Abstract
B cell depletion with rituximab is routinely used for a wide range of autoimmune conditions, including a number of haematology conditions. While there is extensive literature on the use of rituximab in adults in both malignant and non-malignant conditions, there is less data on its use in children. We report the use of rituximab in 5 children with haemophilia and persistent FVIII (n=4) or FIX (n=1) inhibitors.
Methods: 5 children, median age 5 (range 4 to 16) years with haemophilia A (n=4) or haemophilia B (n=1) and persistent inhibitors were treated with rituximab 375mg/m2 weekly × 4. Patients received pre-medication with paracetamol, hydrocortisone and piriton. All had failed immune tolerance (IT) as defined by the UKHCDO guidelines. 1 patient relapsed and received re-treatment.
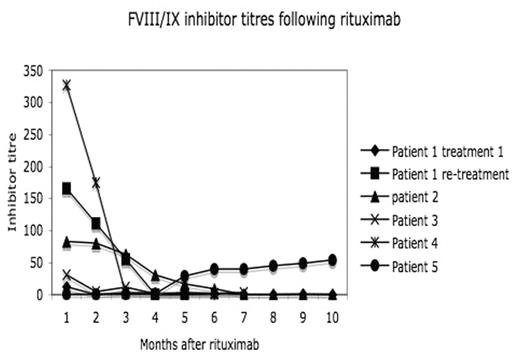
Results: 4 of 5 patients (80%) had a response to treatment with 3 complete responses (CR) with disappearance of inhibitor 4, 6, and 10 months from the start of rituximab; the 4th patient had a partial response (PR) with a fall from 31 to 1.4BU (still in PR at 6 months). Background details are shown in the table. One CR patient relapsed 25 months from treatment and had a second CR within 3 months of re-treatment. Inhibitor titres following rituximab are shown in the figure. 2 CR patients are still in CR at 5 and 15 months. All completed the 4 doses of rituximab with the expected infusion related side effects to the first dose. Patient 5 (haemophilia B) developed grade 3 symptoms with rigors, temperatures and abdominal pain following all 4 doses. B cells (detected by CD19 positivity on flow cytometry) were monitored in all patients. 4/5 had complete depletion of B cells within 1 month of treatment with return of B cells within 6 months (n=3) to 1 year (n=1). One patient (pt 5) did not deplete his B cells following rituximab therapy. This patient did not have a response to treatment (with an increase in the inhibitor levels after re-challenge with FIX) and suffered from the grade 3 reactions. In three patients IgM levels were slightly lower than normal (0.44 (NR >0.5)). There was no significant fall in IgG levels, no unusual infections and no fall in anti-Hep B antibodies titres. In summary, this case series shows that rituximab is useful in children with haemophilia with persistent inhibitors and failure of IT. It appears well tolerated with no immediate adverse effects. While there is a role for rituximab in patients refractory to IT, whether it should be used earlier in the disorder is not known. Further controlled trials are warranted. Finally, the lack of B cell depletion and subsequent lack of response in pt 5 is difficult to explain. We assume but have not confirmed that this patient has an inhibitor to rituximab.
Patient details
| Patient/haemophilia type . | Age (years) . | Mutation . | Other diagnoses . | response to rituximab . | timing of response/duration of response (mos) . | depletion of B cells . |
|---|---|---|---|---|---|---|
| 1/A | 4 | stop codon | excema | Y, CR | 9/23 | Y |
| 2/A | 5 | inversion int22h | excema, ashma | Y, CR | 6/10+ | Y |
| 3/A | 4 | amino acid substitution | Y, PR | 1/6+ | Y | |
| 4/A | 11 | deletion exon14 | Y, CR | 3/5+ | Y | |
| 5/B | 16 | deletion | N | N |
| Patient/haemophilia type . | Age (years) . | Mutation . | Other diagnoses . | response to rituximab . | timing of response/duration of response (mos) . | depletion of B cells . |
|---|---|---|---|---|---|---|
| 1/A | 4 | stop codon | excema | Y, CR | 9/23 | Y |
| 2/A | 5 | inversion int22h | excema, ashma | Y, CR | 6/10+ | Y |
| 3/A | 4 | amino acid substitution | Y, PR | 1/6+ | Y | |
| 4/A | 11 | deletion exon14 | Y, CR | 3/5+ | Y | |
| 5/B | 16 | deletion | N | N |
Figure
Disclosures: Honoraria received from Roche.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal