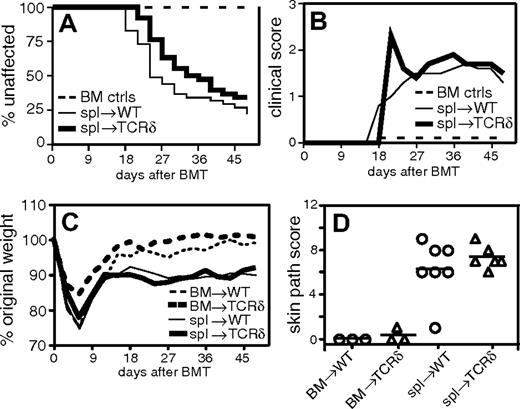
We read with interest the paper by Maeda et al,1 which concluded that host γδ T cells exacerbated graft-versus-host disease (GVHD) by promoting maturation of host antigen-presenting cells (APCs). In the “Introduction,” the authors state that the role of host γδ T cells (in GVHD) is not known. However, we published a study in Blood examining the role of host lymphocytes in GVHD, including that of γδ T cells, 9 months prior to the article by Maeda et al.2 Our work was not referenced by Maeda et al nor by Geoff Hill in his commentary.3 Nonetheless, we do realize that our article appeared in print only 52 days before the article by Maeda and colleagues was submitted and thus, at least at the time of submission, they may have been unaware of our work. Because our published data differ considerably from the plenary paper, we think it is important to clarify the differences for readers of Blood. In contrast to the results of Maeda et al, we found no role for host γδ T cells in that the incidence and severity of skin disease (Figure 1A-B, reproduced from Anderson et al2 ), degree of weight loss (Figure 1C), and pathologic GVHD (Figure 1D) in wild-type and γδ T-cell-deficient recipients were indistinguishable. Also, unlike Maeda et al, we found that recipients deficient in αβ T cells had more severe and rapid GVHD relative to T-replete hosts, and on the basis of further experimentss we attributed this result to missing host CD4+CD25+ T regulatory cells.2
γδ T-cell-deficient recipients do not have increased incidence or severity of GVHD. Combined data from 3 experiments. On day 0, BALB/c (H-2d) recipient mice were lethally irradiated and reconstituted with 8 × 106 T-cell-depleted B10.D2 (H-2d) bone marrow (BM) cells alone (n = 28, both recipient types) or BM plus 107 B10.D2 spleen cells (spl): wild-type (WT) recipients (n = 41) or TCRδ-/- recipients (n = 38). (A) Incidence of clinical skin GVHD. (B) Clinical skin score. Average clinical score for mice affected with GVHD (unaffected mice are excluded). BM control mice did not get GVHD and are represented on the graph as scoring 0. (C) Weight loss. (D) Pathologic skin disease. Representative mice were killed for pathologic analysis, and histologic cutaneous GVHD was scored by a dermatopathologist blinded to experimental groups.
γδ T-cell-deficient recipients do not have increased incidence or severity of GVHD. Combined data from 3 experiments. On day 0, BALB/c (H-2d) recipient mice were lethally irradiated and reconstituted with 8 × 106 T-cell-depleted B10.D2 (H-2d) bone marrow (BM) cells alone (n = 28, both recipient types) or BM plus 107 B10.D2 spleen cells (spl): wild-type (WT) recipients (n = 41) or TCRδ-/- recipients (n = 38). (A) Incidence of clinical skin GVHD. (B) Clinical skin score. Average clinical score for mice affected with GVHD (unaffected mice are excluded). BM control mice did not get GVHD and are represented on the graph as scoring 0. (C) Weight loss. (D) Pathologic skin disease. Representative mice were killed for pathologic analysis, and histologic cutaneous GVHD was scored by a dermatopathologist blinded to experimental groups.
The model systems used in the 2 papers were quite different, possibly explaining the divergent results. We used a major histocompatibility complex (MHC)-matched model (B10.D2→BALB/c; H-2d) akin to the majority of human allogeneic stem cell transplantations, whereas Maeda et al used 3 MHC-mismatched models. These MHC-mismatched models resulted in hyperacute GVHD with substantial early lethality, likely due at least in part to massive cytokine release.4,5 In contrast, the B10.D2→BALB/c model yields a more chronic form of GVHD that is nonlethal and characterized by a T-cell-infiltrative pathology involving the skin with less penetrance in the liver and bowel. Furthermore, the γδ T-cell-deficient mice we used were backcrossed to BALB/c, whereas those used by Maeda et al were on the C57Bl/6 background, and it is possible that the 2 strains may have different responses in the absence of γδ T cells. We wonder whether Maeda et al have examined GVHD in C57Bl/6 γδ T-cell-deficient recipients with MHC-matched, minor histocompatibility antigen-mismatched donors. Also, since Maeda et al suggest that γδ T cells promote host APC maturation, a potential mechanistic explanation for the different experimental findings could be that the B10.D2→BALB/c model is less reliant on host APCs than are MHC-disparate models.5-7 Nonetheless, our data clearly indicate that the roles of γδ T cells differ by situation, and particularly, since we studied an MHC-matched situation that resembles the vast majority of human transplantations, and considering that γδ T cells may provide antipathogen immunity,8,9 the conclusion that γδ T cells should be targeted as a means of GVHD reduction should be made with caution.
The role of γδ T cells in graft-versus-host disease
Anderson et al are right to remind us of the important limitations of mouse models in the dissection of complex graft-versus-host disease (GVHD) biology, particularly when results from different models conflict. Although our conclusions that host γδ T cells regulated intestinal acute GVHD1 are also supported by another group,2 we agree with Anderson and colleagues' elegant summary of potential causes for discrepancies between the models. We were unaware of their work prior to the submission of our manuscript, and we did not examine the role of host γδT cells in minor histocompatibility antigen-mismatched strain combinations. Since GVHD of the skin dominates their model,3 their data might be reconciled with ours if host γδ T cells contribute to the inflammation of intestinal acute GVHD but not to fibrotic skin changes characteristic of chronic GVHD. In any event, we wholeheartedly concur that all insights from animal models must be extrapolated to human patients with caution, and that the efficacy of any approach must be verified in well-designed, carefully controlled clinical trials.4
Correspondence: James L. M. Ferrara, University of Michigan Cancer Center, 1500 E Medical Center Drive, Ann Arbor, MI 48109; e-mail: ferrara@umich.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal