Abstract
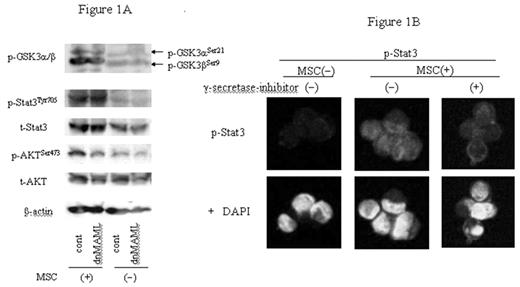
We have previously demonstrated that the bone marrow (BM) microenvironment plays a crucial role in the pathogenesis of acute myeloid leukemia (AML) by influencing tumor growth, survival, and drug resistance. Integrin-linked kinase (ILK) directly interacts with β integrins and phosphorylates AKT in a PI3-kinase(PI3K)-dependent manner. HES1 encodes a basic helix-loop-helix transcription factor downstream of the Notch receptor, and functions as a positive regulator of hematopoietic stem cell self-renewal. In this study, we investigated the functional role of Notch/HES1 signaling in leukemic cell survival stimulated by BM stromal interactions. Direct co-culture of human mesenchymal stem cell (MSC) and leukemic NB4 (AML) or REH (preB-ALL) cells activated ILK kinase activity and enhanced phosphorylation of AKT and GSK3β along with increased Notch1 and HES1 expression. Both, ILK inhibitor QLT0267 or PI3K inhibitor LY294002 inhibited MSC induced p-AKT, p-GSK3β and HES1 expression, while GSK3 inhibitor BIO induced HES1 expression, suggesting that activation of Notch signaling in stromal co-cultures is at least in part mediated via ILK/GSK3β pathway. Because the co-factor Mastermind-like (MAML) is required to transcribe downstream target genes of Notch pathway, we introduced a dominant-negative form of MAML to prevent Notch signaling. dnMAML blocked both basal and MSC-induced expression of cleaved intracellular Notch1 and HES1 and unexpectedly prevented MSC-induced phosphorylation of AktSer473, but not of GSK3βSer9, as documented by Western blot and confocal microscopy analyses (Figure 1A). Co-culture with MSC induced Stat3Tyr705 phosphorylation in NB4 cells, and this effect was abrogated by γ-secretase inhibitor (Fig. 1B). In contrast, Stat3 was still activated in leukemic cells harboring dnMAML (Fig. 1A), suggesting that Stat3Tyr705 phosphorylation is mediated via non-transcriptional effects of Notch or non-Notch effects of γ-secretase inhibition. Co-culture with MSC or with HS27A cells expressing Notch ligand Jagged1 stimulated REH cells proliferation under serum-limited conditions, which was partially blocked by γ-secretase inhibitor and completely abrogated in dnMAML REH cells. Interestingly, dnMAML NB4 cells acquired cell growth ability upon serum limitation.
Altogether, these results suggest that interaction of leukemic and bone marrow stromal cells results in activation of PI3K/ILK/GSK3β signaling, the latter in turn activating Notch pathway. Notch activation enhances cell-type dependent leukemia cell survival upon interaction with BM-derived stromal cells. These effects of Notch signaling are at least in part mediated by a feedback activation of AKT pathway in a transcription-dependent manner, and via activation of Stat3 signaling independent of MAML. Our data show Notch-mediated regulation of AKT/Stat3 pathways and provide a novel role for activated Notch signaling in the context of bone marrow microenvironment.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal