Abstract
Breast cancer cells (BCCs) show preference for the bone marrow (BM). An animal model showed 2 populations of BCCs in the BM with regard to their cycling states. An in vitro model of early BC entry into BM showed normal hematopoiesis. Here, we show a critical role for BCC-derived SDF-1α in hematopoietic regulation. The studies used a coculture of BM stroma and BCCs (cell lines and stage II BCCs). Northern blots and enzyme-linked immunosorbent assay (ELISA) showed gradual decreases in SDF-1α production in BCCs as they contact BM stroma, indicating partial microenvironmental effects caused by stroma on the BCCs. SDF-1 knock-down BCCs and increased exogenous SDF-1α prevented contact inhibition between BCCs and BM stroma. Contact inhibition was restored with low SDF-1α levels. Long-term culture-initiating assays with CD34+/CD38–/Lin– showed normal hematopoiesis provided that SDF-1α levels were reduced in BCCs. Gap junctions (connexin-43 [CX-43]) were formed between BCCs and BM stroma, with concomitant interaction between CD34+/CD38–/Lin– and BM stroma but not with the neighboring BCCs. In summary, SDF-1α levels are reduced in BCCs that contact BM stroma. The low levels of SDF-1α in BCCs regulate interactions between BM stroma and hematopoietic progenitors, consequently facilitating normal hematopoiesis.
Introduction
Despite aggressive efforts to detect breast cancer (BC) early, many survivors develop bone metastasis long after remission.1,2 Furthermore, early detection of cancer does not guarantee improved survival.3 Bone metastasis has been reported in the absence of primary BC,4 suggesting the migration of BC cells (BCCs) during the early phase of the disease and perhaps prior to clinical detection. An alternate explanation is that the cancer cells may have migrated without ever developing into a detectable tumor at the primary site. Others have proposed the possibility of malignancy occurring at a distant site where the cancer cells have adapted the properties of primary BCCs.3
New information on cancer stem cells might partly explain BC resurgence years after remission.5-7 There are reports of BCCs in the bone without a history of primary tumors.8 While there is no explanation for this observation, it has been suggested that this could occur through the process of transdifferentiation by bone marrow (BM) cells to BCCs or from malignant cells of other tissues.8 Regardless of the mechanism, metastasis to the BM remains a clinical dilemma. Because BCCs are heterogeneous, an understanding of this disease would require in-depth research studies incorporating specific cellular subsets in the context of various areas within the BM microenvironment.9
SDF-1α belongs to the chemokine family and is constitutively produced in BM stroma.10,11 Both types of SDF-1 (α and β) act as chemoattractants and have been shown to exhibit antiapoptotic functions.12-15 Both SDF-1 subtypes interact with the CXCR4 receptor.16 During late-stage metastasis, CXCR4-expressing BCCs migrate toward organs with high SDF-1α.12,13,17,18
This study determined whether, at an early phase of BC development, gap junctions are formed between BCCs and BM stroma and also studied the role of SDF-1α in hematopoiesis and the integration of BCCs within stroma. The premise that BM stroma supports the survival of BCCs is partly based on their ability to produce growth-promoting cytokines, secretion of extracellular matrix proteins, expression of ligands for adhesion molecules, and elaboration of gap junctions.19-21 BCCs have been found close to the endosteum of nude mice where stroma and hematopoietic stem cells reside, underscoring a role for stroma.22 Endosteal-resident BCCs have relatively long doubling times as compared with those in the central region of BM.21 These in vivo studies support the present hypothesis of gap junction formation between BCCs and stroma, because this would deter the proliferation of BCCs. In vitro studies have also shown functional changes in BCCs upon contact with BM stroma.21
The significance of this report centers on the sensitivity of BM cells to toxic effects by current therapeutic agents. Targeting of BCCs in the BM could be detrimental to the resident stem cells—hematopoietic and mesenchymal.23 Understanding the molecular mechanisms facilitating early entry of BCCs in BM could lead to potential therapy and/or preventive strategies in BC metastasis. In addition, this report describes studies that are relevant to events within the BM during the early period of BC or during cancer remission.
We report that a low level of SDF-1α is critical to the quiescent nature of BCCs and amenable to forming gap junctions with BM stroma and is critical for appropriate interaction between immature hematopoietic progenitors and BM stromal cells. This report clearly shows that BCCs remain quiescent within BM stroma provided that they produce a low level of SDF-1α, thereby preventing BM disruption. The studies were done with a coculture model on the early events occurring in BCC integration with BM stroma.24
Materials and methods
Reagents, cytokines, and antibodies
Nonimmune rabbit and murine IgG were purchased from Sigma (St Louis, MO); GM-CSF, rhSDF-1α, and rabbit anti–SDF-1 from R&D Systems (Minneapolis, MN); rabbit anti-CXCR4 from Affinity BioReagents (Golden, CO); FITC goat anti–rabbit IgG, PE rat anti–mouse kappa, and FITC-cytokeratin monoclonal antibody (mAb) from BD Biosciences (San Jose, CA); antifibroblast microbeads and FITC-fibroblast mAb from Miltenyi Biotec (Auburn, CA); rabbit anti–connexin-43 (anti–CX-43) from Invitrogen (Carlsbad, CA); prolyl-4-hydroxylase mAb and anti–human fibrobast (clone 5B5) from Dako (Glostrup, Denmark); Texas Red-X phalloidin from Molecular Probes (Eugene, OR); and APC-rabbit anti–mouse IgG from Open Biosystems (Huntsville, AL).
Cell lines and BM stroma
MCF7, ZR-75-30, T47D, DU4475, and BT 483 were purchased from American Type Culture Collection (Manassas, VA). BM stroma was cultured from BM aspirates of healthy individuals as described.21 Use of BM aspirates followed the guidelines of a protocol approved by the Institutional Review Board (IRB) of the University of Medicine and Dentistry of New Jersey (UMDNJ)–Newark campus, and informed consent was provided according to the Declaration of Helsinki.
Primary breast tissue and selection method
Breast tissues were obtained from stage IIA (P1, P2, P3) and IIB (P4, P5, P6) BC patients from 46 to 78 years old.25 At the time of surgery, patients were not given any treatment. The use of breast tissues followed an approved protocol from the IRB and Brookdale Hospital (Brooklyn, NY). Malignant cells were selected as described.24
SDF-1 knockdown BCCs
Because SDF-1α and SDF-1β differed by 4 amino acids, siRNA could not be specific. Thus, SDF-1 siRNA cells are referred as SDF-1 knockdown cells. The siRNA sequence was inserted in pPMSKH1 22 with sequences from 2 regions of the cDNA (accession no. L36034): 5′ cgt caa gca tct caa aat t 3′ (+206/+224; pPMSKH1–SDF-1/KC) or 5′ cag aca agt gtg cat tgac3′ (+287/+305; pPMSKH1–SDF-1/SR). BCCs were cotransfected with pTK-Hyg (Clontech, Palo Alto, CA) and pPMSKH1–SDF-1/KC, pPMSKH1–SDF-1/SR, or pPMSKH1 (no insert). Stable transfectants were selected with 25 μg/mL hygromycin and evaluations done by reverse transcriptase–polymerase chain reaction (RT-PCR) with primers spanning +342/+748: 5′ aca agt aag cac aac agc c 3′ (forward) and 5′ gct cca aac aag cca aat tc 3′ (reverse). Knockdown clones were maintained in media containing hygromycin.
Cocultures of BCCs and BM stroma
Cocultures of BCCs and BM stromal cells were previously described.24 Briefly, cocultures were propagated in stromal media with equal numbers of BM stroma and BCCs as untransfected cells or transfected with pPMSKH1, pPMSKH1–SDF-1/KC, or pPMSKH1–SDF-1/SR. At confluence, BCCs were positively selected with anticytokeratin-conjugated Dynabeads (Dynal Biotech, Oslo, Norway) while BM stroma was retained in the negative fraction. Flow cytometry determined more than 99% purity for each cell subset. BCCs were analyzed with PE-cytokeratin mAb and stromal cells with FITC-fibroblast mAb. Nonspecific labeling was determined in parallel with PE- and FITC-conjugated isotype control. The selected BCCs could survive up to 1 week in stromal media without growth supplement.21
Cell proliferation
BCC proliferation was determined in confluent cocultures containing 5 ng/mL SDF-1α. Cell counts were done by 2 methods. (1) Twenty-four hours after adding SDF-1α, 1 μCi/mL (37 000 Bq/mL) methyl-3H-thymidine (TdR) (70 to 90 Ci/mmol [3.0 × 1012 Bq/mmol]; Dupont/NEN, Boston, MA) was added to cultures. After 24 hours, the cytokeratin-positive cells were harvested onto glass fiber filters and then counted on a scintillation counter. (2) Forty-eight hours after adding SDF-1α, both the adherent and nonadherent cells were collected, separated according to cytokeratin positivity and fibroblast positivity, and the former counted.
Northern analysis
Northern blots were performed for SDF-1α in BCCs and BM stroma obtained from cocultures or cultured alone as described.26 Each cell subset was separated from cocultures at different levels of confluence.24 The analyses were done with 10 μg total RNA. Membranes were hybridized with SDF-1α cDNA probe and randomly labeled with [α-32P]-dATP, 3000 Ci/mM (1017 Bq), (Dupont/NEN) as described. Normalizations were done for 18S rRNA.26 Complementary DNA was generated by RT-PCR with primers spanning +341/+729 of the SDF-1 cDNA (accession no. L36034). PCR products were ligated into pCR2.1 (Invitrogen).
Cytokine microarray
Cytokine production in BM stroma was performed by combining the culture media with cell extracts to include cellbound cytokines. At 24 hours prior to analyses, culture media from cocultures were replaced with fresh media, and the studies were performed with human cytokine protein array II (RayBiotech, Norcross, GA) as described.22 Background levels obtained with media alone were subtracted. The densitometers of internal positive controls were arbitrarily assigned a value of 10 and unknowns calculated using 10 as a point of reference.
Isolation of CD34+/CD38–/Lin– cells
BM cell subsets were selected as previously described.27 Briefly, bone marrow mononuclear cells (BMNCs) were resuspended at 107/mL in RPMI 1640 containing 2% FCS (R2 media). CD34-conjugated Dynabeads M-450 (Dynal Biotech) were added to BMNCs at 100 μL/mL, and the cells were incubated on ice for 2 hours. The coupled cells were positively selected on a magnetic separator, and the uncoupled cells (CD34–) were divided according to CD38 negativity or positivity by consecutive incubation with CD38 mAb at 1:200 dilution and Dynabeads M-450 goat anti–mouse IgG at 100 μL/mL. Three-color flow cytometry with PE-CD34 mAb, APC-CD38 mAb, and tricolor-CD45 mAb indicated greater than 95% cell purity. Cell labeling was performed for 30 minutes with cells at 105 in 200 μL serum-free RPMI 1640. Each analysis contained compensation tubes of single antibody. Nonspecific labeling was determined by incubation with fluorochrome-conjugated isotype IgG.
Cocultures of PKH26-labeled CD34+/CD38+ cells, stroma, and BCCs
CD34+/CD38–/Lin– cells were obtained from healthy BM aspirates and then labeled with the red lipophilic membrane dye, PKH26. Labeling was performed at room temperature using a kit purchased from Sigma. Cells at 106/mL were resuspended with diluent C (provided in the kit) and then diluted further with equal volume of 2 × dye. Cells were incubated for 5 minutes at room temperature with periodic mixing. The reaction was stopped with equal volume of serum. The cells were centrifuged and then washed 3 times with PBS (pH 7.4). Microscopic examination indicated more than 95% labeling. Cells were immediately added to cocultures of stroma and BCCs. At different levels of confluence, 20 PKH26-labeled cells were added in the presence anti–SDF-1α, 2 ng/mL SDF-1α, or 2 ng/mL nonimmune rabbit IgG. Parallel cultures contained media alone.
In parallel cultures, PKH26-labeled cells were added to cocultures at 10% to 20% confluence. After 16 hours, the cells were labeled for stromal cells and BCCs by immunofluorescence. Stromal cells were detected with antifibroblast followed by APC-rabbit anti–mouse IgG. BCCs were detected with FITC-anticytokeratin. Cell labeling was detected by 3-color fluorescence.
Modified (LTC-IC) assays
Modified long-term culture-initiating cell (LTC-IC) assays were performed as described.22 The assay used supporting layers of stroma or cocultures with BCCs from 2 cell lines and 2 primary cells. At confluence, the supporting cells were γ-irradiated with 150 Gy, as previously reported in a dose-response curve ranging from 50 to 500 Gy.21 After 16 hours, media were replaced with 5 mL of fresh media containing BMNCs at 107/mL. Media (50%) were replaced weekly, and at week 12, the adherent and nonadherent cells were combined and then studied in clonogenic cultures for granulocytic-monocytic progenitors (CFU-GM) as described.26 Briefly 105 cells were resuspended in 1.2% methylcellulose supplemented with 3 U/mL GM-CSF and then assayed in duplicate. Colonies with more than 15 cells were counted at day 10.
Immunofluorescence for connexin-43 and tracer dye exchange
Cocultures were established in Nunclon delta plates (Nalge Nunc International, Rochester, NY) with T47D and MCF7. After overnight incubation, cells were washed and then fixed with 1% paraformaldehyde. The cells were permeabilized with 0.1% Triton X-100 and then incubated for 90 minutes with Texas Red–X-phalloidin at 28 U/mL. This was followed by consecutive incubation with 2 μg/mL rabbit anti–CX-43 and 2 μg/mL FITC-goat anti–rabbit IgG. Cells were immediately examined under visible green and red wavelengths using an inverted immunofluorescence microscope (Nikon Eclipse TS100). Functional studies were performed with the Vybrant CFDA SE cell tracer kit (Molecular Probes).
Data analyses
Statistical evaluations of the data were done with analysis of variance and Tukey-Kramer multiple comparisons test. A P value of less than .05 was considered significant.
Results
SDF-1α production in cocultures of BM stroma and BCCs
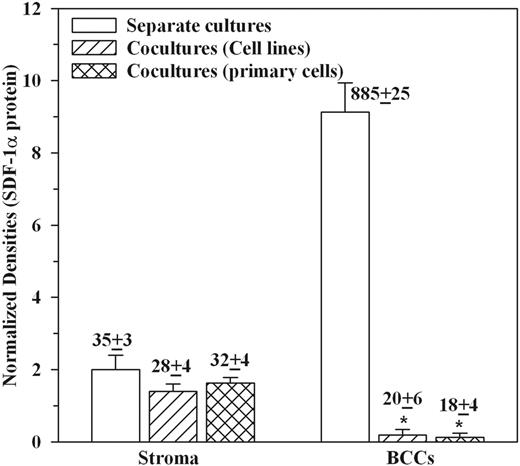
Microarray studies showed decreased production of SDF-1α production in cocultures.22 Because both BM stroma and BCCs constitutively produce SDF-1α,22,28 we asked which subset is involved. Enzyme-linked immunosorbent assay (ELISA) from media of cocultures showed a significant decrease in SDF-1α levels (Table 1). SDF-1α levels represented a 24-hour release because the culture media were replaced 24 hours prior to the determination. We next assayed individual cell subsets for SDF-1α production by replating each separately at 106 per milliliter. Media collected after 24 hours were studied for SDF-1α by microarray and ELISA. The studies used T47D, ZR-75-30, and BT 483 and 3 primary BCCs (P1, P2, P4), each studied with stroma from a different BM donor. The densities of internal positive controls were arbitrarily assigned values of 10 to determine unknown values. The ELISA results, shown above each bar in Figure 1, represent the mean picograms per milliliter ± SD (n = 6). While no change was observed for BM stroma, there was significant (P < .05) reduction in BCCs (Figure 1).
SDF-1α levels in cocultures of BM stroma and BCCs
Cells . | Coculture . | SDF-1α, pg/mL . |
|---|---|---|
| BC cell lines | - | 80 ± 8 |
| Primary BCCs | - | 56 ± 6 |
| BM stroma | - | 22 ± 4 |
| BCCs + stroma | ||
| 4 cell lines | + | 10 ± 3* |
| 4 primary cells | + | 15 ± 3* |
Cells . | Coculture . | SDF-1α, pg/mL . |
|---|---|---|
| BC cell lines | - | 80 ± 8 |
| Primary BCCs | - | 56 ± 6 |
| BM stroma | - | 22 ± 4 |
| BCCs + stroma | ||
| 4 cell lines | + | 10 ± 3* |
| 4 primary cells | + | 15 ± 3* |
BC cell lines (n = 4: T47D, DU4475, BT 483, ZR-75-30) and primary BCCs (P1, P2, P4) were established as cocultures with BM stroma or cultured alone as described.21 Each BCC was studied with stroma from a different donor. At 80% confluence, culture media were collected and then studied for levels of SDF-1α using ELISA. The results are presented as mean ± SD; n = 7.
- indicates cells cultured alone; +, cocultures.
P < .5 versus BCCs (cell lines and primary cells) cultured alone.
SDF-1 mRNA in BM stroma and BCCs from cocultures
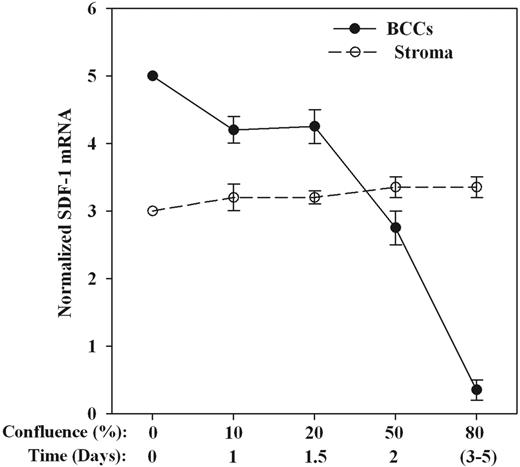
Because the 2 forms of SDF-1 differed by 4 amino acids,16 Northern blots did not discern the 2 subtypes, thus referring to the message as SDF-1 mRNA. We determined whether the changes in SDF-1α production mirrored steady state mRNA by Northern blots. The RNA was obtained from separate cultures or at different levels of confluence. The mean densities of SDF-1 mRNA (±SD) for 2 BC cell lines (T47D, DU4475) and primary BCCs (P3, P4) are shown in Figure 2. The results showed no change in SDF-1α mRNA for stroma while there was a steady decrease in BCCs up to 80% confluence.
CXCR4 expression in BM stroma and BCCs in cocultures
Flow cytometry for CXCR4 expression was studied with cocultures at 80% confluence. BC cell lines (T47D, DU4475, ZR-75-30, BT 483) and stage II BCCs (P1, P2, P5, P6) were studied with BM stroma, each with a different donor. Cells were colabeled with anti-CXCR4 and anticytokeratin (BCCs) or antifibroblasts (stroma). The mean fluorescence intensities (MFI ± SD, n = 8) showed increases in CXCR4 expression in BCCs from cocultures as compared with BCCs cultured alone (Table 2).
CXCR4 expression in BCCs cultured alone and as cocultures
. | MFI . | . | . | ||
|---|---|---|---|---|---|
| BCCs . | Confluency at 20% . | Confluency at 50% . | Confluency at 80% . | ||
| Cell lines* | 672 ± 12 | 625 ± 18 | 620 ± 9 | ||
| Primary BCCs† | 660 ± 8 | 576 ± 6 | 610 ± 8 | ||
| Cocultures with cell lines | 548 ± 14 | 688 ± 12 | 1050 ± 35‡ | ||
| Cocultures with primary cells | 464 ± 12 | 528 ± 10 | 1074 ± 22‡ | ||
. | MFI . | . | . | ||
|---|---|---|---|---|---|
| BCCs . | Confluency at 20% . | Confluency at 50% . | Confluency at 80% . | ||
| Cell lines* | 672 ± 12 | 625 ± 18 | 620 ± 9 | ||
| Primary BCCs† | 660 ± 8 | 576 ± 6 | 610 ± 8 | ||
| Cocultures with cell lines | 548 ± 14 | 688 ± 12 | 1050 ± 35‡ | ||
| Cocultures with primary cells | 464 ± 12 | 528 ± 10 | 1074 ± 22‡ | ||
BCCs were studied for membrane CXCR4 by immunofluorescence. The results are presented as the mean MFI ± SD.
T47D, DU4475, BT 483, ZR-75-30.
P1, P2, P5, P6.
P < .05 versus BCCs (cell lines and primary cells) cultured alone.
SDF-1 production in cocultures. Cocultures with BC cell lines (T47D, ZR-75-30, BT383) and primary BCCs (P1, P2, P4). Each BCC was studied with stroma from a different donor. At 80% confluence, BCCs were positively selected and each (106/mL) replated separately in stromal media. After 24 hours, the culture media were analyzed by protein microarrays and by ELISA for SDF-1α. The densities of internal positive controls were arbitrarily assigned values of 10 and then used as the base for unknowns. The ELISA values, in mean picograms per milliliter ± SD (n = 6), are placed above the respective bars. *P < .05 versus individual BCC culture.
SDF-1 production in cocultures. Cocultures with BC cell lines (T47D, ZR-75-30, BT383) and primary BCCs (P1, P2, P4). Each BCC was studied with stroma from a different donor. At 80% confluence, BCCs were positively selected and each (106/mL) replated separately in stromal media. After 24 hours, the culture media were analyzed by protein microarrays and by ELISA for SDF-1α. The densities of internal positive controls were arbitrarily assigned values of 10 and then used as the base for unknowns. The ELISA values, in mean picograms per milliliter ± SD (n = 6), are placed above the respective bars. *P < .05 versus individual BCC culture.
SDF-1α mRNA in BCCs and BM stroma at different coculture confluence. Cocultures were added established with 100 cells each and at different confluency; each cell subset was separated and then studied for SDF-1 mRNA by Northern analyses. Normalized bands are presented as the mean densities ± SD for P3, P4, T47D, and DU4475. Each BCC was studied with BM stroma from a different donor. The x-axis shows the confluence (%) and also the average time to attain the respective confluence. Zero confluence indicates the time of coculture initiation. Northern analyses at 0% confluence (time 0) showed the values of BCCs and stroma as cells that have never been placed in coculture. To prevent overlap of bands, we arbitrarily assigned densities of 5 and 3 for confluent BCCs and BM stroma cultured alone, respectively.
SDF-1α mRNA in BCCs and BM stroma at different coculture confluence. Cocultures were added established with 100 cells each and at different confluency; each cell subset was separated and then studied for SDF-1 mRNA by Northern analyses. Normalized bands are presented as the mean densities ± SD for P3, P4, T47D, and DU4475. Each BCC was studied with BM stroma from a different donor. The x-axis shows the confluence (%) and also the average time to attain the respective confluence. Zero confluence indicates the time of coculture initiation. Northern analyses at 0% confluence (time 0) showed the values of BCCs and stroma as cells that have never been placed in coculture. To prevent overlap of bands, we arbitrarily assigned densities of 5 and 3 for confluent BCCs and BM stroma cultured alone, respectively.
Role of SDF-1α in monolayers of BCC and BM stroma and BCC proliferation
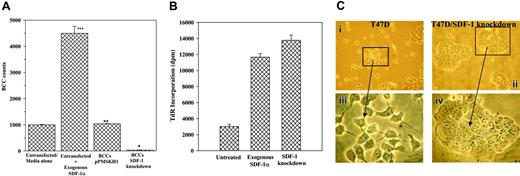
This section studied the cause-effect relationship between SDF-1α levels and BCC proliferation in monolayers. SDF-1α levels were decreased in cocultures (Figure 1), and BCC proliferation was blunted upon contact with BM stroma.17 We therefore determined whether the decrease in SDF-1α was responsible for the cell cycle quiescence of cocultured BCCs. BCC proliferation was determined by 2 methods in confluent cocultures stimulated for 48 hours with 5 ng/mL SDF-1α as follows: (1) The adherent and nonadherent cells were separated. The adherent cells represented monolayers/adherent cells (BCCs and stroma), whereas the nonadherent cells represented the proliferating BCCs. The adherent population was divided into cytokeratin-positive and cytokeratin-negative subsets. The latter stained bright for prolyl-4-hydroxylase, indicating stromal cells. All cell subsets were counted, but only the BCC counts found within the adherent group are presented (Figure 3A). (2) Twenty-four hours after adding SDF-1α, TdR was added to the cocultures. After an additional 24 hours, the cytokeratin-positive cells from both the adherent and nonadherent populations were analyzed for TdR incorporation. BCC counts from the adherent population with SDF-1α indicated a significant (P < .05) increase as compared with similar cultures without exogenous SDF-1α (media alone) (Figure 3A, first and second bars). Stromal cell counts were reduced in cultures with exogenous SDF-1α (not shown), indicating BCC expansion at the “expense” of stromal cells. After analysis of monolayer/adherent cells (Figure 3A), we next determined total BCC proliferation by TdR incorporation. The results showed significant (P < .05) TdR incorporation with exogenous SDF-1α and in SDF-1 knockdown BCCs as compared with untreated BCCs (Figure 3B). Based on the data shown in Figure 3A-B, decreased SDF-1 (knockdown cells) and increased SDF-1α (exogenous) both showed reduced ability to form cocultures (decreased adherence to plastic).
The role of SDF-1 in the quiescence of coculture BCCs was verified by siRNA. T47D, DU4475, and ZR-75-30 cells were stably transfected with pPMSKH1–SDF-1/KC, pPMSKH1–SDF-1/SR, or pPMSKH1 alone. Cells transfected with SDF-1 siRNA showed undetectable SDF-1α (Table 3), whereas pPMSKH1 transfectants produced SDF-1α similar to levels of untransfected cells (Table 3). We observed a loss in monolayer formation in SDF-1 knockdown BCCs. Figure 3C shows cultures of T47D alone—untransfected and SDF-1 knockdown. Noted is multilayer by siRNA (Figure 3C, lower right panel) as compared with untransfected cells that show only monolayer (Figure 3C, lower left panel). Similar observations were noted for DU4475 and ZR-75-30 BCCs (not shown). Because we were interested in coculture monolayers, we removed the nonadherent SDF-1 knockdown BCCs prior to cell counts and found that at 80% confluency pPMSKH1-transfected BCCs showed counts similar to untransfected cells (Figure 3A, first and third bars). SDF-1 knockdown BCCs showed a significant (P < .05) decrease in cells that form monolayer (Figure 3A, third and fourth bars). The altered “behavior” of SDF-1 knockdown BCCs cannot be explained by change in CXCR4 expression, as determined by flow cytometry (not shown). We next determined the total cell proliferation of the knockdown cells by TdR incorporation. The results showed a significant (P < .05) increase in TdR incorporation (Figure 3, right bar), similar to cultures with exogenous SDF-1α (Figure 3B, middle bar), indicating increased proliferation of coculture BCCs by exogenous SDF-1α and by SDF-1 knockdown. The results show a critical role for the concentration of SDF-1α in the formation of monolayer between BCCs and BM stroma in cocultures and increased proliferation of BCCs. In addition, knockdown of SDF-1 caused BCCs to lose their ability to form monolayer.
Efficiency of SDF-1 knockdown in BCCs
BCCs . | SDF-1α, pg/mL . |
|---|---|
| Untransfected | 84 ± 4 |
| pPMSKH1 transfected | 75 ± 3 |
| pPMSKH1-SDF-1/SR | None detected |
| pPMSKH1-SDF-1/KC | None detected |
BCCs . | SDF-1α, pg/mL . |
|---|---|
| Untransfected | 84 ± 4 |
| pPMSKH1 transfected | 75 ± 3 |
| pPMSKH1-SDF-1/SR | None detected |
| pPMSKH1-SDF-1/KC | None detected |
At 80% confluence, culture media were replaced with fresh media. At confluence (48 hours), the culture media were collected and then quantitated for SDF-1α levels by ELISA. The results are presented as the mean ± SD, n = 5.
Effects of exogenous SDF-1α in monolayer formation between SDF-1 knockdown BCCs and BM stroma
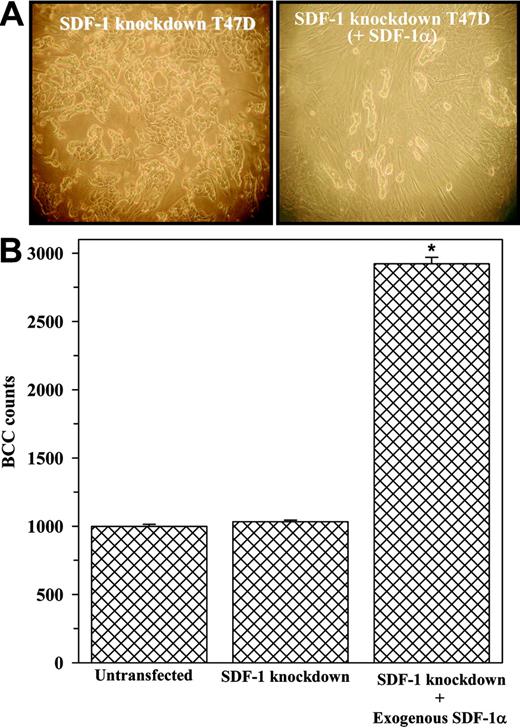
The loss of monolayers by SDF-1 knockdown BCCs (Table 3) caused difficulty in the formation of cocultures. We asked whether supplementation with SDF-1α could reestablish cocultures with SDF-1 knockdown BCCs (T47D, ZR-75-30, DU4475) in the presence or absence of 0.1 ng/mL SDF-1α. This concentration was based on a dose-response study and corresponds to the range of SDF-1α levels produced in cocultures with untransfected T47D, ZR-75-30, and DU4475 (data not shown). Representative cocultures (Figure 4A) with T47D showed foci formation of the BCCs, which appear to prevent the growth of stromal cells (Figure 4A, left panel: nonspindle cells). This contrasts similar cocultures with exogenous SDF-1α, which restored monolayer cocultures, in the absence of foci formation (Figure 4A, right panel: spindle-shape cells–stroma). The BCCs shown in Figure 4 were counted, and the results showed a significant increase in BCC proliferation when exogenous SDF-1α was present as compared with cultures without exogenous SDF-1α (Figure 4B).
Role of SDF-1α in the proliferation of BCCs from cocultures. (A) Cocultures were performed with SDF-1 knockdown T47D, DU4475, and ZR-75-30. Control cocultures were untransfected or transfected with vector alone (pPMSKH1). When the untransfected cells achieved 80% confluence, 5 ng/mL SDF-1α was added to each culture. After 48 hours, confluence was achieved and, at that time, the nonadherent cells were discarded and the BCC subset was selected from the adherent population and then counted. The results are presented as the mean cell counts ± SD, n = 8. *P < .05 versus cultures with untransfected BCCs or cultures with untransfected cells and exogenous SDF-1α. **P > .5 versus untransfected BCCs. ***P < .05 versus untransfected/media alone. (B) The experiments described in panel A were repeated with the following modifications: 24 hours after the addition of SDF-1α,1 μCi/mL TdR was added and, after an additional 24 hours, the BCCs from both the adherent and nonadherent population were analyzed for TdR incorporation. The total disintegrations per minute (dpm) for untreated, SDF-1α–treated, and SDF-1 knockdown cells are presented as the mean ± SD, n = 5. (C) Representative cultures with T47D alone are shown for untransfected cells (i) and similar cells knockdown for SDF-1 (ii); panels iii-iv show the same clusters of cells at a higher magnification. At 80% confluence, cocultures, BCCs were selected and then counted. The results are presented as the mean ± SD of total cell counts. Images were visualized using a Nikon Eclipse TE 300 microscope (Nikon, Tokyo, Japan) equipped with a 10×/0.45 numerical aperture (NA; i-ii) or a 40×/0.8 NA (iii-iv) objective. Images were acquired using an RE Color 2.2.1 camera and SPOT for Windows version 3.5.9 (Diagnostic Instruments, Sterling Heights, MI), and were processed using Adobe Creative Site 2 Premium (Adobe Systems, San Jose, CA).
Role of SDF-1α in the proliferation of BCCs from cocultures. (A) Cocultures were performed with SDF-1 knockdown T47D, DU4475, and ZR-75-30. Control cocultures were untransfected or transfected with vector alone (pPMSKH1). When the untransfected cells achieved 80% confluence, 5 ng/mL SDF-1α was added to each culture. After 48 hours, confluence was achieved and, at that time, the nonadherent cells were discarded and the BCC subset was selected from the adherent population and then counted. The results are presented as the mean cell counts ± SD, n = 8. *P < .05 versus cultures with untransfected BCCs or cultures with untransfected cells and exogenous SDF-1α. **P > .5 versus untransfected BCCs. ***P < .05 versus untransfected/media alone. (B) The experiments described in panel A were repeated with the following modifications: 24 hours after the addition of SDF-1α,1 μCi/mL TdR was added and, after an additional 24 hours, the BCCs from both the adherent and nonadherent population were analyzed for TdR incorporation. The total disintegrations per minute (dpm) for untreated, SDF-1α–treated, and SDF-1 knockdown cells are presented as the mean ± SD, n = 5. (C) Representative cultures with T47D alone are shown for untransfected cells (i) and similar cells knockdown for SDF-1 (ii); panels iii-iv show the same clusters of cells at a higher magnification. At 80% confluence, cocultures, BCCs were selected and then counted. The results are presented as the mean ± SD of total cell counts. Images were visualized using a Nikon Eclipse TE 300 microscope (Nikon, Tokyo, Japan) equipped with a 10×/0.45 numerical aperture (NA; i-ii) or a 40×/0.8 NA (iii-iv) objective. Images were acquired using an RE Color 2.2.1 camera and SPOT for Windows version 3.5.9 (Diagnostic Instruments, Sterling Heights, MI), and were processed using Adobe Creative Site 2 Premium (Adobe Systems, San Jose, CA).
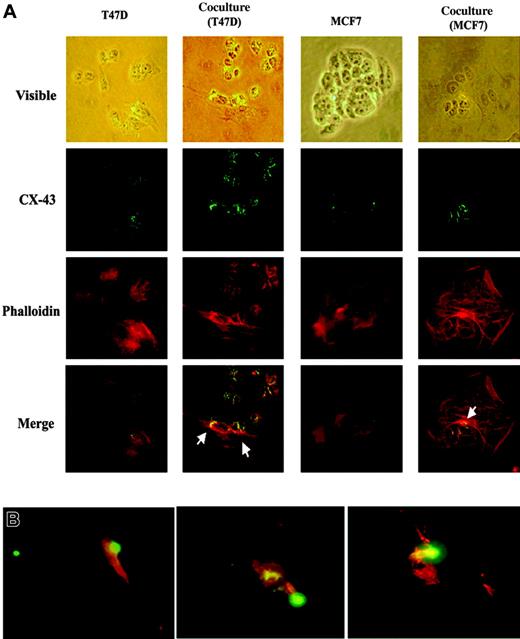
Formation of gap junctions between BCCs and BM stroma
BCCs reverted to cell-cycle quiescence on contact with BM stromal cells.22 Furthermore, BM stromal cells as well as low metastatic BCCs can form gap junctions.29-31 In vitro studies with cocultures of BM stroma and BCCs have shown normal hematopoietic functions.22 We now ask whether BM stroma and BCCs form gap junctions. Cocultures were established with T47D and BM stromal cells, and at 50% confluence the cultures were studied for gap junction by colabeling with Texas Red-X-phalloidin (actin filaments) and FITC-labeled anti–connexin-43 (gap junction). The low metastatic MCF7 was included as a positive control for gap junction (Figure 5A, row 2, first and third columns). Phalloidin allowed visibility of cellular structures. Representative results (Figure 5A) demonstrate connexin formation between BM stroma and BCCs (arrows). Because connexin-43 does not prove functional gap junctions, we next performed 3 cocultures, each with stroma from a different donor and T47D labeled with CFDA SE tracer (green). The dye could be tracked in the contacting BM stroma as yellow because the cocultures were labeled with anti–connexin-43 (red). In each experiment the green tracer was shown in the stroma (Figure 5B, yellow). The dye shown in stroma could not be from an extracellular source because the dye becomes membrane impermeable after it has entered the cells. The diffuse red shown in stromal cells for connexin-43 served as our internal positive control because these cells express connexin.30,31 Trypan blue exclusion indicated no evidence of BCC death. Together, these findings demonstrate gap junction formation after the BCCs have contacted BM stroma.
Relevance to hematopoiesis
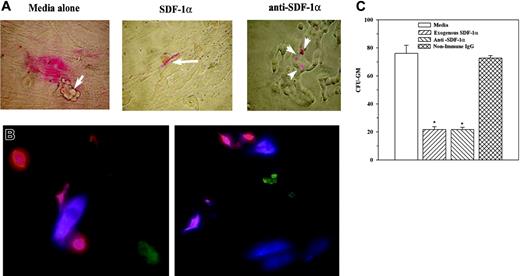
The coculture model represents early integration of BCCs within stroma when hematopoietic stem cells are expected to interact with BM stroma instead of BCCs, as previously reported.18,21,22 We have now shown 2 sets of data that might explain how BCCs take advantage of the BM microvironment without disrupting hematopoietic activity: (1) gap junction formation between stroma and BCCs (Figure 5) and (2) reduced SDF-1α in BCCs contacting BM stroma (Figures 1, 2). We now ask whether reduced SDF-1α in BCCs could be relevant for the interaction between stroma and CD34+/CD38–/Lin– cells, which include the most primitive BM progenitors.32 CD34+/CD38–/Lin– cells were labeled with the red lipophilic PKH26 membrane dye and then added to cocultures with T47D, ZR-75-30, DU4475, BT 483, and stage II BCCs (P1, P6), each with BM stroma from a different donor. Representative cocultures (Figure 6A) show BM cells interacting with stromal cells in media alone (Figure 6A, left panel; arrow depicts BCCs), whereas exogenous SDF-1α (0.1 ng/mL) interacts with BCCs (Figure 6A, middle panel, arrow). Figure 3C shows multilayered BCCs with exogenous SDF-1α. Because SDF-1α has been shown to function in a gradient-dependent mechanism, we developed a premise that a low SDF-1α level in cocultures might be relevant to BCC-stroma interactions. We pretreated the labeled cells with anti–SDF-1α and then added anti–SDF-1α at 0.1 to 10 ng/mL throughout coculture. Anti–SDF-1α at 2 ng/mL allowed coculture formation with the BM cells interacting with BCCs rather than stroma (Figure 6A, right panel, arrows). Nonimmune rabbit IgG at 2 ng/mL showed no change (not shown).
Effects of exogenous SDF-1α in the growth and generation of monolayer cocultures.SDF-1 knockdown BCCs (SDF-1 negative) were established as cocultures in the presence or absence of 0.1 ng/mL SDF-1α. (A) Cocultures with SDF-1–negative BCCs (left panel) without SDF-1α or with 0.1 ng/mL SDF-1α (right panel). Images were visualized, acquired, and processed as in Figure 3Ci-ii. (B) BCC counts in cocultures at 80% confluence (9 experiments with 3 different cell lines). *P < .05 versus cocultures with SDF-1 knockdown BCCs. Error bars indicate mean ± SD; n = 9.
Effects of exogenous SDF-1α in the growth and generation of monolayer cocultures.SDF-1 knockdown BCCs (SDF-1 negative) were established as cocultures in the presence or absence of 0.1 ng/mL SDF-1α. (A) Cocultures with SDF-1–negative BCCs (left panel) without SDF-1α or with 0.1 ng/mL SDF-1α (right panel). Images were visualized, acquired, and processed as in Figure 3Ci-ii. (B) BCC counts in cocultures at 80% confluence (9 experiments with 3 different cell lines). *P < .05 versus cocultures with SDF-1 knockdown BCCs. Error bars indicate mean ± SD; n = 9.
Gap junctions between BCCs and BM stroma. (A) T47D or MCF7 was cultured alone or as cocultures. Cultures were colabeled with CX-43 and the F-actin label, phalloidin. Figure shows representative labeling at magnification × 100. Cells shown in the top row (visible light) are presented as merged staining with anti–CX-43 and phalloidin on the bottom row. Arrows show CX-43 between BM stroma and BCCs. (B) Cocultures were established with the following modification: the BCCs were labeled with Vybrant CFDA SE cell tracer (green) and then added to BM stroma. After 24 hours, the cells were labeled with anti–CX-43 (red). Images were visualized, acquired, and processed as in Figure 3C, except that a 50 ×/0.9 NA objective was used.
Gap junctions between BCCs and BM stroma. (A) T47D or MCF7 was cultured alone or as cocultures. Cultures were colabeled with CX-43 and the F-actin label, phalloidin. Figure shows representative labeling at magnification × 100. Cells shown in the top row (visible light) are presented as merged staining with anti–CX-43 and phalloidin on the bottom row. Arrows show CX-43 between BM stroma and BCCs. (B) Cocultures were established with the following modification: the BCCs were labeled with Vybrant CFDA SE cell tracer (green) and then added to BM stroma. After 24 hours, the cells were labeled with anti–CX-43 (red). Images were visualized, acquired, and processed as in Figure 3C, except that a 50 ×/0.9 NA objective was used.
Because exogenous SDF-1α and anti–SDF-1α both caused the hematopoietic stem cells to interact with stroma, it was concluded that the SDF-1α level is important for interactions between hematopoietic stem cells and stroma. These observations (Figure 6A) were examined at a single-cell level with PKH26-labeled CD34+/CD38–/Lin– cells. The interacting cells were determined by immunofluorescence for stroma (blue) and BCCs (green). Representative figures, with T47D and BM stroma from 2 different donors, show CD34+/CD38–/Lin– cells (red) interacting with stroma (blue). No red cell was observed close to the BCCs (green) (Figure 6B).
We next determined how the observations shown in Figure 6A-B are related to hematopoietic activity. Cocultures served as support for LTC-IC assays to determine whether the BCCs within the coculture could affect hematopoietic activity up to 12 weeks. Fifty percent of culture media containing fresh antibody were replaced weekly. LTC-IC assays showed a significant (P < .05) reduction in the number of CFU-GM colonies for cultures with exogenous SDF-1α and in the presence of anti–SDF-1α (Figure 6C). In summary, low levels of SDF-1α are sufficient for interaction between CD34+/CD38–/Lin– cells and BM stroma. In the absence of SDF-1α or at high levels of SDF-1α, CD34+/CD38–/Lin– cells interact with BCCs. This interaction correlates with a significant decrease in hematopoietic activity.
Discussion
This study reports on a critical role for the SDF-1α level in the integration of BCCs within BM stroma and hematopoietic regulation. A previous report showed normal hematopoietic effects despite support by cocultures.21 We now report on the formation of gap junctions between BCCs and BM stroma, with concomitant reduction in SDF-1α. Reduced SDF-1α is critical for interaction between hematopoietic progenitors and BM stroma rather than BCCs (Figures 5, 6). The influence of BM stroma on the production of SDF-1α by BCCs is consistent with the hypothesis by Welch stating that changes in gene expression in the endosteal region are relevant to bone BC metastasis.33
SDF-1α levels in hematopoietic regulation. (A) Cocultures were performed with T47D, ZR-75-30, DU4475, BT 483, or 2 primary BCCs from stage II BC (P1, P6). At 60% confluence, the following were added to cocultures: 102 PKH26-labeled CD34+/CD38–/Lin– cells and/or 0.1 ng/mL SDF-1α or 2 ng/mL anti–SDF-1α. At confluence, cells were examined microscopically; representative figures are shown. Arrows indicate location of BCCs. (B) Cocultures were established as described for panel A. At 10% to 20% confluence, PHK26-labeled CD34+/CD38–/Lin– cells were added, and after 16 hours the cells were studied by double immunofluorescence for stroma (APC/blue) and BCCs (FITC/green). Representative studies are shown for cocultures with T47D and stroma from 2 different donors. The cells were examined by fluorescence microscopy as for Figure 5B. (C) LTC-IC assays were performed in the presence or absence of 2 ng/mL anti–SDF-1α, 2 ng/mL nonimmune rabbit IgG, media alone, or 0.1 ng/mL SDF-1α. CFU-GM colonies are for 12-week cultures and are presented as the mean ± SD (n = 6). *P < .05 versus media alone or nonimmune IgG.
SDF-1α levels in hematopoietic regulation. (A) Cocultures were performed with T47D, ZR-75-30, DU4475, BT 483, or 2 primary BCCs from stage II BC (P1, P6). At 60% confluence, the following were added to cocultures: 102 PKH26-labeled CD34+/CD38–/Lin– cells and/or 0.1 ng/mL SDF-1α or 2 ng/mL anti–SDF-1α. At confluence, cells were examined microscopically; representative figures are shown. Arrows indicate location of BCCs. (B) Cocultures were established as described for panel A. At 10% to 20% confluence, PHK26-labeled CD34+/CD38–/Lin– cells were added, and after 16 hours the cells were studied by double immunofluorescence for stroma (APC/blue) and BCCs (FITC/green). Representative studies are shown for cocultures with T47D and stroma from 2 different donors. The cells were examined by fluorescence microscopy as for Figure 5B. (C) LTC-IC assays were performed in the presence or absence of 2 ng/mL anti–SDF-1α, 2 ng/mL nonimmune rabbit IgG, media alone, or 0.1 ng/mL SDF-1α. CFU-GM colonies are for 12-week cultures and are presented as the mean ± SD (n = 6). *P < .05 versus media alone or nonimmune IgG.
Others reported on BM microenvironmental influences on the expressions of cytokines and other genes in BCCs.22 Change in SDF-1α production in BCCs that contact BM stroma is an example of BCCs using the BM microenvironment for survival (Figure 1; Table 1). Extrapolation of SDF-1α–CXCR4 on hematopoietic stem cells (Gazitt 34 and this report) leads to assumptions for the reduced SDF-1α in BCCs contacting stroma. If SDF-1α production in BCCs is high, CXCR4-expressing hematopoietic stem cells might interact with the BCCs rather than stroma. This premise is supported by exogenous SDF-1α causing interactions between CD34+/CD38–/Lin– cells and BCCs rather than stroma (Figure 6A). While this observation is unclear, we developed a premise that SDF-1α could act through direct and indirect methods by inducing the expression of other molecules, such as cytokines. Regardless, SDF-1α levels in the region where stromal cells reside in BM could account for interaction between stroma and hematopoietic cells.
The studies showed an increase in the expression of CXCR4 receptor in the BCCs contacting BM stroma (Table 2). This increase in CXCR4 might be critical because endogenous SDF-1α production in the BCCs could be bound through an autocrine mechanism. If SDF-1α coming from BCCs is retained on the surface as unbound, it could potentially attract CXCR4-expressing hematopoietic stem cells. We therefore propose that high expression of CXCR4 and low levels of SDF-1α production in BCCs are critical to prevent hematopoietic stem cells interacting with BCCs but to interact with stromal cells, which are their normal “cellular partners.”21 BCCs cultured alone show similar levels of SDF-1α although CXCR4 expression correlates with the metastatic potential of BCCs (K.E.C. and P.R., unpublished data, August 2005). Thus, the fact that we have observed changes in SDF-1α levels in BCCs upon contact with BM stroma supports that these studies are unique to BM events and that the model is likely representative of an early event of BM invasion.
This study combined the use of cell lines and primary cells. The latter cells were selected by an established method to select primary BCCs from breast biopsies. Because the model represents an early period of BCC entry into BM, we selected cells from patients with stages IIA and IIB BC. It could be argued that the method used to select malignant cells is biased toward BCCs with preference for BM. If this is the case, the studies would be significant to BCCs showing preference for BM. The formation of gap junctions between BCCs and BM stroma is significant (Figure 5), and this observation could lead to future studies that identify the molecules that are shared between the 2 cells.
It is necessary to discuss the physiological significance of the findings considering that the cocultures lacked growth supplements. Previous studies have shown that BCCs do not require growth supplements in cocultures with stroma.21 It could be argued that the change in SDF-1α production was decreased because of altered culture condition. BCCs can survive in stromal media up to 1 week. Furthermore, transfer of BCCs from cocultures to a new culture without stroma, but in stromal media for 24 hours, SDF-1α production reverted to the levels in BCCs (not shown). This argues against the microenvironment as a cause for changes in the production of SDF-1α in BCCs.
Despite the role for SDF-1α in the integration of BCCs within stroma (Figures 3, 4), in its absence or at high concentration, BCCs have lost their ability to remain quiescent (Figures 3, 4, 5, 6). We deduced that SDF-1α is likely to regulate the expressions of oncogenes and/or tumor suppressor genes. Regardless, SDF-1 appears to be the limiting factor in the protection of BCCs within BM. Because all studies incorporated stage II BC, it would be interesting to determine in future studies if similar mechanisms are operative in all stages of BC.
SDF-1α has been linked to osteogenic development.35 High levels of SDF-1α caused an increase in coculture BCC growth (Figure 3B) and could also lead to the development of osteoclasts. If these 2 events occur simultaneously, the BCCs would invade the bone. Because bone metastasis is common in BC, these are intriguing thoughts that require future studies. A particularly exciting finding for SDF-1α in the quiescence of BCCs contacting BM relates to a recent report describing CXCR4-positive tumor stem cells as those entering the bone from periphery.36
The interrelated information shown by molecular and cellular studies can be linked to BCC metastasis. We propose that if the steps involved in this integration are disrupted, the BCCs will begin to proliferate and metastasize to the bone and distant organs. The question, then, is how an increase in SDF-1α would lead to the proliferation of BCCs. Our unpublished studies show that SDF-1α regulates the production of substance P,37 which stimulates hematopoiesis and induces the production of multiple cytokines.37 It is possible that these cytokines could induce BCC growth with the loss of gap junctions and changes in adhesion molecules. It is unclear why exogenous SDF-1α causes interactions of hematopoietic stem cells with BCCs rather than stroma. Because the hematopoietic cells also express other adhesion molecules, perhaps excess SDF-1α causes changes in their expressions allowing them to interact with BCCs.
The studies presented in this report could form the impetus for future in-depth research to understand how BCCs integrate within stroma and also to understand the mechanisms that lead to cancer resurgence from BM. Future studies need to examine BM biopsies from patients at different stages of BC, including cancer remission. The functional studies for gap junctions (Figure 5B) need to be branched into a new area to identify what molecules are shared between the BCCs and BM stroma. These studies will require proteomics, genomics, and microRNA analyses.
Prepublished online as Blood First Edition Paper, July 20, 2006; DOI 10.1182/blood-2006-01-017459.
Supported in part by the United States Department of Defense (grant W81XWH) and University Hospital Cancer Center, New Jersey Medical School, Newark.
The authors declare no competing financial interests.
A.L.M., M.T., and K.E.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal