Normal erythropoiesis critically depends on the balance between the renewal of precursor cells and their differentiation. If the renewal phase is shortened, the decrease in the precursor pool results in anemia; conversely, impaired differentiation increases the number of proliferating progenitors and the potential risk of leukemic transformation. Using gene ablation, we have discovered 2 self-sustaining signal transduction loops that antagonize each other and regulate erythroid progenitor proliferation and differentiation, respectively. We identify Raf-1 as the main activator of the MEK/ERK cascade and as the key molecule in maintaining progenitor proliferation. Differentiation, in contrast, is mediated by Fas via the activation of both the ASK1/JNK/p38 module and the caspase cascade. The point of convergence between the 2 cascades is activated ERK, which positively feeds back on the proliferation pathway by maintaining the expression of Raf-1, while inhibiting the expression of Fas and therefore differentiation. In turn, Fas, once expressed, antagonizes proliferation by exerting a negative feedback on ERK activation and Raf-1 expression. Simultaneously, Fas-mediated caspase activation precipitates differentiation. These results identify Raf-1 and Fas as the key molecules whose expression finely tunes erythropoiesis and the extent of ERK activation as the switch that tips the balance between them. (Blood. 2006;108:152-159)
Introduction
Erythropoiesis is a complex multistage process, whose success depends on the correct balance among cell proliferation, differentiation, and apoptosis. Disruption of this equilibrium is associated with hematologic diseases such as myelodysplasia, aplastic anemia, and thalassemia.1 During normal erythropoiesis, the committed erythroid progenitors give rise to erythroid burst-forming unit (BFU-E) and then to erythroid colony-forming unit (CFU-E) cells. These, in turn, mature into pronormoblasts and basophilic, polychromatic, and orthochromatic normoblasts. In the late stages of differentiation, cells shrink and undergo nuclear condensation followed by nuclear extrusion to finally turn into enucleated reticulocytes and eventually mature into erythrocytes. Cell shrinkage, nuclear condensation, and extrusion are also prominent features of programmed cell death, or apoptosis. During apoptosis, a family of cysteine/aspartic acid proteases (caspases) dismantle the cells by cleaving proteins involved in the maintenance of cell shape, the integrity of the nucleus, and of DNA itself. Caspases, however, play an important nonapoptotic role in the differentiation of cell types such as lens fiber cells,2 keratinocytes,3 megakaryocytes,4 and erythroid cells,5 all associated with enucleation.
Among the classic proapoptotic activators of the caspases are the so-called death receptors, such as Fas or TNF-RI, which drive the oligomerization of initiator caspases and thereby cause the cleavage that initiates the activation of the caspase cascade and the demise of the cell.6 The stimuli responsible for differentiation associated caspase activation are presently unknown. Among the death receptors capable of activating caspases, TNF-RI and TRAIL-RI do not seem to play a central role in erythropoiesis.6 In contrast, the lpr mutation, which results in loss of Fas expression, is associated with a major increase of myeloid but also erythroid precursors in liver and spleen.7-9
Caspase activation can be counteracted by prosurvival stimuli. In this context, we have shown that the Raf-1 kinase, previously implicated in the control of erythroid proliferation by growth factors,10,11 restrains differentiation-associated caspase activation in erythroblasts. Raf-1-deficient erythroblasts differentiate much faster than their wild-type (WT) counterparts. This results in the depletion of erythroid precursors from the fetal liver and in the anemia that characterizes Raf-1-deficient embryos.5,12 In addition, ablation of Raf-1 sensitizes embryonic fibroblasts to Fas-induced, but not TNF-α-induced apoptosis,12 and we have recently shown that this is due to the accumulation of Fas on the surface of Raf-1-deficient fibroblasts.13 In this paper, we analyze the relationship among Fas, Raf-1, and their downstream signaling pathways in the context of erythroid differentiation. We identify Fas as the upstream regulator responsible for differentiation-associated caspase activation and show that lack of Raf-1 expression correlates with the up-regulation of Fas and of the ASK1/JNK/p38 pathway in erythroid progenitors. Unlike its functions in the survival and migration of cultured embryonic fibroblasts,14,15 the role of Raf-1 in erythropoiesis depends on its ability to activate the MEK/ERK pathway. Surprisingly, activated ERK is necessary to maintain the expression of Raf-1 in differentiating erythroblasts, thus creating a positive feedback loop that ensures the correct timing of Fas expression and, ultimately, of erythroid differentiation.
Materials and methods
Cell isolation, culture, and differentiation
Primary fetal liver cells were isolated from 129/SvHsd:Bl6 F2 embryos obtained from F1 crosses of Raf-1+/-129/SvHsd mice12 and lpr/lpr (C57/Bl6; courtesy of A. Martin-Villalaba, DKFZ, Heidelberg, Germany) animals housed in the animal facility of the Max F. Perutz Laboratories (MFPL; Vienna, Austria). Polymerase chain reaction (PCR) analysis of offspring was performed as previously described.12,16,17 Primary fetal liver cells and immortal I/11 erythroblasts were cultured in serum-free erythroid medium (StemPro 34 plus nutrient supplement; Life Technologies, Karlsruhe, Germany) containing 1 U/mL human recombinant erythropoietin (Epo; Janssen-Cilag, Titusville, NJ), 100 ng/mL murine recombinant stem-cell factor (SCF; R&D Systems, Wiesbaden, Germany), 10-6 M dexamethasone (Dex; Sigma-Aldrich, Deisenhofen, Germany), and 40 ng/mL insulin-like growth factor 1 (Promega, Mannheim, Germany). Primary fetal liver cells were expanded for 8 days to obtain homogenous populations of proliferating erythroid progenitors. Differentiation was induced by reseeding the erythroid progenitors (2 × 106 cells/mL) in differentiation medium (Stem-Pro 34 plus nutrient supplement) and 10 U/mL Epo, insulin (4 × 10-4 IE = 10 ng/mL, Actrapid HM; Bagvaerd, Denmark), 3 × 10-6 M glucocorticoid receptor antagonist ZK112.993, and 1 mg/mL iron-saturated human transferrin (Sigma-Aldrich). When indicated, 10-6 M Dex was added to the complete differentiation medium. Cultures were maintained at densities of 2to4 × 106 cells/mL and analyzed daily for cell numbers and cell-size distribution in an electronic cell counter (CASY-1; Schärfe-System, Reutlinger, Germany) to determine proliferation kinetics. Cumulative cell numbers were calculated as previously described.5 When indicated, the MEK inhibitor PD98059 (40 μM; Calbiochem, Bad Soden, Germany) was added to the cultures and replenished every second day.
Cell morphology, FACS analysis, and determination of hemoglobin content
Erythroblasts at various stages of differentiation were cytocentrifuged onto glass slides and stained with hematoxylin-eosin (HemaQuick; Biomed, San Diego, CA) and neutral benzidine (Sigma-Aldrich) to detect hemoglobin as previously described.5 The percentage of nucleated and enucleated erythrocytes, partially mature or immature cells, and apoptotic/dead cells was determined by 4 investigators evaluating 500 cells per sample on at least 2 randomly selected fields.
For fluorescence-activated cell sorting (FACS) analysis, differentiating erythroblasts were harvested at the indicated times and stained with antibodies against Fas or isotype IgG as a control (PharMingen BD Biosciences, San Diego, CA).
Hemoglobin content was analyzed as previously described.5 Values obtained from triplicate determinations were averaged and normalized to cell number and cell volume.
Immunoblotting and reverse transcription-PCR
Erythroblasts were harvested, counted, and lysed as previously described.5 Protein concentration was determined using the Bradford method. However, because hemoglobin, present in different amounts in the cells at various stages, interferes with standard methods of protein determination, we normalized the values according to the hemoglobin content and the decrease in cell size, independently monitored during erythroid differentiation. The normalized samples were immunoblotted as previously described5 and probed with the following primary antibodies: α-JNK, α-phospho-JNK (both from Cell Signaling Technology, Beverly, MA); α-Raf-1 (N-terminal), α-ERK, α-phospho-ERK (all from Transduction Labs, Lexington, KY); α-ASK1, α-lamin B, α-Raf-1 (C-terminal), α-p38, α-phospho-p38 (all from Santa Cruz Biotechnology, Santa Cruz, CA); and α-Fas and α-FasL (both from PharMingen BD Biosciences) prior to incubation with peroxidase-conjugated secondary antibodies and detection by enhanced chemiluminescence (ECL; Pierce Chemical, Rockford, IL).
Total RNA was isolated from erythroid cells by TRI reagent method (MRC, Cincinnati, OH). RNA concentration was determined by measuring the optical density at 260 nm and 100 ng RNA was reverse-transcribed into first-strand cDNA using Superscript (Invitrogen, Paisley, United Kingdom). The resulting cDNA was subjected to 24 amplification cycles (denaturation at 94°C for 1 minute, annealing at 56°C for 1 minute, and extension at 72°C for 1 minute).
The following forward (F) and reverse (R) primers were used: Raf-1, F: 5′-GCACGCTGACTACATC, R: 5′-ACAAAGTTCTGTAGTACC; Fas, F: 5′-CGCGGATCCACCATGCTGTGGATCTG, R: 5′-CGCGAATTCTCACTCCAGACA TTGTCC; ASK1, F: 5′-CCAACCTTCGACTTGGC, R: 5′-ACCAGGAAATCCATCC; ribonuclease inhibitor (RI), F: 5′-TCCAGTGTGAGCAGCTGA, R: 5′-TGCAGGCACTGAAGCACC.
Results
Fas expression is up-regulated during erythroid differentiation and is increased in cells lacking Raf-1
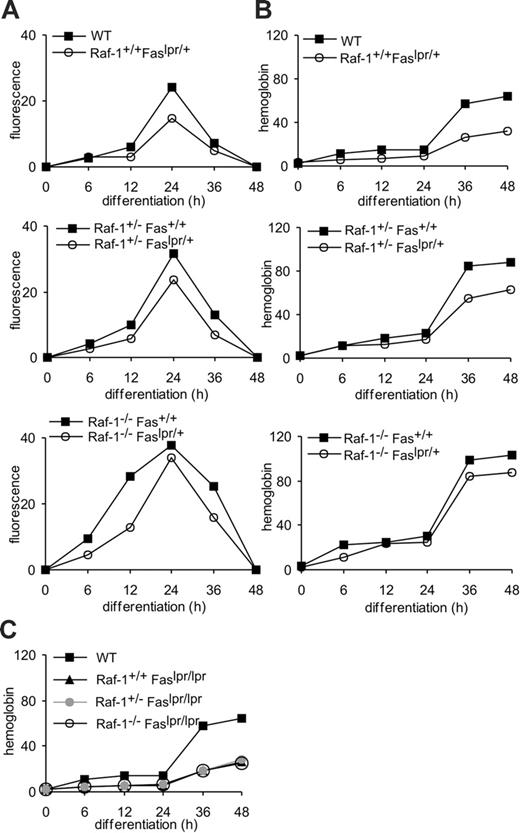
We have previously demonstrated that Raf-1 restrains caspase activation in at least 2 situations: in primary erythroblasts undergoing differentiation5 and in embryonic fibroblasts treated with Fas agonists.12 More recently, we have determined that Raf-1-deficient fibroblasts show increased Fas surface expression and that reducing the amount of Fas by the introduction of one lpr allele on a Raf-1-deficient background suffices to rescue lethality13 as well as the anemic phenotype (Figure 1A) of the Raf-1-/- embryos. These observations prompted us to analyze whether the faster differentiation kinetics displayed by Raf-1-/- erythroblasts5 correlated with an increase in Fas surface expression. FACS analysis showed that Fas surface expression is up-regulated during the differentiation of primary WT erythroblasts in vitro, peaking at 24 hours after induction and disappearing on completion of differentiation (48 hours). Fas was up-regulated with faster kinetics and to higher levels in Raf-1-deficient erythroblasts (Figure 1C). In contrast, TNF-RI was stably expressed throughout differentiation, becoming undetectable at 48 hours (not shown).
Raf-1+/- erythroblasts showed an intermediate behavior in the kinetics of differentiation and Fas expression (not shown and Figure 2A-B). Thus, Fas is selectively up-regulated during erythroid differentiation, and Raf-1 reduction/ablation selectively increases this up-regulation.
Fas mutation delays erythroid differentiation
We next tested whether Fas up-regulation was necessary for erythroid differentiation by reducing Fas expression in WT, heterozygous, and Raf-1-deficient erythroblasts (Figure 2A). To do this, we introduced one or 2 lpr alleles on these backgrounds and monitored the differentiation of erythroblasts derived from E10.5 fetal livers by assessing the 3 classic parameters of differentiation, that is, proliferation rate, volume decrease, and hemoglobin accumulation. All 3 parameters gave similar results. For the sake of brevity, only hemoglobin accumulation is shown in Figures 2B and 5, 6. The presence of one lpr allele resulted in the expected reduction of Fas expression in all 3 backgrounds. Fas expression was identical to WT in Raf-1+/-;lpr/+ cells, whereas the Raf-1-/-; lpr/+ cells still showed slightly elevated Fas levels (Figure 2A). These results correlated perfectly with the kinetics of erythroid differentiation (Figure 2B), which were indistinguishable from the WT in double heterozygous cells, but still slightly faster than normal in Raf-1-/-;lpr/+ erythroblasts. Furthermore, heterozygous Fas levels significantly delayed differentiation of WT erythroblasts. Thus, the levels of Fas expressed by the erythroblasts positively correlate with the speed of differentiation in Raf-1-deficient and heterozygous, but also in WT cells, suggesting a causal link between Fas and erythroid differentiation.
Up-regulation of Fas expression correlates with anemia in Raf-1-deficient erythroblasts and embryos. (A) Anemia in E10.5 Raf-1-deficient embryos is rescued by the introduction of one lpr allele. (B) Faster differentiation correlates with increased Fas surface expression in WT and Raf-1-/-fetal liver erythroblasts. Erythroblasts were cultured in erythroid medium. The progress of differentiation (shown as hemoglobin content per cell and cell volume; B) and Fas expression (C; determined by FACS) were determined at the indicated times during differentiation. For panels B and C, one representative experiment of 6 is shown. F2 embryos and embryo-derived cells originate from crosses of F1 Raf-1+/-;lpr/+ 129/SvHsd:Bl6 mice. Embryo images were acquired using an MZ-Apo stereomicroscope equipped with a PLAN-APO 1.0 ×/12.5 NA bottom objective (Leica, Vienna, Austria). A DKC-5000 CCD camera (Sony, Cologne, Germany) was used to capture images, and Image Access v4.5 software (Imagic, Glattbrugg, Switzerland) was used to acquire them.
Up-regulation of Fas expression correlates with anemia in Raf-1-deficient erythroblasts and embryos. (A) Anemia in E10.5 Raf-1-deficient embryos is rescued by the introduction of one lpr allele. (B) Faster differentiation correlates with increased Fas surface expression in WT and Raf-1-/-fetal liver erythroblasts. Erythroblasts were cultured in erythroid medium. The progress of differentiation (shown as hemoglobin content per cell and cell volume; B) and Fas expression (C; determined by FACS) were determined at the indicated times during differentiation. For panels B and C, one representative experiment of 6 is shown. F2 embryos and embryo-derived cells originate from crosses of F1 Raf-1+/-;lpr/+ 129/SvHsd:Bl6 mice. Embryo images were acquired using an MZ-Apo stereomicroscope equipped with a PLAN-APO 1.0 ×/12.5 NA bottom objective (Leica, Vienna, Austria). A DKC-5000 CCD camera (Sony, Cologne, Germany) was used to capture images, and Image Access v4.5 software (Imagic, Glattbrugg, Switzerland) was used to acquire them.
We confirmed this by introducing 2 lpr alleles on the Raf-1-/-, Raf-1+/-, and on WT background. As expected, Fas surface expression could not be detected in lpr/lpr cells (not shown). Regardless of the status of the c-raf-1 locus, the homozygous lpr cells showed an almost complete block of differentiation, measured as cell proliferation, volume decrease, and hemoglobin accumulation (Figure 2C).
To gain more insight about the differentiation steps affected, we analyzed the cellular composition of the differentiating cultures at different time points. Maturation of WT cells was complete (90% mature erythrocytes, E) within the standard 48 hours (Table 1). As previously described,5 Raf-1-/- cells reached the same percentage (89.5%) of red cells already 36 hours after induction of differentiation. The presence of one lpr allele delayed differentiation of WT, Raf-1 heterozygous, and Raf-1-deficient cells, resulting in the rescue of the Raf-1 phenotype in the heterozygous cells and restoring differentiation of the Raf-1-/- cells to the level of the Raf-1 heterozygotes. lpr/lpr cells, on the other hand, were unable to terminally differentiate even 60 hours after induction of differentiation, regardless of the status of the c-raf-1 locus (Table 1 and data not shown). Fas-induced apoptosis reportedly occurs during erythroid differentiation in the presence of low Epo levels.18 Under our culture conditions (10 U/mL Epo), very few apoptotic/dead cells were observed throughout differentiation. Their number was not reduced in the lpr/+ or lpr/lpr animals. In the compound knock-out (Raf-1-/-;lpr/lpr), the number of apoptotic/dead cells was increased. The exact reason for this increase is unknown, but it can be speculated that if both differentiation and survival are disturbed, apoptosis will increase.
Heterozygosity and homozygosity at the lpr locus rescue the accelerated erythroid differentiation caused by lack of Raf-1
. | Genotype . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Differentiation cell type . | +/+ . | . | . | Ipr/+ . | . | . | Ipr/Ipr . | . | . | ||||||||
| . | Raf-1+/+ . | Raf-1+/− . | Raf-1−/− . | Raf-1+/+ . | Raf-1+/− . | Raf-1−/− . | Raf-1+/+ . | Raf-1+/− . | Raf-1−/− . | ||||||||
| 0 h | |||||||||||||||||
| PB | 83.0 | 83.0 | 80.0 | 77.8 | 82.0 | 76.8 | 94.0 | 87.0 | 81.1 | ||||||||
| DB | 8.6 | 14.7 | 14.4 | 18.7 | 8.0 | 18.2 | 2.0 | 9.3 | 13.0 | ||||||||
| E | 7.0 | 1.0 | 4.0 | 2.4 | 8.5 | 3.5 | 3.0 | 2.7 | 0.5 | ||||||||
| DC | 1.4 | 1.3 | 1.6 | 1.1 | 1.5 | 1.5 | 1.0 | 1.0 | 5.4 | ||||||||
| 24 h | |||||||||||||||||
| PB | 45.0 | 17.0 | 10.0 | 57.3 | 43.0 | 13.7 | 71.6 | 72.0 | 60.1 | ||||||||
| DB | 43.5 | 31.1 | 25.0 | 37.1 | 41.9 | 29.8 | 26.4 | 23.7 | 29.5 | ||||||||
| E | 10.0 | 50.2 | 63.7 | 4.4 | 13.4 | 55.4 | 1.1 | 3.1 | 5.2 | ||||||||
| DC | 1.5 | 1.7 | 1.3 | 1.2 | 1.7 | 1.1 | 0.9 | 1.2 | 5.2 | ||||||||
| 36 h | |||||||||||||||||
| PB | 16.7 | 2.7 | 0.5 | 21.4 | 16.7 | 1.7 | 57.0 | 55.0 | 7.0 | ||||||||
| DB | 30.0 | 17.9 | 9.0 | 58.6 | 35.0 | 17.5 | 37.6 | 40.2 | 79.8 | ||||||||
| E | 52.0 | 78.2 | 89.5 | 18.7 | 46.8 | 79.6 | 4.0 | 3.7 | 7.0 | ||||||||
| DC | 1.3 | 1.2 | 1.0 | 1.3 | 1.5 | 1.2 | 1.4 | 1.1 | 6.2 | ||||||||
| 48 h | |||||||||||||||||
| PB | 1.5 | 1.6 | 0.2 | 3.2 | 2.4 | 1.2 | 27.0 | 23.0 | 2.0 | ||||||||
| DB | 7.0 | 5.6 | 2.5 | 22.3 | 5.0 | 3.2 | 56.0 | 60.0 | 73.9 | ||||||||
| E | 90.0 | 91.3 | 95.6 | 73.4 | 91.0 | 94.2 | 15.5 | 15.6 | 17.0 | ||||||||
| DC | 1.5 | 1.5 | 1.7 | 1.1 | 1.6 | 1.4 | 1.5 | 1.4 | 7.1 | ||||||||
. | Genotype . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Differentiation cell type . | +/+ . | . | . | Ipr/+ . | . | . | Ipr/Ipr . | . | . | ||||||||
| . | Raf-1+/+ . | Raf-1+/− . | Raf-1−/− . | Raf-1+/+ . | Raf-1+/− . | Raf-1−/− . | Raf-1+/+ . | Raf-1+/− . | Raf-1−/− . | ||||||||
| 0 h | |||||||||||||||||
| PB | 83.0 | 83.0 | 80.0 | 77.8 | 82.0 | 76.8 | 94.0 | 87.0 | 81.1 | ||||||||
| DB | 8.6 | 14.7 | 14.4 | 18.7 | 8.0 | 18.2 | 2.0 | 9.3 | 13.0 | ||||||||
| E | 7.0 | 1.0 | 4.0 | 2.4 | 8.5 | 3.5 | 3.0 | 2.7 | 0.5 | ||||||||
| DC | 1.4 | 1.3 | 1.6 | 1.1 | 1.5 | 1.5 | 1.0 | 1.0 | 5.4 | ||||||||
| 24 h | |||||||||||||||||
| PB | 45.0 | 17.0 | 10.0 | 57.3 | 43.0 | 13.7 | 71.6 | 72.0 | 60.1 | ||||||||
| DB | 43.5 | 31.1 | 25.0 | 37.1 | 41.9 | 29.8 | 26.4 | 23.7 | 29.5 | ||||||||
| E | 10.0 | 50.2 | 63.7 | 4.4 | 13.4 | 55.4 | 1.1 | 3.1 | 5.2 | ||||||||
| DC | 1.5 | 1.7 | 1.3 | 1.2 | 1.7 | 1.1 | 0.9 | 1.2 | 5.2 | ||||||||
| 36 h | |||||||||||||||||
| PB | 16.7 | 2.7 | 0.5 | 21.4 | 16.7 | 1.7 | 57.0 | 55.0 | 7.0 | ||||||||
| DB | 30.0 | 17.9 | 9.0 | 58.6 | 35.0 | 17.5 | 37.6 | 40.2 | 79.8 | ||||||||
| E | 52.0 | 78.2 | 89.5 | 18.7 | 46.8 | 79.6 | 4.0 | 3.7 | 7.0 | ||||||||
| DC | 1.3 | 1.2 | 1.0 | 1.3 | 1.5 | 1.2 | 1.4 | 1.1 | 6.2 | ||||||||
| 48 h | |||||||||||||||||
| PB | 1.5 | 1.6 | 0.2 | 3.2 | 2.4 | 1.2 | 27.0 | 23.0 | 2.0 | ||||||||
| DB | 7.0 | 5.6 | 2.5 | 22.3 | 5.0 | 3.2 | 56.0 | 60.0 | 73.9 | ||||||||
| E | 90.0 | 91.3 | 95.6 | 73.4 | 91.0 | 94.2 | 15.5 | 15.6 | 17.0 | ||||||||
| DC | 1.5 | 1.5 | 1.7 | 1.1 | 1.6 | 1.4 | 1.5 | 1.4 | 7.1 | ||||||||
Erythroid progenitors of the indicated genotypes were cultured in differentiation medium. At the indicated times, cytospins stained with neutral benzidine plus histologic dyes were examined to determine the progress of differentiation. The values represent the mean of 4 determinations, each carried out by an independent investigator evaluating 500 cells per sample on at least 2 randomly selected fields.
PB indicates proliferating blast; DB, differentiating blast; E, mature erythrocytes; DC, apoptotic/dead cells.
Together, these data show that Fas plays a significant role in promoting erythroid differentiation and that the increased expression of Fas in the Raf-1-/- cells is the reason for their faster differentiation.
Increased Fas expression is the molecular basis of the increased differentiation observed in Raf-1-deficient erythroblasts. Fas surface expression (A) and erythroid differentiation (B) are increased by Raf-1 reduction/ablation. Fas surface expression and hemoglobin content were determined at the indicated times during differentiation in erythroid cells of indicated genotypes. (C) The lpr/lpr mutation prevents terminal differentiation of WT, Raf-1+/-, and Raf-1-/-erythroblasts. Hemoglobin accumulation is shown as a differentiation parameter. One representative experiment of 4 is shown in the 3 panels.
Increased Fas expression is the molecular basis of the increased differentiation observed in Raf-1-deficient erythroblasts. Fas surface expression (A) and erythroid differentiation (B) are increased by Raf-1 reduction/ablation. Fas surface expression and hemoglobin content were determined at the indicated times during differentiation in erythroid cells of indicated genotypes. (C) The lpr/lpr mutation prevents terminal differentiation of WT, Raf-1+/-, and Raf-1-/-erythroblasts. Hemoglobin accumulation is shown as a differentiation parameter. One representative experiment of 4 is shown in the 3 panels.
Fas is responsible for differentiation-associated caspase activation
These data clearly show the importance of Fas in erythroblast differentiation. To determine whether Fas activation is the stimulus that induces differentiation-associated caspase activation, we monitored the cleavage of the death substrate lamin B during erythroid differentiation of WT and lpr/lpr cells, which did not express any detectable Fas (Figure 3A). Lamin B cleavage is needed to dissolve the nuclear membrane and is therefore likely connected with enucleation.19 As previously reported,5 lamin B cleavage was clearly detectable in differentiating WT cells (36-48 hours after differentiation induction) and Raf-1-/- cells (24-48 hours after differentiation induction), but was essentially absent in cells of either genotype bearing the lpr/lpr mutation (Figure 3A). The down-regulation of Raf-1 protein and mRNA levels also proceeded much less efficiently in lpr/lpr than in WT cells (Figure 3A-B). In addition, and confirming the results described in Figure 1, Raf-1-/- cells contained more Fas protein and mRNA than WT cells, showing that Raf-1 ablation up-regulates Fas protein levels as well as Fas surface expression (Figures 1C and 3A-B). However, in contrast to Fas surface expression, the amount of Fas detectable by immunoblotting or reverse transcription (RT)-PCR (Figure 3A-B) was stable (WT) or even increased (protein, Raf-1-/- cells) up to 48 hours after differentiation induction. These data indicate that at late stages of differentiation, Fas surface expression is regulated not only at the stage of mRNA/protein expression, but also at the level of protein transport to/from the cell surface. The mechanism by which this latter step is accomplished is currently unknown; its existence, however, supports the hypothesis that a tight regulation of Fas surface expression is necessary to maintain normal erythropoiesis.
The lpr mutation abrogates lamin B cleavage and Raf-1 downregulation in differentiating erythroblasts. Lamin B cleavage and the expression of Raf-1, Fas, and FasL were determined by immunoblotting (A) or by RT-PCR (B). The experiments were repeated 4 times (A) or twice (B) with comparable results. In panel B, the ribonuclease inhibitor (RI) was amplified in parallel reaction as a reference gene.
The lpr mutation abrogates lamin B cleavage and Raf-1 downregulation in differentiating erythroblasts. Lamin B cleavage and the expression of Raf-1, Fas, and FasL were determined by immunoblotting (A) or by RT-PCR (B). The experiments were repeated 4 times (A) or twice (B) with comparable results. In panel B, the ribonuclease inhibitor (RI) was amplified in parallel reaction as a reference gene.
In contrast to the tight regulation of Fas expression, similar amounts of FasL were constitutively produced by the cultures, irrespective of the differentiation stage or genotype (Figure 3A). Thus, the effects of Raf-1 ablation on erythroid differentiation are connected with the ability of Raf-1 to regulate Fas expression, and Fas, in turn, promotes the differentiation-associated down-regulation of Raf-1.
Effect of Raf-1 or Fas ablation on MAPK signaling during erythroid differentiation
To gain more insight into the signaling events occurring downstream of Raf-1 or Fas during erythroid differentiation, we analyzed the phosphorylation status of the MAPKs ERK (the best studied downstream target of Raf-1), JNK, and p38. All 3 MAPK pathways were activated during differentiation of fetal liver-derived erythroblasts, albeit with different kinetics. Phosphorylation of ERK was evident as early as 6 hours after differentiation induction and subsided by 36 hours (Figure 4A), a time course that parallels the initial expansion of the precursors in differentiation medium and consistent with previous work showing the importance of this pathway in sustaining proliferation and inhibiting differentiation.20 Raf-1 ablation significantly reduced both the strength and the duration of ERK phosphorylation. Conversely, ERK phosphorylation was markedly increased and prolonged in lpr/lpr erythroid precursors (Figure 4A). These data are consistent with the postulated roles of Raf-1 in antagonizing, and of Fas in promoting, terminal differentiation.
The pattern of JNK and p38 phosphorylation was opposite to that of the ERKs. In WT cells, phosphorylation started at 24 hours and persisted throughout the observation period on to terminal differentiation. This is in line with the previously proposed function of these MAPKs in erythroid differentiation rather than proliferation.21 JNK and p38 were hyperphosphorylated in Raf-1 knock-out (KO) erythroblasts; in addition, JNK phosphorylation appeared earlier (12 hours) than in WT cells (Figure 4A). Thus, Raf-1 supports ERK activation and antagonizes JNK/p38 phosphorylation during erythroblasts differentiation. Raf-1 could impinge on the phosphorylation of the JNK and p38 via its inhibition of ASK1,22 or of Rok-α,15 both of which reportedly activate JNK, p38, or both.23,24 Rok-α expression was undetectable throughout erythroblast differentiation (not shown). ASK1 mRNA and protein levels, however, were expressed in increasing amounts during differentiation and were increased in Raf-1 KO (Figure 4A-B). In contrast, mutation of Fas virtually abrogated ASK1 expression and reduced both the extent and the duration of JNK/p38 phosphorylation (Figure 4A). These results are consistent with the hypothesis that Raf-1 regulates erythroid differentiation by controlling the levels of Fas expression and that Fas, in turn, maximizes the activation of the differentiation-promoting JNK/p38 cascade via the expression of ASK1. Double KO cells showed a compound phenotype and ERK activation was decreased in a manner similar to the Raf-1 KO, demonstrating that the increase in ERK phosphorylation in the lpr/lpr cells was due to Raf-1; ASK1 expression was abrogated and JNK/p38 were diminished compared to the Raf-1 KO, indicating that these events depend on intact Fas signaling (Figure 4A). However, the residual JNK activation and, to a lesser extent, p38 activation, was increased with respect to the lpr/lpr, demonstrating that Raf-1 inhibits both Fas-dependent and -independent JNK/p38 phosphorylation (Figure 4A).
Raf-1 and Fas have opposite effects on MAPK signaling during the differentiation of primary fetal liver-derived erythroblasts. (A) Phosphorylation of ERK, JNK, and p38 as well as the amount of ERK, JNK, p38, and ASK1 expressed by differentiating erythroblasts were determined by immunoblotting. The experiments were reproduced 4 times with comparable results. (B) ASK1 mRNA increases during erythroblast differentiation in a Fas-dependent manner. RT-PCR was repeated twice.
Raf-1 and Fas have opposite effects on MAPK signaling during the differentiation of primary fetal liver-derived erythroblasts. (A) Phosphorylation of ERK, JNK, and p38 as well as the amount of ERK, JNK, p38, and ASK1 expressed by differentiating erythroblasts were determined by immunoblotting. The experiments were reproduced 4 times with comparable results. (B) ASK1 mRNA increases during erythroblast differentiation in a Fas-dependent manner. RT-PCR was repeated twice.
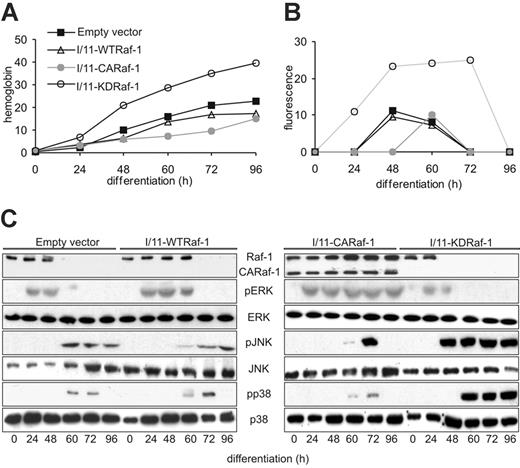
Constitutively active Raf-1 delays, and kinase-dead Raf-1 accelerates, erythroid differentiation and Fas expression in immortalized fetal liver erythroblasts. (A) The differentiation of I/11 cells transfected with empty vector was compared with that of I/11 cells expressing WTRaf-1, CARaf-1, and KDRaf-1, respectively. Hemoglobin content was determined between 0 and 96 hours after induction of differentiation. (B) Fas up-regulation during differentiation of I/11 erythroblasts expressing murine stem-cell virus-EGFP (empty vector) with full-length Raf-1 (WTRaf-1), a constitutively active Raf-1 (CARaf-1) or a kinase-dead mutant (KDRaf-1) was monitored by FACS analysis at the indicated time points. (C) CARaf-1 enforces, and KD-Raf-1 reduces, ERK phosphorylation in differentiating I/11 cells. The converse is observed for JNK/p38 phosphorylation. One representative experiment of 5 is shown in panels A-C.
Constitutively active Raf-1 delays, and kinase-dead Raf-1 accelerates, erythroid differentiation and Fas expression in immortalized fetal liver erythroblasts. (A) The differentiation of I/11 cells transfected with empty vector was compared with that of I/11 cells expressing WTRaf-1, CARaf-1, and KDRaf-1, respectively. Hemoglobin content was determined between 0 and 96 hours after induction of differentiation. (B) Fas up-regulation during differentiation of I/11 erythroblasts expressing murine stem-cell virus-EGFP (empty vector) with full-length Raf-1 (WTRaf-1), a constitutively active Raf-1 (CARaf-1) or a kinase-dead mutant (KDRaf-1) was monitored by FACS analysis at the indicated time points. (C) CARaf-1 enforces, and KD-Raf-1 reduces, ERK phosphorylation in differentiating I/11 cells. The converse is observed for JNK/p38 phosphorylation. One representative experiment of 5 is shown in panels A-C.
To gain more insight in the role played by Raf-1 in erythroid differentiation, Fas expression, and MAPK activity, we used I/11 immortalized erythroblasts, a cell line derived from the fetal liver of p53 KO animals, which can be easily transfected. As previously described, I/11 cells undergo terminal differentiation, albeit with slower kinetics than primary fetal liver erythroblasts. Interestingly, this delay in differentiation correlates with a reduction in the expression of Fas (Figure 5A-B). This is likely due to the absence of p53, which positively regulates Fas expression in a number of ways.25-27 I/11 cells expressing WTRaf-1 differentiated with kinetics similar to I/11 cells, probably due to the fact that the exogenous protein was down-regulated along with the endogenous during differentiation (Figure 5A,C). Consistently, Fas expression and MAPK activation were hardly affected, with the exception of a slight increase in ERK phosphorylation levels (Figure 5C). In contrast, cells expressing a kinase-dead Raf-1 (I/11-KDRaf-1) differentiated faster (Figure 5A). This was accompanied by more sustained Fas expression and stronger JNK and p38 activation, and JNK activation occurred with earlier kinetics. Additionally, ERK activation was reduced (Figure 5C). This situation was strongly reminiscent of that observed in the Raf-1 KO primary erythroblasts (Figure 4A), and implied an essential function of Raf-1 as a MEK/ERK activator during erythroid differentiation. In addition to the changes in Fas expression and MAPK phosphorylation, however, expression of KDRaf-1 accelerated the down-regulation of both the exogenous and the endogenous Raf-1 protein and mRNA, both of which disappeared after 24 hours instead of 48 hours (WT) or 60 hours (I/11-WTRaf-1) of differentiation (Figure 5C and data not shown). To investigate whether the kinase function of Raf-1 or ERK activation (or both) determined the kinetics of Raf-1 downregulation during erythroid differentiation, we monitored the fate of the endogenous Raf-1 protein in cells expressing a truncated, constitutively active form of Raf-1 (I/11-CARaf-1), which differentiate extremely slowly5 (Figure 5A). In these cells, ERK activation was high and persisted throughout the observation period. Consistent with a potential role of ERK in maintaining Raf-1 expression, neither the exogenous CARaf-1 nor the endogenous protein was down-regulated in these cells, and Raf-1 mRNA was present throughout differentiation (Figures 5C and 6C-D). In line with the inverse relationship between Raf-1 and Fas expression, the cells expressed little Fas (Figure 5B). In addition, activation of JNK, and, less so, of p38, was weaker and shortened compared to that observed in I/11 and I/11-WTRaf-1 cells (Figure 5C).
ERK activation regulates Raf-1 and Fas expression during erythroid differentiation. (A-B) Chemical inhibition of MEK accelerates erythroid differentiation and increases Fas surface expression. I/11 cells were treated with the MEK inhibitor PD98059 (40 μg, 72 hours) during differentiation. Hemoglobin content (A) and Fas expression (B) were monitored between 0 and 96 hours of differentiation. (C) Chemical inhibition of MEK decreases Raf-1 protein expression and ERK phosphorylation in differentiating I/11 cells. Protein expression and phosphorylation were determined by immunoblotting. (A-C) One representative experiment of 5 is shown. (D) Chemical inhibition of MEK decreases Raf-1 mRNA levels and increases Fas mRNA levels in differentiating I/11 cells. mRNA levels were determined by RT-PCR, which was repeated twice with comparable results.
ERK activation regulates Raf-1 and Fas expression during erythroid differentiation. (A-B) Chemical inhibition of MEK accelerates erythroid differentiation and increases Fas surface expression. I/11 cells were treated with the MEK inhibitor PD98059 (40 μg, 72 hours) during differentiation. Hemoglobin content (A) and Fas expression (B) were monitored between 0 and 96 hours of differentiation. (C) Chemical inhibition of MEK decreases Raf-1 protein expression and ERK phosphorylation in differentiating I/11 cells. Protein expression and phosphorylation were determined by immunoblotting. (A-C) One representative experiment of 5 is shown. (D) Chemical inhibition of MEK decreases Raf-1 mRNA levels and increases Fas mRNA levels in differentiating I/11 cells. mRNA levels were determined by RT-PCR, which was repeated twice with comparable results.
Effect of ERK on Raf-1 and Fas expression during erythroid differentiation
To assess whether the correlation between ERK activation and Raf-1 expression was causal, we treated I/11-WTRaf-1 and I/11-CARaf-1 cells with the MEK inhibitor PD98059 and monitored Raf-1 expression throughout differentiation. The inhibitor completely abrogated ERK phosphorylation and accelerated the loss of Raf-1 during differentiation. Surprisingly, the endogenous and the exogenous protein were equally affected, as shown by the disappearance of CARaf-1 (Figure 6C). Consistent with a role for Raf-1 in antagonizing Fas-induced erythroid differentiation, Fas surface expression increased as Raf-1 decreased (Figure 6B). As observed in primary erythroblasts, the effects on Raf-1 and Fas expression could also be detected by RT-PCR (Figure 6D). Consistent with all of these aspects, the inhibitor accelerated differentiation in both cell lines (Figure 6A).
The glucocorticoid receptor maintains Raf-1 expression in proliferating erythroblasts
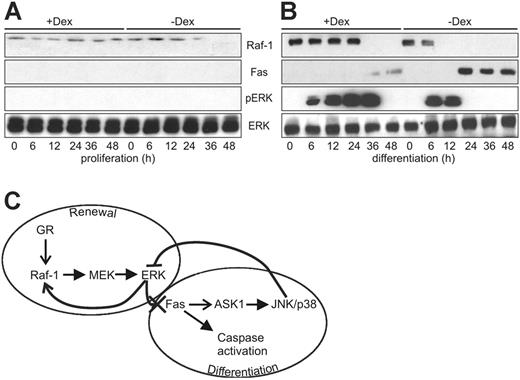
The data summarized are consistent with a model in which the down-regulation of Raf-1 sets the pace of erythrocyte differentiation by reducing ERK activity and allowing the expression of Fas. However, the question remained how Raf-1 expression was maintained in proliferating erythroblasts and gradually ablated during differentiation. In most of the cell types studied, Raf-1 is constitutively expressed, and its expression is not increased by growth factor stimulation. Recently, however, transcriptional upregulation of Raf-1 by glucocorticoids has been reported in the mouse hippocampus during fear conditioning.28 Glucocorticoids play an important role in erythropoiesis in vivo29-31 and in erythroblast cultures, where they are necessary to sustain the renewal of the erythroid precursors.31,32 In our culture system, Dex is a component of the proliferation medium, but it is not included in the differentiation medium. Indeed, withdrawal of Dex and SCF is sufficient to initiate a differentiation program that depends on the presence of Epo exclusively to inhibit apoptosis.33 We investigated whether Dex withdrawal determined the decay of Raf-1 expression in erythroblast cultures by monitoring the levels of Raf-1 in cultures kept in proliferation medium in the presence or absence of Dex, and in cultures kept in differentiation medium supplemented or not with Dex. The results in Figure 7 show that Raf-1 expression was constitutive in erythroblasts kept in proliferation medium, but Dex withdrawal was sufficient to decrease, and eventually ablate, Raf-1 expression. Conversely, in differentiation medium, adding back Dex significantly prolonged the expression of Raf-1. Consistent with the proposed regulation of Fas by Raf-1, Fas expression followed the converse pattern, being repressed by the presence of Dex and increased by its withdrawal (Figure 7).
Dex maintains Raf-1 expression in proliferating and differentiating primary erythroblasts. (A) Effect of Dex on the amount of Raf-1, Fas, and phosphorylated ERK detected in proliferating (A) and differentiating (B) erythroblasts. Protein expression and phosphorylation were determined by immunoblotting. The experiment was repeated twice with comparable results. (C) Regulation of Raf-1 and Fas expression by activated ERK maintains the equilibrium between renewal and differentiation of erythroid precursor cells—a working model. In differentiating erythroblasts, Raf-1 is required for ERK activation. Activated ERK is responsible for maintaining Raf-1 expression and suppressing Fas expression. Glucocorticoids maintain Raf-1 expression and therefore renewal. Withdrawal of the renewal stimulus causes the down-regulation of the Raf-1/ERK axis and allows the expression of Fas, which in turn up-regulates the ASK1/JNK/p38 module, suppresses ERK activation and brings about differentiation. See text for details. → stimulation of expression; —-▸ enzymatic activation; —X suppression of expression; — suppression of enzymatic activity.
Dex maintains Raf-1 expression in proliferating and differentiating primary erythroblasts. (A) Effect of Dex on the amount of Raf-1, Fas, and phosphorylated ERK detected in proliferating (A) and differentiating (B) erythroblasts. Protein expression and phosphorylation were determined by immunoblotting. The experiment was repeated twice with comparable results. (C) Regulation of Raf-1 and Fas expression by activated ERK maintains the equilibrium between renewal and differentiation of erythroid precursor cells—a working model. In differentiating erythroblasts, Raf-1 is required for ERK activation. Activated ERK is responsible for maintaining Raf-1 expression and suppressing Fas expression. Glucocorticoids maintain Raf-1 expression and therefore renewal. Withdrawal of the renewal stimulus causes the down-regulation of the Raf-1/ERK axis and allows the expression of Fas, which in turn up-regulates the ASK1/JNK/p38 module, suppresses ERK activation and brings about differentiation. See text for details. → stimulation of expression; —-▸ enzymatic activation; —X suppression of expression; — suppression of enzymatic activity.
Discussion
The data demonstrate the existence of 2 self-amplifying loops in the differentiation of erythroid precursors: the first involves the Raf-1/MEK/ERK pathway and is important in maintaining the undifferentiated state; the second loop hinges on the death receptor Fas, which is responsible for caspase activation and differentiation, but also for the expression of ASK1 and, at least in part, for the activation of JNK/p38 in this system. There is extensive cross-talk between these 2 loops: the activation of the ERK cascade prevents/delays the expression of Fas and thereby the activation of both the caspases and the JNK/p38 pathway; on the other hand, Fas expression and the ensuing activation of the differentiation program down-regulate ERK, thereby stabilizing Fas and the downstream signaling that sustains differentiation (Figure 7C). In our system, Raf-1 expression is maintained by the renewal factor Dex during erythroblast proliferation. This is reminiscent of the positive effect of glucocorticoids on Raf-1 expression reported in the hippocampus of mice exposed to fear conditioning.28 In erythroid differentiation medium, which does not contain Dex, Raf-1 downregulation can be delayed, but not entirely blocked, by sustained ERK activation. Unlike in other experimental systems,14,15 Raf-1 is necessary for ERK activation during erythroblast differentiation (Figures 4, 6). Therefore ERK, by inducing Raf-1 expression, maintains its own activation and the proliferation of differentiating erythroblasts. At the same time, ERK prevents terminal differentiation by inhibiting the expression of Fas. Expression of Fas on ERK inhibition has been observed before in both lymphomas and in solid tumor cells.34,35 In these cases, however, the outcome of Fas expression is apoptosis rather than differentiation. In our system, Fas induction has multiple effects: it triggers the differentiation-associated caspase activation responsible, for instance, for lamin B cleavage (Figure 3); in addition, it stimulates the expression of the JNK/p38 kinase ASK1 and the activation of both JNK and p38; concomitantly, it triggers the repression of the ERK cascade, thereby perpetuating its own expression and downstream signaling (Figure 4, 6). We have not investigated the mechanism of Fas-induced ERK repression. However, sustained JNK activation like the one that follows Fas expression in differentiating erythroblasts has previously been shown to inhibit ERK activation by mitogenic factors.36
Our work identifies the regulation of Raf-1 and Fas expression as the crucial switch that determines whether an erythroid precursor cell will proliferate or differentiate. Failure to down-regulate Raf-1 or constitutive activation of Raf-1 results in a differentiation block (Figures 3, 6). Similarly, lack of Fas expression will not allow differentiation to proceed and will, instead, maintain the Raf-1/MEK/ERK cascade-driven proliferation of erythroid precursors (Figures 3, 6). In line with this, Raf-1 is activated by erythroleukemia inducing strains of the Friend spleen focus-forming virus,37 and oncogenic, constitutively active v-raf in combination with myc38 or with loss of p5339 efficiently transforms erythroid cells. On the other hand, lack of Fas is associated with increased erythroid progenitor-cell numbers,8,9 whereas an increase in Fas correlates with defects in erythroid progenitor growth and abnormal erythroid differentiation during myelodysplasia.40,41
Activated ERK is the neuralgic convergence point that sustains Raf-1 expression and the proliferation of erythroid progenitors, while concomitantly repressing Fas expression and putting the brakes on differentiation. So how does it do that? At present, we have no information about the mechanism of Fas repression. In the case of Raf-1, we already know that down-regulation occurs at the mRNA level (Figures 3B and 6D). However, the fact that the endogenous and the exogenous Raf-1 mRNA and protein are coregulated in I/11 cells expressing either CARaf-1 or KDRaf-1 strongly suggests that the down-regulation happens in a promoter-independent manner. It is tempting to speculate that activated ERK may repress the expression of a small noncoding RNA (microRNA) that targets the Raf-1 gene. In the last few years, regulation of gene expression by microRNAs has been demonstrated in many developmental processes, including hematopoiesis.42 Indeed, one of the transcription factors targeted by the ERK pathway, serum response factor (SRF), regulates microRNA expression during formation of cardiac muscle.43 It should also be noted, in this context, that the expression of Ras, the small GTPase upstream of the Raf/MEK/ERK cascade, is regulated by micro-RNAs in Caenorhabditis elegans, and, possibly, in human cancer.44 The possibility of feedback loops involving microRNAs to regulate Raf-1 and Fas expression during erythroid differentiation is attractive and deserves further investigation.
Prepublished online as Blood First Edition Paper, March 9, 2006; DOI 10.1182/blood-2005-09-3866.
Supported by grants P15784-MOB of the Austrian Research Fund and LSH-CT-2003-506803 of the European Community (EC) (M.B.) and by grants of the Federal Ministry for Education, Science and Culture (J.C.H.).
C.R., D.P., and K.M. performed the research and analyzed the data; H.B. and J.C.H. contributed reagents and analytical tools; A.K. and M.B. jointly designed the research and analyzed the data; M.B. wrote the paper.
C.R. and D.P. contributed equally to this work. A.K. and M.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Philipp Leuschner for performing the initial experiments on Fas detection; Matthias Hamerl, Claudia Khrla, Alexander Dangl, Katja Hiesböck, and Ladislaus Szabó for excellent technical help; and Thomas Decker (MFPL, Vienna Biocenter) for critically reading this manuscript. C. R. and D. P. are PhD and K. M. is a PhD candidate of the University of Vienna and this work is submitted in partial fulfillment of the requirement for the PhD.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal