AMD3100, a bicyclam antagonist of the chemokine receptor CXCR4, has been shown to induce rapid mobilization of CD34+ hematopoietic cells in mice, dogs, and humans, offering an alternative to G-CSF mobilization of peripheral-blood hematopoietic stem cells. In this study, AMD3100-mobilized CD34+ cells were phenotypically analyzed, marked with NeoR-containing retroviral vectors, and subsequently transplanted into myeloablated rhesus macaques. We show engraftment of transduced AMD3100-mobilized CD34+ cells with NeoR gene marked myeloid and lymphoid cells up to 32 months after transplantation, demonstrating the ability of AMD3100 to mobilize true long-term repopulating hematopoietic stem cells. More AMD3100-mobilized CD34+ cells are in the G1 phase of the cell cycle and more cells express CXCR4 and VLA-4 compared with G-CSF-mobilized CD34+ cells. In vivo gene marking levels obtained with AMD3100-mobilized CD34+ cells were better than those obtained using CD34+ cells mobilized with G-CSF alone. Overall, these results indicate that AMD3100 mobilizes a population of hematopoietic stem cells with intrinsic characteristics different from those of hematopoietic stem cells mobilized with G-CSF, suggesting fundamental differences in the mechanism of AMD3100-mediated and G-CSF-mediated hematopoietic stem cell mobilization. Thus, AMD3100-mobilized CD34+ cells represent an alternative source of hematopoietic stem cells for clinical stem cell transplantation and genetic manipulation with integrating retroviral vectors.
Introduction
Mobilized peripheral-blood (PB) CD34+ cells have become the preferred source of repopulating hematopoietic stem cells (HSCs), replacing bone marrow (BM) cells for autologous or allogeneic transplantation in patients with hematolymphoid malignancies or solid tumors. Mobilized PB collections contain much larger numbers of CD34+ cells, are obtained with less intervention and pain to donors, shorten engraftment time and hospital stay for recipients, and result in lower overall cost.1 Conventional strategies for HSC mobilization include administration of cytokines, namely G-CSF, alone or in combination with myelosuppressive chemotherapy. PB HSC mobilization and collection by leukapheresis have been optimized in numerous clinical trials, but a proportion of patients and donors still fail mobilization. In addition, in some patient populations, cytokine mobilization appears to have unacceptable risks, for instance in patients with sickle cell anemia, severe sickle cell crises and even death have been reported following the use of G-CSF for mobilization.2-4 Thus, new mobilizing agents with high efficacy and a low side effect profile are needed.
In recent years, some of the mechanisms underlying PB HSC mobilization have been elucidated, leading to the development of new mobilization strategies and better understanding of the processes of egress and ingress of primitive hematopoietic cells from the marrow microenvironment. The interaction between CXCR4, a CXC chemokine receptor, and its ligand stromal-derived factor-1α (SDF-1α) has been found to play a key role in PB HSC mobilization.5 The bicyclam molecule AMD3100, shown to reversibly block the interaction between CXCR4 and SDF-1α, was initially developed for selective inhibition of CXCR4-facilitated human immunodeficiency virus (HIV) entry into cells.6,7 During pharmacokinetic studies with single-dose intravenous AMD3100 administration in healthy volunteers, an unexpected increase in white blood cell (WBC) count was noted.8 These observations prompted studies to evaluate the effects of AMD3100 on mobilization of PB HSCs. Broxmeyer et al9 demonstrated rapid mobilization of murine hematopoietic progenitor cells (HPCs) and long-term repopulating (LTR) cells after AMD3100 administration in various strains of mice. Those studies led to the first clinical trials of AMD3100 for mobilization of CD34+ cells and HPCs in healthy human volunteers.10 Maximum increase in circulating HPCs occurred in humans 6 hours after intravenous injection of AMD3100. Using limiting dilution analyses in the nonobese diabetic/severe combined immune deficiency (NOD/SCID) xenograft model, the frequency of NOD/SCID repopulating cells (SRCs) per 106 CD34+ cells in the PB of healthy donors was 4.5-fold higher than the frequency detected in comparable G-CSF-mobilized PB collections.9 AMD3100 has also been combined with G-CSF in mice9 and healthy volunteers,9,11 resulting in a synergistic increase in CD34+ cell mobilization. The frequency of SRCs per kilogram in apheresis samples mobilized with G-CSF and AMD3100 was higher compared with samples mobilized with G-CSF or AMD3100 alone.9 Recently, AMD3100, alone12 or in combination with G-CSF,13 has been shown to be safe and effective for the rapid mobilization of CD34+ cells in patients with multiple myeloma and non-Hodgkin lymphoma who had received prior chemotherapy, suggesting the utility of this agent in various clinical stem cell transplantation settings.
Retroviral vectors have been used to introduce genetic markers into HSCs as a means to follow HSC progeny after in vivo reconstitution of an ablated recipient. Detection of retrovirally marked hematopoietic cells in the PB of a recipient several months after transplantation is an indication of hematologic reconstitution with marked long-term repopulating HSCs. Over the past 3 decades, this approach has allowed quantitative elucidation of the hallmark properties of HSCs and the development of a comprehensive systemic picture of in vivo stem-cell behavior over time.14-21 The nonhuman primate model has proven a valuable in vivo assay for evaluation of HSC-based gene transfer technologies because of stem-cell dynamics, cytokine responsiveness, and retroviral receptor properties approximating those found in humans.22,23
In this study, we used retroviral gene marking to demonstrate long-term repopulating capacity of AMD3100-mobilized CD34+ cells in the autologous transplantation rhesus macaque model. We also compared cell-cycling status, surface phenotype, and in vitro migration toward SDF-1α of CD34+ cells collected from rhesus macaque AMD3100-mobilized PB to G-CSF-mobilized PB and to steady state BM to gain insights into the mechanisms of mobilization with this agent, and potential differences in the characteristics of cells mobilized with AMD3100 compared with G-CSF.
Materials and methods
Collection of PB HSCs from rhesus macaques
Young rhesus macaques (Macaca mulatta) used in these studies were housed and handled in accordance with the guidelines set by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (DHHS publication No. NIH 85-23), and the protocol was approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute. In 2 animals (RC909 and RQ2851), PB cells were harvested 3 hours after administration of a single subcutaneous dose of 1 mg/kg AMD3100 (AnorMED, Langley, Canada), and collected cells were used for transplantation. Previous dose-response studies showed a maximum increase in circulating CD34+ cells between 3 and 6 hours after 1 mg/kg AMD3100 in rhesus macaques (data not shown). In 4 additional animals (RQ3636, RQ3590, RQ3565, RQ4380), BM was aspirated from the posterior iliac crest for analysis. One week later, a single dose of AMD3100 1 mg/kg was administered, and PB cells were collected by leukapheresis.24 One month after the mobilization with AMD3100, recombinant human (rhu) G-CSF (10 μg/kg; Amgen, Thousand Oaks, CA) was administered as daily subcutaneous injections for 3 days, and twice daily on the fourth day. Mobilized PB cells were collected by leukapheresis on day 5 as described.24 AMD3100-mobilized and G-CSF-mobilized CD34+ cells were transduced with 2 different retroviral vectors and used simultaneously for transplantation into 2 of these 4 latter animals (RQ3636 and RQ3590).
For each cell fraction (BM, AMD3100-mobilized PB cells, and G-CSF-mobilized PB cells), peripheral-blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation over lymphocyte separation media (LSM) (ICN Biomedicals/Cappel, Aurora, OH). CD34+ cells were enriched using the 12.8 IgM anti-CD34 biotinylated antibody and magnetic cell sorting (MACS) streptavidin microbeads (Miltenyi Biotec, Auburn, CA) per manufacturer's instructions. Purities of 80% to 95% CD34+ cells were typically obtained. In 2 animals (RQ3565 and RQ4380), CD34+ enrichment was further increased to greater than 97% by sorting using flow cytometry (Coulter EPICS Altra; Beckman Coulter, Miami, FL).
CFU assays
CFU assays were performed using MethoCult M4230 methylcellulose (MC) media (StemCell Technologies, Vancouver, BC) supplemented with 5 U/mL rhu erythropoietin (Amgen), 10 ng/mL rhuGM-CSF (Sandoz, East Hanover, NJ), 10 ng/mL rhuIL-3 (Sandoz), and 100 ng/mL rhuSCF (Amgen) at 37°C in 5% CO2. Between days 10 and 14, colonies of more than 50 cells were counted, and the degree of progenitor enrichment was calculated by enumerating the colonies in CFU assays on cells cultured before and after CD34+ enrichment.
Vectors and transduction procedure
Moloney murine leukemia virus-derived retroviral vectors (G1Na and LNL6) carrying a bacterial NeoR gene were used in this study.25,26 We assessed the biologic titers of these vectors by making serial dilutions of the vectors and then monitoring the transfer of G418 resistance to HeLa cells. The biologic titers of both vectors were equivalent and between 1 and 5 × 106 biologically active vector particles/mL. For transduction, retroviral supernatant was harvested from subconfluent producer cells cultured for 12 to 18 hours in DMEM (Mediatech, Herndon, VA) supplemented with 10% FBS (HyClone, Logan, UT), and 4 mM l-glutamine, penicillin (50 mg/mL), and streptomycin (50 mg/mL) at 37°C in 5% CO2. Fresh vector supernatant was passed through a 22-μm nylon filter (Millipore, Bedford, MA) to remove cellular debris before transduction. CD34+ cells were cultured at a starting concentration of 1 to 2 × 105 cells/mL in filtered vector supernatant, supplemented with 100 ng/mL recombinant human stem cell factor (rhuSCF; Amgen), 100 ng/mL rhuFlt-3 ligand (Immunex, Seattle, WA), and 100 ng/mL megakaryocyte growth and development factor (MGDF; Kirin-Amgen, Thousand Oaks, CA), and 4 μg/mL protamine sulfate (Sigma, St Louis, MO) in flasks previously coated with the CH-296 fragment of fibronectin (Retronectin; TaKaRa, Shiga, Japan) per manufacturer's instructions. Every 24 hours, nonadherent cells were collected, spun down, resuspended in fresh vector supernatant and cytokines, and added back to the same fibronectin-coated flasks. At the end of the 96-hour incubation period, cultured cells were removed from the plates with trypsin. Each animal received 2 doses of 500 cGy total body irradiation and was reinfused with one (AMD3100-mobilized PB cells; RC909 and RQ2851) or both (AMD3100- and G-CSF-mobilized PB cells; RQ3636 and RQ3590) aliquots of transduced cells via a central venous catheter, 1 day after the last irradiation. Twenty-four hours later, G-CSF at 5 μg/kg was initiated intravenously daily until the total WBC count reached 6000/μL. Standard supportive care was administered, including blood-product transfusions, fluid and electrolyte management, and antibiotics as needed. Hematopoietic recovery was monitored by daily complete blood counts.
Sample collection
PB samples were collected at the time of recovery, monthly through 6 months after transplantation, and then every 3 months. From each blood sample, PBMCs and granulocytes (GRANs) were isolated by density gradient centrifugation over LSM as previously described.27
Analysis of transduction efficiency
The transduction efficiency in progenitor cells (CFU) was calculated by dividing the number of colonies positive for the NeoR gene after 35 cycles of a nested polymerase chain reaction (PCR) amplification by the total number of colonies20 analyzed for each transduction experiment. The primers used were NeoR outer forward primer, 5′-GGCCAGACTGTTACCACTCC-3′; NeoR outer reverse primer, 5′-CAGCCGATTGTCTGTTGTGC-3′; NeoR inner forward primer, 5′-CGGATCGCTCACAACCAGTC-3′; NeoR inner reverse primer, 5′-AGCCGAATAGCCTCTCCACC-3′. To determine in vivo gene marking, genomic DNA was extracted from PBMCs and GRANs collected at different time points after transplantation using the QIAamp DNA blood Midi kit (Qiagen, Valencia, CA). At one time point after transplantation, DNA was also extracted from B and T cells purified from the PBMC fraction from each animal by sorting using flow cytometry (Coulter EPICS Altra). Real-time PCR was performed using an ABI Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA) for quantification of NeoR gene transfer efficiency. The primers and probes used were specific for the G1Na or LNL6 retroviral vectors: G1Na forward primer, 5′-TCGGTAGTCGACGGATCC-3′; G1Na reverse primer, 5′-TTGCCAAACCTACAGGTGG-3′; G1Na probe, 5′-FAM/AGAAGCTTGGGCCCATCGA/TAMRA-3′; LNL6 forward primer, 5′-AAGGGACCTCAAGGCTTTCC-3′; LNL6 reverse primer, 5′-CCTGTCTTTAACAAATTGGACTAATCG-3′; LNL6 probe, 5′-JOE/AGGGACACTAGGCTGACTCCATCGAGC/TAMRA-3′. Real-time PCR results were normalized to the amounts of albumin DNA: albumin forward primer, 5′-CTTCACAGAATAGGGTTGAAGATTGA-3′; albumin reverse primer, 5′-AGCACAGGTTTTGTGGTTTTTAAAT-3′; albumin probe, 5′-FAM/CATAACTATCCCAAAGACCTATCCATTGCACTATGCTT/TAMRA-3′.
Cell-cycle analysis
Cell-cycle analysis was performed using the fluorochrome acridine orange as previously described.28 Briefly, 1 to 2 × 105 CD34+ cells were resuspended in ice-cold PBS containing 1% BSA (ICN Biomedicals). A volume of 0.4 mL was gently added of solution A (Triton X-100, 0.1% [vol/vol], HCl 0.08 M, NaCl 0.15 M). The cells were allowed to chill on ice for 15 seconds, and 1.2 mL ice-cold solution B was subsequently added (acridine orange 6 μg/mL [Molecular Probes, Eugene, OR], EDTA-Na 1 mM, NaCl 0.15 M, phosphate-citric acid buffer pH 6.0). Cell luminescence was measured during the next 2 to 10 minutes using flow cytometry (Coulter EPICS XL-MCL cell analyzer; Beckman Coulter). A single 15 mW argon ion laser operating at 488 nm was used. The green fluorescence signal (DNA detection) was selected with a 525 ± 25 nm bandpass filter (FL1), and the red luminescence (RNA detection) was selected with a 675 ± 25 nm bandpass filter (FL4). The fraction of cells in the G0, G1, S, and G2/M phases of the cell cycle was determined by gating on cell populations defined by their DNA and RNA contents as previously described.28
Flow cytometry
CD34+ cells (1 × 105) were incubated for 30 minutes at 4°C with monoclonal antibodies all cross-reactive to rhesus macaque cells, including FITC-conjugated anti-human CD49d (VLA-4) (Clone HP2/1; Beckman Coulter), PE-conjugated anti-human CD34 (clone 563; BD Biosciences Pharmingen, San Diego, CA), and PE-Cy5-conjugated anti-human CXCR4 (clone12G5; eBioscience, San Diego, CA). A Coulter EPICS XL-MCL cell analyzer was used for analysis, and 10 000 to 20 000 cells were recorded for each sample. B- and T-cell sorting was performed using FITC-conjugated anti-human CD3 (clone J606; BD Biosciences Pharmingen) and PE-conjugated anti-human CD20 (clone B1-RD1; Beckman Coulter) both cross-reactive to rhesus macaque cells. Cells were sorted using a fluorescence-activated cell sorting (FACS) Vantage SE DiVA instrument (Becton Dickinson, San Jose, CA).
SDF-1α migration assay
Migration assays were performed in duplicate in transwell plates (Costar, Cambridge, MA) of 6.5-mm diameter, with 5-μm pore uncoated filters. Before adding cells to the upper compartment, the transwells were washed 3 times with assay medium (RPMI with 0.25% BSA). A total of 1 × 105 CD34+ cells or positive control Jurkat cells were resuspended in 0.1 mL assay medium, and these cells were seeded in the upper compartment of the transwells. A volume of 0.6 mL assay medium was added in the presence or absence of 100 ng/mL SDF-1α to the lower compartment. A 0.1-mL sample containing cells was kept in assay medium as an input control for the quantitation of the number of migrated cells. The transwell plates were incubated at 37°C, in 5% CO2, for 4 hours. Migrated cells were quantitated using polystyrene beads 6.79 μm (Spherotech, Libertyville, IL) and flow cytometry analysis by determining the ratio between the number of cells before and after migration per 5000 polystyrene beads counted. Using this method, a minimum number of 100 migrated cells could be determined reliably.
Statistical analysis
Analysis of significance was carried out using the 2-tailed Student t test and regression analysis using SigmaPlot (SPSS Science, Chicago, IL) and Excel (Microsoft, Seattle, WA).
Results
AMD3100 mobilizes HSCs with long-term repopulating capacity in the rhesus macaque autologous transplantation model
We used retroviral gene marking to demonstrate long-term repopulating capacity of AMD3100-mobilized CD34+ cells in the rhesus macaque autologous transplantation model. In 4 animals (RC909, RQ2851, RQ3636, and RQ3590), PB cells were harvested by apheresis after administration of 1 or 2 subcutaneous doses of AMD3100 1 mg/kg. Animals RQ3636 and RQ3590 were also mobilized with G-CSF 1 month after AMD3100 mobilization for competitive repopulation experiments (see “In vivo gene marking levels obtained with AMD3100-mobilized CD34+ cells are better than levels obtained with G-CSF-mobilized CD34+ cells”). CD34+ cells were enriched and transduced for 4 days with retroviral vectors containing the neomycin resistance (NeoR) gene (G1Na or LNL6 producer cell lines), in fibronectin (FN)-coated flasks in the presence of cytokines. NeoR gene transfer efficiency in progenitor cells (CFUs) derived from cells cultured at the end of the transduction period was 80% to 100%. Animals were conditioned with 500 cGy daily for 2 days, and cells were reinfused after transduction. Table 1 summarizes the CD34+ cell counts obtained after each mobilization procedure for these 4 animals and 2 additional monkeys mobilized for in vitro studies (see “More AMD3100-mobilized CD34+ cells are in the G1 phase of the cell cycle compared with G-CSF-mobilized CD34+ cells” and “More AMD3100-mobilized CD34+ cells express CXCR4 and VLA-4 compared with G-CSF-mobilized CD34+ cells”). As observed in clinical practice, there was variability in the efficiency of mobilization between individual monkeys; we mobilized between 3.6 × 106 and 2.6 × 107 CD34+ cells per kilogram of body weight after a single dose of AMD3100. In 1 of 4 animals (RC909), a second dose of AMD3100 and repeat apheresis 24 hours after the first apheresis were required to increase the yield of collected PB CD34+ cells. None of the animals showed evidence of adverse effects to AMD3100 administration, including fever, vomiting, diarrhea, gastrointestinal bleeding, loss of appetite, or decreased activity levels. The time to hematopoietic recovery, as defined by an absolute neutrophil count greater than 500/μL, varied between 7 and 14 days (Table 1).
Summary of CD34+ cell counts and engraftment kinetics of rhesus macaques mobilized with AMD3100 and/or G-CSF
Animal and mobilizing agent . | No. of CD34+ cells* . | Time to engraftment, d† . |
|---|---|---|
| RC909 | 14 | |
| AMD3100 | 3.6 × 106 | |
| AMD3100‡ | 4.8 × 106 | |
| RQ2851 | 7 | |
| AMD3100 | 1.9 × 107 | |
| RQ3636 | 11 | |
| AMD3100 | 2.6 × 107 | |
| G-CSF | 8.3 × 106 | |
| RQ3590 | 8 | |
| AMD3100 | 4.0 × 106 | |
| G-CSF | 2.4 × 106 | |
| RQ3565§ | N/A | |
| AMD3100 | 1.6 × 107 | |
| G-CSF | 3.2 × 106 | |
| RQ4380§ | N/A | |
| AMD3100 | 1.9 × 107 | |
| G-CSF | 1.6 × 106 |
Animal and mobilizing agent . | No. of CD34+ cells* . | Time to engraftment, d† . |
|---|---|---|
| RC909 | 14 | |
| AMD3100 | 3.6 × 106 | |
| AMD3100‡ | 4.8 × 106 | |
| RQ2851 | 7 | |
| AMD3100 | 1.9 × 107 | |
| RQ3636 | 11 | |
| AMD3100 | 2.6 × 107 | |
| G-CSF | 8.3 × 106 | |
| RQ3590 | 8 | |
| AMD3100 | 4.0 × 106 | |
| G-CSF | 2.4 × 106 | |
| RQ3565§ | N/A | |
| AMD3100 | 1.6 × 107 | |
| G-CSF | 3.2 × 106 | |
| RQ4380§ | N/A | |
| AMD3100 | 1.9 × 107 | |
| G-CSF | 1.6 × 106 |
N/A indicates not applicable.
Number of CD34+ cells per kilogram body weight collected by apheresis after mobilization with AMD3100 or G-CSF
Days to reach an absolute neutrophil count greater than 500/μL
Animal RC909 required a second dose of AMD3100 and repeat apheresis 24 hours after the first apheresis to increase the yield of collected PB CD34+ cells
Animals RQ3565 and RQ4380 did not receive transplants; CD34+ cells obtained from these animals were used for in vitro studies only
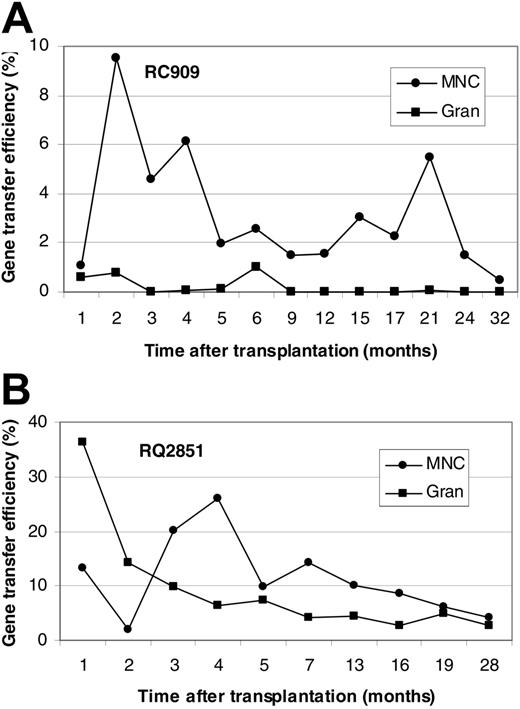
Figure 1 shows in vivo NeoR gene marking levels in PBMCs and GRANs at different time points after transplantation for animals RC909 and RQ2851. Levels of PBMC, B-cell, T-cell, and GRAN NeoR gene marking at steady state were 0.5%, 0.2%, 0.5%, and 0.05%, respectively, in animal RC909 up to 32 months after transplantation (Figure 1A). In animal RQ2851, levels of PBMC, B-cell, T-cell, and GRAN NeoR gene marking at steady state were 5%, 1%, 4%, and 3%, respectively, up to 28 months after transplantation (Figure 1B). A third animal (RQ3636) was analyzed up to 24 months after transplantation, and steady-state marking levels of 3%, 2%, 3% and 0.6% were detected in PBMCs, B cells, T cells, and GRANs, respectively (Figure 2A). Animal RQ3590 showed steady-state marking levels of 0.1%, 0.3%, 0.1%, and 0.01% in PBMCs, B cells, T cells, and GRANs, respectively, up to 24 months after transplantation (Figure 2B). These long-term in vivo marking results using retrovirally marked AMD3100-mobilized CD34+ cells confirm the ability of AMD3100 to mobilize true long-term repopulating HSCs, and the ability of these cells to be successfully transduced with retroviral vectors.
More AMD3100-mobilized CD34+ cells are in the G1 phase of the cell cycle compared with G-CSF-mobilized CD34+ cells
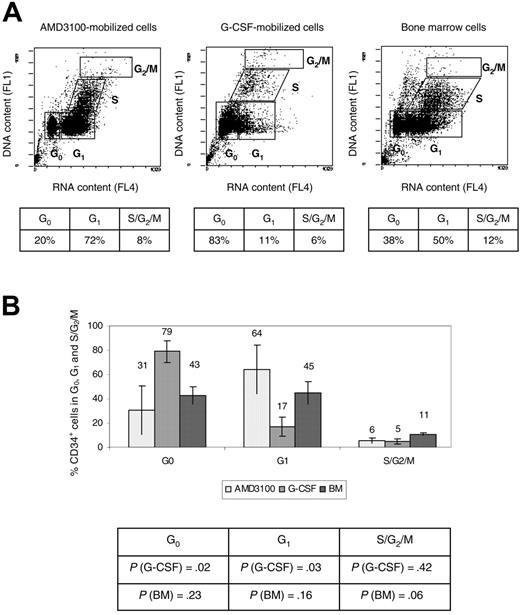
CD34+ cells obtained from 4 animals (RQ3590, RQ3636, RQ3565, and RQ4380) (Table 1) were analyzed using the fluorochrome acridine orange for quantification of the fraction of cells in the G0, G1, S, and G2/M phases of the cell cycle.28 A representative experiment (animal RQ3636) is shown (Figure 3A). Overall, 31% ± 20% AMD3100-mobilized CD34+ cells were in the G0 phase of the cell cycle, compared with 79% ± 9% G-CSF-mobilized CD34+ cells (P = .02), and 43% ± 7% for the BM CD34+ cells (P = .23) (Figure 3B). In contrast, 64% ± 20% AMD3100-mobilized CD34+ cells were in G1 compared with 17% ± 8% G-CSF-mobilized CD34+ cells (P = .03), and 45% ± 9% for the BM CD34+ cells (P = .16) (Figure 3B). There were no significant differences when comparing the S/G2/M phases of the cell cycle of AMD3100-mobilized CD34+ cells with BM or G-CSF-mobilized CD34+ cells. However, as previously reported,24,29 we observed more cells in the G2/S/M phase of the cell cycle in the BM fraction compared with the G-CSF-mobilized CD34+ cells (P = .005). Overall, these data demonstrate that more AMD3100-mobilized CD34+ cells are in the G1 phase of the cell cycle compared with G-CSF-mobilized CD34+ cells and approximate more closely the cycling status of steady-state BM CD34+ cells. These results suggest a different mechanism of mobilization with AMD3100 that results in the egress of cells from the BM with properties distinct from G-CSF-mobilized CD34+ cells.
In vivo gene marking levels derived from retroviral transduction of AMD3100-mobilized CD34+ cells. Presence of vector in PBMCs and GRANs at various times (in months) after transplantation of retrovirally transduced AMD3100-mobilized CD34+ cells in rhesus macaques was detected by real-time PCR. Results are presented for animal RC909 (A) and RQ2851 (B). Gene marking levels were also determined in B and T cells 32 months (RC909; B cells, 0.2%; T cells, 0.5%) or 28 months (RQ2851; B cells, 1%; T cells, 4%) after transplantation.
In vivo gene marking levels derived from retroviral transduction of AMD3100-mobilized CD34+ cells. Presence of vector in PBMCs and GRANs at various times (in months) after transplantation of retrovirally transduced AMD3100-mobilized CD34+ cells in rhesus macaques was detected by real-time PCR. Results are presented for animal RC909 (A) and RQ2851 (B). Gene marking levels were also determined in B and T cells 32 months (RC909; B cells, 0.2%; T cells, 0.5%) or 28 months (RQ2851; B cells, 1%; T cells, 4%) after transplantation.
Competitive repopulation experiments. In 2 animals (RQ3636 [A] and RQ3590 [B]), a single dose of AMD3100 (1 mg/kg) was administered subcutaneously. PB cells were collected by leukapheresis, enriched for CD34+ cells, transduced with G1Na retroviral vectors (animal RQ3590) or a second distinguishable NeoR-containing vector, LNL6 (animal RQ3636), and frozen for subsequent reinfusion in the animals. One month after the first mobilization, recombinant human G-CSF was administered, and hematopoietic cells were collected by leukapheresis on day 5. CD34+ cells were subsequently enriched and transduced with the alternative distinguishable retroviral vectors (LNL6 for animal RQ3590 and G1Na for animal RQ3636). Cells were frozen and soon after reinfused in the animals along with the previously transduced AMD3100-mobilized fraction. Gene marking levels were determined at different time points after transplantation by real-time PCR in PBMCs and GRANs. Gene marking levels were also determined in B and T cells 24 months after transplantation in animal RQ3636 (B cells, 2%; T cells, 3%) and animal RQ3590 (B cells, 0.3%; T cells, 0.1%).
Competitive repopulation experiments. In 2 animals (RQ3636 [A] and RQ3590 [B]), a single dose of AMD3100 (1 mg/kg) was administered subcutaneously. PB cells were collected by leukapheresis, enriched for CD34+ cells, transduced with G1Na retroviral vectors (animal RQ3590) or a second distinguishable NeoR-containing vector, LNL6 (animal RQ3636), and frozen for subsequent reinfusion in the animals. One month after the first mobilization, recombinant human G-CSF was administered, and hematopoietic cells were collected by leukapheresis on day 5. CD34+ cells were subsequently enriched and transduced with the alternative distinguishable retroviral vectors (LNL6 for animal RQ3590 and G1Na for animal RQ3636). Cells were frozen and soon after reinfused in the animals along with the previously transduced AMD3100-mobilized fraction. Gene marking levels were determined at different time points after transplantation by real-time PCR in PBMCs and GRANs. Gene marking levels were also determined in B and T cells 24 months after transplantation in animal RQ3636 (B cells, 2%; T cells, 3%) and animal RQ3590 (B cells, 0.3%; T cells, 0.1%).
More AMD3100-mobilized CD34+ cells express CXCR4 and VLA-4 compared with G-CSF-mobilized CD34+ cells
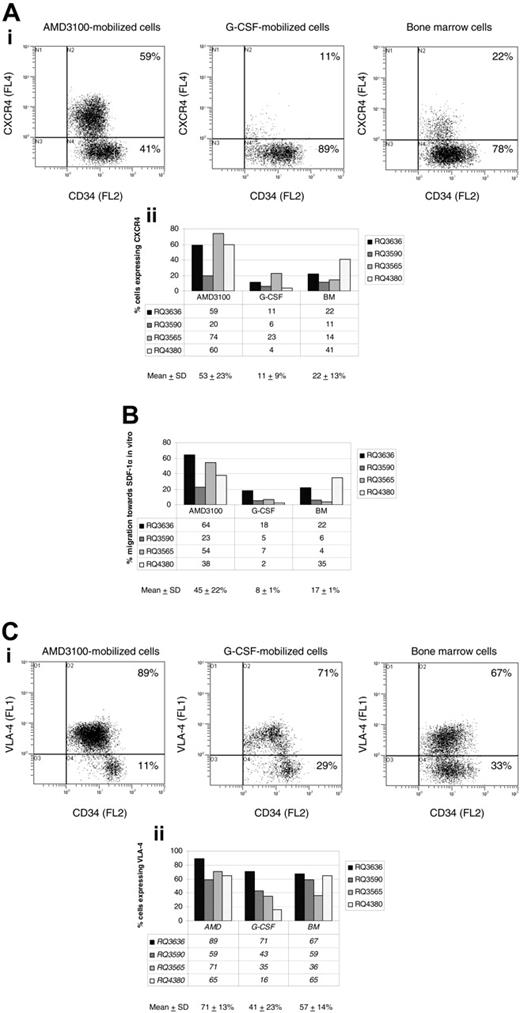
In the 4 animals discussed in “More AMD3100-mobilized CD34+ cells are in the G1 phase of the cell cycle compared with G-CSF-mobilized CD34+ cells” (RQ3590, RQ3636, RQ3565, and RQ4380), we conducted studies to investigate phenotypic and functional characteristics hypothesized to be important in mobilization and homing of hematopoietic progenitor and stem cells. We compared the cell-surface phenotype and the in vitro capacity of migration toward SDF-1α of steady-state BM, AMD3100-, and G-CSF-mobilized CD34+ cells. A representative experiment (animal RQ3636) comparing the expression of CXCR4 is shown (Figure 4A, top). Overall, flow cytometry analysis revealed CXCR4 expression on 53% ± 23% of AMD3100-mobilized CD34+ cells, compared with 11% ± 9% G-CSF-mobilized CD34+ cells (P = .02) and 22% ± 13% BM CD34+ cells (P = .07) (Figure 4A, bottom).
Cell-cycle analysis. CD34+ cells were analyzed using flow cytometry after staining with acridine orange to measure the G0, G1, S, and G2/M contents of each cell source (AMD3100-mobilized PB cells, G-CSF-mobilized PB cells, and BM) cells). (A) Representative experiment (animal RQ3636). The percentage of cells in each phase of the cell cycle is shown in the accompanying table below each flow cytometry panel. (B) Summary of the percentage of CD34+ cells in each phase of the cell cycle. The P values comparing G-CSF-mobilized or BM CD34+ cells with AMD3100-mobilized CD34+ cells are shown in the accompanying table below the summary chart.
Cell-cycle analysis. CD34+ cells were analyzed using flow cytometry after staining with acridine orange to measure the G0, G1, S, and G2/M contents of each cell source (AMD3100-mobilized PB cells, G-CSF-mobilized PB cells, and BM) cells). (A) Representative experiment (animal RQ3636). The percentage of cells in each phase of the cell cycle is shown in the accompanying table below each flow cytometry panel. (B) Summary of the percentage of CD34+ cells in each phase of the cell cycle. The P values comparing G-CSF-mobilized or BM CD34+ cells with AMD3100-mobilized CD34+ cells are shown in the accompanying table below the summary chart.
The increased number of AMD3100-mobilized CD34+ cells expressing CXCR4 also correlated with a marked increase in their ability to migrate toward SDF-1α in vitro (45% ± 22%) compared with G-CSF-mobilized CD34+ cells (8% ± 1%, P = .01) (Figure 4B). The difference in migration to SDF-1α was not statistically significant when comparing AMD3100-mobilized CD34+ cells with BM CD34+ cells (17% ± 1%, P = .08) (Figure 4B). The difference in level of expression of CXCR4 on positive cells as assessed by mean fluorescence intensity was not statistically significant between the 3 cell populations analyzed.
A representative experiment (animal RQ3636) comparing the expression of VLA-4 is shown (Figure 4C, top). Overall, more AMD3100-mobilized CD34+ cells expressed the cell-surface marker VLA-4 (71% ± 13%) compared with G-CSF-mobilized CD34+ cells (41% ± 23%, P = .03). The difference was not statistically significant when compared with BM CD34+ cells (57% ± 14%, P = .2) (Figure 4C, bottom). The difference in level of expression of VLA-4 on positive cells as assessed by mean fluorescence intensity was not statistically significant between the 3 cell populations analyzed.
In vivo gene marking levels obtained with AMD3100-mobilized CD34+ cells are better than levels obtained with G-CSF-mobilized CD34+ cells
We conducted competitive repopulation experiments in 2 animals (RQ3636 and RQ3590) to compare levels of genetically modified cells attainable after retroviral transduction of AMD3100- and G-CSF-mobilized CD34+ cells (Figure 2A-B). In both animals, levels of in vivo PBMC and GRAN NeoR gene marking at steady state originated predominantly from the AMD3100-mobilized fraction rather than the G-CSF-mobilized cells. Animal RQ3636 showed approximately 2.5% and 0.6% in vivo gene marking in PBMCs and GRANs, respectively, derived from the AMD3100-mobilized fraction and undetectable marking in PBMCs and GRANs derived from the G-CSF-mobilized cells 24 months after transplantation (Figure 2A). In animal RQ3590, 0.1% and 0.01% in vivo gene marking in PBMCs and GRANs, respectively, was derived from AMD3100-mobilized cells compared with undetectable marking from the G-CSF-mobilized cells 24 months after transplantation (Figure 2B).
Phenotypic analysis and SDF-1α migration assay. CD34+ cells were obtained via bone marrow aspiration, or following mobilization with AMD3100 or G-CSF from 4 rhesus macaques. CD34+ cells were analyzed by flow cytometry following staining with monoclonal antibodies, including FITC-conjugated anti-human CD49d (VLA-4), PE-conjugated anti-human CD34, and PE-Cy5-conjugated anti-human CXCR4. (A) CXCR4 cell-surface marker expression on CD34+ cells in a representative experiment (animal RQ3636) is shown in the top panel, and a summary of CXCR4 expression on CD34+ cells in all 4 rhesus macaques is shown in the bottom panel. (B) SDF-1α migration assay. CD34+ cells from 4 rhesus macaques were incubated in transwells in the presence or absence of SDF-1α. The percentage migration of CD34+ cells toward SDF-1α in vitro is summarized for each cell population analyzed (BM, AMD3100-, and G-CSF-mobilized cells). (C) VLA-4 cell-surface marker expression on CD34+ cells in a representative experiment (animal RQ3636) is shown in the top panel, and a summary of VLA-4 expression on CD34+ cells in all 4 rhesus macaques is shown in the bottom panel. SD indicates standard deviation.
Phenotypic analysis and SDF-1α migration assay. CD34+ cells were obtained via bone marrow aspiration, or following mobilization with AMD3100 or G-CSF from 4 rhesus macaques. CD34+ cells were analyzed by flow cytometry following staining with monoclonal antibodies, including FITC-conjugated anti-human CD49d (VLA-4), PE-conjugated anti-human CD34, and PE-Cy5-conjugated anti-human CXCR4. (A) CXCR4 cell-surface marker expression on CD34+ cells in a representative experiment (animal RQ3636) is shown in the top panel, and a summary of CXCR4 expression on CD34+ cells in all 4 rhesus macaques is shown in the bottom panel. (B) SDF-1α migration assay. CD34+ cells from 4 rhesus macaques were incubated in transwells in the presence or absence of SDF-1α. The percentage migration of CD34+ cells toward SDF-1α in vitro is summarized for each cell population analyzed (BM, AMD3100-, and G-CSF-mobilized cells). (C) VLA-4 cell-surface marker expression on CD34+ cells in a representative experiment (animal RQ3636) is shown in the top panel, and a summary of VLA-4 expression on CD34+ cells in all 4 rhesus macaques is shown in the bottom panel. SD indicates standard deviation.
Discussion
AMD3100 mobilizes HSCs with long-term repopulating capacity in the rhesus macaque autologous transplantation model
Similar to findings in mice,9 healthy human volunteers,8-11 and patients with multiple myeloma or non-Hodgkin lymphoma,12,13 administration of AMD3100 induces rapid mobilization of CD34+ cells in rhesus macaques. Using a competitive repopulation assay, Broxmeyer et al9 have previously shown that AMD3100-mobilized murine cells contained repopulating activity, with self-renewal capacity as assessed by transplantation into lethally irradiated secondary mice. Studies in NOD/SCID xenograft models have also suggested that short-term repopulating cells are mobilized in humans by this agent,9 but unequivocal evidence for the presence of long-term repopulating cells in the blood of large animals following AMD3100 mobilization has not been shown previously and is important before the application of AMD3100-mobilized cells in allogeneic or autologous hematopoietic stem cell transplantation.
Autologous transplantation of rhesus macaque AMD3100-mobilized CD34+ cells transduced with retroviral vectors resulted in rapid (7-14 days) engraftment and long-term (up to 32 months of follow-up) in vivo gene marking. These findings demonstrate the ability of AMD3100 to induce mobilization of true long-term repopulating HSCs with adequate homing and engraftment capacity following transplantation. These results predict a clinical value for AMD3100 as a novel HSC mobilization agent for autologous or allogeneic stem cell transplantation. It is conceivable that AMD3100 will be of particular clinical benefit in combination with G-CSF as suggested by the recent demonstration of the synergistic mobilization effect of AMD3100 with G-CSF9,11,13 or that AMD3100 will be beneficial in mobilizing cells from patients unable to safely receive G-CSF or found to be poor mobilizers with G-CSF or other mobilization regimens.
Mechanisms of AMD3100- and G-CSF-mediated HSC mobilization are different
Our results demonstrate a statistically significant increase in the number of AMD3100-mobilized CD34+ cells expressing the chemokine receptor CXCR4 and the cell-adhesion molecule VLA-4 compared with G-CSF-mobilized CD34+ cells. The expression of CXCR4 and VLA-4 on AMD3100-mobilized CD34+ cells, and their ability to migrate toward SDF-1α in vitro, more closely resemble those of steady-state BM and may have important implications for their homing and engraftment abilities. These results are consistent with previous reports indicating a reduction in expression of VLA-4 and CXCR4 on G-CSF-mobilized cells compared with steady-state BM.5,30,31 The very rapid time frame of AMD3100 mobilization compared with cytokine mobilization suggests a much more direct pathway of release, and the similarities of these cells in phenotype and function to BM CD34+ cells also argues for a direct and nonselective release mechanism. A model has been proposed wherein G-CSF stimulation induces down-regulation of SDF-1α in BM stroma and up-regulates matrix metalloproteinase-9, neutrophil elastase, and cathepsin G.5 In the presence of proteases, cleavage of CXCR4 occurs, resulting in mobilization of HSCs and progenitors from BM into PB. In our study, the lower number of G-CSF-mobilized CD34+ cells expressing CXCR4 (11% ± 9%) is consistent with this model and could be the result of CXCR4 inactivation by proteolytic cleavage. A study also showed decreased staining with the CXCR4 monoclonal antibody 12G5 in G-CSF-mobilized CD34+ cells compared with BM CD34+ cells.32 In contrast, the higher number of AMD3100-mobilized CD34+ cells expressing CXCR4 (53% ± 23%) suggests an alternative mode of mobilization wherein direct reversible inhibition without proteolytic cleavage of CXCR4 receptors by AMD3100 leads to rapid mobilization. The increased number of AMD3100-mobilized CD34+ cells expressing VLA-4 (71% ± 13%) suggests that AMD3100-induced disruption of downstream signaling through CXCR4/SDF-1α pathway could also alter other adhesion receptor pathways (eg, VCAM-1/VLA-4) leading to mobilization of stem/progenitor cells. Integrin signaling can change adhesion characteristics through this class of receptors, potentially resulting in release of cells without proteolytic cleavage of the integrin receptor or its ligand VCAM-1. Collectively, these observations are consistent with a recent study of the mechanisms of stem-cell mobilization in transgenic mice deficient in one or more hematopoietic proteases, suggesting a complex model in which both protease-dependent and -independent pathways may contribute to stem-cell mobilization.33
AMD3100-mobilized CD34+ cells represent an alternative source of HSCs for transplantation and gene therapy applications
Our results support the notion that HSCs collected from alternative sources might have very different properties when used for transplantation or gene therapy. In vivo gene marking levels using AMD3100-mobilized CD34+ cells are comparable to in vivo levels previously achieved in the rhesus macaque model using PB CD34+ cells mobilized with a combination of G-CSF and SCF and are significantly better than those obtained using PB CD34+ cells mobilized with G-CSF alone.34 We considered possible explanations for the higher marking levels achieved using AMD3100-mobilized CD34+ cells compared with G-CSF-mobilized CD34+ cells. Assuming similar transduction efficiency between AMD3100- and G-CSF-mobilized CD34+ cells, it was conceivable that the larger number of CD34+ cells consistently obtained after AMD3100 mobilization (Table 1) and reinfused to the animals after transduction would result in higher in vivo markings in this competitive repopulation design. However, we found significantly higher levels of PBMC and GRAN NeoR gene markings originating from the AMD3100-mobilized CD34+ cells even after normalization for the number of CD34+ cells infused per kilogram weight of the animal. Alternatively, a relative increased frequency of repopulating HSCs within the total AMD3100-mobilized CD34+ population may have accounted for the increase in vivo NeoR gene marking. However, the 4.5-fold increase in SRC activity noted in AMD3100-mobilized compared with G-CSF-mobilized CD34+ cells9 cannot solely account for the increase in NeoR gene marking obtained with AMD3100-mobilized cells. Therefore, intrinsic differences in the target HSCs mobilized with AMD3100 likely permit more efficient transduction with retroviral vectors.
We hypothesize that the increase in vivo NeoR gene marking observed in AMD3100-compared with G-CSF-mobilized CD34+ cells may be due, in part, to a difference in cell-cycling status. It is well established that oncoretroviral vectors require cell cycling for efficient transduction of their target cells. The increased proportion of cells in the G1 phase of the cell cycle is consistent with better in vivo marking levels obtained with AMD3100-mobilized CD34+ cells. In one study, Liles et al10 used a high-specific activity [3H]-thymidine kill assay to assess the percentage of AMD3100-mobilized hematopoietic progenitor cells in the S phase of the cell cycle. In their study, 0% to 5% kill was obtained, consistent with our finding of 4% ± 1.7% AMD3100-mobilized CD34+ cells in the S phase of the cell cycle using acridine orange.
Overall, our data indicate that AMD3100 mobilizes a population of HSCs with long-term repopulating capacity and intrinsic characteristics different from those of HSCs mobilized with G-CSF. The increased expression of cell-surface molecules implicated in retention and homing of primitive cells in the BM following transplantation may significantly increase the efficacy of these cells in diverse transplantation applications, providing an alternative source of HSCs for autologous and allogeneic stem cell transplantation in patients with hematolymphoid malignancies or solid tumors. AMD3100-mobilized CD34+ cells are amenable to genetic manipulation with integrating retroviral vectors and likely will also be better targets for lentiviral vectors, which preferentially transduce cells in G1 as compared with G0 phase of the cell cycle,35,36 In particular, use could be considered for HSC gene therapy approaches in patients with sickle cell anemia in whom documented complications, including severe sickle cell crises and even death, have precluded mobilization using G-CSF. A full understanding of the mechanisms controlling stem-cell and progenitor-cell mobilization with AMD3100 awaits further experimentation. Unveiling regulatory pathways in mobilization with AMD3100 will not only offer clinical benefits but will greatly enhance our basic understanding of survival, proliferation, and differentiation of hematopoietic stem cells.
Prepublished online as Blood First Edition Paper, January 26, 2006; DOI 10.1182/blood-2005-09-3592.
Two of the authors (S.F. and G.B.) are employed by a company (AnorMED, Inc) whose product (AMD3100) was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Keyvan Keyvanfar, Stacie Anderson, and Philip McCoy for their help with flow cytometry analysis, and the veterinary and support staff at 5 Research Court for excellent primate care.

![Figure 2. Competitive repopulation experiments. In 2 animals (RQ3636 [A] and RQ3590 [B]), a single dose of AMD3100 (1 mg/kg) was administered subcutaneously. PB cells were collected by leukapheresis, enriched for CD34+ cells, transduced with G1Na retroviral vectors (animal RQ3590) or a second distinguishable NeoR-containing vector, LNL6 (animal RQ3636), and frozen for subsequent reinfusion in the animals. One month after the first mobilization, recombinant human G-CSF was administered, and hematopoietic cells were collected by leukapheresis on day 5. CD34+ cells were subsequently enriched and transduced with the alternative distinguishable retroviral vectors (LNL6 for animal RQ3590 and G1Na for animal RQ3636). Cells were frozen and soon after reinfused in the animals along with the previously transduced AMD3100-mobilized fraction. Gene marking levels were determined at different time points after transplantation by real-time PCR in PBMCs and GRANs. Gene marking levels were also determined in B and T cells 24 months after transplantation in animal RQ3636 (B cells, 2%; T cells, 3%) and animal RQ3590 (B cells, 0.3%; T cells, 0.1%).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/9/10.1182_blood-2005-09-3592/2/m_zh80090694780002.jpeg?Expires=1770990752&Signature=b1Q8l9CwE73CLLENw32cw-u289d5~s9SGNTDZO~UwgffoIWUX17lNmH1LUVsKbcXP7i7dmH88FLbuGbOFwWQHyEhKCvLHOgxSdmg~m2QBmuvRqLTqZVij1cfVqe1PM-FfEGVksIrIFXQdyQTsMoPXpzGyfbZi68VN9APvJyHQye71nc53D9YkXfEBJMqO4PSg3rBdxe9CA3FOVsZHDCtJzFUp2f9GQV-uOTfgEFe6-Ey2kAF6qG7I9Djjld0lOShMgaD12Q0XS9K4aX-5bbOGUH-oDJkjZKNk7TCQxbt29yiE3lu-XecmpVY-qotiU1HV3rWzrcWQ6mJDqLZvUxx5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal