Induction of antigen-specific tolerance is critical for autoimmunity prevention and immune tolerance maintenance. In addition to their classical role as sentinels of the immune response, dendritic cells (DCs) play important roles in maintaining peripheral tolerance through the induction/activation of regulatory T (Treg) cells. The possibility of generating tolerogenic DCs opens new therapeutic perspectives in autoimmune/inflammatory diseases. Characterizing endogenous factors that contribute to the development of tolerogenic DCs is highly relevant. We here report that the immunosuppressive neuropeptide vasoactive intestinal peptide (VIP) induces the generation of human tolerogenic DCs with the capacity to generate CD4 and CD8 Treg cells from their respective naive subsets. The presence of VIP during the early stages of DC differentiation from blood monocytes generates a population of IL-10-producing DCs unable to fully mature after the effects of inflammatory stimuli. CD4 Treg cells generated with VIP-differentiated DCs resemble the previously described Tr1 cells in terms of phenotype and cytokine profile. CD8 Treg cells generated with tolerogenic VIP DCs have increased numbers of IL-10-producing CD8+CD28--CTLA4+ T cells. CD4 and CD8 Treg cells primarily suppress antigen-specific TH1-mediated responses. Therefore, the possibility of generating or expanding ex vivo tolerogenic DCVIPs opens new therapeutic perspectives for treating autoimmune diseases and graft-versus-host disease after allogeneic transplantation in humans.
Introduction
Dendritic cells (DCs) are a heterogeneous population of antigen-presenting cells (APCs) that contribute to innate immunity and that initiate adaptive immune responses to antigens associated with infection and inflammation.1 Successful initiation of the adaptive immune response requires DC maturation after signaling through the toll-like receptors and CD40. However, in addition to their classical role as sentinels of the immune response, DCs play an important role in immune homeostasis by inducing and maintaining tolerance.2 The maturation/activation state of DCs might be the control point for the induction of peripheral tolerance by promoting the generation/activation of regulatory T (Treg) cells. Mature DCs (mDCs) are potent APCs that enhance T cell immunity, whereas immature DCs (iDCs) are involved in the induction of peripheral T cell tolerance.1-5 Although the clinical use of iDCs may not be suitable in autoimmune diseases and transplantation, because iDCs are likely to mature in inflammatory conditions,5 tolerogenic DCs prevent lethal graft-versus-host disease (GVHD) in hosts who undergo transplantation with allogeneic bone marrow cells while maintaining the graft-versus-tumor response.3,6-8 Immunosuppressive therapy, traditionally focused on lymphocytes, has been revolutionized by targeting DCs, and the in vitro generation of tolerogenic DCs has become the focus of new therapies.9
Vasoactive intestinal peptide (VIP), an immunoregulatory neuropeptide released in inflammatory/autoimmune conditions,10 affects innate and adaptive immune responses.11 Recently, we have shown that VIP affects mouse bone marrow-derived DCs differently, depending on the DC maturation state.12 iDCs treated with VIP up-regulate CD86 expression, stimulate T cell proliferation, and promote TH2-type responses. In contrast, VIP down-regulates CD80 and CD86 expression of mDCs and inhibits their capacity to activate allogeneic T cells. However, VIP administration during the early phases of DC differentiation induces the generation of murine regulatory/tolerogenic DCs with the capacity to induce CD4 Treg cells, to restore tolerance in vivo, to prevent the progression of autoimmune disorders,13 and to reduce the deleterious consequences of acute GVHD after allogeneic transplantation.14 To exploit a novel strategy involving the use of tolerogenic DCs for the prevention and treatment of human immunopathogenic diseases, we investigated the effect of VIP in the generation of human regulatory DCs that affect allogeneic T cell responses.
Materials and methods
Cell isolation and cultures
Human DCs were generated from leukapheresis products of healthy blood donors, as described.15 In brief, peripheral blood mononuclear cells were isolated by Ficoll density gradient centrifugation, and monocytes were isolated by plastic adherence and with the use of monocyte enrichment mixture and magnetic colloid (StemCell, Maylan, France). Monocytes (2 × 106) were cultured with complete medium (RPMI 1640 supplemented with 100 U/mL penicillin-streptomycin, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, and 10% heat-inactivated fetal calf serum) containing GM-CSF (800 U/mL; PeproTech, Rocky Hill, NJ) and IL-4 (500 U/mL; PeproTech) in the absence (DCcontrols) or the presence of VIP (DCVIPs; 10-8 M; Calbiochem, San Diego, CA). After 6 days, nonadherent cells were collected and subjected to negative selection with anti-CD2 and anti-CD19 mAbs conjugated with immunomagnetic beads (Miltenyi Biotec, Auburn, CA). Resultant cells were cultured for 48 hours with LPS (1 μg/mL) or human TNFα (10 ng/mL) to induce activation/maturation.
Human naive CD4 and CD8 T cells were purified from peripheral blood mononuclear cells (PBMCs) obtained from different donors with use of the CD4/CD45RA and CD8 Multisort kit (Miltenyi Biotec) according to the manufacturer's recommendations and were typically more than 99% pure, as indicated by flow cytometry analysis (CD4+CD45RO-CD62L+ or CD8+CD45RO-CD62L+, respectively).
Human TH1 cells were generated from naive CD4 T cells, as described.15 To generate human tetanus toxin (TT)-specific CD4 T cells and allogeneic fibroblast-specific CD8 T cells, PBMCs (107) were primed with TT (1 μg/mL) or necrotic allogeneic fibroblasts (106) for 3 weeks in medium containing IL-2 (100 U/mL), and CD4+ or CD8+ T cells were negatively selected, as described.16 Resultant cells (greater than 95% CD3+CD4+ cells or greater than 95% CD3+CD8+ cells) were cultured in medium with IL-2 (10 U/mL) for 5 days and were used for subsequent experiments. For isolation of different T-cell populations (CD4+, CD4+CD25+, CD4+CD25-), cells were labeled with PE-anti-CD25 and PerCP-anti-CD4 antibodies, as described, and the different populations were gated and sorted using a FACSCalibur flow cytometer (Becton Dickinson, San Diego, CA).
Flow cytometry
Cells were incubated with various PerCP-, FITC-, and PE-labeled mAbs (BD PharMingen, San Diego, CA) diluted at optimal concentration for immunostaining, fixed in 1% paraformaldehyde, and analyzed on a FACSCalibur flow cytometer (Becton Dickinson). We used isotype-matched antibodies as controls and IgG block (Sigma) to avoid nonspecific binding to Fc receptors. For analysis of intracellular CTLA4, cells were stained first for surface CD4 with PerCP-anti-CD4 mAb, fixed with Cytofix/Cytoperm solution (BD PharMingen), incubated with PE-anti-CTLA4 mAb diluted in 0.5% saponin, and analyzed by flow cytometry.
Cytokine assays
Cytokine contents in the supernatants of DC cultures or DC-CD4 T cell cocultures were determined by specific sandwich ELISA using capture/biotinylated detection antibodies (BD PharMingen).
Endocytosis assay
Mannose receptor-mediated endocytosis was measured as the cellular uptake of FITC-dextran (Sigma) and was quantified by flow cytometry, as described.16
Mixed leukocyte reaction
Naive CD4 T cells (2 × 105) were cultured with allogeneic DCcontrols or DCVIPs at various T/DC ratios in the presence or absence of IL-2 (100 U/mL) for 3 days. Cell proliferation in primary cultures was evaluated by using a cell proliferation assay (BrdU) from Roche Diagnostics GmbH (Mannheim, Germany). TT-primed CD4 T cells (5 × 105) were cultured with unprimed, OVA-primed, or TT-primed DCcontrols or DCVIPs at various T/DC ratios. In some experiments, DCs (105) were cultured with purified syngeneic or allogeneic unprimed or primed CD4 T cells (5 × 105). After 3 days of culture, T cells were recovered by immunodepletion of CD11c+ DCs, rested for 3 days in complete medium supplemented with IL-2 (20 U/mL), and restimulated (5 × 105) with different numbers of allogeneic LPS-matured DCs or syngeneic TT-pulsed DCs (105).
Human naive CD8 T cells (5 × 105) were cocultured with allogeneic DCcontrols or DCVIPs at various T/DC ratios for 5 days, and CD8+ T cells were negatively selected by the depletion of DCs. Subsequently, CD8+CD28+ and CD8+CD28- T cells were selected using anti-human CD28 mAb (BD PharMingen) plus goat anti-mouse IgG mAb-conjugated immunomagnetic beads, cocultured with allogeneic LPS-matured DCs (104), and assayed for proliferation as described.
Analysis of Treg cell function
Purified naive CD4 or CD8 T cells were exposed to allogeneic DCcontrols or DCVIPs as described. Five days later, CD4 or CD8 T cells were recovered by immunodepletion of DCs and were cultured with syngeneic TH1 cells (5 × 105) in the presence of allogeneic mDCs (105). Some cultures were performed in the presence of blocking anti-IL-10 (10 μg/mL), anti-TGFβ1 (40 μg/mL), or anti-CTLA4 (10 μg/mL) mAbs or of IL-2 (100 U/mL). To determine the cell-contact dependence of the regulatory response, we placed TH1 cells (5 × 105) with allogeneic mDCs (105) in the bottom well of a Transwell system (Millipore, Auburn, CA) and the recovered CD4 or CD8 T cells (2 × 105) with allogeneic mDCs (105) in the upper Transwell chamber. After 72 hours, we measured the proliferative response of the bystander reactive TH1 cells in the bottom well.
mRNA analysis
Cytotoxicity assay
In vivo-primed CD8 T cells and allogeneic fibroblast-specific CD8 T cells were cultured with Na251CrO4 (100 μCi (3.7 MBq)/106 cells; NEN Life Science Products, Boston, MA)-labeled human allogeneic fibroblasts (104) for 4 hours at various effector-to-target cell ratios (E/T ratios). The radioactivity released in the supernatants was measured, and the percentage of specific lysis was calculated.7
Results
VIP interferes with the differentiation of human DCs and their subsequent maturation
To determine whether exposure to VIP during human DC differentiation results in DC phenotypic and functional changes, we compared human monocyte-derived DCs generated in the presence or absence of VIP in terms of surface markers, phagocytic capacity, and cytokine production. As previously described, human monocytes cultured with GM-CSF plus IL-4 for 6 days differentiated into iDCs (Figure 1A). With LPS stimulation, iDCs matured to DCs expressing high levels of DC maturation markers (CD83), MHC molecules (classes 1 and 2), and costimulatory molecules (CD40, CD80, CD86) (Figure 1A; DCcontrols). However, DCs generated in the presence of VIP (DCVIPs) were resistant to the LPS-induced up-regulation of the costimulatory molecules (CD40, CD80, CD86) and the maturation-associated changes (CD83) (Figure 1A). We observed similar results by quantitative real-time PCR and after stimulation of DCVIPs with TNFα, anti-CD40 antibodies, or an inflammatory cocktail containing TNFα/IL-6/IL-1β/PGE2 (not shown). Because iDCs and mDCs differ in their phagocytic capacity, we assessed the endocytic ability of DCVIPs by measuring the FITC-dextran uptake through mannose receptors. DCVIPs exhibit higher phagocytic activity than DCcontrols do (Figure 1B). With Toll-like receptor activation, iDCs matured into cells capable of producing high levels of inflammatory cytokines. In contrast to DCcontrols, which produce TNF, IL-12, IL-6, IL-8, and low levels of IL-10, DCVIPs produce very low levels of proinflammatory cytokines (TNF, IL-12, IL-6) but release significant levels of the anti-inflammatory cytokine IL-10 (Figure 1C). Taken together, these results indicate that the DCs generated in the presence of VIP differ from those generated with GM-CSF/IL-4 alone in terms of costimulatory molecule expression, phagocytic capacity, and cytokine profile. The characteristics expressed by DCVIPs are similar to those reported for tolerogenic DCs.1-3,6,8,19
VIP interferes with the differentiation of human DCs and their subsequent maturation. Human DCs generated in the absence (DCcontrols) or presence (DCVIPs) of VIP were stimulated with LPS to induce DC activation/maturation. (A) Cell surface, maturation, and costimulatory markers were analyzed by flow cytometry. Dashed lines in top histograms represent the staining profile with isotype-matched control antibodies. Numbers represent the mean channel fluorescence intensity (MFI) for each phenotypic marker. Histograms are representative of 4 independent experiments. (B) DCs differentiated in the presence of VIP show augmented antigen uptake, evaluated by endocytosis, of FITC-dextran at different times. Results are expressed as fluorescence intensity and are the mean ± SD of 3 experiments performed in duplicate. (C) Differentiation of DCs with VIP modifies their cytokine production after activation. Results are the mean ± SD of 4 experiments performed in duplicate.
VIP interferes with the differentiation of human DCs and their subsequent maturation. Human DCs generated in the absence (DCcontrols) or presence (DCVIPs) of VIP were stimulated with LPS to induce DC activation/maturation. (A) Cell surface, maturation, and costimulatory markers were analyzed by flow cytometry. Dashed lines in top histograms represent the staining profile with isotype-matched control antibodies. Numbers represent the mean channel fluorescence intensity (MFI) for each phenotypic marker. Histograms are representative of 4 independent experiments. (B) DCs differentiated in the presence of VIP show augmented antigen uptake, evaluated by endocytosis, of FITC-dextran at different times. Results are expressed as fluorescence intensity and are the mean ± SD of 3 experiments performed in duplicate. (C) Differentiation of DCs with VIP modifies their cytokine production after activation. Results are the mean ± SD of 4 experiments performed in duplicate.
VIP differentiates tolerogenic DCs that induce IL-10/TGFβ-producing anergic CD4 T cells
Tolerogenic DCs are poor stimulators of T-cell proliferation and cytokine production.3,7,8,15,18,19 To examine the capacity of the DCVIPs to prime and differentiate CD4 T cells, we cocultured DCcontrols or DCVIPs with alloreactive CD4 T cells. Priming with DCcontrols results in a strong proliferation of allogeneic CD4 T cells, whereas DCVIPs induce only weak proliferation (Figure 2A; primary culture). To determine whether the CD4 T cells exposed to DCVIPs are indeed anergic, we restimulated CD4 T cells primed with DCcontrols or DCVIPs with fresh LPS-matured DCs (mDCs) generated from the same donor as the DCcontrols/DCVIPs. T cells exposed initially to DCcontrols proliferated and produced a typical TH1 cytokine profile (large amounts of IFNγ and IL-2 but no IL-4, IL-5, or IL-10). In contrast, T cells primed with DCVIPs did not proliferate after successive restimulation, and they exhibited a cytokine profile characteristic of regulatory Treg1 cells—that is, production of high amounts of IL-10 and TGFβ and no or negligible synthesis of IFNγ, IL-2, IL-4, and IL-5 (Figure 2A, restimulation). We next examined the effectiveness of DCVIPs for the presentation of processed antigen to antigen-specific T cells. In a first coculture, TT-pulsed DCcontrols, but not unpulsed and ovalbumin-pulsed DCcontrols, induced antigen-specific proliferation and IFNγ production in TT-specific CD4 T cells. After restimulation with syngeneic TT-pulsed mDCs, the CD4 T cells responded strongly in terms of proliferation and IFNγ production (Figure 2B). In contrast, TT-pulsed DCVIPs elicited weak responses (Figure 2B), suggesting that DCVIPs pulsed with soluble proteins induce a state of tolerance in antigen-specific CD4 T cells.
VIP generates human tolerogenic DCs, which induce IL-10-producing anergic CD4 T cells. Human DCs generated in the absence (DCcontrols, ▪) or presence (DCVIPs, □) of VIP were stimulated with LPS to induce DC activation/maturation. (A) Purified naive CD4 T cells were cultured with graded doses of allogeneic DCcontrols (▪) or DCVIPs (□) in primary cultures (panel i). CD4 T cells from primary cultures were recovered, rested, and restimulated with LPS-matured allogeneic DCs (panel ii). Cultures of DCcontrols or DCVIPs without T cells did not proliferate. Cytokine production was determined in secondary cultures at DC/T cell ratios of 1:10 (panel iii). Each result is the mean ± SD of 3 experiments performed in duplicate. (B) TT-primed TH1 cells were cocultured with TT-pulsed syngeneic DCcontrols (▪) or DCVIPs (□)in primary cultures (panel i). After 3 days of culture, TH1 cells were rescued, rested, and restimulated with TT-pulsed, LPS-matured syngeneic DCs, and proliferation (panel ii) and IFNγ production (panel iii) were determined. Unpulsed or ovalbumin-pulsed DCcontrols or DCVIPs did not induce the proliferation of TT-primed TH1 cells. Each result is the mean ± SD of 3 experiments performed in duplicate.
VIP generates human tolerogenic DCs, which induce IL-10-producing anergic CD4 T cells. Human DCs generated in the absence (DCcontrols, ▪) or presence (DCVIPs, □) of VIP were stimulated with LPS to induce DC activation/maturation. (A) Purified naive CD4 T cells were cultured with graded doses of allogeneic DCcontrols (▪) or DCVIPs (□) in primary cultures (panel i). CD4 T cells from primary cultures were recovered, rested, and restimulated with LPS-matured allogeneic DCs (panel ii). Cultures of DCcontrols or DCVIPs without T cells did not proliferate. Cytokine production was determined in secondary cultures at DC/T cell ratios of 1:10 (panel iii). Each result is the mean ± SD of 3 experiments performed in duplicate. (B) TT-primed TH1 cells were cocultured with TT-pulsed syngeneic DCcontrols (▪) or DCVIPs (□)in primary cultures (panel i). After 3 days of culture, TH1 cells were rescued, rested, and restimulated with TT-pulsed, LPS-matured syngeneic DCs, and proliferation (panel ii) and IFNγ production (panel iii) were determined. Unpulsed or ovalbumin-pulsed DCcontrols or DCVIPs did not induce the proliferation of TT-primed TH1 cells. Each result is the mean ± SD of 3 experiments performed in duplicate.
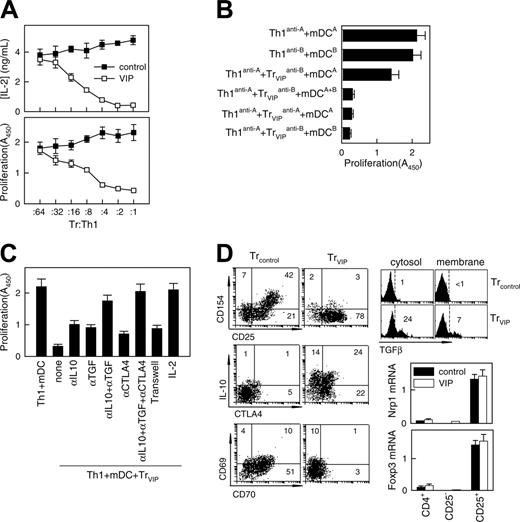
DCVIPs induce functional human CD4 Treg cells in vitro
After TCR stimulation, Treg cells suppressed the proliferation and IL-2 production of antigen-specific effector T cells. To determine whether T cells exposed to DCVIPs become functional CD4 Treg cells, we restimulated alloreactive TH1 cells with mDCs in the presence of CD4 T cells derived from the same donor and previously primed with allogeneic DCcontrols (CD4Treg controls) or DCVIPs (CD4Treg VIPs). Treg VIPs inhibited the proliferation of syngeneic TH1 cells in response to allogeneic mDCs in a dose-dependent manner, whereas CD4Treg controls were not suppressive (Figure 3A). Similar results were obtained with respect to IL-2 production (Figure 3A). CD4Treg VIP did not suppress the costimulatory capacity of allogeneic mDCs because mDCs pretreated with CD4Treg VIPs for 48 hours had the same stimulatory capacity as untreated mDCs (not shown). We next investigated whether CD4Treg VIPs and effector TH1 cells have to interact with target structures expressed by the same mDCs. We generated TH1 and CD4Treg VIPs from the same donor (donor C) by priming with allogeneic mDCs from 2 different donors (donors A and B). Donor A-specific CD4Treg VIPs (Treg VIPsanti-A suppressed the proliferation of donor A-specific TH1 (TH1anti-A) cells in coculture, and donor B-specific CD4Treg VIPs (Treg VIPsanti-B) also suppressed the proliferation of TH1anti-A cells in the presence of mDCs from donors A and B (mDCsA+B) (Figure 3B), suggesting that the TH1-inhibitory capacity of CD4Treg VIPs does not require the simultaneous presentation of the target antigen on the same mDCs. Nevertheless, the activation of CD4Treg VIPs by the respective target mDCs is an essential prerequisite because the exposure of donor A-specific TH1 cells with donor B-specific CD4Treg VIPs solely in the presence of donor A-derived mDCs did not lead to an inhibition of TH1 proliferation (Figure 3B).
VIP-differentiated DCs generate human regulatory T cells in vitro. Purified naive CD4 T cells exposed to allogeneic DCcontrols (Treg controls) or DCVIPs (Treg VIPs) were evaluated for suppressive/regulatory activity. (A) Treg VIPs (□) or Treg controls (▪) were cocultured at different ratios with syngeneic TH1 cells in the presence of allogeneic mature DCs (mDCs). Proliferation of T cells and IL-2 production was determined. Treg VIPs, alone or with allogeneic mDCs, did not proliferate. (B) TH1 cells and Treg VIPs were generated by priming of CD4 T cells from donor C with allogeneic DCcontrols or DCVIPs from donor A (TH1anti-A/Treg VIPsanti-A) or donor B (TH1anti-B/Treg VIPsanti-B) and were cocultured in the presence of mDCs from donor A or donor B, or both, as indicated. Proliferation of responder T cells was determined. Each result is the mean ± SD of 3 experiments performed in duplicate. (C) Polarized TH1 cells were cocultured with Treg VIPs and allogeneic mDCs in the presence or absence of blocking anti-IL-10, anti-TGFβ, anti-CTLA-4, or IL-2 antibody. Additionally, TH1 + mDCs were separated from Treg VIPs+mDCs in a Transwell system. The proliferative response of TH1 cells was determined. (D) Phenotype of Treg VIPs and Treg controls. Expression of surface markers (CD25, CD69, CD70, CD154), intracellular IL-10 and CTLA-4, and cytosolic and membrane-bound TGFβ were determined by flow cytometry. Numbers represent the percentage of positive cells in each quadrant. Foxp3 and neuropilin 1 (Nrp1) mRNA expression in sorted CD4+, CD4+CD25+, and CD4+CD25- Treg controls (▪) or Treg VIPs (□) was determined by real-time RT-PCR. Results are representative of 5 independent experiments.
VIP-differentiated DCs generate human regulatory T cells in vitro. Purified naive CD4 T cells exposed to allogeneic DCcontrols (Treg controls) or DCVIPs (Treg VIPs) were evaluated for suppressive/regulatory activity. (A) Treg VIPs (□) or Treg controls (▪) were cocultured at different ratios with syngeneic TH1 cells in the presence of allogeneic mature DCs (mDCs). Proliferation of T cells and IL-2 production was determined. Treg VIPs, alone or with allogeneic mDCs, did not proliferate. (B) TH1 cells and Treg VIPs were generated by priming of CD4 T cells from donor C with allogeneic DCcontrols or DCVIPs from donor A (TH1anti-A/Treg VIPsanti-A) or donor B (TH1anti-B/Treg VIPsanti-B) and were cocultured in the presence of mDCs from donor A or donor B, or both, as indicated. Proliferation of responder T cells was determined. Each result is the mean ± SD of 3 experiments performed in duplicate. (C) Polarized TH1 cells were cocultured with Treg VIPs and allogeneic mDCs in the presence or absence of blocking anti-IL-10, anti-TGFβ, anti-CTLA-4, or IL-2 antibody. Additionally, TH1 + mDCs were separated from Treg VIPs+mDCs in a Transwell system. The proliferative response of TH1 cells was determined. (D) Phenotype of Treg VIPs and Treg controls. Expression of surface markers (CD25, CD69, CD70, CD154), intracellular IL-10 and CTLA-4, and cytosolic and membrane-bound TGFβ were determined by flow cytometry. Numbers represent the percentage of positive cells in each quadrant. Foxp3 and neuropilin 1 (Nrp1) mRNA expression in sorted CD4+, CD4+CD25+, and CD4+CD25- Treg controls (▪) or Treg VIPs (□) was determined by real-time RT-PCR. Results are representative of 5 independent experiments.
The observation that CD4Treg VIPs produced high levels of the immunosuppressive cytokines IL-10 and TGFβ suggests that the inhibitory effect of CD4Treg VIPs on TH1 proliferation might be mediated through soluble factors. When CD4Treg VIPs and TH1 cells were separated in transwell experiments by a semipermeable polycarbonate membrane that allows the free exchange of soluble factors but excludes direct cell contact of TH1 and CD4Treg VIPs, the proliferation of effector TH1 cells was still inhibited but to a lesser degree, indicating that direct cellular contacts and soluble factors mediated the inhibitory effect (Figure 3C). In regular cocultures, adding anti-TGFβ, anti-IL-10, or anti-CTLA4 antibodies reversed inhibition only modestly. However, adding both anti-IL-10 and anti-TGFβ had a more pronounced effect, and adding all 3 antibodies reversed the inhibitory effect completely (Figure 3C). In addition, as previously described for CD4 Treg cells, high amounts of exogenous IL-2 reduced the inhibitory activity of CD4Treg VIPs considerably (Figure 3C). Given that the proliferation of CD4Treg VIPs could not be induced with similar amounts of IL-2, this result suggests that CD4Treg VIPs cells suppress the proliferation of TH1 cells through the inhibition of endogenous IL-2 production. To correlate the regulatory activity of CD4Treg VIPs with the expression of various surface molecules and cytokines, we further characterized CD4Treg controls and CD4Treg VIPs (Figure 3D). Both T-cell populations up-regulated CD25, but only CD4Treg controls showed enhanced expression of the activation molecules CD69, CD70, and CD154 (CD40 ligand). CD70 costimulated CD4 T cells to produce IL-2 and IFNγ. Cross-linking of CD70 regulated the expression of CD154 on activated T cells, and CD154-CD40 interactions were a critical signal for T-cell and DC activation. In contrast, only CD4Treg VIPs showed high levels of intracellular IL-10 and CTLA4 (CD152). Indeed, we observed constitutive expression of CTLA4, an inhibitor of T cell proliferation, IL-2 production, and cell cycle progression,20,21 in CD4Treg VIPs for at least 2 weeks (not shown). Furthermore, CD4Treg VIPs, but not CD4Treg controls, expressed high levels of intracellular (soluble) and membrane-bound TGFβ. The increased levels of membrane-associated CTLA4 and TGFβ were relevant for the partial dependence on direct cellular contact of the CD4Treg VIPs suppression function. Therefore, the phenotype of CD4Treg VIPs correlated with their regulatory T cell activity. However, CD4Treg VIPs and CD4Treg controls did not significantly differ in the expression of other markers characteristic of CD4+CD25+ regulatory T cells, such as Nrp1 and Foxp3 (Figure 3D).
DCVIPs induce CD8+CD28- Treg cells
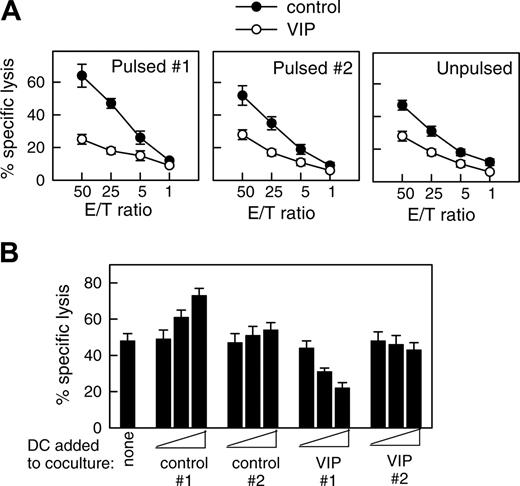
DCs internalize and process cellular fragments for cross-presentation to T cells.1 Therefore, we examined whether DCVIPs pulsed with allogeneic cellular fragments prepared from necrotic fibroblasts induced a similar state of tolerance in allogeneic fibroblast-specific cytotoxic CD8 T cells. Fibroblast-specific CD8 T cells exposed to DCVIPs showed a dose-dependent decrease in their cytotoxic capacity against antigen-specific targets compared with those primed with DCcontrols (Figure 4). Less reduction in the CD8 T lytic activity was observed when they were cocultured with unpulsed DCVIPs or DCVIPs pulsed with allogeneic cellular fragments prepared from unrelated donor 2 (Figure 4A). Little or no change in their cytotoxicity capacity was observed when CD8 T cells were treated with allogeneic DCVIPs derived from donor 2, who is unrelated to the recipient of allogeneic fibroblasts used in the priming of CD8 T cells (Figure 4B). This indicates that the regulation of CD8 T cells by DCVIPs was antigen specific. We next examined the phenotype of the human CD8 T cells generated in the presence of allogeneic DCVIPs. The coculture of naive CD8+ T cells with allogeneic DCVIPs induced CD8+CD28- T cells, whereas allogeneic DCcontrols preferentially generated CD8+CD28+ T cells (Figure 5A). In addition, whereas higher numbers of IFNγ-producing CD8+ T cells were observed for CD8+ T cells primed with allogeneic DCcontrols than with unprimed naive CD8+ T cells, the number of IL-10-producing CD8+ T cells was increased in the CD8+ T cells primed with DCVIPs (Figure 5A). Furthermore, naive CD8+ T cells exposed initially to allogeneic DCcontrols strongly proliferated in response to second restimulation with allogeneic mDCs, whereas CD8+ T cells primed with DCVIPs proliferated only weakly after successive restimulation with mDCs (Figure 5B). Isolated CD8+CD28- T cells exposed to DCcontrols or DCVIPs did not proliferate on restimulation, and priming with DCVIPs only slightly decreased the proliferative capacity of CD8+CD28+ T cells (Figure 5B), suggesting that the increased anergy observed in the DCVIPs-primed CD8+ T cells was caused mainly by the increase in the number of CD8+CD28- T cells. Taken together, these results indicate that DCVIPs induced a state of tolerance in antigen-specific CD8 T cells, characterized by low proliferation and cytotoxicity, increased IL-10 production, and a high proportion of CD8+CD28- T cells. These characteristics are similar to those reported for CD8 Treg cells.8,22,23
DCVIPs induce T cell anergy in alloreactive cytotoxic CD8 T cells. (A) Human CD8 T cells (5 × 106) obtained from the PBMCs primed with allogeneic fibroblasts (donor 1) were cultured with or without syngeneic DCcontrols or DCVIPs (5 × 105) obtained from the same donor pulsed without (unpulsed) or with the necrotic fibroblasts used in the priming culture (pulsed 1) or unrelated donor-derived necrotic fibroblasts (pulsed 2). After 3 days of coculture, CD8+ cells (5 × 105) were recovered, rested, and assayed for cytotoxicity against the allogeneic fibroblasts used in the priming culture (donor 1) at different effector-target (E/T) cell ratios. The established allogeneic fibroblast-specific CD8 T cells showed cytotoxicity only against allogeneic fibroblasts used in their generation (donor 1), indicating that their cytotoxicity was antigen specific, because none of the CD8+ cells showed cytotoxicity against allogeneic fibroblasts from an unrelated donor (donor 2). Each result is the mean ± SD of 3 experiments performed in duplicate. (B) Human allogeneic fibroblast-specific CD8 T cells (5 × 106) were cocultured without (none) or with unpulsed DCcontrols or DCVIPs (5 × 103, 5 × 104, or 5 × 105 cells) derived from the indicated donors (allogeneic 1 and syngeneic 2) for 3 days. These CD8 T cells were then rescued and subsequently assayed (5 × 105 cells) for cytotoxicity against the allogeneic fibroblasts (104 cells) used in the priming culture (donor 1). The value of spontaneous release cpm was less than 10% of the total release cpm. Each result is the mean ± SD of 4 experiments performed in duplicate.
DCVIPs induce T cell anergy in alloreactive cytotoxic CD8 T cells. (A) Human CD8 T cells (5 × 106) obtained from the PBMCs primed with allogeneic fibroblasts (donor 1) were cultured with or without syngeneic DCcontrols or DCVIPs (5 × 105) obtained from the same donor pulsed without (unpulsed) or with the necrotic fibroblasts used in the priming culture (pulsed 1) or unrelated donor-derived necrotic fibroblasts (pulsed 2). After 3 days of coculture, CD8+ cells (5 × 105) were recovered, rested, and assayed for cytotoxicity against the allogeneic fibroblasts used in the priming culture (donor 1) at different effector-target (E/T) cell ratios. The established allogeneic fibroblast-specific CD8 T cells showed cytotoxicity only against allogeneic fibroblasts used in their generation (donor 1), indicating that their cytotoxicity was antigen specific, because none of the CD8+ cells showed cytotoxicity against allogeneic fibroblasts from an unrelated donor (donor 2). Each result is the mean ± SD of 3 experiments performed in duplicate. (B) Human allogeneic fibroblast-specific CD8 T cells (5 × 106) were cocultured without (none) or with unpulsed DCcontrols or DCVIPs (5 × 103, 5 × 104, or 5 × 105 cells) derived from the indicated donors (allogeneic 1 and syngeneic 2) for 3 days. These CD8 T cells were then rescued and subsequently assayed (5 × 105 cells) for cytotoxicity against the allogeneic fibroblasts (104 cells) used in the priming culture (donor 1). The value of spontaneous release cpm was less than 10% of the total release cpm. Each result is the mean ± SD of 4 experiments performed in duplicate.
As with CD4 Treg cells, CD8 Treg cells have been shown to suppress the proliferation/activation of antigen-specific effector CD4 T cells.8,21-23 To determine whether CD8 T cells exposed to DCVIPs become functional CD8 Treg cells, we restimulated human TH1 cells with alloreactive mDCs in the presence of CD8 T cells derived from the same donor and previously primed with allogeneic DCcontrols (CD8Treg controls) or DCVIPs (CD8Treg VIPs). CD8Treg VIPs inhibited the proliferation of syngeneic TH1 cells in response to allogeneic mDCs in a dose-dependent manner, whereas CD8Treg controls are not suppressive (Figure 5C, left panel). Similar results were obtained for IL-2 production (not shown). The suppressive capacity of CD8Treg VIPs was abolished when CD8+CD28+ cells were used (Figure 5C, middle panel). The CD8+CD28- T cells generated with DCVIPs were more inhibitory than those generated with DCcontrols on a per cell basis for TH1 proliferation in response to allogeneic mDCs (Figure 5C, right panel). The suppression of TH1 proliferation was abrogated when CD8Treg VIPs and TH1 cells were separated, and it was bypassed by the addition of IL-2 (Figure 5D). In agreement with contact dependence, blockage of CTLA4, but not of IL-10 or TGFβ1, reversed the suppressive action of CD8Treg VIPs on TH1 proliferation and IFNγ production (Figure 5D). Phenotypic analysis showed that CD8 T cells exposed to DCVIPs contain a high percentage of cells expressing constitutive CTLA4 compared with untreated or DCcontrols-treated naive CD8 T cells (Figure 5E). These results suggest that DCVIPs lead to the generation of CD8+CD28-CTLA4+ Treg cells from naive CD8+ T cells.
Discussion
The induction of antigen-specific tolerance is critical for the prevention of autoimmunity and the maintenance of immune tolerance. In addition to their classical role as sentinels of the immune response inducing T cell reactivity, increasing evidence indicates that DCs can induce specific T cell tolerance. Although the underlying mechanisms are not fully understood, the capacity to induce Treg cells is an important property of tolerogenic/regulatory DCs. The in vitro generation of tolerogenic “designer” DCs is a desirable goal and represents the subject of intensive investigations. We report here the use of the neuropeptide VIP as a new approach to induce human tolerogenic DCs with the capacity to generate CD4 and CD8 Treg cells. The presence of VIP during the early stages of DC differentiation from human blood monocytes leads to the development of DCs that cannot mature after inflammatory stimuli. These DCVIPs exhibit a tolerogenic phenotype, characterized by low expression of costimulatory molecules (CD40, CD80, CD86), low production of proinflammatory cytokines, and increased production of IL-10. These cells do not prime T-cell responses, and they suppress previously primed immune responses. Exposure of T cells to DCVIPs results in the induction of CD4 and CD8 Treg cells.
DCVIP generate human CD8 Treg. (A) Human naive CD8+ T cells (5 × 106) were cultured with allogeneic DCcontrols or DCVIPs (5 × 105 cells). After 5 days, CD8 T cells were isolated from the cocultures and assayed for phenotype (left panel) and cytokine profile (right panel) by flow cytometry. Human naive CD8+ T cells were used as controls. Numbers represent the percentage of positive cells in each quadrant. Cytokine levels in cultured supernatants (determined by ELISA) were consistent with the intracellular staining (not shown). Results are representative of 4 experiments with similar findings. (B) Total CD8+, CD8+CD28+, or CD8+CD28- T cells were isolated from the coculture of human naive CD8+ T cells (5 × 106) with allogeneic DCcontrols or DCVIPs (5 × 105 cells) for 5 days and were cultured with human allogeneic mDCs (104). Proliferation of CD8 T cells was determined. CD8 T cells without allogeneic mDCs did not proliferate. Each result is the mean ± SD of 3 experiments performed in duplicate. (C) Total CD8+, CD8+CD28+, or CD8+CD28- were isolated from the coculture of human naive CD8+ T cells (5 × 106) with allogeneic DCcontrols or DCVIPs (5 × 105 cells) for 5 days and were cocultured at different ratios with human syngeneic TH1 cells (105) in the presence of allogeneic mDCs (104). (D) Human TH1 cells were cultured with allogeneic mDCs (104) and CD8+ T cells (CD8VIP, 2 × 104) isolated from cocultures with DCVIPs, in the presence or absence of blocking anti-IL-10, anti-TGFβ, anti-CTLA-4, or IL-2. Isotype IgG was used as control. Additionally, TH1 + mDCs were separated from CD8VIP + mDCs in a Transwell system. The proliferative response of TH1 cells and the production of IFNγ were determined after 4 days of culture. mDCs alone did not proliferate. Each result is the mean ± SD of 3 experiments performed in duplicate. (E) CTLA-4 expression of naive CD8 T cells or CD8 T cells isolated from cocultures of human naive CD8 T cells and allogeneic DCcontrols or DCVIPs was determined by flow cytometry. Dashed lines correspond to isotype IgG controls. Results are representative of 4 independent experiments.
DCVIP generate human CD8 Treg. (A) Human naive CD8+ T cells (5 × 106) were cultured with allogeneic DCcontrols or DCVIPs (5 × 105 cells). After 5 days, CD8 T cells were isolated from the cocultures and assayed for phenotype (left panel) and cytokine profile (right panel) by flow cytometry. Human naive CD8+ T cells were used as controls. Numbers represent the percentage of positive cells in each quadrant. Cytokine levels in cultured supernatants (determined by ELISA) were consistent with the intracellular staining (not shown). Results are representative of 4 experiments with similar findings. (B) Total CD8+, CD8+CD28+, or CD8+CD28- T cells were isolated from the coculture of human naive CD8+ T cells (5 × 106) with allogeneic DCcontrols or DCVIPs (5 × 105 cells) for 5 days and were cultured with human allogeneic mDCs (104). Proliferation of CD8 T cells was determined. CD8 T cells without allogeneic mDCs did not proliferate. Each result is the mean ± SD of 3 experiments performed in duplicate. (C) Total CD8+, CD8+CD28+, or CD8+CD28- were isolated from the coculture of human naive CD8+ T cells (5 × 106) with allogeneic DCcontrols or DCVIPs (5 × 105 cells) for 5 days and were cocultured at different ratios with human syngeneic TH1 cells (105) in the presence of allogeneic mDCs (104). (D) Human TH1 cells were cultured with allogeneic mDCs (104) and CD8+ T cells (CD8VIP, 2 × 104) isolated from cocultures with DCVIPs, in the presence or absence of blocking anti-IL-10, anti-TGFβ, anti-CTLA-4, or IL-2. Isotype IgG was used as control. Additionally, TH1 + mDCs were separated from CD8VIP + mDCs in a Transwell system. The proliferative response of TH1 cells and the production of IFNγ were determined after 4 days of culture. mDCs alone did not proliferate. Each result is the mean ± SD of 3 experiments performed in duplicate. (E) CTLA-4 expression of naive CD8 T cells or CD8 T cells isolated from cocultures of human naive CD8 T cells and allogeneic DCcontrols or DCVIPs was determined by flow cytometry. Dashed lines correspond to isotype IgG controls. Results are representative of 4 independent experiments.
Although the precise mechanisms remain unknown, several possibilities may account for the generation of Treg cells by DCVIPs. The activation of naive CD4 T lymphocytes requires 2 signals delivered by mDCs, one mediated through antigen/MHC 2-TCR interaction (signal 1) and another mediated by the interaction of costimulatory molecules such as CD80/CD86-CD28 and CD40-CD40L. Costimulatory molecules, especially CD40, appear to be key determinants of the decision between tolerance and immunity.24-26 The characteristic phenotype of DCVIPs—high levels of MHC plus poor expression of costimulatory molecules, which will deliver stimulatory but not costimulatory signals—is in agreement with their tolerance-inducing ability. In addition, the observation that DCVIPs secrete IL-10 may be linked to the stability of their tolerogenic-like phenotype. IL-10 has been shown to inhibit the expression of costimulatory molecules on APCs, and the addition of IL-10 to bone marrow precursors resulted in enrichment in tolerogenic DCs.8,27 Furthermore, DCVIPs-derived IL-10 could be involved in the development of Tr1-like cells because adding IL-10 to primary T cells resulted in the generation of IL-10-producing T cells.28 However, blocking IL-10 produced by DCVIPs partially, but not totally, reversed their capacity to induce human CD4 Treg cells (not shown), suggesting the involvement of other mechanisms in the tolerogenic capacity of DCVIPs.
NF-κB activity is required for myeloid DC differentiation. The connection among NF-κB transactivating activity, tolerance— particularly the generation of tolerogenic DCs and IL-10-producing regulatory T cells—and lack of CD40, CD80, and CD86 expression or signaling has been demonstrated recently.27,29,30 As previously reported for monocytes/macrophages and mouse bone marrow-derived DCs,11,31 VIP reduces the nuclear translocation of NF-κB in human monocyte-derived DCs (not shown), supporting the involvement of this mechanism in the generation of tolerogenic DCs by VIP.
Our data indicate that the stimulation of naive CD4 T cells with allogeneic DCVIPs generates T cells that display the typical properties of Tr1 cells, including a characteristic cytokine production profile (high IL-10 and TGFβ and little or no IFNγ, IL-2, or IL-4), intrinsic low-proliferative capacity, and suppression of the antigen-specific proliferation/activation of other CD4 TH1 cells, even when TH1 cells were restimulated with mDCs. The mechanism of Tr1-induced suppression is still controversial. For example, evidence shows that suppression is primarily mediated by IL-10 and TGFβ in a cell contact-independent manner,28 whereas other studies describe a cytokine-independent, cell contact-mediated mechanism.32 Indeed, antigen-specific IL-10-producing Tr1 cells suppress inflammation in colitis and allergic models in an IL-10-dependent manner,28 whereas the blockade of TGFβ in vivo abrogates T cell-mediated suppression of severe colitis.33 DCVIPs-induced Tr1-like cells act through soluble factors and direct cellular contact, suggesting the presence of different CD4 Treg cell subpopulations. A characteristic marker of Treg cells is the constitutive expression of CTLA4, a negative regulatory factor critical for the induction and function of Treg cells.33,34 In agreement with these reports, CD4Treg VIPs express high levels of CTLA4, which explains their partial dependence on cell contact for suppressive activity. In addition, CD4Treg VIPs express high levels of membrane-bound TGFβ, which has been shown to participate in the suppressive activity of Treg cells.35 Some populations of regulatory DCs mediate their tolerogenic activity through the generation of peripheral CD4+CD25+ Treg cells.3,8,27 Although the CD4+CD25+ population is slightly increased in CD4Treg VIPs, the fact that CD4Treg VIPs did not express significant levels of the Treg markers Foxp3 and Nrp1 argues against the possibility that DCVIPs induce the generation of CD4+CD25+ Treg cells.
Our study also shows that priming of naive CD8+ T cells with allogeneic DCVIPs reduced their proliferative and lytic activity in an antigen-dependent manner. In contrast to DCcontrols, which activate CD8 T cells through the delivery of signal 1 plus signal 2, DCVIPs deliver a potent signal 1 and a weak signal 2 to antigen-specific CD8+ T cells, resulting in anergy. The anergic state is associated with an increased number of IL-10-producing CD8+CD28- T cells. CD8 T cells exposed to DCVIPs suppress syngeneic TH1 effector cells, a characteristic that seems to reside in the CD8+CD28- population. CD8+CD28- T cells generated in various ways were shown to suppress CD4 T cells.8,22,23,36 Although CD8 Treg cells with regulatory properties have received less attention than CD4 Treg cells, several studies have demonstrated their importance in tolerance, and tolerogenic DCs were shown to play an essential role in the induction of CD8Treg.8,22,37,38 Consistent with previous reports,8,22,39 CD8Treg VIPs mediated their suppressive capacity by direct cell contact with CD4 T effector cells, and increased CTLA4 expression in CD8Treg VIPs appears to play a major role. The lack of CD28 expression on the CD8Treg VIPs may reflect expansion of CD8+CD28- precursor cells or the loss of CD28 expression during in vitro culture. Several studies in humans suggest that CD8+ T cells with suppressive function can be derived from human CD8+CD28- T cells but not from CD8+CD28+ T cells.22,23,40
Interestingly, DCVIPs retained their capacity to induce Treg under inflammatory conditions. This observation is particularly relevant for conditions in which ongoing antigen presentation is associated with chronic inflammation, including autoimmune diseases, allograft rejection, and GVHD. We have recently found that murine regulatory DCVIPs showed a prominent therapeutic action on models of rheumatoid arthritis, multiple sclerosis,13 and allogeneic bone marrow transplantation,14 even when administered after disease onset. The in vivo efficacy of DCVIPs was antigen specific and depended on their compatibility with the host MHC antigens. We found that DCVIPs directly suppressed not only the effector functions of autoreactive T cells and in vivo-primed allogeneic CD4 and CD8 T cells but also their responsiveness to in vitro restimulation. Therefore, the mechanism responsible for the DCVIPs therapeutic effect in autoimmunity and acute GVHD involves the induction of tolerant T cells and direct suppression of effector T cells in vivo. In agreement with this hypothesis, treatment with CD4Treg VIPs abrogated, in a haplotype/antigen- and a TGFβ/IL-10-dependent manner, acute GVHD in mice after bone marrow transplantation and in arthritic mice after disease progression.13,14
It has been proposed that tolerance induction by DCs requires maturation signals different from microbial or inflammatory stimuli. In steady state conditions, VIP could represent one of the endogenous maturation signals driving the differentiation of tolerogenic DCs with a semimature phenotype. VIP is secreted in the lymphoid microenvironment, mainly by TH2 cells after antigen stimulation, and VIP levels are increased in immunopathologic conditions such as autoimmunity and inflammation.10,11 Therefore, DCVIPs may represent a population of DCs that have matured to display a stable tolerogenic phenotype. Under steady state conditions, DCVIPs could be loaded with self-antigens and commonly encountered antigens; after migration to the lymphoid organs, they could induce CD4 and CD8 Treg differentiation and tolerance. We have recently demonstrated that the administration of VIP on TCR transgenic mice induced the in vivo emergence of DCs in draining lymph nodes with the capacity to generate antigen-specific CD4 T cells with regulatory capacity.31 However, as far as we know, the presence of cells with a DCVIPs phenotype has not been reported in humans in physiologic or pathologic conditions.
The possibility of generating human tolerogenic DCVIPs opens new therapeutic avenues for the treatment of autoimmune diseases and allogeneic transplantation. In animal models, the in vitro pulsing of tolerogenic DCVIPs with self-antigens or alloantigens, followed by in vivo injection, led to the differentiation of antigen-specific Treg cells. Therefore, the inclusion of tolerogenic DCVIPs in future therapeutic regimens may minimize the dependence on nonspecific immunosuppressive drugs used currently for the treatment of autoimmune disorders and transplant rejection.
Prepublished online as Blood First Edition Paper, January 5, 2006; DOI 10.1182/blood-2005-11-4497.
Supported by grants from the Spanish Ministry of Health (PI04/0674; M.D.), the National Institutes of Health (2RO1A047325; D.G., M.D.), and the Ramon Areces Foundation (M.D.) and by fellowships from Junta de Andalucia (M.D., E.G.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal