We compared the safety and efficacy of allogeneic stem cell transplantation (allo-SCT) after reduced-intensity conditioning using either unrelated umbilical cord blood (UCB) donors or matched-sibling donors (MSDs) for 21 adults at high risk with advanced Hodgkin lymphoma (UCB, n = 9; MSD, n = 12). Both groups were comparable except for younger age in the UCB cohort (median, 28 vs 42 years; P = .02). Neutrophil recovery occurred earlier in the MSD group (median, 7 vs 10 days; P = .02). All patients had sustained donor engraftment by day 60. Cumulative incidence of acute severe graft-versus-host-disease (33% vs 33%; P = .99), chronic graft-versus-host-disease (11% vs 33%; P = .24), and 100-day treatment-related mortality (11% vs 17%; P = .80) were comparable. With median follow-up periods of 17 and 24 months, the 2-year progression-free survival rates were 25% (95% confidence interval [95% CI], 0%-55%) for UCB and 20% (95% CI, 0%-44%) for MSD allo-SCT (P = .67). Our results suggest comparable outcomes for reduced-intensity allo-SCT using UCB or MSD in adults at high risk with advanced Hodgkin lymphoma.
Introduction
Patients with relapsed or primary-refractory Hodgkin lymphoma have very poor outcomes with conventional salvage chemotherapy regimens. High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) leads to prolonged progression-free survival in nearly half of these patients at high risk.1-3 Although a clinical graft-versus-lymphoma effect has been reported in patients with Hodgkin lymphoma, results of allogeneic stem cell transplantation (allo-SCT) with myeloablative conditioning have been disappointing because of the high treatment-related mortality (TRM) rate observed in this typically heavily pretreated group of patients.4-6 Allo-SCT using nonmyeloablative or reduced-intensity conditioning (RIC) regimens is feasible, leads to long-term engraftment, exhibits a graft-versus-malignancy effect, and results in significantly lower TRM in patients with a variety of hematologic and nonhematologic malignancies.7,8
For patients who lack a human leukocyte antigen (HLA)-matched sibling donor (MSD), unrelated umbilical cord blood (UCB) offers certain advantages over unrelated donor bone marrow. In addition to rapid availability, UCB has the potential benefit of lower risk for severe acute graft-versus-host disease (GVHD) despite higher donor-recipient HLA disparity.9-11 We have previously reported that UCB allo-SCT after RIC conditioning produces high rates of engraftment with tolerable toxicity levels and acceptable incidences of acute GVHD.12
We report a pilot study comparing the safety and efficacy of allo-SCT after RIC using either UCB or peripheral blood MSD in adults with advanced Hodgkin lymphoma who were ineligible for myeloablative conditioning.
Study design
Patient characteristics
Data were collected prospectively on 21 consecutive patients with advanced Hodgkin lymphoma (UCB, n = 12; MSD, n = 12) who underwent allo-SCT after RIC between July 2000 and May 2005. Patients were considered for UCB allo-SCT if they had no HLA-compatible related donors (5 of 6 or 6 of 6 HLA-A, HLA-B, or DRB1 matches).
Indications for using RIC rather than a conventional myeloablative preparative regimen were (1) patient older than 55 years with MSD (n = 4) or older than 45 years with UCB donor (n = 1), (2) extensive previous therapy (previous ASCT [n = 14] or more than 12 months of alkylator chemotherapy [n = 16]), or (3) poor performance status including major comorbidity (n = 6); 14 patients had 2 or more of these high-risk features.
Patient, disease, and transplantation characteristics were comparable except for younger age and shorter duration of first complete remission in the UCB group (Table 1). One patient was in complete remission, and all patients had chemosensitive disease before allo-SCT.
Patient, disease, and transplantation characteristics and posttransplantation outcomes
Characteristics . | UCB . | MSD . | P . |
|---|---|---|---|
| Patients | |||
| No. | 9 | 12 | |
| Age, y, median (range) | 28 (19-45) | 42 (23-58) | .02 |
| Male, no. (%) | 4 (44) | 8 (67) | .31 |
| Histology, no. (%) | |||
| Nodular sclerosing | 9 (100) | 11 (92) | .38 |
| Lymphocyte predominant | 0 | 1 (8) | |
| Disease characteristics at initial diagnosis | |||
| Ann Arbor stages, no. (%) | |||
| 1-2 | 3 (33) | 2 (17) | .27 |
| 3-4 | 6 (67) | 10 (83) | |
| B symptoms, no. (%) | 6 (67) | 7 (58) | .68 |
| Extranodal disease, no. (%) | 2 (22) | 1 (8) | .37 |
| Duration of CR1, mo, median (range)* | 4 (3-9) | 9 (4-60) | .03 |
| Disease characteristics at pretransplantation relapse | |||
| Ann Arbor stages, no. (%) | |||
| 1-2 | 4 (44) | 4 (33) | .70 |
| 3-4 | 5 (56) | 8 (67) | |
| B symptoms, no. (%) | 3 (33) | 6 (50) | .45 |
| Extranodal disease, no. (%) | 3 (33) | 5 (42) | .70 |
| No. total previous chemo regimens, median (range) | 4 (3-5) | 4 (3-7) | .97 |
| Previous ASCT, no. (%) | 7 (78) | 7 (58) | .35 |
| Post-ASCT CR duration, mo, median (range) | 6 (3-36) | 11 (4-54) | .56 |
| Transplantation characteristics | |||
| Time from diagnosis to allo-SCT, mo, median (range) | 28 (16-83) | 22 (11-154) | .78 |
| Disease status at allo-SCT, no. (%) | |||
| Primary refractory | 2 (22) | 2 (17) | .90 |
| Relapses 1-2 | 4 (44) | 5 (42) | |
| Relapse 3+ | 3 (33) | 5 (42) | |
| Residual disease present at allo-SCT, no. (%) | 8 (89) | 12 (100) | .24 |
| Conditioning regimen, no. (%) | |||
| Bu/Flu/TBI | 2 (22) | 6 (50) | .20 |
| Cy/Flu/TBI | 7 (78) | 6 (50) | |
| HLA 1-2 antigen mismatch, no. (%) | 9 (100) | 1 (8) | < .01 |
| Cell dose × 107 NC/kg, median (range) | 3.8 (2.3-5.3) | 10.0 (7.9-16.4) | < .01 |
| Posttransplantation outcomes | |||
| Time to neutrophil engraftment, d, median (range) | 10 (6-28) | 7 (5-12) | .02 |
| Complete donor chimerism, no. (%) | |||
| Day 21 | 6 (67) | 12 (100) | .06 |
| Day 60 | 9 (100) | 12 (100) | |
| Acute GVHD, no. (%) | |||
| Grades 2-4 | 6 (67) | 7 (58) | .70 |
| Grades 3-4 | 3 (33) | 4 (33) | .99 |
| Chronic GVHD, no. (%) | 1 (11) | 4 (33) | .24 |
| CR after allo-SCT, no. (%) | 8 (89) | 9 (75) | .42 |
| TRM, no. (%) | |||
| 100 d | 1 (11) | 2 (17) | .80 |
| 180 d | 2 (22) | 3 (25) | .88 |
| Follow-up after allo-SCT, mo, median (range) | 17 (4-51) | 24 (9-53) | |
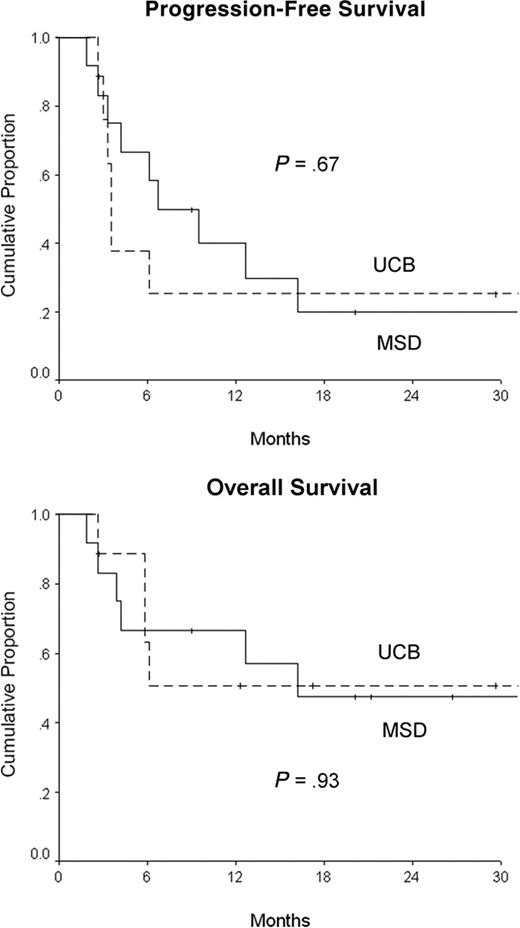
| 2-y PFS rate, % (95% CI) | 25 (0-55) | 20 (0-44) | .67 |
| 2-y OS rate, % (95% CI) | 51 (16-86) | 48 (19-77) | .93 |
Characteristics . | UCB . | MSD . | P . |
|---|---|---|---|
| Patients | |||
| No. | 9 | 12 | |
| Age, y, median (range) | 28 (19-45) | 42 (23-58) | .02 |
| Male, no. (%) | 4 (44) | 8 (67) | .31 |
| Histology, no. (%) | |||
| Nodular sclerosing | 9 (100) | 11 (92) | .38 |
| Lymphocyte predominant | 0 | 1 (8) | |
| Disease characteristics at initial diagnosis | |||
| Ann Arbor stages, no. (%) | |||
| 1-2 | 3 (33) | 2 (17) | .27 |
| 3-4 | 6 (67) | 10 (83) | |
| B symptoms, no. (%) | 6 (67) | 7 (58) | .68 |
| Extranodal disease, no. (%) | 2 (22) | 1 (8) | .37 |
| Duration of CR1, mo, median (range)* | 4 (3-9) | 9 (4-60) | .03 |
| Disease characteristics at pretransplantation relapse | |||
| Ann Arbor stages, no. (%) | |||
| 1-2 | 4 (44) | 4 (33) | .70 |
| 3-4 | 5 (56) | 8 (67) | |
| B symptoms, no. (%) | 3 (33) | 6 (50) | .45 |
| Extranodal disease, no. (%) | 3 (33) | 5 (42) | .70 |
| No. total previous chemo regimens, median (range) | 4 (3-5) | 4 (3-7) | .97 |
| Previous ASCT, no. (%) | 7 (78) | 7 (58) | .35 |
| Post-ASCT CR duration, mo, median (range) | 6 (3-36) | 11 (4-54) | .56 |
| Transplantation characteristics | |||
| Time from diagnosis to allo-SCT, mo, median (range) | 28 (16-83) | 22 (11-154) | .78 |
| Disease status at allo-SCT, no. (%) | |||
| Primary refractory | 2 (22) | 2 (17) | .90 |
| Relapses 1-2 | 4 (44) | 5 (42) | |
| Relapse 3+ | 3 (33) | 5 (42) | |
| Residual disease present at allo-SCT, no. (%) | 8 (89) | 12 (100) | .24 |
| Conditioning regimen, no. (%) | |||
| Bu/Flu/TBI | 2 (22) | 6 (50) | .20 |
| Cy/Flu/TBI | 7 (78) | 6 (50) | |
| HLA 1-2 antigen mismatch, no. (%) | 9 (100) | 1 (8) | < .01 |
| Cell dose × 107 NC/kg, median (range) | 3.8 (2.3-5.3) | 10.0 (7.9-16.4) | < .01 |
| Posttransplantation outcomes | |||
| Time to neutrophil engraftment, d, median (range) | 10 (6-28) | 7 (5-12) | .02 |
| Complete donor chimerism, no. (%) | |||
| Day 21 | 6 (67) | 12 (100) | .06 |
| Day 60 | 9 (100) | 12 (100) | |
| Acute GVHD, no. (%) | |||
| Grades 2-4 | 6 (67) | 7 (58) | .70 |
| Grades 3-4 | 3 (33) | 4 (33) | .99 |
| Chronic GVHD, no. (%) | 1 (11) | 4 (33) | .24 |
| CR after allo-SCT, no. (%) | 8 (89) | 9 (75) | .42 |
| TRM, no. (%) | |||
| 100 d | 1 (11) | 2 (17) | .80 |
| 180 d | 2 (22) | 3 (25) | .88 |
| Follow-up after allo-SCT, mo, median (range) | 17 (4-51) | 24 (9-53) | |
| 2-y PFS rate, % (95% CI) | 25 (0-55) | 20 (0-44) | .67 |
| 2-y OS rate, % (95% CI) | 51 (16-86) | 48 (19-77) | .93 |
Excludes 2 patients with primary refractory disease in each group
Treatment plan
Until September 2001, patients (n = 8) underwent conditioning with a busulphan, fludarabine, total body irradiation (Bu/Flu/TBI) regimen consisting of busulfan 2 mg/kg orally every 12 hours for 4 doses (days -8, -7), fludarabine 40 mg/m2 intravenously daily (days -6 through -2), and 200 cGy total body irradiation (TBI) (day -1). Subsequently, all patients underwent a cyclophosphamide, fludarabine, total body irradiation (Cy/Flu/TBI) regimen in which cyclophosphamide 50 mg/kg intravenously (day -6) was substituted for busulfan. All patients received GVHD prophylaxis with cyclosporin A (days -3 to 180) and mycophenolate mofetil (days -3 to 30). The treatment protocol was approved by the University of Minnesota institutional review board, and all patients gave written informed consent before transplantation.
UCB grafts
UCB grafts had at least 4 of 6 HLA-A, HLA-B, or DRB1 antigens that were matched to the recipient and—if 2 donor units were infused—to each other as well. Each unit had a cryopreserved cell dose of at least 1.5 × 107 nucleated cells per kilogram of recipient body weight (NC/kg). Seven (78%) patients received grafts consisting of 2 U UCB to optimize cell dose. All patients undergoing UCB allo-SCT received 1 to 2 HLA antigen-mismatched grafts. Each patient with 1 U UCB received 4 of 6 HLA antigen-matched grafts. Five patients with double UCB transplants received at least one 6 of 6 (n = 1) or 5 of 6 (n = 4) HLA-matched unit, whereas 2 patients received a graft with both units 4 of 6 HLA antigen-matched to the recipient. The median infused cell dose was 3.8 × 107 NC/kg.
Donor chimerism analysis
Donor chimerism was performed on days 21 to 28, 60, 100, 180, and 360 and then annually using quantitative polymerase chain reaction of informative polymorphic variable number tandem repeat or short tandem repeat regions.
Statistical analyses
Sustained donor engraftment was defined as sustained neutrophil recovery and donor hematopoiesis beyond day 42 after allo-SCT. Time of neutrophil engraftment was the first of 3 consecutive days with an absolute neutrophil count exceeding 0.5 × 109/L. Complete donor chimerism was defined as bone marrow reconstitution consisting of greater than 90% donor bone marrow. The Kaplan-Meier method was used to calculate survival curves for progression-free survival (PFS) and overall survival (OS).13 Cumulative incidence calculations were performed for TRM, engraftment, GVHD, and relapse. Event times were measured from the date of transplantation to the date of death or last contact. Comparisons of patient and transplant characteristics were performed using the χ2, Fisher exact, or Wilcoxon rank sum test as appropriate. Analysis was performed as of August 2005.
Results and discussion
Neutrophil engraftment (Table 1) was observed to occur sooner in the MSD than in the UCB cohort (median, 7 vs 10 days; P = .02). The cumulative incidence of sustained donor engraftment was 100% for both donor groups; there were no graft failures. Complete donor chimerism was seen in all patients by day 60.
The cumulative incidence of severe acute (grade 3/4) GVHD was 33% (95% confidence interval [95% CI], 2%-64%) for the UCB group and 33% (95% CI, 6%-60%) for the MSD group (P = .99). The cumulative incidence of chronic GVHD at 1 year was 11% (95% CI, 0%-31%) for the UCB and 33% (95% CI, 7%-59%) for the MSD group (P = .24).
Two-year PFS rates were 25% (95% CI, 0%-55%) in patients receiving UCB and 20% (95% CI, 0%-44%) in those receiving MSD (Table 1; Figure 1). Two patients who underwent MSD allo-SCT received donor-lymphocyte infusion (DLI) for posttransplantation relapse, with resultant partial responses lasting 3 and 6 months. TRM was low and comparable between the 2 groups (Table 1). Seven patients died within 180 days of allo-SCT. Infection and organ failure were the predominant causes of death (n = 3), followed by progressive disease (n = 2), diffuse alveolar hemorrhage (n = 1), and severe acute GVHD (n = 1, MSD group).
For the entire cohort, allo-SCT for primary refractory disease and short duration of complete remission (CR) after ASCT were associated with poor PFS. The 2-year PFS rates for patients who underwent transplantation for primary refractory and relapsed disease were 0% and 29% (95% CI, 5%-53%) (P < .01); patients who experienced 12 months or less of CR after ASCT had a 2-year PFS rate of 0% compared with 67% (95% CI, 30%-100%) for those who experienced CR for more than 12 months (P = .04).
These results indicate comparable outcomes with RIC allo-SCT for patients with advanced Hodgkin lymphoma using either UCB or MSD as a donor source. Patients with advanced Hodgkin lymphoma typically receive extensive chemotherapy, including ASCT, before consideration for allo-SCT, thereby making them poor candidates for myeloablative conditioning. The lower regimen-related toxicity of RIC preparative regimens extends the opportunity for allo-SCT and its graft-versus-lymphoma effect to this group of patients. Because many patients lack matched related or unrelated donors, UCB grafts, despite the limitations of low cell dose and lack of donor availability for DLI, can serve to expand the donor pool and provide an effective and safe alternative.
Progression-free and overall survival for patients undergoing unrelated UCB and MSD allogeneic stem cell transplantation with reduced-intensity conditioning.
Progression-free and overall survival for patients undergoing unrelated UCB and MSD allogeneic stem cell transplantation with reduced-intensity conditioning.
In this heavily pretreated patient group at high risk, 5 patients had durable and prolonged PFS (median follow-up, 30 months; range, 9-53 months). This observation, along with the responses after DLI, further suggests the presence of a graft-versus-lymphoma effect in patients with advanced Hodgkin lymphoma. More studies are needed to identify patients who would benefit most from this treatment approach.
Prepublished online as Blood First Edition Paper, December 29, 2005; DOI 10.1182/blood-2005-09-3827.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal