical differences in survival outcome according to the number of HLA antigens mismatched were noted. The relative risk of overall mortality for 3 mismatched HLA antigens compared with 1 or 2 mismatched HLA antigens was 1.20 (CI, 0.63-2.26; P = .58). The probability of 2-year overall survival in G-BM (n = 35), G-PB (n = 25), and G-BMPB (n = 233) groups was 66%, 64%, and 71%, respectively (P = .38). No significant influence on the outcome was evidenced from donor-recipient pair relationships. Two-year overall survival probabilities from different mismatched donor sources were 71.3% in sibling donors, 71.1% in father donors, 63.7% in mother donors, and 57.7% in children donors (data not shown).
Relapse and TRM after transplantation. Cumulative incidence of relapse (A) and TRM (B) after HLA-identical sibling or HLA-mismatched related transplantations (P = .77 and P = .05, respectively).
Relapse and TRM after transplantation. Cumulative incidence of relapse (A) and TRM (B) after HLA-identical sibling or HLA-mismatched related transplantations (P = .77 and P = .05, respectively).
Results of multivariate analysis for aGVHD, cGVHD, TRM, relapse, treatment failure (death in complete remission or relapse), and overall survival are shown in Table 4. Every variable listed in Table 1 was considered in multivariate analysis. The analyses suggested that the patients diagnosed with CML, MDS, and/or in early disease stage prior to HCT had a lower incidence of relapse and treatment failure and had a better survival (Table 4). Tests in the multivariate analyses indicated that based on current data analyses, CD3+, CD4+, and CD8+ T-cell dose, patient age, as well as different mismatched donor sources had no significant effect on any of the studied outcomes.
Discussion
Our results using the present regimen of conditioning, grafting, and posttransplantation supportive care for HLA-mismatched/haploidentical HCT are encouraging and not significantly worse than results during the same period at the same institution with matched sibling donors. ATG was used during conditioning in our mismatched transplant recipients for the following reasons. (1) ATG has a relatively prolonged half-life in vivo. It can be detected even 30 days or longer after its administration.24,25 Thus, it potently deletes T lymphocytes long term in vivo, preventing GVHD with no increase in incidence of relapse.26,27 (2) ATG included in conditioning results in a faster donor chimerism after HCT, especially for transplantations from alternative donors.28,29
LFS and OS after transplantation. Adjusted probability (derived from multivariate regression models) of LFS (A) and OS (B) after HLA-identical sibling or HLA-mismatched related transplantations (P = .32 and P = .55, respectively).
LFS and OS after transplantation. Adjusted probability (derived from multivariate regression models) of LFS (A) and OS (B) after HLA-identical sibling or HLA-mismatched related transplantations (P = .32 and P = .55, respectively).
But ATG can also damage hematopoietic stem/progenitor cells to some degree and retard immune reconstitution for several months after BMT.30 To overcome these problems, high-dose stem cell numbers were given, using combined G-mobilized marrow and PBSC grafts. With the use of G-BMPB as source of grafts, more hematopoietic cells may be harvested than via use of BM or PB alone. Qualitatively, G-CSF–mobilized PB grafts have more T-polarized cells (Th2), which modulate the cytokine profile of type-2 dendritic cells and could potentially protect the host from aGVHD.31-33 Besides, mesenchymal stem cells (MSCs)/mesenchymal (stroma) progenitor cells (MPCs) from G-BM may possess immunoregulatory activity,34 reduce the incidence of GVHD,35,36 and allow crossing of major histocompatibility (MHC) barriers. Larger amounts of hematopoietic cells and lymphocytes from G-BMPB can partially overcome the harmful effect of ATG on hematopoiesis and immune reconstitution.37,38
We had only 11% grades III to IV aGVHD in the patients who underwent matched transplantations and 16% in the patients who underwent mismatched HCTs. These incidences of grade III to IV aGVHD are lower than some previous reports.39 The reasons for the decreased incidence of severe aGVHD may be multifactorial, including in vivo T depletion with ATG40 in the mismatched patients; the use of G-CSF–mobilized BMPB41-43 and the combination of CSP, MTX, and MMF44 could have resulted in a low incidence of severe aGVHD.
ATG is associated with delayed immune reconstitution and is often accompanied by severe infections, particularly viral disease. In our study, the high rate of CMV serologic–positive patients prior to transplantation and the high incidence of CMV antigenemia after HCT were evident in both matched and mismatched patients. We attempted to prevent serious complications due to CMV by giving ganciclovir before transplantation, and then promptly and preemptively treating any CMV antigenemia after transplantation. This remarkably decreased the incidence of lethal CMV disease. The incidence of hemorrhagic cystitis (HC) varies according to type of transplantation,44 age groups,45 and type of immunosuppression. Our data suggest that HC is more frequent in patients who underwent mismatched than in those who underwent matched transplantations (35% versus 13%, respectively, P < .001). The excessive rate of CMV serologic positivity before transplantation and CMV antigenemia after transplantation as well as the use of ATG might contribute to the high rate of HC after HCT. The majority of patients had manifestation of HC at +30 days or later after HCT. This is in agreement with previous reports that viral infections are a major contributor to morbidity during this period.46,47 Further efforts may identify a more optimal dosing or timing of ATG,25,29,40 or even a better in vivo T-cell–depleting agent.48,49
OS by disease stage. Adjusted probability (derived from multivariate regression models) of OS after HLA-identical sibling or HLA-mismatched related transplantations for patients diagnosed with early or intermediate disease (A) and advanced disease (B) (P = .55 and P = .9, respectively).
OS by disease stage. Adjusted probability (derived from multivariate regression models) of OS after HLA-identical sibling or HLA-mismatched related transplantations for patients diagnosed with early or intermediate disease (A) and advanced disease (B) (P = .55 and P = .9, respectively).
Multivariate analyses from our updated results of patients who underwent mismatched transplantations showed, as might be expected, that advanced leukemia was the strongest prognostic factor for a poor outcome. In children with advanced leukemia and a female donor the outcome was unfavorable, while in adult recipients a cousin or father donor appeared to be unfavorable (D.-P.L., manuscript in preparation). Generally, our results were not entirely consistent with previous studies of inherited maternal antigen/inherited paternal antigen (NIMA/NIPA) mismatched transplantation.50-52 The use of ATG in the conditioning regimen, which produces intensive T-cell depletion in vivo, may partially conceal the histocompatibility barriers due to HLA disparity and the minor histocompatibility effects due to NIMA/NIPA in the haploidentical transplantation setting. For better evaluation of the effect of maternal grafts/paternal grafts on haploidentical transplantation, further studies are necessary.
In conclusion, with the current protocol, we believe that we have identified a sufficiently safe regimen allowing engraftment and acceptable TRM for family HLA-mismatched transplantation in appropriate patients. Our comparisons in this relatively large study found that every major end point of allogeneic HCT including relapse, TRM, and overall and leukemia-free survival compared between HLA-matched and -mismatched HCT did not statistically differ. The current study also shows that patients with advanced disease stage but absence of a family or unrelated HLA-matched donor might achieve nearly comparable therapeutic effects from family-mismatched/haploidentical transplantation. Further reduction of relapse and other complications in the future will lead to better clinical outcomes, as will more information on choosing the best haploidentical donor from within a family.
Prepublished online as Blood First Edition Paper, December 27, 2005; DOI 10.1182/blood-2005-05-2146.
Supported by grants from National “211 project” (no. 92000-242156014), Peking University evidence-based medicine (EBM) group.
D.-P.L. served as the principal investigator and director over the entire period of the study. All coauthors, except D.L., had Ward duty as attending doctors. X.-J.H., L.D., T.W., K.-Y.L., and H.-Y.R. were all associate investigators. D.L. was responsible for the HLA laboratory. L.D. and T.W. compiled clinical data. L.D. and D.-P.L. were writers of the paper. M.-J.Z. and L.D. performed all of the statistical analysis. M.-J.Z. also checked the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Cynthia Dunbar (National Institutes of Health, Bethesda, MD) for critically reviewing the paper and her important advice in science and in English. We thank Prof Rainer F. Storb (Fred Hutchinson Cancer Research Center, Seattle, WA) for his helpful comments on the paper. We thank every faculty member who has participated in these studies. We also thank Dr Yan-Rong Liu for excellent laboratory support for the FACS analysis.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal